Note: Descriptions are shown in the official language in which they were submitted.
2Q~126~
1 Field of the Invention
2 The present invention relates to a urea-formaldehyde
3 reaction product for use as a sustained nitrogen-release source
4 and to a process for the preparation thereof.
BACKGROUND OF THE INVENTION
6 Ammonia-based fertilizers have long been utilized as
7 means for augmenting the nitrogen availability within the
nitrogen cycle. Deleteriously, however, bacterial oxidation of
9 the ammonia therein converts it initially to nitrite and then to
nitrate before it can be utilized by the plants. As is well
11 known in the art, nitrites are toxic. The nitrates are usually
12 lost as a nitrogen source by leaching out and ultimate reduction
13 to nitrogen.
14 In order to eliminate such nitrification processes,
urea has long been used as a slow, or sustained, nitrogen-
16 release source. However, the enzyme urease is present in the
17 soil. Urease is functional to convert urea to ammonia extremely
18 rapidly.
19 Similarly, urea has been utilized as an ammonium source
for ruminants. But, urease is also present in the rumen of such
21 animals. Rapid release of ammonia within the rumen will cause
22 the animal undue distress, and therefore is not desirable.
23 In order to circumvent the problems associated with
24 urea the prior art processes have provided various condensation
products of the reaction between urea and formaldehyde
26 (hereinafter referred to as the urea-formaldehyde reaction).
27 The reaction of urea with formaldehyde and the
28 resultant products are well documented.
1 The reaction products will vary depending upon the
2 reaction conditions. Under basic conditions the yield of
3 methylol compounds predominates. Under acidic conditions,
4 methylene-bonded compounds are produced.
U.S. Patent 3,759,687, issued to A. Nobell, and U.S.
6 2,644,806, issued to M. A. Kise, exemplify the prior art
7 teachings on the urea-formaldehyde reaction undertaken in aqueous
8 solution and the properties of the condensation products thereof.
9 It will be readily appreciated that such condensation
products comprise urea-formaldehyde polymers having high
11 molecular weights. The control of the degree of polymerization
12 however, is difficult using these prior art processes.
13 Typically, the polymer chains may comprise from 4 to 7 urea
14 units. It is undesirable to have such high molecular weight
polymers because bacteria have difficulty in breaking down
16 polymer chains which contain more than 4 or 5 urea units therein.
17 Additionally, such compositions exhibit solubility only in hot
18 water. Therefore, when these prior art compositions are utilized
19 as, for example, fertilizers, in order to be effective they must
be ploughed into the ground.
21 There exists, therefore the need for a sustained
22 nitrogen-release urea-formaldehyde product possessing the
23 following characteristics:
24 - low molecular weight polymer chains preferably
containing no more than four urea units; and
26 - cold water solubility whereby the need for
27 ploughing the product in would be eliminated as
28 the product would go into solution readily in rain
29 water or the like;
2~1 2~
1 and for a process characterized by the following:
2 - simplicity;
3 - inexpensiveness; and
4 - imparting a degree of controllability to the
polymerization process.
6 DESCRIPTIO~ QF__THE DRAWINGS
7 Figure 1 is a plot of the concentration of ammonia in
8 the rumen of sheep verses time which is included to demonstrate
9 the slow release properties of the products of the present
invention.
11 SUMMARY QF THE INVENTION
12 In accordance with the present invention it has been
13 discovered that if the reaction between urea and formaldehyde is
14 conducted under alkaline conditions (preferably utilizing either
NaOH or KOH) in an alcohol (specifically ethanol or methanol) it
16 is possible, within limits, to control the degree of
17 polymerization, and thereby provide low molecular weight reaction
18 products which are substantially cold water soluble. By cold
19 water æoluble is meant soluble at 20C.
Advantageously, by utilizing either of said alcohols
21 as the reaction media it is possible to recover substantially all
22 the alcohol for recycling. Additionally, the reaction is
23 conducted at a lower temperature than in the aqueous reactions,
24 with concomitant reduction in heating costs.
Broadly stated, the invention is a process for
26 preparing a urea-formaldehyde condensation reaction product for
27 use as a sustained nitrogen-release source, comprising the steps
2~ 21~
l of: (a) reacting urea and at least one of gaseous formaldehyde
2 and solid paraformaldehyde in an alkaline non-aqueous solution
3 of methanol or ethanol with heat, the molar ratio of urea to
4 formaldehyde or paraformaldehyde being in the range of between
about 2.1:1 - 3:1; (b) distilling off the methanol or ethanol
6 solvent to thereby yield a low melting point mixture in molten
7 form; (c) adding an acid or acid-producing substance to the melt
8 and heating the mixture to a temperature in the range of about
9 110C until solidification thereof takes place; and (d) further
heating the product of step (c) to remove the water of reaction
11 to thereby yield a urea-formaldehyde reaction product comprising
12 urea, diureaformaldehyde, triureaformaldehyde and cold water
13 insoluble urea formaldehyde resin.
14 DESCRIPTION OF THE PREFERRED EMBODIMENT
The invention involves a three-stage process. The
16 process is defined by the following reactions:
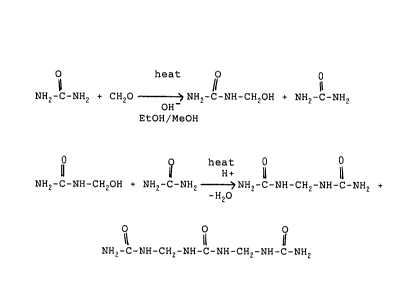
17 O heat O 0
11 11
18 NH2-C-NH2 + CH2O OH? NH2-C-NH-CH2OH + NH2-C-NH2 (1)
EtOH/MeOH
21 Q heat 0
22 0 U H+ U a
23 NH2-C-NH-CH2OH + NH2-C-NH2 ~NH2-C-NH-CH2-NH-C-NH2 +
24 -H2O
O O O
26 NH2-C-NH-CH2-NH-C-NH-CH2-NH-C-NH2 (2)
27 In the first stage of the process urea and gaseous
28 formaldehyde or paraformaldehyde are reacted in a non-aqueous,
2 ~ ~
1 alkaline, alcoholic solution to form methylol urea and urea as
2 outlined in reaction 1 supra.
3 The reactants are of commercial grade. The ratio of
4 urea to formaldehyde or paraformaldehyde is critical to ensure
formation of the desired reaction products. The ratio of urea
6 to formaldehyde (or paraformaldehyde) should be in the range of
7 between 2.1:1 to about 3:1 because the product would be insoluble
8 at less than 2 to 1.
9 The alcohol utilized in the process must be ethanol or
methanol. The concentration of alcohol would be 4 mls of alcohol
11 to each gm of urea.
12 The base is utilized as a reaction catalyst. The base
13 can be selected from either sodium hydroxide or potassium
14 hydroxide. The concentration of the hydroxide should be in the
range of about 0.5% or sufficient to adjust the alcohol solution
16 pH to between about 10 - 12 and maintain the alkalinity of the
17 solution until the last stage of the process. In the absence of
18 the base the reaction produces, undesirably, large amounts of hot
19 water insoluble adducts.
The reaction is conducted at the temperature of the
21 boiling point of the particular alcohol solvent.
22 The reaction of the first stage is carried out as
23 follows.
24 The urea is first added to the alcohol solution made
alkaline by addition of the hydroxide thereto. The formaldehyde
26 or paraformaldehyde is added gradually with heating until
27 reaching the boiling point of the alcohol. The amount of alcohol
28 utilized is sufficient to permit the solvation of the urea at
29 this temperature. The urea gradually dissolves as the methylol
- 2~:1. 2~
1 groups are formed. Upon addition of all the para or formaldehyde
2 at the solvent boiling point all the urea should be in solution.
3 The first stage reaction yields approximately 50
~ percent methylol urea and slightly more than 50 percent urea.
The second stage of the process comprises distilling
6 off the alcohol, leaving a low melting point solid in the form
7 of a melt. Vacuum may optionally be used to remove the alcohol.
8 The temperature is controlled to leave the solid as a melt. It
9 will be noted that by recovering the alcohol being evaporated
off, it may be recycled.
11 The third staye of the process involves raising the
12 melt to an elevated temperature and adding an acid or acid-
13 producing substance thereto, to form the diureaformaldehyde and
14 higher molecular weight polymer chain compounds. The formed solid
is heated further to drive off the water of reaction.
16 Specifically, the melt from the second stage is heated
17 to a temperature of about 110C. This temperature is selected
18 to ensure fluidity of the melt and a rapid reaction with the
19 acid. It will be noted that if the temperature is increased the
content of hot water insoluble urea formaldehyde resin formed
21 will deleteriously increase concomitantly.
22 An acid or acid-releasing substance is added to the
23 melt with rapid stirring. The addition of the former initiates
24 the reaction of the methylol groups with the excess unreacted
urea. The acid may be a mineral acid, for example hydrochloric.
26 Alternatively, an organic acid, for example, glacial acetic acid
27 or an acid-releasing substance such as ammonium chloride may be
28 utilized. The amount of acid should be sufficient to neutralize
29 the base. The amount of acid utilized has been found to
2 ~
1 accelerate the rate of polymerlzation to the final product.
2 However, the reaction will take place, albeit less rapidly
3 without the addition of an acid-releasing substance. For
4 example, with addition 1 gm. of ammonium chloride to 100 gm urea
at 110C , solidification will take place within five minutes.
6 The formed solid is then heated to a temperature of
7 130C to drive off the water of reaction and produce the final
8 product.
9 Example I
100 gm of urea were added to a solution containing 400
11 ml methanol and 1 ml of concentrated potassium hydroxide. 23.8
12 gm of paraformaldehyde were added with heating until the boiling
13 point of methanol, 65C, was reached. Boiling was continued
14 until all of the urea went into solution. The solvent was then
evaporated leaving a melt. The melt was heated to 110C for a
16 period of approximately 2 minutes and 1 gm of ammonium chloride
17 in 3 ml of water was added with rapid stirring. Upon
18 solidification, the solid was heated to 130C to drive off the
19 water of the reaction.
Analysis of the solid showed the nitrogen content was
21 as follows:
22 % N = 42.3
23 Results
24 25 gm of the solid were ground and added to 500 ml
water. The solution was heated to boiling, filtered and the solid
26 dried and weighed. The solution was cooled to room temperature
27 and filtered. The solid product was dried and weighed.
~ ~9 ~
1 The solid product was tested to determine its
2 solubility.
3 % solubility in hot water = 90
4 % solubility in cold water = 79
Example II
6 100 gm of urea were added to a solution containing 400
7 ml ethanol and 1 ml of concentrated potassium hydroxide. 22.2
8 gm of paraformaldehyde were added slowly with heating until the
9 boiling point of ethanol (78.3C) was achieved. The solution was
heated until all the urea went into solution. The solvent was
11 evaporated, leaving a melt. The melt was heated to 110C for
12 about 2 minutes and 1 ml of glacial acetic acid was added with
13 rapid stirring. Upon solidification, the product solid was
14 heated to 130C to drive off the water of the reaction.
Analysis of the solid showed the nitrogen content was
16 as follows:
17 % N = 42.6
18 Results
19 25 gm of the solid were ground and added to 500ml
water. The solution was heated to boiling and filtered. The
21 solid was dried and weighed. The solution was cooled to room
22 temperature and filtered. The solid was dried and weighed.
23 The solid product was tested to determine its
24 solubility:
% solubility in hot water = 92
26 % solubility in cold water = 82
1 Example III
2 The following table presented herebelow provides
3 characterization data of the urea-formaldehyde reactlon products
4 produced in accordance with the process of the invention. An
analysis of a commercial product prepared by a area-formaldehyde
6 reaction process which was carried out using aqueous conditions
7 is provided for comparison purposes. As well, theoretical values
8 calculated from statistical distribution at the 2.1:1 urea to
9 formaldehyde ratio are indicated.
All constituent values are given as percent of total
11 nitrogen.
12 ~atio of Reactants Urea MDU DMTU
13 Theoretical 28 25 19 28
14 Commercial 27 25 14.6 29.5
Product
16 2.1:1 UF
17 Invention 26.7 39.7 12.2 21.4
18 Product
19 Commercial 34 25 10.6 24.5
Product
21 2.25:1 UF
22 Invention 32.4 34.7 11.5 21.4
23 Product
24 where UF is parts of urea to formaldehyde
MDU is diureaformaldehyde
26 DMTU is triureaformaldehyde
27 CWIN is cold water insoluble urea-formaldehyde
28 resin