Note: Descriptions are shown in the official language in which they were submitted.
204129~
2-115,279 comb.
PIGMENT DISPERSING AGENT
This invention relates to a pigment dispersing
agent used when a pigment is mixed with a coating
composition in the manufacture of paints.
In the manufacture of paints, it is an important
05 factor to mix a pigment with a coating composition.
In this case, the pigment to be usually used in the paint
is dispersed into only a part of a vehicle constituting
the paint together with a proper solvent, diluent or
other additives by means of a mill used in the paint
lo industry to form a dispersion paste. Then, the result-
ing paste is mixed with the remaining amount of the
vehicle and another necessary additives to obtain a
pigment dispersing composition as a paint. However, a
greatest problem of such a pigment dispersing composi-
tion lies in that pigment particles are apt to beagglomerated. Such an agglomeration may occur in
dispersing step, dissolving step, storing step or
painting step. As a result, it is known to cause un-
favorable phenomena such as decrease of paint stability,
occurrence of troubles in the painting, reductions of
color strength, gloss and distinctness of image in the
finally obtained paint film, flooding, floating, color
change with time and the like. Such a phenomenon that
2041292
the pigment particles are apt to be agglomerated is
explained by London-van der Waals force attracting the
particles each other. In order to overcome such an
attracting force, it is necessary to provide an
adsorption layer onto the surf~ce of the particle, and
consequently many pigment dispersing agents and grinding
aids are proposed and various methods of improving
pigment dispersion are attempted. For instance, there
are known (1) a method of dispersing the pigment with a
non-ionic, anionic or cationic surfactant, or a wetting
agent such as aliphatic polyvalent carboxylic acid or
the like as an assistant; (2) a method of dispersing the
pigment with an ampholytic substance obtained by
reacting a substance having an affinity with a pigment
1~ with a substance having a solvent affinity as disclosed
in British Patent No. 1,108,261, No. 1,159,252 and No.
1,346,298; (3) a method of decreasing surface tension
with a surfactant such as alkyl silicone to prevent the
floating; (4) a method of mixing the pigment with a
certain substituted derivative of the pigment to conduct
pigment dispersion as disclosed in Japanese Patent laid
open No. 51-18736; (5) a method of using as a dispersing
agent a compound obtained by reacting an organic
compound having two or more isocyanate groups, an
organic compound having one or.more functional group to
be reacted with isocyanate group (amino group, hydroxyl
2041292
group) and a polymer having substantially one functional
group to be reacted with isocyanate group as disclosed
in US Patent No. 3,684,711 and British Patent No.
1,393,401; (6) a method of dispersing the pigment with a
high molecular weight dispersing agent obtained by
reacting a basic substance with a polyester compound as
disclosed in EP-A-0208041, Japanese Patent laid open
No. 60-166318 and No. 61-174939, and US Patent No.
4,933,417; (7) a method of dispersing the pigment with a
high molecular weight dispersing agent obtained by
reacting a basic substance with an acrylic polymer as
disclosed in Japanese Patent laid open No. 46-7294; (8)
a method of dispersing the pigment with a high molecular
weight dispersing agent obtainQd by reacting a basic
l~ substance with an acrylic polymer and a polyester
compound as disclosed in EP-A-0358358; and the like.
In the grinding aids or dispersing agents used
in the above methods (1)-(3), however, the adsorption
layer adsorbed on the particle surface is thin and does
not develop a satisfactory stabilizing effect and hence
the pigment dispersing performances are not improved.
The pigment derivative used in the method (4) is
naturally colored, so that it can not generally be used
for various pigments. The fundamental thought of the
W pigment dispersing agent used in the methods (5)-(8) is
a technical idea of making a block structure comprised
2~41292
of a polymer portion capable of solvating with a solvent
and an anchor portion adsorbed on the pigment as
described by A. Topham in Progress in Organic Coatings,
vol. 5, (1977) pp 237-243. In case of utilizing such a
technique, it is important that the polymer portion
capable of solvating with the solvent is excellent in
the compatibility with a resin used as a film forming
component of the paint film and that the number of
adsorption points to the pigment is increased and the
adsorption state of the dispersing agent to the pigment
is rendered into a tail-like state, whereby an effective
steric repulsion layer is formed in the polymer portion
of the dispersing agent adsorbed on the pigment to
stabilize the dispersed particles, and the like. If the
16 polymer portion solvating with the solvent is poor in
the compatibility with the resin added as the film
forming component of the paint film, the solvated
polymer portion agglomerates to cause the degradation of
pigment dispersing performances. Furthermore, when the
number of adsorption points to the pigment is small, the
resorption of the dispersing agent from the pigment is
easily caused to degrade the pigment dispersing
performances, while if the ads~rption state of the
dispersing agent to the pigment is loop, the formation
of the steric repulsion layer in the solvated polymer
portion is insufficient and the degradation of pigment
2041292
dispersing performances is caused. In the method (5),
the polyester compound made from dialcohol and
dicarboxylic acid starting from monoalcohol is used,
which includes many compounds such as polyester compound
having substantially one hydroxyl group in its terminal,
polyester compound having hydroxyl groups in both
terminals, polyester compound containing no hydroxyl
group. When the dispersing agent is made by using such
a polyester compound, the adsorption state to the pigment
l~ is tail or loop and hence the satisfactory pigment
dispersing performances are not obtained. In the
pigment dispersing agent obtai~ed by the method (6),
when the dispersing agent is good in the compatibility
with the resin added as a film forming component of the
paint film, the considerable improvement of the pigment
dispersing performances is observed, but when it is poor
in the compatibility with the resin, the protection
layer of the dispersing agent adsorbed on the pigment
agglomerates to undesirably degrade the pigment
dispersing performances. Particularly, since a
dispersing agent consists mainly of the polyester
compound, it is poor in the compatibility with an
acrylic resin or the like being important as a painting
resin, so that it is difficult to obtain good pigment
dispersing performances in case of acrylic resin series
paints. In the dispersing agent obtained by the method
2041292
(7), since an acrylic resin is.used in the polymer
portion, it acts as a dispersing agent for usual acrylic
resin series paints, but it is difficult to provide good
pigment dispersing performances in case of polyester
series paints because the compatibility with the
polyester resin or the like is poor. In the dispersing
agent obtained by the method (8), almost of the
aforementioned problems are solved, but the yellowing is
undesirably caused at the curing in case of
m thermosetting resin type paints.
Under the above circumstances, the inventors
have made various studies with respect to a method of
providing adsorption points onto a pigment and
effectively incorporating a polymer component into a
16 pigment dispersing agent so as to have excellent
compatibility with acrylic resin and polyester resin
frequently used in the art and pigment dispersing
performances and also the development of pigment
dispersing agent causing no yellowing even when being
applied to thermosetting type paints, and as a result
the invention has been accompl-shed.
That is, the invention provides the following
first, second and third pigment dispersing agents.
The first pigment dispersing agent according to
the invention is represented by the following general
formula (I) or (II):
2041292
-7-
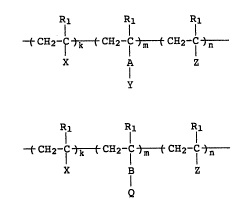
Rl Rl Rl
~CH2-1C )k ( CH2-lc )m ( CH2-f )n ( I )
X A Z
71 71 71
~CH2-f )k ( CH2-lc )m ( CH2-f )n ( II )
X Z
[wherein Rl is a hydrogen atom or a methyl group, X is a
tertiary amino group and/or a nitrogen-containing
heterocyclic ring group with a basicity selected from
R3 R3 R3
-C-O-R2-N/ -C-N-R2-N/ -O-R2-N/
O R3 , O H R3 , R3
(wherein R2 is an alkylene group having a carbon number
of 1-8, and R3 is an alkyl group having a carbon number
of 1-4), an imidazole group, a pyridine group, a
carbazole group, a quinoline group and an N-alkyl
piperidine group, each of A and B is an organic bonding
group, each of Y and Q is a polyester compound residue
and/or a polyester polyether compound residue, Z is a
hydrogen atom, an alkyl group having a carbon number of
1-4, a halogen atom, a phenyl group, a nitrile group,
R5
--C--O--R4, --C--R4, --O--C--R4, --O--R4, --C--N/
~ ~ O o R5
21~41292
-8-
-C-N-R2-OH , -C-OH , -C-O-R2-CH-OH ,
Il 1 11 11 1
O H O O Rl
-C-o-R2-7H-o-tc-R2-o)o~4 H or -C-O-R2-0-R3
O Rl O O
(wherein R4 is an alkyl group having a carbon number of
1-18, R5 is a hydrogen atom, or an alkyl group or an
alkoxy group having a carbon number of 1-4), k is an
integer of 1-200, m is an integer of 1-100 and n is an
integer of 0-200], and has a number average molecular
weight of 1,000-100,000 and an amine value of
10-200 mgKOH/g.
In the first pigment dispersing agent, as to the
range of k in the formulae (I) and (II), when k exceeds
200, the adsorption group to the pigment is too long and
may act as an agglomerating agent and hence the
degradation of the pigment dispersing performances may
undesirably be caused.
Then, A in the formula (I) is an organic bonding
group represented by the following general formula (1),
(2) or (3):
1 1
V-R2-CH-O-~C-R2-O)o~4 C-N-W-N-C-O-y (1)
O O H H O
-C-O-R2-O-C-CH C-N-W-N-C-O-Y
Il ll I ll I I ll (2)
~ o c=o o H H o
CH3
2041292
g
-CH2-O-R2-lCH-CH2-O-C-R6-C-Y
OH o O
(wherein V is
-C-O , -C-N- or -O- ,
Il 11 1
O O H
W is a residue of diisocyanate compound and R6 is a
residue of acid anhydride having a carbon number of 2-6),
and B in the formula (II) is an organic bonding group
represented by the following general formula (4) or (5)
V-R2-CIH-Otc-R2-o )0~4 Q (4)
Rl O
-C-O-CH2-CH-O-Q
O R7
[wherein R7 is a phenyl group, or an alkyl group or an
aromatic group having a carbon number of 3-19,
-CH2 -O-C -R8
o
(wherein R8 is an alkyl group or an aromatic group having
a carbon number of 3-18) or -CH2-O-Rg (wherein Rg is an
alkyl group or an aromatic group having a carbon number
of 1-18)].
In the formula (I), Y is a residue of a
polyester polyether compound obtained by ring-opening
copolymerization of a lactone compound and a monoepoxy
compound using monovalent alcohol and/or monocarboxylic
2041292
- 10-
acid as an initiator, and having a number average
molecular weight of 500-20,000 and/or a residue of a
polyester compound obtained by ring-opening copolymer-
ization of a lactone compound, a monoepoxy compound and
an acid anhydride using monovalent alcohol and/or
monocarboxylic acid as an initiator, and having a number
average molecular weight of 500-20,000, and may have
block structure represented by the following formulae
(6)-(10) as well as a structure of polymer residue
containing randomly bonded various components:
Rlo-C-O ~ CH2-CH-O-C-R6-C-0) CH2-CH-O-
11 1 11 11 P
O R7 0 0 R7
tC-R2 ~~ ~ (6)
o
Rll-o~c-R6-c-o-cH2-7H-o )r ( C-R2-~~ (7)
O O R7 0
Rll-~~C-R2-0 )s ( C-R6-c-o-cH2-cH-o~ (8)
O O O R7
Rll-O~C-R2-0 ~ (CH2-fH-O ~ (g
O R7
Rll-O~CH2- ICH-O~ ( C-R2-0 )t (10)
R7 0
(wherein Rlo is an alkyl group or an aromatic group
having a carbon number of 1-20 and Rll is a saturated
. .
20~1292
aliphatic, alicyclic or aromatic group having a carbon
number of 1-18).
In the formula (II), Q is a residue of a
polyester polyether compound obtained by ring-opening
copolymerization of a lactone compound and a monoepoxy
compound and having a number average molecular weight of
500-20,000 and/or a residue of a polyester compound
obtained by ring-opening copolymerization of a lactone
compound, a monoepoxy compound and an acid anhydride and
having a number average molecular weight of 500-20,000,
and may have block structure represented by the following
formulae (11)-(14) as well as a structure of polymer
residue containing randomly bonded various components:
H ~ O - R2 -C ) s ( O -CH - CH2 ~ O - C - R6 ~ C ) r ( 11 )
O R7 0 0
H~o-cH-cH2 -0-C-R6-c~o-R2 -C~ (12)
R7 0 0 ~
H~O - R2 -C ) t ( O -CIH - CH2 )u (13)
O R7
H~o-lcH-cH2 )u ( O-R2-1CI )t (14)
R7 0
When the number average molecular weight in the
compound having the above Y group (hereinafter referred
~041292
-12-
to as component Y) and the compound having the above Q
group (hereinafter referred to as component Q) is less
than 500, the sufficient formation of the steric
repulsion layer is difficult and the stable pigment
dispersing performances are degraded. On the other
hand, when the number average molecular weight exceeds
20,000, it is difficult to produce these components with
a good reproducibility and hence the components are not
practical as an industrial material.
As to the ranges of m and n in the formulae (I)
and (II), when m exceeds 100 and n exceeds 200, respec-
tively, the molecular weight becomes too higher and the
pigment dispersing performances undesirably lower.
The second pigment dis~ersing agent according to
1~ the invention is a product obtained by reacting an
acrylic polymer having a tertiary amino group
concentration of 0.9-18 mol/kg, an active hydrogen
concentration of 0.18-9.0 mol/kg and/or an active
methylene group concentration of 0.18-9.0 mol/kg and a
number average molecular weight of 800-30,000 (herein-
after referred to as component S) with a polymer compound
having one isocyanate g~oup in its terminal and a number
average molecular weight of 500-20,000 obtained through a
reaction of a ring-opened copolymer between lactone
compound having a carbon number of 3-17 and monoepoxy
compound using monovalent alcohol as an initiator, a
2~41292
-13-
ring-opened copolymer among lactone compound, monoepoxy
compound and acid anhydride using monovalent alcohol
and/or monocarboxylic acid as an initiator and a
diisocyanate compound (hereinafter referred to as
component T) at a mol ratio of component S to component
T of l:l-1:40, and has a number average molecular weight
of l,000-100,000, a sum of hydroxyl value and active
methylene value of 5-200 mgKOH/g and an amine value of
10-200 mgKOH/g.
l~ In the component S, when the tertiary amino
group concentration is less 0.9 mol/kg, the adsorption
force to the pigment is weak and the dispersing perform-
ance is degraded. While, when it exceeds 18 mol/kg, the
water resistance and weather resistance of the resulting
16 paint film are damaged. When the number average
molecular weight is less than 800, the adsorption force
is lacking and the dispersing performance is degraded.
While, when it exceeds 30,000, the adsorption group is
too long and may act as an agglomerating agent and hence
the pigment dispersing performances are degraded.
The active hydrogen group and/or active methylene group
in the component S forms a crosslinking point in the
paint film and provides a bonding point to the component
T, so that when the concentration of each of the active
hydrogen group and active methylene group is less than
0.18 mol/kg, the steric stability is lacking and the
2041292
-14-
dispersing stability lowers, while when it exceeds 9.0
mol/kg, the pigment dispersing agent is apt to be
desorbed from the surface of the pigment and the
dispersing performance undesirably lowers.
As mentioned above, the second pigment
dispersing agent is constituted with the components S
and T and obtained by reacting these components S and T
at a mol ratio of 1:1-1:40. However, when the mol ratio
of S/T is less than 1:1, the steric repulsion layer is
not sufficiently formed and the pigment dispersing
performances are degraded, while when it exceeds 1:40,
the desorption from the pigment surface is apt to be
caused, and the dispersion performance lowers.
The second pigment dispersing agent consists of
the component S and the component T bonded by reacting
the active hydrogen and/or active methylene group in the
component S with the isocyanate group in the component T.
The bonded state is illustrated below when the reaction
group of the component S is hydroxyl group:
¦Component S ~ OH+OCN 1 Component T ¦
¦Component S ~ O-C-IN 1 Component T ¦
O H
Furthermore, it is illustrated below when the
reaction group of the component S is active methylene
group:
20~1292
-15-
O O
¦Component S ~ C-CH2-C-CH3+OCN 1 Component T ¦
ICH3
C=O
¦ Component S ~ C-CH-ICl-lN 1 Component T ¦
O O H
Since the second pigment dispersing agent is a
reaction product between the component S and the
component T as mentioned above, it is desirable that the
sum of hydroxyl value and active methylene value in the
pigment dispersing agent itself is within a range of
5-200 mgKOH/g. When the sum of hydroxyl value and active
methylene value is less than 5 mgKOH/g, the amount of
the pigment dispersing agent to be crosslinked with a
crosslinking agent in the film forming component of the
paint film is lacking, so that it is not fixed in the
paint film through the crosslinking reaction but may
serve as a plasticizer to injury the performances of
paint film, particularly humidity resistance, water
resistance and weather resistance and the like. Further-
more, when being used to multi-painting specification,
troubles in the adhesion between the paint films may be
caused. On the other hand, when the sum of hydroxyl
value and active methylene value exceeds 200 mgKOH/g, the
polarity becomes too high and hence the water resistance
and flexibility of the paint film are degraded and the
... . .. ..
2041292
-16-
pigment dispersing performances are lowered.
The third pigment dispersing agent according to
the invention is a copolymer having a number average
molecular weight of l,000-100,000 and an amine value of
10-200 mgKOH/g, which is obtained by copolymerizing (a)
40-97% by weight of a macromer having vinyl group or
isopropenyl group in its terminal and a number average
molecular weight of 500-20,000 obtained by blocking a
half of (i) a polymer compound having one hydroxyl group
in its terminal with (ii) a diisocyanate compound and
then blocking the remaining half of the polymer compound
with (iii) a hydroxyl group containing polymerizable
monomer and/or an active methylene group containing
polymerizable monomer with (b) 3-60% by weight of a
tertiary amino group containing copolymerizable monomer
and/or a copolymerizable monomer having nitrogen-
containing heterocyclic ring with a basicity and (c) 0-
47% by weight of copolymerizable monomer other than the
above components (a) and (b), in which the above polymer
compound (i) having one hydroxyl group in its terminal
is a polymer compound represented by the following
general formula (III), (IV) or (V):
Rlo-C-O~CH2-CH-O-C-R6-C-0 )p CH2-CH-O-
O R7 O O R7
t Cl - R2 ~ ~ ( m )
.. ... ......
20~1292
- 17-
Rll-O~C-R6-C-O-CH2-7H-O )r ( C-R2-Ot~H (IV)
O O R7 0
Rll-O~C-R2-O )t ( CH2-CH-O k~H (V)
O R7
(wherein Rlo is an alkyl group or an aromatic group
having a carbon number of 1-20, Rll is a saturated
aliphatic, alicyclic or aromatic group having a carbon
number of 1-18, p and q are l_p+q'200, r and s are
l_r+s_200, and t and u are l't+u-'200).
When the number average molecular weight of the
component (a) is less than 500, the sufficient formation
of the steric repulsion layer is difficult and the
pigment dispersing performances lower. While, when the
number average molecular weight exceeds 20,000, the
component (a) is difficult to be produced with a good
reproducibility and is not practical as an industrial
material. Furthermore, when the amount of the component
(a) exceeds 97% by weight, it is considered that the
adsorption force to the pigment surface is lower than an
affinity of the polymer component (a) with the resin
added as a film forming component of the paint film, so
that the desorption of the pigment dispersing agent from
the pigment surface is caused and hence the pigment
dispersing performances undesirably lower. While, when
it is less than 40% by weight, the polymer component (a)
2041292
-18-
is lacking for the sufficient formation of the steric
repulsion layer and hence the pigment dispersing
performances undesirably lower.
As the component (b), the tertiary amino group
containing copolymerizable monomer and/or the copolymer-
izable monomer having a nitrogen-containing heterocyclic
ring with a basicity are necessary to be compounded in
an amount of 3-60% by weight.
When the amount of the component (b) is less
than 3% by weight, the adsorption force to the pigment
is weak and the pigment dispersing performances
considerably lower, while when it exceeds 60% by weight,
the properties of paint film such as water resistance,
weather resistance, adhesion property and the like are
16 undesirably degraded.
Furthermore, the copolymerizable monomer other
than the above components (a) and (b) may be compounded
in an amount of 0-47% by weight as the component (c).
When the amount of the component (c) exceeds 47
by weight, the ratio of the macromer as the component
(a) in the pigment dispersing agent becomes lower and
hence the steric repulsion layer is not sufficiently
formed and the degradation of the pigment dispersing
performances is undesirably caused.
When the number average molecular weight in each
of the first, second and third pigment dispersing agents
20~1292
- 19-
according to the invention is ~ess than l,000, the
polymer component of the pigment dispersing agent
adsorbed on the pigment surface can not develop the
satisfactory effect as the steric repulsion layer and
creates a phenomenon such as reagglomeration of
dispersed particles or caking thereof to undesirably
cause the remarkable degradation of the pigment dispers-
ing performances. If it is intended to render the
number average molecular weight into more than l00,000,
it is difficult to produce the pigment dispersing agent
with a good reproducibility and the pigment dispersing
agent may inversely act as an agglomerating agent.
Therefore, the number average molecular weight
is required to be within a range of l,000-l00,000 in the
16 pigment dispersing agent according to the invention.
Furthermore, when the amine value in the pigment
dispersing agent according to the invention is less than
10 mgKOH/g, the adsorption force onto the pigment surface
is poor but the affinity with the resin added as a film
forming component is good, so that the desorption of the
pigment dispersing agent from the pigment surface is
caused to undesirably degrade the pigment dispersing
stability, while when it exceeds 200 mgKOH/g, the ratio
of the steric repulsion layer to the portion of the
pigment dispersing agent adsorbed on the pigment surface
becomes too small and hence the sufficient pigment
2041292
-20-
dispersing stability is not obtained and the properties
of paint film such as water resistance, weather
resistance, adhesion property and the like are
undesirably degraded.
Therefore, the amine value in the pigment
dispersing agent according to the invention is required
to be within a range of 10-200 mgKOH/g.
Moreover, the term "active hydrogen" used herein
means a hydrogen atom directly bonded to oxygen, sulfur,
nitrogen or the like and havin~ a reactivity larger than
that of hydrogen directly bonded to carbon, and the term
"active methylene" means a methylene group in which
alfa-hydrogen atom is adjacent to two or more carbonyl
groups and/or nitrile groups and has a large reactivity.
16 The active methylene value is calculated from an
amount of KOH required for the neutralization in a mixed
solvent of dioxane and isopropyl alcohol with an
alcoholate of KOH as a titrating reagent through
potential difference titration, and the amine value is
calculated from an amount of p-toluene sulfonic acid
required for the neutralization in a solvent of acetic
acid with a solution of p-toluene sulfonic acid in
acetic acid through anhydrous ~otential difference
titration.
Furthermore, the number average molecular weight
in the components Y, Q, S, T and (a) is measured by gel
,
20412~2
-21-
permeation chromatography using tetrahydrofuran as a
developing solvent, and the number average molecular
weight of the pigment dispersing agent is measured by
gel permeation chromatography using dimethyl formamide
as a developing solvent.
In the pigment dispersing agent according to the
invention, the tertiary amino group and/or nitrogen-
containing heterocyclic ring group with the basicity
included in each of the component X according to the
first invention and the components S and (b) according to
the second invention has an excellent adsorbing effect
to the pigment surface, and the polyester compound
residue and/or polyester polyether compound residue in
the components Y and Q according to the first invention
16 and the components T and (c) according to the second
invention form a sufficient steric repulsion layer on
the pigment surface in an organic solvent, whereby the
reagglomeration of pigment particles is prevented and
the improved dispersing stability can be provided to the
pigment dispersing agent composition. Furthermore, the
pigment dispersing agent according to the invention has
polyester structure and acryl structure in its molecule,
so that it exhibits an excellent compatibility with both
polyester resin and acrylic resin and can be used to both
acrylic resin series and polyester resin series paints
without causing reagglomeration of pigment particles.
2041292
-22-
Moreover, in the pigment dispersing agent
according to the invention, the tertiary amino group
and/or the nitrogen-containing heterocyclic ring with
the basicity is an adsorption point to the pigment
surface, so that even when it is used in the
thermosetting paint, there is caused no yellowing of
paint film during the baking and curing.
The production of the pigment dispersing agent
according to the invention will be described in detail
below.
In the first pigment dispersing agent, there are
the following two methods for the pigment dispersing
agent of the formula (I).
In the first method, at least one monocarboxylic
or aromatic monocarboxylic acid represented by Rlo-COOH
(Rlo is an alkyl group having a carbon number of 1-20) is
first used as an initiator, and then at least one mono-
epoxy compound represented by the following formula (15):
R7-CH-CH2
O (15)
(R7 is a phenyl group, or an alkyl group or an aromatic
group having a carbon number of 3-19), at least one acid
anhydride represented by the following formula (16):
~R6~
O=C \ C=O (16)
0/
20~1292
-23-
(R6 is a residue of acid anhydride having a carbon
number of 2-6) and at least one lactone represented by
the following formula (17):
o
R2-C (17)
(R2 is an alkylene group having a carbon number of 1-8)
are subjected to a ring-opening addition reaction at a
temperature of 100~C-200~C, preferably 120~C-160~C to form
a polyester compound having one hydroxyl group in its
terminal.
As the monocarboxylic or aromatic monocarboxylic
acid, use may be made of acetic acid, propionic acid,
caprilic acid, nonanoic acid, capric acid, lauric acid,
myristic acid, palmitic acid, stearic acid, isononanoic
acid, 2-ethyl hexanoic acid, arachic acid, benzoic acid,
p-tert-butyl benzoic acid and the like. As the monoepoxy
compound of the formula (15), use may be made of styrene
oxide having phenyl group; phenyl glycidyl ether, p-
tolyl glycidyl ether, n-butyl glycidyl ether and
dibromocresyl glycidyl ether, each containing an alkyl
group or aromatic group with a carbon number of 4-19;
glycidyl ester of lauric acid, glycidyl ester of stearic
acid, glycidyl ester of versatic acid and glycidyl ester
of p-tert-butyl benzoic acid, each containing an alkyl
group or aromatic group with a carbon number of 3-17 and
2041292
-24-
glycidyl ester group; ~-olefin oxide and the like
containing an alkyl group with a carbon number of 5-10.
As the acid anhydride of the formula (16), use may be
made of phthalic anhydride, succinic anhydride, phthalic
anhydride hexahydride, phthalic anhydride tetrabromide,
phthalic anhydride tetrachloride, 3,6-endomethylene
tetrahydrophthalic anhydride, maleic anhydride,
chlorendic anhydride and the like. As the lactone of
the formula (17), use may be made of ~-caprolactone, ~-
propione lactone, ~-valerolactone and the like.
In this case, there may be used a method wherein
the monoepoxy compound and the acid anhydride are ring-
opening polymerized with the monocarboxylic acid as an
initiator and then subjected to ring-opening addition
1~ reaction with the lactone to form a polymer of block
structure, and a method wherein the monoepoxy compound,
acid anhydride and lactone are randomly reacted with the
monocarboxylic acid as an initiator to form a polymer of
random structure.
A solvent used in the above reaction is not
particularly restricted. As the solvent, mention may be
made of aromatic hydrocarbons such as toluene, xylene,
ethylbenzene, Solveso #100 (registered trade mark, made
by Exxon Chemical Co.), Solveso #150 (registered trade
mark, made by Exxon Chemical Co.), turpentine oil,
tetrline and the like; aliphatic hydrocarbons such as
20~1292
-25-
n-hexane, cyclohexane, methyl cyclohexane, n-heptane, n-
octane, n-decane, mineral spirit, isooctane, nonane,
trimethyl hexane, solvent naphtha, Isoper (registered
trade mark, made by Exxon Chemical Co.), Newsol Delax
(registered trade mark, made by Nippon Oil Co., Ltd.)
and the like; esters such as methyl acetate, ethyl
acetate, n-butyl acetate, isobutyl acetate and the like;
ethers such as ethyl ether, tetrahydrofuran, dioxane,
diglyme and the like; ketones such as acetone, methyl
ethyl ketone, methyl isobutyl ketone, methyl amyl
ketone, cyclohexanone, isophorone and the like;
cellosolves and their esters such as ethylene glycol
monomethyl ether, ethylene glycol monethyl ether,
ethylene glycol monobutyl ether, propylene glycol
1~ monomethyl ether, propylene glycol monoethyl ether,
propylene glycol monobutyl ether and the like;
chlorinated hydrocarbons such as methylene chloride,
trichloroethylene, perchloroethylene,
orthodichlorobenzene and the like; dimethylformamide,
dimethylsulfoxide and so on. These solvents may be used
alone or in admixture.
Moreover, the above reaction may be carried out
in the presence or absence of a catalyst. As the
catalyst, use amy be made of a well-known catalyst
selected from tertiary amines such as triethylamine,
dimethyl benzylamine and the like; quaternary amine
20~1292
-26-
salts such as tetramethyl ammonium chloride and the
like; organic metal salts such as tetrabutoxy titanate,
dibutyl tin dilaurate and the like; phosphorus compounds
such as triphenyl phosphine and the like; organic
carboxylic acid salts such as potassium benzoate, sodium
benzoate and the like; inorganic salts such as potassium
iodide and the like.
Similarly, a polyester compound having a given
molecular weight and one hydroxyl group in its terminal
1~ is obtained by using at least one monovalent alcohol
represented by the formula of Rll-OH (Rll is a saturated
aliphatic, alicyclic or aromatic group having a carbon
number of 1-18) as an initiator and then subjecting at
least one acid anhydride of the formula (16), at least
1~ one monoepoxy compound of the formula (15) and further
at least one lactone of the formula (17) to a ring-
opening addition reaction in the same manner as
described above. As the monovalent alcohol, use may be
made of saturated aliphatic alcohols such as methyl
alcohol, ethyl alcohol, n-propyl alcohol,isopropyl
alcohol, n-butyl alcohol, isobutyl alcohol, t-butyl
alcohol, amyl alcohol, n-hexyl alcohol, n-heptyl
alcohol, lauryl alcohol, stearyl alcohol and the like;
alicyclic alcohols such as cyclopentanol, cyclohexanol
and the like; and aromatic alcohols such as benzyl
alcohol, p-tert-butyl benzyl alcohol and the like.
2041292
-27-
Even in the latter case, there may be used a
method wherein the monoepoxy compound and the acid
anhydride are ring-opening polymerized with the
monovalent alcohol as an initiator and then subjected to
ring-opening addition reaction with the lactone to form
a polymer of block structure, and a method wherein the
monoepoxy compound, acid anhydride and lactone are
randomly reacted with the monovalent alcohol as an
initiator to form a polymer of random structure.
IO Moreover, the same solvent and catalyst as described
above may be used in this reaction.
Furthermore, a polyester polyether compound
having a given molecular weight and one hydroxyl group
in its terminal is obtained by subjecting at least one
Ih lactone of the formula (17) and at least one monoepoxy
compound of the formula (15) to a ring-opening addition
reaction with the above monovalent alcohol as an
initiator.
Even in this case, there may be used a method
wherein the lactone is ring-opening polymerized with the
monovalent alcohol as an initiator and then subjected to
ring-opening addition reaction with the monoepoxy
compound to form a polymer of block structure, a method
wherein the monoepoxy compound is ring-opening
polymerized with the monovalent alcohol as an initiator
and then subjected to ring-opening addition reaction
~ 20~1292
with the lactone to form a polymer of block structure,
and a method wherein the monoepoxy compound and lactone
are randomly reacted with the monovalent alcohol as an
initiator to form a polymer of random structure.
Moreover, the same solvent as described above may be
used in this reaction.
As the ring-opening addition reaction for the
production of the polyester polyether compound, there
are anion polymerization, coordinate anion polymer-
ization cation polymerization and the like as mentionedbelow.
The anion polymerization is carried out at 10-
200~C in the presence of a well-known catalyst selected
from alkali metals such as Li, Na, K and the like; metal
lfi alcolates such as NaOR', LiOR' and the like and tertiary
amines for 1-15 hours.
The coordinate anion polymerization is carried
out at 10-200~C in the presence of a well-known catalyst
such as Al(R)3, Zn(R)2, (R)2Al(OR'), Al(OR')3, Ti(OR')4
or the like for 1-15 hours.
Moreover, R' is a hydrogen atom or an alkyl
group having a carbon number of 1-18 and R is a halogen
atom or an alkyl group having a carbon number of 1-18.
The cation polymerization is carried out at
10-200~C in the presence of a well-known catalyst
selected from metal halides such as AlC13 and the like
2041292
and acids such as p-toluene sulfonic acid, phosphoric
acid and the like for 1-15 hours.
Then, at least one polyester or polyester
polyether compound obtained by anyone of the above
W methods is reacted with at least one diisocyanate
compound of the formula OCN-W-NCO (W is a residue of
diisocyanate compound) at a mol ratio of the polyester
compound to diisocyanate compound of 0.7-1.3, preferably
0.9-1.1 and at 0~C-80~C for 0.5-6 hours to obtain a
polyester and/or polyester polyether compound having one
isocyanate group in its terminal.
As the diisocyanate compound, mention may be
made of tolylene diisocyanate, isophorone diisocyanate,
4,4'-diphenylmethane diisocyanate, hexamethylene
1~ diisocyanate, lysine diisocyanate, methylcyclohexane-2,4
(2,6) diisocyanate, 4,4'-
methylenebis(cyclohexylisocyanate), 1,3-
(isocyanatemethyl)cyclohexane, trimethylhexamethylene
diisocyanate, diisocyanate of dimer acid and the like.
Further, the polyester and/or polyester
polyether compound obtained by the above method is
reacted with at least one hydroxyl group containing
copolymerizable monomer and/or at least one active
methylene group containing copolymerizable monomer at a
mol ratio of the compound to the copolymerizable monomer
of 0.7-1.5, preferably 0.8-1.2 and at 0~C-140~C for
~ -' 204 1 2~2
-30-
1-10 hours to obtain a macromer having vinyl group or
isopropenyl group in its terminal. The formation of the
macromer may be carried out in the absence or presence
of a well-known catalyst selected from tertiary amines
such as triethylamine, dimethyl benzylamine and the like
and organic metal salts such as dibutyl tin dilaurate,
zinc naphthenate, zinc octylate and the like.
As the hydroxyl group containing copolymerizable
monomer, mention may be made of 2-hydroxyethyl acrylate,
IO 2-hydroxyethyl methacrylate, hydroxypropyl acrylate,
hydroxypropyl methacrylate, N-methylol acrylamide, N-
methylol methacrylamide, allyl alcohol, ~-caprolactone-
modified hydroxyalkyl (metha)acrylate (e.g. Placcel FM-
2, FM-4, trade -mark, made by Daicel Chemical Industries)
1~ and the like. As the active methylene group containing
copolymerizable monomer, mention may be made of aceto-
acetoxyethyl acrylate, acetoacetoxyethyl methacrylate,
acetoacetoxypropyl acrylate, acetoacetoxypropyl
methacrylate, allyl acetoacetate and the like.
On the other hand, the polyester and/or
polyester polyether compound having one hydroxyl group
in its terminal is subjected to a ring-opening addition
reaction with the acid anhydride of the formula (16) at
100~C-200~C, preferably 120-160~C to form a polyester
and/or polyester polyether compound having one carboxyl
group in its terminal. Then this polymer compound is
2041292
-31-
reacted with glycidyl group containing copolymerizable
monomer such as glycidyl (metha)acrylate, allylglycidyl
ether or the like at a mol ratio of 0.7-1.5, preferably
0.9-1.1 and at 100~C-200~C, preferably 120-160~C for 2-
20 hours to obtain a macromer having vinyl group or
isopropenyl group in its terminal. In the formation of
the macromer, it is desirable to add a polymerization
inhibitor such as hydroquinone, 4-methoxy phenol or the
like.
The thus obtained macromer of the polyester
and/or polyester polyether compound represented by the
following formula (18):
~ 1
CH2=C \ (18)
A-Y
is copolymerized with at least one of a tertiary amino
group containing copolymerizable monomer and/or a
copolymerizable monomer having a nitrogen-containing
heterocyclic ring with a basicity represented by the
following formula (19):
~ 1
CH2=C~ (19)
and, if necessary, at least one copolymerizable monomer
represented by the following formula (20):
~ 1
CH2=C \ (20)
2041292
-32-
in the presence of a polymerization initiator at
40~C-180~C in the aforementioned solvent for 3-20 hours,
whereby the first pigment dispersing agent can be
produced.
As the copolymerizable monomer of the formula
(19), mention may be made of aminoalkyl (metha)acrylates
such as N,N-dimethylaminoethyl (metha)acrylate, N,N-
diethylaminoethyl (metha)acrylate, N,N-
dimethylaminopropyl (metha)acrylate, N,N-
diethylaminopropyl (metha)acrylate, N,N-
dimethylaminobutyl (metha)acrylate, N,N-
diethylaminobutyl (metha)acrylate, N,N-
dimethylaminohexyl (metha)acrylate, N,N-
diethylaminohexyl (metha)acrylate and the like;
16 aminoalkyl (metha)acrylamides such as N,N-
dimethylaminoethyl (metha)acrylamide, N,N-
diethylaminoethyl (metha)acrylamide, N,N-
dimethylaminopropyl (metha)acrylamide, N,N-
diethylaminopropyl (metha)acrylamide, N,N-
dimethylaminobutyl (metha)acrylamide, N,N-
diethylaminobutyl (metha)acrylamide and the like;
aminoalkyl vinyl ethers N,N-dimethylaminoethyl vinyl
ether, N,N-diethylaminoethyl vinyl ether, N,N-
dipropylaminoethyl vinyl ether, N,N-dibutylaminoethyl
vinyl ether, N,N-dimethylaminopropyl vinyl ether, N,N-
diethylaminopropyl vinyl ether, N,N-dipropylaminopropyl
'- 2041292
-33-
vinyl ether, N,N-dimethylaminobutyl vinyl ether, N,N-
diethylaminobutyl vinyl ether, N,N-dipropylaminobutyl
vinyl ether, N,N-dibutylaminobutyl vinyl ether, N,N-
dimethylaminopentyl vinyl ether, N,N-diethylaminopentyl
vinyl ether, N,N-dipropylaminopentyl vinyl ether, N,N-
dimethylaminohexyl vinyl ether, N,N-diethylaminohexyl
vinyl ether, N,N-dipropylaminohexyl vinyl ether, N,N-
dibutylaminohexyl vinyl ether, N,N-dimethylaminooctyl
vinyl ether, N,N-diethylaminooctyl vinyl ether and the
like; vinyl pyridines such as 2-vinyl pyridine, 4-vinyl
pyridine, 5-ethyl-2-vinyl pyridine and the like; vinyl
imidazoles such as l-vinyl imidazole, l-vinyl-2-methyl
imidazole and the like; vinyl quinolines such as 2-vinyl
quinoline and the like; and vinyl piperidines such as N-
16 methyl-3-vinyl piperidine and the like.
As the copolymerizable monomer of the formula
(20), mention may be made of alkyl esters of
(metha)acrylic acid such as methyl (metha)acrylate,
ethyl (metha)acrylate, propyl (metha)acrylate, butyl
(metha)acrylate, hexyl (metha)acrylate, octyl
(metha)acrylate, lauryl (metha)acrylate, stearyl
(metha)acrylate, cyclohexyl (metha)acrylate, benzyl
(metha)acrylate and the like; alkoxyalkyl esters such as
methoxyethyl (metha)acrylate, methoxybutyl
(metha)acrylate, ethoxyethyl (metha)acrylate,
ethoxybutyl (metha)acrylate and the like;
2041292
-34-
(metha)acrylamides such as (metha)acrylamide, N-methyl
(metha)acrylamide, N-ethyl (metha)acrylamide, N-butyl
(metha)acrylamide, N,N-dimethyl (metha)acrylamide, N,N-
diethyl (metha)acrylamide, N,N-dipropyl
(metha)acrylamide, N-methylol (metha)acrylamide, N-
ethoxymethyl (metha)acrylamide, N-butoxymethyl
(metha)acrylamide, N,N-dimethylaminopropyl acrylamide
and the like; hydroxy (metha)acrylates such as
and the like; hydroxy (metha)acrylates such as 2-
hydroxyethyl (metha)acrylate, hydroxypropyl(metha)acrylate, Placcel FM-2, Placcel FM-4 and the
like; vinyl aromatics such as styrene, ~-methylstyrene,
vinyl toluene and the like; ~,~-ethylenically
unsaturated carboxylic acids such as acrylic acid,
16 methacrylic acid and the like; alkylvinyl ethers such as
methylvinyl ether, ethylvinyl ether, butylvinyl ether,
isobutylvinyl ether, cyclohexylvinyl ether, tert-
butylcyclohexylvinyl ether, 2-chloroethylvinyl ether, n-
propylvinyl ether, l-propylvinyl ether, n-butylvinyl
ether, l-butylvinyl ether, tert-butylvinyl ether, n-
pentylvinyl ether n-hexylvinyl ether, n-octylvinyl
ether, 2-ethylhexylvinyl ether and the like; alicyclic
vinyl ethers such as cyclopentyl vinyl ether, cyclohexyl
vinyl ether, methylcyclohexyl vinyl ether and the like;
aromatic vinyl ethers such as benzyl vinyl ether and the
like; hydroxyalkyl vinyl ethers such as hydroxyethyl
2041292
-36-
vinyl ether, hydroxypropyl vinyl ether, hydroxyisopropyl
vinyl ether, hydroxybutyl vinyl ether, hydroxyhexyl
vinyl ether, hydroxyoctyl vinyl ether, 2-hydroxyethyl
vinyl ether, 3-hydroxypropyl vinyl ether, 2-
hydroxypropyl vinyl ether and the like; vinyl acetate,
vinyl chloride, acrylonitrile, allyl ethers and the like.
As the polymerization initiatoruse may be made
of all compounds usually used in the vinyl type
polymerization, which includes, for example,
azobisisobutyronitrile, 2,2'-azobis-2,4-
dimethylvaleronitrile, l,l'-azobiscyclohexane-l-
carbonitrile, di-t-butyl peroxide, t-butylperoxy
benzoate, t-butyl peroctoate, cumene hydroperoxide,
lauroyl peroxide, acetyl peroxide, methyl ethyl ketone
16 peroxide, dicumyl peroxide and the like.
As the copolymerization method, the well-known
methods such as solution polymerization, bulk
polymerization, suspension polymerization and the like
may be applied, and among them the solution
polymerization method is desirable.
As the second production method, the tertiary
amino group containing copolymerizable monomer and/or
copolymerizable monomer having a nitrogen-containing
heterocyclic ring with a basicity represented by the
formula (19), at least one of the above hydroxyl group
containing copolymerizable monomer and active methylene
20~1292
-36-
group containing copolymerizable monomer and, if
necessary, the copolymerizable monomer of the formula
(20) are previously copolymerized in the presence of the
polymerization initiator at 40-180~C in the solvent for
3-10 hours to obtain an acryl polymer. Then, the acryl
polymer is reacted with the polyester and/or polyester
polyether compound having one isocyanate group in its
terminal obtained by the first production method at
30-100~C for 3-20 hours, whereby the first pigment
1~ dispersing agent can be produced.
As a production method of the pigment dispersing
agent represented by the general formula (II), at least
one hydroxyl group containing copolymerizable monomer or
carboxyl group containing copolymerizable monomer such
16 as (metha)acrylic acid or the like is used as an
initiator and then at least one monoepoxy compound of
the formula (15), at least one acid anhydride of the
formula (16) and at least one lactone of the formula
(17) are subjected to a ring-opening addition reaction
according to the first production method in the presence
of the polymerization inhibitor to obtain a macromer
having a given molecular weight and containing hydroxyl
group in one terminal and vinyl group or isopropenyl
group in the other terminal. In this case, there may be
W used a method wherein the hydroxyl group containing
copolymerizable monomer or carboxyl group containing
20~1292
-37-
copolymerizable monomer is first used as an initiator
and then the monoepoxy compound and acid anhydride are
ring-opening copolymerized and subjected to a ring-
opening addition reaction with the lactone to form a
polymer of block structure, a method wherein the
hydroxyl group containing copolymerizable monomer is
used as an initiator and then the lactone is subjected
to a ring-opening addition reaction and ring-opening
copolymerized with the epoxy compound and acid anhydride
to form a polymer of block structure, a method wherein
the hydroxyl group containing copolymerizable monomer or
carboxyl group containing copolymerizable monomer is
first used as an initiator and then the monoepoxy
compound, acid anhydride and lactone are randomly
16 addition reacted to from a polymer of random structure.
On the other hand, at least one lactone of the formula
(17) and at least one monoepoxy compound of the formula
(15) are subjected to a ring-opening addition reaction
with the hydroxyl group containing copolymerizable
monomer as an initiator in the presence of the
polymerization inhibitor according to the above first
production method to obtain a macromer of polyester
polyether compound having a given molecular weight and
containing hydroxyl group in one terminal and vinyl
group or isopropenyl group in the other terminal.
In this case, there may be used a method wherein the
2041292
-38-
hydroxyl group containing copolymerizable monomer is
first used as an initiator and then the lactone is
subjected to a ring-opening addition reaction and ring-
opening copolymerized with the monoepoxy compound to
form a polymer of block structure, a method wherein the
hydroxyl group containing copolymerizable monomer is
used as an initiator and then the monoepoxy compound is
ring-opening copolymerized and subjected to a ring-
opening addition reaction with the lactone to form a
polymer of block structure, a method wherein the
hydroxyl group containing copolymerizable monomer is
first used as an initiator and then the monoepoxy
compound and lactone are randomly addition reacted to
from a polymer of random structure.
Then, the thus obtained macromer of polyester or
polyester polyether compound represented by the
following formula (21);
~ 1
CH2=C \ (21)
B-Q
is copolymerized with at least one copolymerizable
monomer selected from the tertiary amino group
containing copolymerizable monomer and copolymerizable
monomer having a nitrogen-containing heterocyclic ring
with a basicity represented by the formula (19) and, if
necessary, at least one copolymerizable monomer
20~1292
-39-
represented by the formula (20) in the presence of the
polymerization initiator at 40-180~C for 3-20 hours,
whereby the first pigment dispersing agent can be
produced.
The second dispersing agent according to the
invention can be produced according to the second method
for the production of the first pigment dispersing agent
of the formula (I).
The third dispersing agent according to the
m invention can be produced according to the first method
for the production of the first pigment dispersing agent
of the formula (I).
The pigment dispersing agents according to the
invention are used for paints, inks, toners and magnetic
l~ tape coating materials as well as various colorants.
As a dispersing resin to be applied, use may be made of
alkid resin, oil-free polyester resin, acrylic resin,
epoxy resin, polyurethane resin, silicone resin,
fluorine resin, melamine resin, benzoguanamine resin,
urea resin and the like. Further, as an objective
pigment, mention may be made of inorganic pigments such
as titanium dioxide, zinc oxide, cadmium sulfide, iron
oxide, calcium carbonate, red lead, zinc sulfide, barium
sulfate, barium carbonate, clay, talc, chrome yellow,
carbon black and the like;organic pigments such as azo
series, diazo series, condensed diazo series, thioindigo
2041292
-40-
series, indanthrone series, anthraquinone series,
benzimidazolone series, phthalocyanine series,
pyranthrone series, anthrapyridine series, isoindolinone
series, perylene series, quiacridone series,
furavanthrone series, dioxazine series, piranthrone
series, perinone series and so on. The pigment
dispersing agents according to the invention exhibit an
effect of improving the pigment dispersibility against
these pigments.
IO The pigment dispersing agent according to the
invention is used in an amount as a solid content of
1-200% by weight, preferably 5-100~ by weight to the
pigment in a so-called pigment dispersion base composi-
tion. The pigment dispersion base composition is
lh comprised of the pigment dispersing agent according to
the invention, the pigment and the organic solvent, or
may further contain a part or whole of a film-forming
resin.
The pigment dispersion base composition is
dispersed by means of dispersing machine usually used in
the art, such as roll mill, ball mill, sand grind mill,
attritor, paint shaker, kneader, high speed dispersing
machine, ultrasonic dispersing machine, dissolver or the
like, which is supplied for use in colorants.
The paints obtained by using the pigment
dispersing agent according to the invention are
2041292
-41-
excellent in the dispersibility of pigment and color
strength and form a paint film having good gloss,
distinctness of image and smoothness. Furthermore, the
pigment dispersing agent according to the invention
strongly adsorbs onto the surface of the pigment to
prevent a tendency of agglomerating the pigment
particles, resulting in the production of the pigment
dispersion base composition having an excellent storage
stability and a high pigment content, and consequently
l~ paints having a high solid content can be produced.
In the pigment dispersing agent according to the
invention, since the ring-opening reaction product of
monocarboxylic acid, monoepoxy compound, acid anhydride
and lactone, the ring-opening reaction product of
1~ monovalent alcohol, acid anhydride, monoepoxy compound
and lactone, or the ring-opening reaction product of
monovalent alcohol, lactone and monoepoxy compound is a
polymer component, the polarity can optionally be
adjusted by changing the monomer composition, so that
the pigment dispersing agent according to the invention
is applicable to both acrylic resin series paint and
polyester series paint frequently used in the art.
Furthermore, in the pigment dispersing agent
according to the invention, it is possible to introduce
hydroxyl group into the structure as a crosslinking
point, so that the pigment dispersing agent can
- 2041292
-42-
chemically be fixed in the paint film by the curing
reaction with a crosslinking agent likewise the case of
the film-forming resin. As a result, the boundary face
between the pigment and the resin is chemically
strengthen, so that there is no damage on the water
resistance, humidity resistance, weather resistance and
the like of the paint film.
In addition, since all amino groups in the
pigment dispersing agent are tertiary amino group, even
when the pigment dispersing agent is applied to
thermosetting paints, no yellowing occurs, so that it is
useful in the colorant field.
The following examples are given in illustration
of the invention and are not intended as limitations
1~ thereof. In these examples, "part" and ll%ll are by weight.
Reference Synthesis Examples 1, 2
<Production of polyester resin>
A polyester resin as a main film-forming
component for polyester resin series paints was produced
as follows.
Into a reaction vessel provided with a reflux
condenser, an inlet tube for nitrogen gas, a thermometer
and a stirring blade were charged starting materials
having a composition shown in Table 1 (Reference
Synthesis Examples 1, 2), which were heated to an upper
limit temperature of 230~C under a stream of nitrogen gas
204 1 292
-43-
with stirring. Water generated in the progress of the
reaction was removed together with xylene through
azeotropic distillation and the heating was further
continued till the acid value was about 10 to complete
the reaction. The resulting resin was diluted with
xylene so as to render the nonvolatile content into 60%,
whereby the objective polyester resins PE-l and PE-2
were obtained.
Table 1
Reference Synthesis
Example
1 2
Polyester resin No. PE-l PE-2
lauric acid 4.5
tall oil fatty acid - 16.2
caster oil - 3.8
phthalic anhydride 5.8 17.7
isophthalic acid 21.8
adipic acid 1.6 5.0
neopentyl glycol 12.8 10.4
trimethylol propane 7.4
pentaerythritol - 8.6
Cardula E-101) 8.3
xylene 1.8
xylene (diluting solvent)36.0 38.3
nonvolatile content (%) 2) 60 60
resin acid value 10 10
1) Cardula E-10:trade~mark, made by Shell
Chemical Co., Ltd.
(glycidylester of versatic acid)
2) according to JIS K 5400 (1979) 8. 2
~ 20 11292
-44-
Reference Synthesis Examples 3, 4
<Preduction of acrylic resin>
An acrylic resin as a main film-forming
component for acrylic resin series paints was produced
as follows.
Into a reaction vessel provided with a reflux
condenser, an inlet tube for nitrogen gas, a
thermometer, a stirring blade and a monomer dropping
device was charged a solvent shown in Table 2 and then
the temperature was raised to a reflux temperature under
a stream of nitrogen gas. A mixture of monomers and t-
butylperoxy benzoate as a polymerization inhibitor as
shown in Table 2 was added dropwise through the dropping
device over 2 hourS under reflux state. After the
1~ completion of the addition, the stirring was further
continued for 5 hours under reflux state to complete the
polymerization, whereby the objective varnishes AC-l,
AC-2 were obtained.
ao
20~129~
-45-
Table 2
Reference Synthesis Example 3 4
Acrylic resin No. AC-l AC-2
solvent xylene 40 40
methyl methacrylate 23.4 20.4
butyl acrylate 13.3 6.4
styrene 10.0 4.0
Monomer
composition 2-hydroxyethyl methacrylate 12.0 3.0
acrylic acid 0.7 0.7
Placcel FM-2 1~ 25.0
t-butylperoxy benzoate 0.6 0.5
nonvolatile content (~) 60.0 60.0
Properties weight average molecular weight 21000 28000
hydroxyl value 86 87
1) Placcel FM-2: trade name, made by Daicel
Chemical Industries
(lactone-modified methacrylate
monomer)
Synthesis Examples 1, 2
A macromer for the pigment dispersing agent was
produced as follows.
Into a reaction vessel provided with a reflux
condenser, an inlet for nitrogen gas, a dropping funnel,
a thermometer and a stirring blade were charged a
solvent and sodium methylate and decanol as (Y+A)
components shown in Table 3 and then the temperature was
raised to 130-140~C under a stream of nitrogen gas.
Further, ~-caprolactone shown in table 3 was added
dropwise through the dropping funnel over 1 hour under
' 2041292
-46-
reflux state. After the stirring was further continued
for 4 hours under reflux state and dried by heating at
140~C for 30 minutes, it was confirmed that the
nonvolatile content arrived at the given value, and then
butylglycidyl ether shown in Table 3 was added dropwise
through the dropping funnel over 1 hour. After the
stirring was further continued for 4 hours under reflux
state and dried by heating at 140~C for 30 minutes, it
was confirmed that the nonvolatile content arrived at
the given value. Next, the resulting varnish was cooled
to a temperature of 30~C and added with tolylene
diisocyanate shown in Table 3, which was stirred at 30~C
for 1 hour, and after it was confirmed that 50% of
isocyanate group was reacted (a case that 2 mol of
16 isocyanate group in tolylene diisocyante was reacted was
100%), 2-hydroxyethyl methacrylate shown in Table 3 was
added. After the addition, the temperature was raised
to 60~C, and the reaction was continued at this
temperature for 3 hours, and the reaction was stopped
after it was confirmed that 100% of isocyanate group was
reacted. Thus, the macromers A-l, A-2 were produced.
Synthesis Examples 3, 4, 7-20
In the same vessel as in Synthesis Example 1 were
charged solvent, and decanol, phthalic anhydride and
dimethyl benzylamine as ~Y+A) components as shown in
Tables 3 and 4 and then the temperature was raised to
20~1292
-47-
130-140~C under a stream of nitrogen gas. Thereafter,
butylglycidyl ether or phenylglycidyl ether shown in
Table 3 was added dropwise through the dropping funnel
over 1 hour under reflux state. After the reaction was
continued for 5 hours under reflux state, it was
confirmed that the acid value of the resin arrived at
not more than 1, and then dibutyl tin dilaurate was
added and further ~-caprolactone was added dropwise
through the dropping funnel over 1 hour. After the
l~ reaction was continued for 5 hours under reflux state
and the drying was carried out by heating at 140~C for
30 minutes, it was confirmed that the nonvolatile
content arrived at the given value. Then, the resulting
varnish was cooled to a temperature of 30~C and added
16 with tolylene diisocyanate as shown in Tables 3, 4,
which was stirred at 30~C for 1 hour. After it was
confirmed that 50~ of isocyanate group was reacted, 2-
hydroxyethyl methacrylate or acetoacetoxyethyl
methacrylate shown in Table 3 was added. After the
addition, the temperature was raised to 60~C and the
reaction was further continued at this temperature for
4 hours, it was confirmed that 100~ of isocyanate group
was reacted. Thus, the macromers A-3, A-4, and A-7 to
A-20 were produced.
Synthesis Examples 5, 6
Into the same vessel as in Synthesis Example 1
204129~
-48-
were charged solvent, and decanol, phthalic anhydride
and dibutyl tin dilaurate as (Y+A) components as shown
in Table 3, which was raised to a reflux temperature of
130-140~C. Then, Cardula E-10 shown in Table 3 was
added dropwise through the dropping funnel over 1 hour.
After the reaction was continued for 5 hours under reflux
state and it was confirmed that the acid value of the
resin arrived at not more than 1, the reaction product
was cooled to 30~C. Then, tolylene diisocyanate shown
in Table 3 was added and stirred at 30~C for 1 hour, and
after it was confirmed that 50% of isocyanate group was
reacted, 2-hydroxyethyl methacrylate shown in Table 3
was added. After the addition, the temperature was
raised to 60~C and the reaction was further continued at
l~ this temperature for 4 hours and completed by confirming
that 100% of isocyanate group was reacted. Thus, the
macromers A-5, A-6 were produced.
Synthesis Example 21
Into the same vessel as in Synthesis Example 1
were charged solvent, and decanol, phthalic anhydride and
tetramethyl ammonium chloride as (Y+A) components as
shown in Table 5, which was raised to a reflux temper-
ature of 130-140~C. Then, butylglycidyl ether shown in
Table 5 was added dropwise through the dropping funnel
over 1 hour. After the reaction was continued for
5 hours under reflux state and it was confirmed that the
20~1292
-49-
acid value of the resin arrived at not more than 1,
dibutyl tin dilaurate shown in table 5 was added and
further ~-caprolactone was added dropwise through the
dropping funnel over l hour. Further, the reaction was
continued for 5 hours under reflux state and it was
confirmed that after the drying was carried out by
heating at 140~C for 30 minutes, the nonvolatile content
arrived at the given value. Next, phthalic anhydride
shown in Table 5 was added and the reaction was continued
for 3 hours under reflux state. After it was confirmed
that the acid value arrived at the given value, 4-
methoxy phenol and glycidyl methacrylate were added and
the reaction was continued for 10 hours under reflux
state. After it was confirmed that the acid value arrived
16 at not more than 2, the reaction product was cooled to
room temperature. Thus, the macromer A-21 was produced.
Synthesis Example 22
Into the same vessel as in Synthesis Example 1
were charged solvent, and acrylic acid, Cardula E-10,
phthalic anhydride, tetramethyl ammonium chloride and 4-
merthoxy phenol as (B+Q) components as shown in Table 5,
which was raised to a reflux temperature of 130-140~C.
After the reaction was continued for 3 hours under
reflux state and it was confirmed that the acid value of
the resin arrived at not more than 1, dibutyl tin
dilaurate was added and further ~-caprolactone was added
2~1292
-50-
dropwise through the dropping funnel over 1 hour and
then the reaction was continued for 5 hours under reflux
state. After the drying was carried out by heating at
140~C for 30 minutes, it was confirmed that the
nonvolatile content arrived at the given value.
Thereafter, the resulting varnish was cooled to
room temperature. Thus, the macromer A-22 was produced.
Synthesis Example 23
The same procedure as in Synthesis Example 22
IQ was repeated except that 2-hydroxyethyl methacrylate was
used instead of acrylic acid in table 5, whereby the
macromer A-23 was produced.
Synthesis Examples 24-28
An acrylic copolymer as a component S for the
1~ pigment dispersing agent was produced as follows.
Into a reaction vessel provided with a reflux
condenser, an inlet tube for nitrogen gas, a dropping
funnel, a thermometer and a stirring blade was charged a
solvent among a composition shown in Table 6, which was
raised to a temperature of 120~C under a stream of
nitrogen gas. A mixture of monomers and polymerization
initiator shown in Table 6 was added dropwise through
the dropping funnel over 2 hours under reflux state and
the stirring was further continued for 5 hours to
complete the polymerization, whereby the acrylic
copolymers S-l to S-5 were produced as a component S.
2041292
-51-
Synthesis Example 29
A polymer compound as a component T for the
pigment dispersing agent was produced as follows.
Into the same vessel as in Synthesis Example 1
were charged solvent, sodium methylate and decanol as
shown in Table 7, which was raised to a temperature of
130-140~C under a stream of nitrogen gas. A mixture of
~-caprolactone and butylglycidyl ether shown in Table 7
was added dropwise through the dropping funnel over
2 hours and the stirring was further continued over
10 hours under reflux state. After the cooling to 30~C,
tolylene diisocyanate was added and the stirring was
continued at 30~C for 2 hours to produce the polymer
compound T-l as a component T.
l~ Synthesis Examples 30-34
The same procedure as in Synthesis Example 29
was repeated according to the composition as shown in
Table 7 to obtain the polymer compounds T-2 to T-6 as a
component T.
Comparative Synthesis Example 1
Into the same vessel as in Synthesis Example 1
were charged solvent, and butanol and dibutyl tin
dilaurate as (Y+A) components as shown in Table 8, which
was raised to a reflux temperature. Then, ~-
caprolactone shown in Table 8 was added dropwise through
the dropping funnel over 1 hour. The reaction was
204129~
-52-
further continued for 4 hours under reflux state.
Thereafter, the reaction product was cooled to 20~C and
added with tolylene diisocyanate shown in Table 8.
The stirring was continued at 20~C for 2 hours and 2-
hydroxyethyl methacrylate shown in Table 8 was added
after it was confirmed that 50% of isocyanate group was
reacted. After the addition, the temperature was raised
to 50~C, and the reaction was continued at this
temperature for 5 hours. After it was confirmed that
100% of isocyanate group was reacted, the reaction was
stopped to obtain a macromer A-24.
Comparative Synthesis Example 2
A macromer A-25 was obtained according to a
compounding recipe shown in Table 8 in the same manner
16 as described in Synthesis Example 5.
Comparative Synthesis Examples 3, 4
Macromers A-26, A-27 were obtained according to
a compounding recipe shown in Table 9 in the same manner
as described in Synthesis Example 21.
Examples 1-24
Into the same reaction vessel as used in
Synthesis Example 1 was charged a greater part of a
solvent as shown in Tables 10 and 11, and then the tem-
perature was raised to 85~C. Next, a monomer mixture of
(Y+A) components and component X and 20% of an initiator
solution as shown in Tables 10 and 11 were added dropwise
2041292
-53-
through separate dropping funnels over 2 hours. Further-
more, the reaction was continued at 85~C for 2 hours to
obtain objective pigment dispersing agents 1-24.
Examples 25-29
Into the same reaction vessel as used in
Synthesis Example 1 was charged a greater part of a
solvent as shown in Table 11, and then the temperature
was raised to 85~C. Next, a monomer mixture of (Y+A)
components, component X and component Z and 20~ of an
m initiator solution as shown in Table 11 were added
dropwise through separate dropping funnels over 2 hours.
Furthermore, the reaction was continued at 85~C for
2 hours to obtain objective pigment dispersing agents
25-29.
16 ExamPles 30-34
Pigment dispersing agents 30-34 were produced
according to a compounding recipe as shown in Table 12
in the same manner as in Example 25.
Example 35-42
A pigment dispersing agent was produced
according to a composition ratio as shown in Table 13 as
follows.
Into the same vessel as in Synthesis Example 1
were charged component S and solvent as shown in Table 13,
to which was added component T with stirring. There-
after, the reaction mass was heated to a temperature
2041292
-~4-
shown in the column "reaction conditions" of Table 13
and reacted at this temperature for a time as shown in
the column of "reaction conditions" to obtain objective
pigment dispersing agents 35-42.
Comparative Examples 1-5
Pigment dispersing agents 43-47 were produced
according to a compounding recipe shown in Table 14 in
the same manner as in Example 1.
Comparative Example 6
Into the same vessel as in Synthesis Example 1
was charged a pleater part of a solvent as shown in
Table 14 and then the temperature was raised to 50~C.
Next, a monomer mixture of (Y+A) components and component
X and a solution of 10~ initiator in xylene as shown in
1~ Table 14 were added dropwise through separate dropping
funnels over 3 hours. The reaction was further
continued at 50~C for 4 hours to produce a pigment
dispersing agent 48.
Comparative Examples 7-10
Pigment dispersing agents 49-52 were produced
according to a compounding recipe as shown in Table 15
in the same manner as in Example 30.
Comparative Example 11
A pigment dispersing agent 53 was produced
according to a compounding recipe as shown in Table 13
in the same manner as in Example 35.
~' 2041292
-55-
Application Examples 1-48
Dispersion pastes and paints each having a
composition as shown in Tables 16-19 were prepared by
using the pigment dispersing agents shown in Tables
10-13, and then the properties of the paste and the film
performances of the paint were measured to evaluate the
effect of the pigment dispersing agent according to the
invention.
That is, the starting materials were uniformly
IO mixed in accordance with a formulation of dispersion
paste shown in Tables 16-19 and a pigment was dispersed
thereinto by means of a paint shaker (made by Red Devil
Co.) to obtain a dispersion paste. The viscosity and
storage stability of the dispersion paste are shown in
16 the column "paste properties" of Tables 16-19.
Then, the starting materials using the above
dispersion paste were mixed in accordance with a
formulation for paint shown in Tables 16-19 and
thoroughly stirred to prepare a paint. To this paint
ao was added a thinner of dilution (a mixed solvent of
cellosolve acetate/xylene=50/50) so as to adjust a
viscosity to 22 seconds (25~C) through Ford Cup No. 4,
whereby a finishing paint for spray coating was
obtained.
Next, a primer coated plate was provided in
order to evaluate the film performances of this paint.
1 ~ 204 1 292
-56-
That is, an SPCC dull steel sheet (size: 0.8x100x150 mm)
subjected to a zinc phosphate treatment was subjected to
na electrodeposition with a general-purpose black cation
electropaint (AQUA Black No. 4200, trade-m~r~, made by
Nippon Oil and Fats Co., Ltd.) and then baked at 170~C
for 30 minutes to obtain a dried paint film of 20 ~m in
thickness. Thereafter, an alkyd/melamine series gray
intermediate paint for automobile (EPICO No. 1500
sealer, trade-mark, made by Nippon Oil and Fats Co.,
Ltd.) was diluted with a thinner (Thinner TR101, trade-
mark, made by Nippon Oil and Fats Co., Ltd.) so as to
adjust a viscosity to 30 seconds (25~C) through Ford Cup
No. 4, which was sprayed onto the above primer coated
sheet and baked at 140~C for 30 minutes to obtain a
1~ dried paint film of 40 ~m in thickness. Then, the
aforementioned finishing paint was sprayed onto the thus
painted steel sheet and baked at 140~C for 30 minutes to
obtain a test film (finished film thickness: 40 ~m).
The specular gloss at 30~ (Drigon goniophotometer, made
by Hunter Laboratories), humidity resistance, weather
resistance, adhesion property and the like were
evaluated with respect to the thus obtained test
specimen. The results are also shown in Tables 16-19.
Application Comparative Examples 1-12
a~ Dispersion pastes and paints each having a
composition as shown in Table 20 were prepared by using
*
~rade-mark
B
- 204 1 292
-57-
the pigment dispersing agents shown in Tables 14 and 15
in the same manner as in Example l, and then the
properties of paste and film performances of paint were
measured.
Application Comparative Example 13
Into a reaction vessel provided with a
condenser, an inlet tube for nitrogen gas, a thermometer
and a stirring blade were charged 400 parts of xylene,
59.4 parts of lauric acid, 222.8 parts of Cardula E-lO
(glycidyl ester of versatic acid, epoxy equivalent: 250,
trade~ma~k, made by Shell Chemicals Co., Ltd.) and
87.9 parts of phthalic anhydride as disclosed in US
Patent No. 4,933,417, which was raised to a temperature
of 150-160~C and stirred in a nitrogen gas atmosphere
1~ for 5-8 hours. When the acid value of the resin arrived
at not more than 2.0, the reaction mass was cooled to
20-30~C and added with 51.7 parts of tolylene
diisocyanate and then the stirring was further continued
at 40~C for 5-8 hours. When 50% of isocyanate grpup was
reacted, 178.2 parts of Epomin Sp-006 (trade-~ar*, made
by Nippon Shokubai Kagaku Kogyo Co., Ltd.) was added and
then the stirring was continued at 30~C for 5 hours to
obtain a pigment dispersing agent 54.
A dispersion paste and paint having a
composition as shown in Table 20 were prepared by using
the above pigment dispersing agent in the same manner as
- 2041292
-68-
in Example l, and then the properties of paste and film
performances of paint were measured to evaluate the
effect of this pigment dispersing agent.
Application Comparative Example 14
Into the same vessel as in Synthesis Example 1
was charged 40.0 parts of xylene and then the
temperature was raised to a reflux state as disclosed in
EP-A-0358358. The, a monomer mixture of 14.15 parts of
methyl methacrylate, 12.0 parts of ethyl acrylate,
12.0 parts of lauryl methacrylate, 9.9 parts of styrene,
8.9 parts of 2-hydroxyethyl methacrylate, 0.85 part of
glycidyl methacrylate and 4.3 parts of t-butylperoxy
benzoate was added dropwise through the dropping funnel
over 2 hours while maintaining the reflux state. After
16 the completion of the addition, the stirring was further
continued for 2 hours under the reflux state to obtain
an acrylic resin Bl-2. In to the same vessel as in
Synthesis Example 1 was charged 81.7 parts of the resin
Bl-2, to which was added a mixed solution of 0.98 part
of Epomine SP-006 (trade name, Nippon Shokubai Kagaku
Kogyo Co., Ltd.) and 17.32 parts of butanol with strong
stirring and held at 120~C for 2 hours to obtain a
pigment dispersing agent 55.
A dispersion paste and paint having a
composition as shown in Table 19 were prepared by using
the above pigment dispersing agent in the same manner as
-- 20~1292
-59-
in Example 1, and then the properties of paste and film
performances of paint were measured to evaluate the
effect of this pigment dispersing agent.
In case of using the pigment dispersing agents
according to the invention, as shown in Tables 16-19,
the storage stability of dispersion paste is excellent
and also it has been confirmed that as to the film
performances, the gloss is excellent in all paint films
and the humidity resistance, weather resistance and
adhesion property are not damaged. Furthermore, there
is observed no yellowing of the paint film due to the
pigment dispersing agent.
However, as shown in Application Comparative
Examples of Table 20, in cases that the number average
1~ molecular weight of (Y+A) components is less than 500
(Application Comparative Example 1) or exceeds 20,000
(Application Comparative Examples 2, 10), and that the
amine value is less than 10 (Application Comparative
Examples 3, 7) or exceeds 200 (Application Comparative
Examples 4, 8), and that the number average molecular
weight of the pigment dispersing agent is less than
1,000 (Application Comparative Examples 5, 9) or exceeds
100,000 (Application Comparative Example 6), and that
the dipsersing agent is not used (Application Compar-
ative Example 12), the stability of the dispersion paste
is bad and poorer than those of Application Examples.
2041292
-60-
In case of US Patent No. 4,933,417 and EP-A-
0358358, the paint film is yellowed during the baking
and is poorer than those of Application Examples.
0~
1~
ao
Table 3 ( a )
Synthesis Example
1 2 3 4 5 6 7 8 9
Macromer No. A-l A-2 A-3 A-4 A-5 A-6 A-7 A-8 A-9
solvent xylene 36.6 38.1 37.0 37.0 39.3 40.3 33.7 33.3 35.4
decanol 4.3 2.33.0 3.0 2.6 1.2 - - 3.0
lauric acid - - - - - - 3.8 3.8
phthalic anhydride - - 8.4 8.4 8.5 8.5 8.4 8.4 8.4
butylglycidyl ether 15.3 22.1 8.8 - - - 11.3 - 8.8
phenylglycidyl ether - - - 10.2 - - - 13.1
Resin (Y~A) Cardula E-10 1) - - - - 16.0 15.9
Composition compOnents ~-caprolaCtone 35.5 33.0 36.6 35.2 28.5 31.7 36.6 35.2 36.6
sodium methylate 0.1 0.1 - - - - - - -
dimethyl benzylamine - - 0.3 0.3 - - 0.3 0.3 0.3
dibutyl tin dilaurate - - 0.1 0.10.1 0.1 0.1 0.1 0.1
tolylene diisocyanate 4.7 2.5 3.3 3.32.9 1.3 3.3 3.3 3.32-hydroxyethyl methacrylate 3.5 1.9 2.5 2.52.1 1.0 2.5 2.5
acetoacetoxyethyl methacrylate - - - - - - - - 4.1_ _ _ - 3.0 3.0 -
q - - - - - - 16.9 16.3
. r - - 3.0 3.03.5 7.6 - - 3.0 ~'
Deslgn
value s _ - 16.9 16.9 15.2 37.0 - - 16.9 2
t 11.5 19.9 - - - - - - -
u 4.3 11.7
number average molecular weight 2300 4300 3300 3300 2600 4100 3400 3500 3350
Properties
nonvolatile content (%) 63.4 61.9 62.7 62.7 60.7 59.7 66.0 66.4 64.3
1) Cardula E-10: trade name, made by Shell Chemical Co., Ltd. (glycidylester of versatic acid)
Table 3 ( b )
Synthesis Example
10 11 12 13 14 15 16 17 18
Macromer No. A-10 A-ll A-12 A-13 A-14 A-15 A-16 A-17 A-18
solvent xylene35.4 37.6 36.1 36.7 35.0 37.3 35.8 36.8 35.0
decanol 3.0 3.0 3.0 3.0 3.03.0 3.0 3.0 3.0
lauric acid
phthalic anhydride 8.4 8.4 8.4 8.4 8.48.4 8.4 8.4 8.4
butylglycidyl ether - 8.8 8.8 8.8 8.88.8 8.8 8.8 8.8
phenylglycidyl ether 10.2
Resin (Y+A) Cardula E-10 1)
Composition compOnents ~-caprolactone 35.2 36.6 36.6 36.6 36.6 36.6 36.6 36.6 36.6
sodium methylate ~ - ~ ~ ~ ~ ~ ~ ~
dimethyl benzylamine 0.3 0.3 0.3 0.3 0.30.3 0.3 0.3 0.3
dibutyl tin dilaurate 0.1 0.1 0.1 0.1 0.10.1 0.1 0.1 0.1
tolylene diisocyanate 3-3 3.0 3.0 3-5 3-5 3-3 3-3 3-3 3-3
2-hydroxyethyl methacrylate - 2.2 - 2.6 - 2.2 - 2.7
acetoacetoxyethyl methacrylate 4.1 - 3.7 - 4.3 - 3.7 - 4.5
p
------ -- O
Design r 3 3.0 3.0 3- ~_~
value s 16.3 16.9 16.9 16.9 16.9 16 9 16 9 16 9 16 9 2
t
u
number average molecular weight 3350 3300 3350 3350 3550 3550 3550 3350 3550
Properties
nonvolatile content (~) 63.3 62.1 63.6 63.0 64.7 62.4 63.9 62.9 64.7
1) Cardula E-10: trade name, made by Shell Chemical Co., Ltd. (glycidylester of versatic acid)
2041292
- 63 -
Table 4
Synthesis
Example
19 20
Macromer No. A-l9 A-20
solvent xylene 39.6 39.5
decanol 10.8 0.6
phthalic anhydride 10.0 2.6
Resin (Y~A) butylglycidyl ether 10.6 2.8
composition components ~-caprolactone 7.8 53.0
dimethyl benzylamine 0.3 0-3
dibutyl tin dilaurate0.1 0.1
tolylene diisocyanate11.9 0.6
2-hydroxyethyl methacrylate 8.9 0.5
Design r 1.0 5.0
value
s 1.0 131.6
Properties number average molecular weight 900 17000
nonvolatile content (~) 60.1 60.2
Table 5
Synthesis Example 21 Synthesis Example 22 23Macromer No. A-21 Macromer No. A-22 A-23
Solvent xylene 397.38 xylene 397.38 398.58
decanol 40.7 acrylic acid 12.7
phthalic anhydride 114.5 methacrylate ~ 22.3
butylglycidyl ether 105.6 Cardula E-10 276.9 270.0
Resin (Y+A) tetramethyl ammonium chloride phthalic anhydride 130.1 132.0
compo- compo- . , , compo- c~
sition nents dlbutyl tln dllaurate 1.2 nents tetramethyl ammonium 0.12 0.12
~-caprolactone 264.5 chloride
phthalic anhydride 38.2 4-methoxy phenol 1.2 1.2
4-methoxy phenol 1.2 dibutyl tin dilaurate 1.2 1.2
glycidyl methacrylate 36.6 ~-caprolactone 180.4 175.8
Proper- number average molecular weight 2300 number average molecular weight 3300 3400
ties nonvolatile content (%) 60 nonvolatile content (~) 60 60
v lue r ~ s 12 r ~ s 15 15
20~1292
- 6~ -
Table 6
Synthesis Example
2425 26 27 28
Component S No. S-l S-2 S-3 S-4 S-5
Solvent Xylene 25 25 25 25 25
toluene 24 24 24 24 24
dimethyl aminoethyl
methacrylate 15.0 - _ - 27.3
dimethyl aminopropyl
methacrylamide 21.7
acrylamide - 15.0 24.4
2-hydroxyethyl methacrylate 6.0 - 6.0 13.5
Monomer
compo- Placcel FM-4 1) 28.0 - 28.0
acetoacetoxyethyl methacrylate - 27.3 - 11.1
methyl methacrylate - - - - 9.0
butyl methacrylate - - - - 12.7
2,2'-azobis(2,4- 2 0
dimethylvaleronitrile)
carbonitrile 2.0 - 2.0 2.0 2.0
nonvolatile content (~) 50 50 50 50 50
concentration of active 1.9 2.6 1.9 2.1 0
ties amincnentration of tertiary1.9 2.6 1.9 3.2 3.5
concentration of active
methylene group ~ 2.6 0 1.0 0
number average molecular 3090 3170 3080 1880 1500
1) Placcel FM-4: trade name, made by Daicel Chemical Industries
(lactone-modified methacrylate monomer)
2041292
- 66-
Table 7
Synthesis Example
29 30 31 32 33 34
Component T No. T-l T-2 T-3 T-4 T-5 T-6
solvent xylene 40 40 39 39 40 40
decanol 4.3 2.3 3.0 3.0 2.6 1.2
s-caprolactone 35.5 33.0 36.6 35.2 28.5 31.7
butylglycidyl ether 15.3 22.1 8.8
phenylglycidyl ether - - - 10.2
Resin Cardula E-10 1) _ _ _ - 16.0 15.9
compo-
sition phthalic anhydride - - 8.4 8.4 8.5 8.5
dibutyl tin dilaurate - - 0.1 0.1 0.1 0.1
dimethyl benzylamine - - 0.8 0.8
sodium methylate 0.1 0.1
tolylene diisocyanate 4.8 2.5 3.3 3.3 4.3 2.6
nonvolatile content (%) 60 60 60 60 60 60
Proper-
ties number average 2200 4200 3200 3200 2500 4000
1) Cardula E-10: trade name, made by Shell Chemical Co., Ltd.
(glycidylester of versatic acid)
2041292
- 67 -
Table 8
Comparative
Synthesis Example
1 2
Macromer No. A-24 A-25
solvent xylene 39.9 39.9
butanol 9.3
decanol - 0.4
phthalic anhydride - 0.7
compo- compo- Cardula E-10 1) 1.5
sition nents ~-caprolactone 14.3 56.7
dibutyl tin dilaurate 0.1 0.1
tolylene diisocyanate 21.8 0.4
2-hydroxyethyl methacrylate14.6 0.3
Design r 0 2.0
value s 1.0 200.0
Proper- number average molecular weight 490 23000
ties nonvolatile content (%) 60.1 60.1
1) Cardula E-10: trade name, made by Shell Chemical Co., Ltd.
(glycidylester of versatic acid)
20~1292
- 68-
Table 9
Comparative Synthesis Comparative Synthesis
Example 3 Example 4
Macromer No.A-26 Macromer No.A-27
Solvent xylene 397.6 xylene 397.48
butanol 92.9 decanol 4.1
phthalic
anhydride152.9
~-caprolactone 143.1
butylglycidyl 0
dibutyl tin 1.2 tetramethyl
dilaurate ammonium0.12
Resin (Y~A) (Y~A) chloride
compo- compo- compo- dibUtyl tin
sition nents phthalic 185.8 nents dilaurate 1.. 2
anhydride
~-caprolactone 294.5
4-methoxy1.2 anhydride3.8
phenol
4-methoxy
phenol 1.2
methacrylate 178.2 glyCidYl 3 7
number average number average
molecular weight478 molecular weight23000
Proper-
ties nonvolatile nonvolatile
content (%) 60 content (%) 60
Design s 1 r ~ s 141
2041292
- 69 -
tX~ tn ~ O CO U~ ~ O
o ~ N I ~ ~ ~ ~ ~
r~ I I I I . I I I I I I I ~ I N I u~ O O O ~o
t~ u~ u~ o
~ I I I I I O t.~
tl~
O N I ~ O o ~ O
~ tr~ N I ~ ~ ~ ~ ~
o tr~ N I ~ ~ ~ ~ ~
N U~ o ~ I I t I N I Ul o O O
tn ~ o t~O N ,~
t~5 ~
tU tU
O _ .,~
a) tlU
~ U t
Q ~ - ~ o
u r~
tu t
~ ~1 ; 4 ~I r
a
J au - r~
~ N ~ L
O ~ ) - ~l . U ~ ~- U _ dP
J U
,_ ~4 ' ~ a
au _ ~ U ~~ _
o au
z ~ u ~
N _I + au r~
C ~ .r . . I I V --- U ~ ~ ~ J
au x u r~ ~ ~ ~ ~ ul -- ~c N
n~ J a~
r r~ r ,-1 J ~ ~,
e ; ~ -. al a
au -- ; ~ o e I ~ .
:~ .e . . . .,.. ,. .,, . .,,
+ ~ E
~ ~ -
., U~ -- ~ X ~ C~ ,- U~
-
P O
,.,- . U~
JJ au
p~ u~
Table 10 (b)
Example
10 11 12 13 14 15 16 17 18
Pigment Dispersing a~ent No. 10 11 12 4 14 15 16 17 18
xylene 38.9 37.8 39.2 38.6 40.2 38.1 39.4 38.6 40.2
solvent
cyclohexane
(Y+A) macromer No. A-10 A-ll A-12 A-13 A-14 A-15 A-16 A-17 A-18
components
compounding amount55.3 56.4 55.0 55.6 54.0 56.1 54.8 55.6 54.0
dimethyl aminoethyl methacrylate
dimethyl aminopropyl methacrylamide
components dimethyl aminopropyl acrylamide 5.0 5.0 5.0 5.05.0 5.0 5.0 5.0 5.0
4-vinyl pyridine
compo- l-vinyl imidazole - - - ~ ~ ~ ~ ~ ~ -~
sition stylene
n-butylmethacrylate
components dimethyl acrylamide
2-hydroxyethyl methacrylate
acrylic acid
initiator 2,2'-azobis(2,4-dimethylvaleronitrile)0.8 0.8 0.80.8 0.8 0.8 0.8 0.8 0.8
l,l'-azobiscyclohexane-l-carbonitrile
solid (Y+A) components87.5 87.5 87.5 87.5 87.5 87.5 87.5 87.5 87.5
content
ratio components X12.5 12.5 12.5 12.5 12.5 12.5 12.5 12.5 12.5
components Z '- ~ - ~ ~ ~ ~ ~ ~
amine value 45 45 45 45 45 45 45 45 45
Proper- hydroxyl value ~ ~ ~ ~ ~ ~ ~ ~ ~
ties acid value 0 0 0 0 0 0 0 0 0
number average molecular weight2450 2500 2400 2500 2350 2300 2400 2500 2500
nonvolatile content (%) 40 40 40 40 40 40 40 40 40
Table 11
Example
19 20 21 22 23 24 25 26 27 28 29
Pigment Dispersing agent No. 19 20 21 22 23 24 25 26 27 28 29
solvent xylene 38.0 38.3 37.0 36.8 37.4 39.2 39.3 39.2 39.0 39.1 19.0
cyclohexane ~ - - - - - - 20.0
(Y+A) macromer No. A-3 A-3 A-3 A-3 A-3 A-3 A-3 A-3 A-3 A-3 A-3
Components Compounding amount 55.8 55.0 58.4 59.0 58.5 53.1 52.3 53.6 53.7 51.8 52.0
dimethyl aminoethyl methacrylate 5.0 - - - - - 5.0
dimethyl aminopropyl methacrylamide - 5-5
compo- dimethyl aminopropyl acrylamide - - - - 3.3 6.7 - 4.4 4.4 4.4 2.2
nents X
4-vinyl pyridine ~ ~ 3-4
compo- l-vinyl imidazole - - - 3.0 - - - - - - -
sition stylene - - - - - - 1.5 - _ _ _
n-butylmethacrylate - - ~ ~ ~ ~ 0-7
compo- dimethyl acrylamide - - - - - - - 2.0
nents Z
2-hydroxyethyl methacrylate - - - - - - - - 1.9
acrylic acid - - - - - - - - - 3.1 5.2
i itiato 2,2'-azobis(2,4-dimethylvaleronitrile) 1.2 1.2 1.2 1.2 0.8 1.0 1.2 0.8 1.0 1.6 1.6
l,l'-azobiscyclohexane-l-carbonitrile
solid (Y+A) components 87.5 86.2 91.5 92.5 91.7 83.2 82.0 84.0 84.2 81.2 81.5
ratio components X 12.5 13.8 8.5 7.5 8.3 16.8 12.5 11.0 11.0 11.0 5.5 ~
components Z ~ ~ ~ ~ ~ - 5.5 5.0 4.8 7.8 13.0 t-~
amine value 44.7 45.4 45.9 44.8 29.7 60.2 44.7 39.6 39.6 39.6 19.8 CS~
Proper- hYdr~XYl value 0 0 0 20.5 0 0 I~
ties acid value ~ ~ ~ ~ ~ ~ ~ ~ 0 60.4 101.3
number average molecular weight 2600 2200 2400 2700 2400 2300 2800 3000 2900 3100 3300
nonvolatile content (%) 40 40 40 40 40 40 40 40 40 40 40
2041292
- 72-
Table 12
Example
3031 32 33 34
Pigment dispersing agent No. 3031 32 33 34
solvent xylene 35.9 35.9 35.9 36.9 37.2
(Y+A)or macromer No. A-l A-2 A-3 A-22 A-23
(Q+B)
components compounding amount 58.3 58.3 58.3 55.8 55.0
compo- dimethyl aminopropyl5 0 5 0 5 0 5 0 5 0
stylene - - - 1.5
compo- compo-
sition nents Z 2-hydroxyethyl 2 0
methacrylate
initiatordim;thylvaleronitrile) 0-8 0-8 0-8 0-8 0-8
solid IQ+B) compon nts 87.5 87.5 87.5 83.7 82.5
content
ratio components X 12.5 12.5 12.5 12.5 12.5
components Z - - - 3.8 5.0
amine value mgKOH/g 45.0 45.0 45.0 45.0 45.0
Proper- hYdr~Xyl value mgKOH/g 0 0 0 0 21.6
ties number average molecular weight 3300 2500 2100 2700 3400
nonvolatile content (~) 40 40 40 40 40
. ~
2041292
- 73 -
~1 ~ ~ ~l ~ I ~
N N ~ o ~ ~ o ~ ~ ~ ~ ~r~
,q N E~ O O 11-
_I_I o ~ U~ o ~ o ~"
O O ~ o ~ er O ~ O
~ ~o~ ~~ I oO ~ o
t~ I N _I
O I ~ O ~ ~ O ~"
o ~ N o ~ ~ N
~ o oN~ ~ .o j o ~
Q 11~ O o ; ~ ~ N
G~ NO E~ 1 2
z z
U~ ~ C
~ o
C r l ~1
zX U 1 r l
C Ul E~ C O
r Or l" ~ _
C _I~
~11 0 ~I) O ~
S ~ ~U
~ o o e
r~ 6 1~
'C V ~rl r o
t~; . .rl
.rl ~ D U
20~1292
- 74-
Table 14
Comparative Example
2 3 4 5 6
Pigment dispersing agent No. 43 44 45 46 47 48
xylene 20.0 20.0 33.2 30.0 25.0 11.3
solvent
cyclohexane 16.4 16.4 - 21.7 24.8 10.0
(Y~A) macromer No. A-21 A-22A-5 A-5 A-l9 A-20
compo-
nents compounding amount57.2 57.2 65.220.143.1 71.8
dimethyl aminoethYl 5 6 5 6
methacrylate
compo- dimethyl aminopropyl
compo- nents X methacrylamide - 4.1 6.8
sition
dimethyl aminopropyl 0.8 27 8
acrylamide
2,2'-azobis(2,4-
dimethyl- 0.80.80.8 0.4 3.0
initi- valeronitrile)
ator 1,1'-
azobiscyclohexane-l- - - - - - 0.1
carbonitrile
solid (Y~A) components 86.0 86.0 98.030.586.3 86.4
content compo e t X 14.0 14.0 2.0 69.513.7 13.6
components Z
amine value 50 50 7.2 250 45.1 44.9
Proper- numibhr average molecular 2000 10000 4000 4000 900 120000
nonvolatile content (~) 40 40 40 40 30 50
2041292
- 76 -
Table 15
Com)arative Example
7 8 9 10
Pigment dispersing agent No. 49 50 51 52
solvent xylene 33.2 51.6 35.9 35.9
(Y+A) macromer No. A-l A-l A-4 A-5
compo-
nents compounding amount65.220.2 58.358.3
sitiPOn compo- dimethyl aminopropyl0.8 27.8 5.0 5.0
nent X acrylamide
initi- 2,2'-azobis(2,4- 0 8 0 0 8
ator dimethyl-valeronitrile)~ ~4 0.8
solid (Y+A) components 98.030.3 87.587.5
content
ratio component X 2.069.7 12.512.5
amine value mgKOH/g 7.2250.0 45.045.0
Proper- hYdr~XYl value mgKOH/g 0 0 0 0
ties number average molecular weight 40001500 850 35000
nonvolatile content (~)40 40 40 40
2041292
- 76-
o , , o . ~ ~ o o ~ o o o , o ~ ~ ~ o ~ o o
~'1 1' ~ N IU~ 1 I N I I I ~ ~ O ~ 1 N O a~ CO O ~10 O O
~l ~~ U I ~ U~ I 1~ ~ I N I ~ N o o O ~ ~ ~ ~1 0 CO O O
~ I I o ~ I~ I I I N C ~ ~ ~ NO ~ O a O O
C ~ N ~ ~ ~ ~ O ~ ~ O ~ O O
-- ~' ~''¢ N 1' 1~ N o
li~D ~ U I ~ I I I O ~ I ~ O ~ O O O ~ O ~~ It7 0 ~' O O
U~ N I 0~ I N ~ ~ O
,~~ I ~ N I1~ N O ~ ~D ~1
~ 1 N I 0 In I~8 1 I N I ~ r ~ ~ ~ N o a~ ~ ~ CO O O
N ~ I N I 0 u~ I ~D I N I I ~ r o o U~ ~ ~ ~
U I NCO Iu~In I N I I ~ N O o ~ I ~ ~ a~ O 11~ 0 0
_
~ O ,~
a) _
E~
C U~
01 _
J~
~ -- ~ ~ ~ C 0 ~n
C ~ ~ C
O O O O O
O C~ O
In ~ I~ ~ 'G r
~~ N
~ U~ C . I ~ O ~J C l'~ r ~J C r~
~ ~l ~ --~ O) O r4 ~ C rn ~,1 r~ v
Z r' rn ~ ,~ O ~rd rr ~,1 C ~' ~ Cr
~ ~rl ~1 ~ OLl ~ ~ U Ul 1~1 L r ~~1 C
V rnLl ~ ~-1 f~ O Orn Ll ~U ~ a
C ., ~ f~l _ Ct: ~E ~,~ Cf~ Ll 1~1 _ Ll IU
4~I ~ ~ OLl~I -- Ll
~ C r r ~ ~o IJ J~
fd ~ r~ r~ o ~- ~, n U J
~ ~rl '~ Ll ~ L _ -rl ~ r , ~r
-~ ~ ~Llr l r l ~ Ll r l _ ~ ~r o
n z C ., c~ o :.~ C ~ U O f ~ ~ ~- -
r rn ~ ~ P~ X r~ sa cr, p~ p~ z ,~ v ~, ~ d
rn Ll --
~r
'~ Ll ~ rV ~ ~r ~
rl 1~ rl V
d J V 1~ r --~ C
r l J - ~r ~11 - 10 fd
Ll r l O ' '-
,e C4 , o rn ~ 0 1~ o ~ o
2041292
I ~ o~ I ~ ~ I I I I I u~ ~ o o u~ o O ~10 ~ o co o o
r~l r~l ~ I ~ Cl~ I I I I ~ r1 ~ ~ ~~
r~ r~ ~ r~ ~ ~ I~ N ~ N ~ ~ O a~
~ I", ~1 1 ~ I I I I ~ I N ~ O ~ ~ I ~ o ~ O C~ O O
- I ~ O ~ ~ ~ O ~ ~ ~ O ~r O O O ~ O O O O
U I ~ ~ I I ~ I I I ~ ~ ~ ~ ~ I ~ ~ ~ ~ I' O ~
~ N a~ I~ I~ I I I I I ~ N O ~ UO~ I r1 ~ U~ ~ ~
r~ ~ ~ U~ U~ I I NO I I I O ri O O O I O O O O
CJ~ N r~l O CO ~~ 00 0 0
--I ~ O _I
~I r:
R
E~
~ _
~ P.
-
~ ~J ~
-- -- -- -- -- c n ~q
~In~o1~-~1 ~ ________
~n Q~ a. N ~1 ~ n ~
~ o o o o o
t~ CO N U~ rl J
o co o I ~_ nn
N-- ~ r~ J ~ V
~rl J ~ O ~ O
~ ~n C -~ ~ o ~ C ~ ~ ~ C ,~ .
~ ~ O 1~ ~ q ~; vn ~,1 r
Z , C vo ~ o ~ J C vn ~,1 C
~ ~ ~ OL¦ ~ ~ Cw IwD. ~rl ~" vn ~ L V r r
vn w ~ ~,~ AJ ~.r G O O vq w a~ ., v
r ., ~ ~ -- ~ ~ ~ ~ q r~ ~ w ~AJ _ W
~ q w w ~ _ cw ~ o w w -- w
r o~ v. ~
,AJ . U ' ~ ~rl .r n t~
t~ ~ r ~ C~ r l ~ I ~ r ~ r ~L .r
C ~ ~_ ~ .-- ~r'-- ~
~ ~ r 'W r~r~ _ ~ , _ _ ~ ~ W r~ _ . -r o
~n Z .. , u o ~ ~-- c) O~ J O
r vn ~ P' x ~J r ~ ~ ~ Z ~ v ~ AJ
.rl ~)
vn w L L
~, ~ ~ I ~ vn ~ vn
~ W W ~r
vn ~,1 ~ I ~
r~ ~r
Q, vn _~,1 .,~
r l ~r ~ -- ,J ,AJ
~, , q r ~ G G~ Q E
_1L, r~l CW ~rl .1 ~1 ~J ,.1 Cw ~rl
~ O 0 1 ~ O ~ O ~
Table 17(a)
Application Ecample
21 22 23 24 25 26
Pigment dispersing agent No. 1) 17 18 19 20 21 22 23
Acrylic resin No. 2) AC-2 AC-2 AC-2 AC-2 AC-2 AC-2 AC-2
Polyester resin No. 3)
pigment dispersion agent 20 20 10 10 2.7 2.7 10
Acrylic resin 28.328.3 35 35 28.228.2 35
polyester resin
xylene 15.915.9 17.517.5 7.6 7.6 17.5
Formulati~n cellosolve acetate 15.8 15.817.5 17.5 7.5 7.5 17.5
of disper-
sion paste butyl cellosolve
Rubicron Red 500RG 4)
Paliogen Violet L-5080 5) 20 20
Paliogen Maroon L-3820 6)
Novoperm Red F3RK-70 7) - - 20 20 - - 20 Co
Titanium dioxide JR-603 8) ~ - ~ ~ 54 54
amount of pigment dipsersing agent added(~)9) 40 40 20 20 2 2 20
Oprfoppastees viscosity of dispersion paste (PS) 10) 5.4 5.2 2.4 2.3 9.8 9.9 2.3
stability of dispersion paste11) good good good good good good good
dispersion paste 40 40 40 40 50 50 40
acrylic resin 30 30 30 30 24.424.4 30 l~
FormulatiOn polyester resin
of paint melamine resin 12) 20 20 20 20 16.916.9 20 ~-~
leveling agent 13) 0.6 0.6 0.6 0.6 0.6 0.6 0.6 2
thinner 14) 9.4 9.4 9.4 9.4 8.1 8.1 9.4
30~ specular gloss 15) 86 86 86 86 84 84 85
Properties humidity resistance 16) good good good good good good good
of paint weather resistance 17) 86 85 87 86 85 84 87
film non-yellowing property 18)good good good good good good good
adhesion property 19) good good good good good good good
Table 17 ( b )
Ap~licaticn Example
27 28 29 30 31 32
Pigment dispersing agent No. 1) 24 25 26 27 28 29
Acrylic resin No. 2) AC-2 AC-2 AC-2 AC-2 AC-2 AC-2
Polyester resin No. 3)
pigment dispersion agent 10 10 10 20 2.7 2.7
Acrylic resin 35. 35 35 28.3 28.2 28.2
polyester resin
xylene 17.5 17.5 17.5 15.9 7.6 7.6
FormulatiOn cellosolve acetate 17.5 17.5 17.5 15.8 7.5 7.5
of disper-
sion paste butyl cellosolve
Rubicron Red 500RG 4)
Paliogen Violet L-5080 5)
Paliogen Maroon L-3820 6) - _ _ 20
Novoperm Red F3RK-70 7) 20 20 20 ~ ~ ~ ~D
Titanium dioxide JR-603 8) - - - - 54 54amount of pigment dipsersing agent added(%)9) 20 20 20 40 2 2
oPf~pPastees viscosity of dispersion paste (PS) 10) 2.1 2.2 2.3 5.5 10.0 9.7
stability of dispersion paste11) good good good good good good
dispersion paste 40 40 40 40 50 50
acrylic resin 30 30 30 30 24.4 24.4
FormulatiOn polyester resin
of paint melamine resin 12) 20 20 20 20 16.9 16.9 ~~~
leveling agent 13) 0.6 0.6 0.6 0.6 0.6 0.6
thinner 14) 9.4 9.4 9.4 9.4 8.1 8.1
30~ specular gloss 15) 86 86 87 87 85 86
Properties hUmidity resistance 16) good good good good good good
of paint weather resistance 17) 86 87 86 86 84 85
film non-yellowing property 18) good good good good good good
adhesion property 19) good good good good good good
Table 18
Ap-lication Example
33 34 35 36 37 38
Pigment dispersing agent No. 1) 30 30 31 32 33 34
Acrylic resin No. 2) AC-l - AC-2 - AC-l AC-l
Polyester resin No. 3) - PE-l - PE-2
pigment dispersion agent 20 20 10 10 20 3
Acrylic resin 28.3 - 35.0 - 28.3 28.3
polyester resin - 28.3 - 35.0
xylene 15.9 15.9 17.5 17.5 15.9 3
Formulati~n cellosolve acetate 15.8 15.8 17.5 17.5 15.8 5.7
of disper-
sion paste Rubicron Red 500RG 4) - - - 20
Paliogen Violet L-5080 5) - ~ 20
Paliogen Maroon L-3820 6) 20 20 - - 20 ~ g
Titanium dioxide JR-603 8) - - ~ - ~ 60
amount of pigment dipsersing agent added(%)9)40 40 20 20 40 5
Properties
of paste viscosity of dispersion paste (PS) 10) 5.7 8.6 3.3 6.4 6.9 9.8
stability of dispersion paste11) good good good good good good
dispersion paste 40 40 40 40 40 50
acrylic resin 30 - 30 - 40 24.4
Formulation polyester resin ~ 30 30
of paint melamine resin 12) 20 20 20 20 20 16.9 hP'
leveling agent 13) 0.6 0.6 0.6 0.6 0.6 0.6 2
thinner 14) 9.4 9.4 9.4 9.4 9.4 8.1 2~3
30~ specular gloss 15) 85 88 89 81 84 89
Properties hUmidity resistance 16) good good good good good good
of paint weather resistance 17) 85 85 85 80 82 85
film
non-yellowing property 18) good good good good good good
adhesion property 19) good good good good good good
Table l9(a)
Comparative pplication E~ample
1 2 3 4 5 6 7
Pigment dispersing agent No. 1) 43 44 45 46 47 48 49
Acrylic resin No. 2) AC-2 AC-2 AC-2 AC-2 AC-2 AC-2 AC-l
Polyester resin No. 3)
pigment dispersion agent 20 20 20 20 27 16 20
Acrylic resin 28.3 28.328.3 28.3 28.328.3 28.3
polyester resin
xylene 15.9 15.915.9 15.9 12.417.9 15.9
Formulati~n cellOsolve acetate 15.815.8 15.8 15.812.3 17.8 15.8
of disper-
sion paste butyl cellosolve
Rubicron Red 500RG 4)
Paliogen Violet L-5080 5)
Paliogen ~aroon L-3820 6) 20 20 20 20 20 20 20
Novoperm Red F3RK-70 7) - - ~ ~ ~ ~ ~
Titanium dioxide JR-603 8)
amount of pigment dipsersing agent added(%)9)40 40 40 40 40 40 40
of paste viscosity of dispersion paste (PS) 10) 65 80 90 70 75 101 87
stability of dispersion paste 11) bad bad bad bad bad bad bad
dispersion paste
acrylic resin
FormulatiOn polyester resin G
of paint melamine resin 12) - - - - - - - ~'
leveling agent 13) - - - - - - -
thinner 14) - - - - - - - CS~
30~ specular gloss 15)
Properties humidity resistance 16)
of paint weather resistance 17)
film
non-yellowing property 18)
adhesion property 19)
Table l9(b)
Comparative pplication E~ample
8 9 10 11 12 13 14
Pigment dispersing agent No. 1) 50 51 52 53 - 54 55
Acrylic resin No. 2)AC-l AC-l AC-l AC-l AC-2 - AC-l
Polyester resin No. 3) - - - - - PE-2
pigment dispersion agent 20 20 20 20 - 1.2 1.4
Acrylic resin 28.328.3 28.3 28.3 41.7 - 20.8
polyester resin - - - - - 20.8 20.2
xylene 15.915.9 15.9 15.9 19.230.4 20.0
Formulati~n cellosolve acetate 15.8 15.8 15.8 15.819.1
of disper-
sion paste butyl cellosolve - - - - - 10.0
Rubicron Red 500RG 4)
Paliogen Violet L-5080 5)
Paliogen Maroon L-3820 6) 20 20 20 20 20
Novoperm Red F3RK-70 7) ~ ~ ~ ~ ~ ~ ~
Titanium dioxide JR-603 8) - ~ ~ ~ ~ 37.6 37.6
amount of pigment dipsersing agent added(~)9) 40 40 40 40 - 2 2
oPf~pasteeS viscosity of dispersion paste (PS) 10) 69 75 108 65 108 5 7
stability of dispersion paste 11) bad bad bad bad bad good good
dispersion paste - - - 40 40 59.1 59.1
acrylic resin - - - 30 30 - 20.1
FormulatiOn polyester resin 20.1 - ~
of paint melamine resin 12) - - - 20 20 13.9 13.9 ~p,
leveling agent 13) - - - 0.6 0.6 0.5 0.5 ~~~
thinner 14) - _ _ 9.4 9.4 6.4 6.4 C~
30~ specular gloss 15) - - - 52 35 87 86 ~i
Properties hUmidity resistance 16) bad good good good
of paint weather resistance 17) - _ _ 53 88 88 87
film non-yellowing property 18) ~ ~ ~ good good bad bad
adhesion property 19) - - - bad good good good
Table 20
Application Example
39 40 41 42 43 44 45 46 47 48
Pigment dispersing agent No. 1) 35 36 37 38 39 40 41 42 35 35
Acrylic resin No. 2) AC-l - AC-2 AC-2 AC-2 AC-2 - AC-2
Polyester resin No. 3) - PE-l - - - - PE-l - PE-l PE-2
pigment dispersion agent20 20 10 20 20 2.7 10 10 20 20
Acrylic resin 28.3 - 35 28.3 28.3 28.2 - 35
polyester resin - 28.3 - - - - 35 - 28.3 28.3
xylene 15.9 25.4 17.5 15.9 15.9 7.6 28 17.5 25.4 25.4
FormulatiOn cellosolve acetate 15.8 - 17.5 15.8 15.8 7.5 - 17.5
of disper-
sion paste butyl cellosolve - 6.3 - - - - 7.0 - 6.3 6.3
Rubicron Red 500RG 4)
Paliogen Violet L-5080 5) - 20 - - 20
Paliogen Maroon L-3820 6) 20 - - 20 - - - - 20
Novoperm Red F3RK-70 7) - - 20 - - - 20 20 - 20 c~
Titanium dioxide JR-603 8) - - - - - 54
amount of pigment dipsersing agent added(%)9)40 40 20 40 40 220 20 40 40
of~pastteeS viscosity of dispersion paste (PS) 10)5.2 6.2 1.5 6.0 5.3 10.0 1.4 2.2 8.3 3.0
stability of dispersion paste11) good good good good good good good good good good
dispersion paste 40 40 40 40 40 50 40 40 40 40
acrylic resin 30 - 30 30 30 24.4 - 30 - - O
Formulation polyester resin ~ 30 - 30
of paint melamine resin 12) 20 20 20 20 20 16.9 20 20 20 20 ~g
leveling agent 13) 0.6 0.6 0.6 0.6 0.6 0.6 0.6 0.6 0.60.6 r~thinner 14) 9.4 9.4 9.4 9.4 9.4 8.1 9.4 9.4 9.49.4 ~g30~ specular gloss 15) 88 87 88 86 85 84 87 83 81 93
Properties humidity resistance 16) good good good good good good good good good good
of paint weather resistance 17) 85 90 86 87 87 84 89 88 85 85
film non-yellowing property 18)good good good good good good good good good goodadhesion property 19) good good good good good good good good good good
20~1292
-84-
Note:
1) Pigment dispersing agents according to the invention
(Tables 10-15)
2) Acrylic resin for film formation in Table 2
3)Polyester resin for film formation in Table 1
4) Rubicron Red 500RG (quinacridone series organic
pigment, made by Toso K.K., trade name)
5) Paliogen Violet L-5080 (thioindigo series organic
pigment, made by BASF AG, trade name)
IO 6) Paliogen Maroon L-3820 (perylene series organic
pigment, BASF AG, trade name)
7) Novoperm Red F3RK-70 (azo series organic pigment,
made by Hoechst, trade name)
8) titanium dioxide JR-603 (made by Teikoku Kako K.K.,
16 trade name)
9) amount as a solid content of pigment dispersing agent
added to pigment (%)
10) paste viscosity: measured by means of B-type
viscometer (trade name, made by Tokyo Keiki K.K.) at
20~C (unit: PS)
11) After the dispersion paste was left to stand at 50~C
for 5 days, the viscosity was measured at 20~C by means
of B-type viscometer. When the ratio of initial
viscosity to viscosity after 5 days is within a range of
0.9-1.4, the storage stability is good, while when it is
not less than 2.0, the property is poor.
2041~92
-85-
12) melamine resin: Uban 220 (made by Mitsui Toatsu
Chemicals, Inc., trade name, nonvolatile content: 60%)
13) leveling agent: Modaflow (made by Monsanto Co.,
trade name, 10% xylene solution)
14) thinner for dilution: cellosolve acetate/xylene =
50/50 (%)
15) Doligon goniophotometer (made by Hunter
Laboratories)
16) Humidity test: After the test sheet was held at
O 50~Cil~C in a moisture atmosphere of not less than 95%RH
for 120 hours, it was taken out from the above
atmosphere and left to stand for 24 hours. The good or
bad humidity resistance was evaluated by the size and
density of the resulting blister.
1~ 17) The 60~ specular gloss was measured by means of a
sunshine carbon weathermeter after the sheet was exposed
for 1,000 hours according to a method for weather
acceleration test of JIS D0205-7.6. The property was
represented by the gloss retention (%).
18) Resistance to yellowing: The good or bad resistance
to yellowing was judged by visually observing the
yellowing of paint film after the sheet was coated with
the paint.
19) Adhesion property: The good or bad adhesion property
was evaluated by a peeling state of a cellophane tacky
tape (JIS Z1522) when a central portion of the test
204129~
-86-
sheet was cut into square meshes of 11 parallel lines at
a distance of lmm so as to arrive to the sheet surface
through the paint film by means of a cutter knife (JIS
K5400 (1979)-6.15) and then the tape was adhered
thereonto and then peeled off upward.
1(~
1~
8~
~ . . ~ .....