Note: Descriptions are shown in the official language in which they were submitted.
The invention relates to new imidazolyl deriv-
atives, their use as curing agents in epoxy-resin
compositions, and curable epoxy-resin compositions
incorporating said imidazolyl derivatives and
comprising an epoxy resin and compounds of the general
formula (I), and optionally commonly used curing agents
and solvents, for the manufacture of molded articles.
In the manufacture of composite materials, two
basic processes are employed today.
One of these is the wet-layup process, a one-step
process in which reinforcing materials are impregnated
with a curable mixture and heat-cured in one step to
the thermoset final state.
In the other process, the two-step process,
so-called prepregs are first produced from reinforcing
materials and a curable mixture, and these prepregs are
then processed into finished parts in a separate second
step. With respect to operating procedure, a
distinction is made between working with and without
solvents.
The prepregs are normally produced in a continuous
operation in which the reinforcing materials are passed
through an impregnating bath of the resin/curing agent
mixture being used, or the impregnant is mixed only
just before it is applied to the base material and then
spread thereon with a special device. The amount of
impregnant to be applied to a given base-material web
is controlled not only through the viscosity of the
impregnant but also through squeeze rolls located
downstream.
With solvent-containing systems, the solvent con-
tained in the impregnating solution is evaporated
through heat input after the impregnating operation,
and the resin system is converted at the same time from
JMK SC~EP~I NG: 2 5 0 5 . APP
2 ~
the A stage to the B stageO Depending on the operating
conditions and the resin system used, the reinforcing
materials impregnated with liquid to highly viscid
impregnant are thus turned into a prepreg that is
slightly tacky to almost dry. In this process step it
is important that the solvent be completely eliminated
from the impregnating mixture and that the latent
curing agent needed to cure the prepreg in the second
process step not be activated just yet, as this would
cause the impregnated reinforcing materials to react
completely, which is not desired.
With solventless systems, depending on the
chemical composition of the resin system the material
either also undergoes a short heat treatment after
impregnation or the reinforcing materials are lined on
both sides with release sheets immediately after
impregnation, without any separate heat treatment, and
placed into intermediate storage appropriate to the
system. During this intermediate storage, either a
gradual transition of the resin system to the B stage
takes place or the impregnant is fixed on the base
materials through physical effects alone and largely
without chemical changes.
The prepregs so obtained can be stored and shipped
as rolls before they are cut to size, as required for
the intended end use, and stacked to the thickness of
the finished part. Under the simultaneous action of
pressure and heat, the prepreg stack is completely
cuxed to give a high-strength molded part, the still
low-molecular-weight, fluid resins being thus converted
to the high-molecular-weight C stage of a thermoset.
While in the one-step process long open times and
short cure times at low cure temperatures are required,
prolonged storage stability of the prepregs is an
JMX-SCH3~RING: 2505 . APP
additional reguirement in the two-step process.
Storage temperatures lower than room temperature have
become steadily less acceptable in practice.
Of importance is further that, depending on the
prepreg manufacturing method, the viscosity of the
ready-to-use curable mixture remains substantially
constant for as long a period as possible. This is
necessary, especially when an impregnating bath of
large volume is used, for achieving constant resin
deposition and an invariant B stage since the
manufacturing conditions cannot be continually adjusted
to changing relationships within the curable mixture
and since fluctuations in the viscosity would have an
adverse effect on the physical properties of the full
cured end product.
What is desired in practice is a curable mixture
whose viscosity remains constant in the impregnating
bath for an extended period of time and which can then
be stored as a prepreg at room temperature for a long
time without undergoing chemical changes.
Regardless of how they are manufactured, the
prepregs should cure completely within a short time at
the lowest possible temperature, the maximum
temperature of the exothermic reaction should remain at
a low level even with moderately thick layersl and the
profile of physical properties of the finished products
should meet practical requirements.
These requirements concerning curing behavior and
profile of properties apply alss to epoxy-resin systems
to be processed by the wet-layup method.
For certain applications, all that is required
and, in fact, desired is a partial cure to the point
where the molded articles are dimensionally stable,
complete curing taking place, optionally after
JMK-SCHERING: 2 505 . APP
2 ~ 3 ~
intermediate storage, in a subsequent tempering
operation at the necessary temperatures. However, it
is important that even during the partial cure, the
thermal stability of the material increase to a level
above the cure temperature or otherwise the temperature
of the molded article will have to be lowered before it
can be removed from the mold.
Dicyandiamide, long used as a latent curing agent
in curable mixtures based on epoxy resins, is usually
combined with co-curing agents and/or accelerators to
obtain the desired properties. A great many
suggestions for its use in this field are known from
the literature.
While dicyandiamide solutions can be used to
produce homogeneous substrates, the use of solvents
gives rise to other problems.
Dicyandiamide is soluble in sufficient amounts in
only a few solvents, particularly dimethylformamide and
methyl glycol. However, these solvents are
toxicologically hazardous and create problems both in
the manufacture of the prepregs, that is, during
impregnation of the reinforcing materials and conver-
sion to the B stage, and in waste disposal.
Since dicyandiamide is only sparingly soluble,
rather large amounts of solvents must be used, and
these affect the impregnating viscosity in such a way
that the binder content on the reinforcing materials
cannot be chosen as desired.
! Inasmuch as these solvents canno~ be removed com-
pletely during the cure, there is, moreover, the danger
that when the finished parts are subjected to thermal
stresses the material will fail prematurely and/or the
solvents will be given off uncontrolled to the ambient
air in the field.
JMR-SCHERING: 2 5 0 5 . APP
When solid crystalline dicyandiamide is used
without solvents in liquid epoxy resins, the necessary
amount of dicyandiamide is either dispersed directly in
the epoxy resin or a highly filled dicyandiamide/epoxy
resin paste is first prepared and later adjusted with
the bulk of the epoxy resin to the desired resin/curing
agent concentration.
In either case, preparation of the dispersions is
not a simple matter. Moreover, when standing for an
extended period of time, particularly under
impregnating conditions, the dispersions tend to
separate.
When solid crystalline dicyandiamide is used
without solvents in epoxy resins which at room
temperature are solid, a paste of dicyandiamide and
liquid epoxy resins is also ~irst prepared and then
worked into the solid-resin melt at elevated
temperature.
Apart from the problems outlined, undesired
amounts o~ liquid epoxy resins are introduced into the
solid resin when this operating procedure is employed.
Moreover, when solid crystalline dicyandiamide is
used, inhomogeneities which are due to undissolved and
unreacted particles are observed in the cured
substrates.
The present invention seeks to overcome the draw-
backs of the prior art and to provide curable mixtures,
based on epoxy compounds and latent curing agents
soluble or homogeneously dispersible in the epoxy
resins, which partially cure to the dimensionally
stable state or completely cure to the thermoset final
state at relatively low temperatures within a short
time and without high peak exotherms, whose thermal
- stability meets practical requirement~, and in which
'
J~C--SC}I}~RING:2505.APP
,~
~,~
prepregs have adequate storage stability at low
temperature.
This goal is attained through the use of a new
curing agent, optionally with the concurrent use of
conventional latent curing agents.
The invention thus, in one respect, relates to
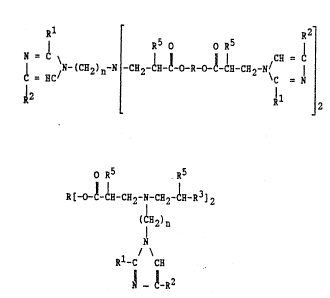
compounds of the general formulas
11 R5 o o R5 ~2
N = ~ ~ ~
¦ ~N-(CH2)~-N -C~-CH-C-0-R-0-C-~-c~2 N\C I (I)
2 11 2
and
0 ~5 R5
R[-o-l-lH-~H2-N-CH2-~H-R3]2
2)n
N (II)
R -C C~
,1 ~-R2
where R is a divalent, optionally branched aliphatic,
10 cyclic or alicyclic hydrocarbon group having from 2 to
20, and more particularly from 4 to 8, carbon atoms and
cptionally containing ether groups; R1 and R2 are,
independently of one another, hydrogen, or aliphatic or
aromatic hydrocarbon groups, and more particularly H,
CH3 or C2H5; R3 is -COOH, CN, -COOC2H4-OH, ~CONH-NH2 or
-CooR4; R4 is an aliphatic hydrocarbon group having~from
JM}C-SCHERING: 2 505 . APP
l to 4 carbon atoms, and more particularly -CH2-CH2-OH;
n is 2 or 3; and R5 is hydrogen or a methyl group.
The invention further relates to curing agents for
glycidyl compounds which can be prepared by reacting
(A) imidazolyl compounds of the general formula
CH =
H2N- (CH2) n~N
(III)
C =
~1
where Rl and R2 are, independently of one another,
aliphatic or aromatic hydrocarbon groups, and more
particularly H, CH3 or C2H5, and n is 2 or
preferably 3, with
(B) diacrylates of the general formula
R(-0-C-C=CH2)~ (IV)
1 15
where R is a divalent, optionally branched
aliphatic, cyclic or alicyclic hydrocarbon group
having from 2 to 20, and more particularly from 4
;~ to 8, carbon ats~ms, and R5 iS hydrogen or a methyl
group,
in a molar ratio of (A~ to (B) of from l:2 to 2:l, and
optionally further reacting these addition compounds
with
(C) (l) acrylic acid or derivatives of acrylic acid
of the
JMX-SCHERING:2505.APP
general fsrmula
CH2=C-R3 ~V)
~5
where R3 is -COOH, -CN, -CONH-NH2, -COOC2H4-OH or
-CooR4 and R4 is an aliphatic hydrocarbon group
having from 1 to 4 carbon atoms when the addition
product of (A) and (B) contains free amine
hydrogen atoms, or with
(2) imidazoles of the general formula
N ~ I
NH (VI)
= CH
where Rl and R2 are, independently of one another,
H, CH3, C2H5 or phenyl when the addition product of (A)
and (B) has terminal double bonds (according to formula
;. [IV]),
the compounds of step (C) being used in such
amounts that all reactive amine hydrogen atoms or
double bonds of the addition products of (A) and (B)
are reacted.
One object of the invention is the use of
compounds of the general formulas
JM~C-SCHERING: 2 51:)5 . APP
Il R5 o ~ R5 ~2
¦ ~C/N-(cH2)n-N -CH2-1H-~-O-R-O~ R-CH2-N~ ~ (I)
2 ~1 2
and
~ R5 R5
Rl-o~ -cH2-N-~2-~N-R3]2
(~2)n
/~\ ~II)
Rl-C CH
N _ ~-R2
where R is a divalent, optionally branched aliphatic,
cyclic or alicyclic hydrocarbon group having from 2 to
20, and more particularly from 4 to 8, carbon atoms and
optionally containing ether groups; R1 and R2 are,
independently of one another, hydrogen, or aliphatic or
aromatic hydrocarbon groups, and more particularly H,
CH3 or C2H5; R3 is -COOH, -CN, -COOC2H4-OH, -CONH-NH2 or
-CooR4; R4 is an aliphatic hydrocarbon group having from
1 to 4 carbon atoms, and more particularly -CH2-CH2-OH;
n is 2 or 3; and R5 is hydrogen or a methyl group,
optionally with the concurrent use of commonly used
nitrogen-containing heterocyclic amino compounds, as
curing agents for epoxy resins.
The invention has as a further object molded
epoxy-resin articles characterized in that in a first
step the reinforcements or embedm~nts are impregnated
J~5K-SCHERING: 2505 .APP
J
at room temperature with a binder, composed o~
(a) an epoxy resin with more than one epoxy group per
molecule on the average;
(b) compounds of the general formulas
Jl R5 o o R5 R2'
N = \ ~ C~ = ~
~N-~C~2)n-N -~2-~H-~-O-R-o-~ c~2-N/ ~ (I)
2 I1 2
and
o ~5 ~5
R[-o-J-CH-C~2-N-CH2-1~-R3]2
(IH2 ) n
II III)
Rl-C Cll
: ~ J ~2
. 5 where R is a divalent, optionally branched
aliphatic, cyclic or alicyclic hydrocarbon group
having from 2 to 20, and more particularly from 4
to 8, carbon atoms and optionally containing ether
YrOUP5; R1 and R2 are, independently of one
another, hydrogen, or aliphatic or aromatic
hydrocarbon groups, and more particularly H CH3
Or C2H5; R3 iS -COOH -CN COOC2H4-OH _CONH-NH2
or -CooR4; R4 is an aliphatic hydrocarbon group
: having from 1 to 4 carbon atoms, and more particu-
~ 15 larly -CH2-CH2-OH; n iS 2 or 3; and R5 is H or CH3;
~ and optionally
JMK-SCHERING: 2505 .APP
2 ~
(c) commonly used solvents, fillers, reinforcements or
embedments, pigments and auxiliaries; and
optionally
(d) commonly used nitrogen-containing heterocyclic
amino compounds,
and in a first step converted to the solid and
dimensionally stable state and then, in a second step,
fully cured at a temperature that is below the
softening point of the molded articles formed in the
first step.
Further objects of the .invention are set forth in
the claims.
The imidazolyl compounds of the invention can be
prepared by addition reactions, the acrylate compounds
being reacted in a first step with N-aminoalkyl-
imidazolyl compounds containing primary amino groups,
in a ratio of mols of acrylate compound to mols of
primary amino groups that may range from 1:2 to 2~
and, in a second step, acrylic acid or derivatives of
acrylic acid being added to the secondary amino groups
formed, and imidazolyl compounds of formula (VI3 to the
freP double bonds. The addition reactions of the first
and second steps generally are carriad out by known
methods.
The acrylate compounds which, in accordance with
the invention, are used in making the addition
compounds are esterification or transesterification
products of polyhydric alcohols and acrylic acids.
These reactions generally are carried out by known
methods.
The alcohols here used may be straight- or
branched-chain polyhydric aliphatic alcohols, and
particularly diols such as butanediol, hexanediol,
octanediol, neopentyl glycol or decanediol, or cyclic
JMK-SCHERING: 2505 .APP
12
or alicyclic diols such as 1,4-cyclohexanediol or
1,4-dihydroxymethylcyclohexane, or diols containing
ether groups, such as tetraethylene glycol or tri-
propylene glycol.
The imidazolyl compounds which, in accordance with
the invention, are used in making the addition products
are compounds of the general formula
R2
CH =
H2N-(CH2)n~Ni ~ I (III~
C =
where R1 and R2 are, independently of one another,
aliphatic or aromatic hydrocarbon groups, and more
particularly H, CH3 or C2H5, and n is 2 or preferably 3.
From l to 2 mols of the imidazolyl compound of formula
(III) are used per mol vf acrylate compound of the
aforesaid acrylate compounds of formula (IV).
The acrylic acid or derivatives of acrylic acid
used in accordance with the invention are compounds of
the ~eneral formula
CH2 ~ C-R3 (V)
where R3 is -COOH, -CN, -COOC2H4-OH, -CONH-NH2 or
-CooR4; and R4 is an aliphatic hydrocarbon group having
from 1 to 4 carbon atoms, and more particularly CH3 or
C2H5 .
SCHERING: 2 505 . APP
One mol of the acrylic acid compounds is used per
secondary amino group of the addition compounds made in
the first step.
The imidazolyl compounds containing only one amine
hydrogen atom which, in accordance with the invention,
are added to the addition compounds with free double
bonds made in a first step are compounds of the general
formula
R
N
¦ NH (VI~
C~l
~2
where R1 and R2 are, independently of one another, H,
CH3, C2H5 or phenyl. One mol of the compounds of
formula (VI) is used per double bond.
The addition products of the invention which can
be prepared by these process steps are compounds of the
general formulas
R1 RS O O ~5
N =, ~ ~ C~ -
(CR2)n-N -CR2-~B-~-O-R-O~ B-C~2~N/ l (I)
~2 ~1 2
and
o R5 R5
Rl-o-c-lH-cR2-N-c~2-l~-R3]2
(1~2)~
~ lII)
/ \
Rl_C CH
,1 ~-R2
where R is a divalent, optionally branched aliphatic,
cyclic or alicyclic hydrocarbon group having from 2 to
: 20, and more particularly from 4 to 8, carbon atoms and
optionally containing ether groups; Rl and R2 are,
independently of one another, hydrogen, or aliphatic or
aromatic hydrocarbon groups, and more particularly H,
CH3 or C2H5; R3 is -COOH, -CN, -COOC2H4-OH, -CONH-NH2 or
-CooR4; R4 is an aliphatic hydrocarbon group having from
1 to 4 carbon atoms, and more particularly -CH2-CH2-OH;
n is 2 or 3; and R5 is hydrogen or a methyl group.
Apart from the preferred compounds of the general
formulas (I) and (II), which are formed when the ratio
between the imidazolyl compounds of formula (III) and
the acrylate compounds of formula (IV) is an
even-numbered 1:2 or 2:1, molar ratios between these
values will yield structured products which contain the
structural unit
.
R5 O O R5
~_N_~H2_~H_I_O_R_O_~_~H_CH2_J m
(1H~)n (VII)
Rl-~ CH
d ~-R2
repeatedly (m) in the chain and have derivatives of
either formula (V) or formula (VI) as end groups.
Both m, which is preferably between 1 and 5, and
more particularly between 1 and 3, and the end groups
can be determined at will through the choice of the
molar ratios of compounds of formula (III) to compounds
JM}C-SCHERING: 2 505 . APP
of formula (IV).
It thus becomes possible to control the catalytic
activity (tertiary or total nitrogen content) and to
adjust the viscosity from low-viscosity to
high-viscosity to solid.
In a first step, the addition products of the
imidazolyl compounds of formula (III) and the acrylate
compounds of formula (IV) are prepared, in molar ratios
that assure that no double bonds are present at the
same time as tertiary aliphatic nitrogen, and in a
second step the derivatives of acrylic acid of formula
(V) are added.
Preferably, however, all components are reacted
simultaneously. A statistical mixture is then obtained
which usually is less homogeneous than is the case when
a stepwise procedure is employed. Care should be taken
to assure that there is no excess of acrylic acid or
derivatives thereof while tertiary nitrogen is also
present. No significant difference has been observed
so far as suitability for the end uses contemplated by
the invention is concerned.
The curing agents of the invention can be used
singly or as a mixture at the rate of from 2 to 35 g,
; and more particularly from 4 to 25 g, but preferably
from 5 to 20 g, of curing agent per 100 g of epoxy
resin.
The imidazolyl compounds of the invention can also
be used in the form of their salts. Use may here be
made of the organic and inorganic salt formers known in
this field. In accordance with the invention, however,
mono- or polybasic organic carboxylic acids are
preferred, branched-chain monocarboxylic acids having
up to 10 carbon atoms, such as 2-ethylhexoic acid,
being particularly well suited.
JM~C-SCHERING: 2505 . APP
The epoxy resins which, in accordance with the
invention, are used as a binder constituent are
glycidyl esters and ethers with two or more epoxy
groups per molecule, and preferably glycidyl ethers
based on mono- or polyhydric phenols. In accordance
with the invention, glycidyl ethers of
2,2-bis(4-hydroxyphenyl)propane (bisphenol A) with
epoxy values of from 0.2 to 0.6, and particularly the
compounds with epoxy values of from 0.45 to 0.55 which
: 10 are liquid at room temperature, are preferred.
The glycidyl ethers based on bisphenol F and the
novolacs have also proved advantageous.
~ Also usable are the commercial halogenated, and
t more particularly brominated, epoxy resins based on the
aforesaid phenols.
The amino compounds which, in accordance with the
invention, may also be used are preferably commonly
used nitrogen-containing heterocyclic amino compoun~s,
that is, N-alkylimidazoles such as N-methyl- or
: 20 N ethylimidazole, and/or imidazoline compounds of the
general formula
: tHINH-CH2-CH21X-N - C-~z R tVlII)
H2
CH2
where R is an optionally branched alkyl or alkylene
group having fewer than 10 carbon atoms, and more
particularly -CH3, -CHOH-CH3 or -(CHR~)y~ R' is H or
-CH3, x is 1, 2 or 3, y is from 4 to 8, and z is equal
to the valence of R, and particularly those where x is
1, z is 1, and R is -CH3 or -CH2-CH3. Other curing
J2~5}C-SCHERING: 2 5 0 5 . APP
2 ~
17
agents commonly used in this field may also be used, if
desired.
For modification of the properties of the end
product, other epoxy resins may be used concurrently,
as may modifiers or auxiliaries such as phenolic
resins, melamine resins, silicone resins, inorganic and
organic fillers such as quartz powders, titanium
dioxide, carbon black, and silicone or butadiene
rubber.
To obtain the desired viscosity, resins of
di~ferent viscosities, diluents, or such commonly used
solvents as dimethyl formamide, acetone, methyl ethyl
ketone, methyl glycol or propylene glycol monomethyl
ether, or mixtures thereof, may be used.
In prepregging, organic and inorganic fibers,
nonwovens and woven fabrics based on aramid, carbon or
cellulose, metals such as boron, steel, etc., ceramics
and especially glass are used.
The solvent-containing prepregs are generally made
by known methods, in which the base materials are
impregnated with the reactive resin mixture in an
impregnating bath and, after the excess resin has been
squeezed off, continuously converted from the A stage
to the B stage with input of energy (mostly heat) and
simultaneous removal of the solvent. Depending on the
desired preprey consistency (viscid to solid), the
prepregs are then provided on both sides with a release
sheet and wound into a roll for storage and shipping.
The further processing involves cutting the individual
prepreg layers to size and assembling them into a
stack, from which a highly crosslinked part is produced
by shaping with simultaneous heat input.
The curing agents of the invention can als~ be
used successfully in solventless prepregs based on
JMR--SCHERING: 2505 .APP
2 ~ r3 ~3
18
epoxy resins and, optionally, commonly used curing
agents. Here the base materials are impregnated at
optionally elevated temperature and by conventional
methods with the binder system and placed into storage
appropriate to the system before they are processed
further like solvent~containing systems.
Further examples of solventless systems are
wet-layup laminates, base materials for the electrical
industry, fiber-reinforced molded parts produced in
situ, heat-curing one-component adhesives for the
bonding of body sections in the automotive industry
(flange-joint adhesives), for example, as well as
epoxy-resin castings, epoxy-resin coatings and
epoxy-resin filament- or tape-wound structures.
Both the imidazolyl compounds and the acrylates
used in the examples are commercial products of BASF
AXtiengesellschaft, Ludwigshafen, and have been used in
that technical grade.
EXAMPLES
(I) PREPARATION OF THE CURING AGENTS OF THE INVENTION
Example 1
41.7 g (0.33 mol) of 1-(3-aminopropyl)imidazole
and S4.7 g (0.66 mol) of 2-methylimidazole are
dissolved in 70.0 g of ethanol at about 60~ C and
slowl~ mixed with 132.0 g ~0.66 mol) of butanediol
diacrylate. The mixture is allowed to react for
another hour at 70 C, and the ethanol is then drawn
of~.
A product is obtained which has the following
characteristics:
Amine value: 342-343
Viscosity/25~ C: 1.2 Pa s
JMK-SCHERING: 2 505 . APP
19
ExamPle 2
250 g (2 mols) of 1-(3-aminopropyl)imi~azole is
introduced as initial charge and slowly mixed at 60-80
C with 198 g (1 mol) of butanediol acrylate with
cooling.
The addition product has the following
characteristics:
Amine value: 494-497
Viscosity/25 C: 1.6 Pa-s
448 g of this addition product is slowly mixed at
60 C with 232 g (2 mols) of 2-hydroxyethyl acrylate
with cooling.
The mixture is allowed to react for another hour
at 80 C. A product is obtained which has the
following characteristics:
Amine value: 326-327
Viscosity/25 C: 10.4 Pa-s
Example 3
375 g ~3 mols) of 1-(3-aminopropyl)imidazole is
introduced as initial charge and slowly mixed at 60-80
C with 396 g (2 mols) of butanediol acrylate with
cooling.
The addition product has the folîowin~
ch~racteristics:
Amine value: 435-437
Viscosity/25 C: 5.1 Pa-s
385.5 g of this addition product is slowly mixed
at Ç0 C with 116 g (1 mol) of 2-hydroxyethyl acrylate
with cooling.
The mixture is allowed to react for another hour
at 80 C. A product is obtained which has the
following characteristics:
JMK-SCHERING: 2 51:) 5 . APP
.
Amine value: 335-337
Viscosity/25 C: 6.7 Pa s
Example 4
125 g (1 mol) of 1-(3-aminopropyl)imidazole and
164 g (2 mols) of 2-methylimidazole are dissolved in
150 g of ethanol at about 60 C and slowly mixed with
452.6 g (2 mols) of hexanediol diacrylate at
temperatures ranging from 60 to 80 C. The mixture is
allowed to react for another hour at 70 C, and the
ethanol is then drawn off.
A product is obtained which has the following
characteristics:
Amine value: 299-301
Viscosity/25 C: 2.5 Pa s
Example 5
300 g of the product of Example 4 is homogeneously
mixed with 58.3 g of 2-ethylhexoic acid at 80 C. A
product is obtained which has the following
characteristics:
Amine value: 252
Viscosity/25 C: 4.3 Pa-s
Example 6
125 g (1 mol) of 1-(3-aminopropyl)imidazole is in-
troduced as initial charge, and 198 g (1 mol~ of
butanediol diacrylate is slowly added at temperatures
ranging from 60 to 80 C. After the exothermic
reaction has subsided, stirring is continued for 3 hr
at 90 C. A product is obtained which has the
following characteristics:
Amine Yalue: 345-346
Viscosity/2~ C: 1290 Pa-s
J~K-SCHE~ING: 2 5 0 5 . APP
2~4~33~
(II) PREPARATIONnOF A PREPREG REACTION MIXTURE
Example 1
100 g of an epoxy resin (epoxide equivalent weight
about 190) is mixed with 14 g of the inventive reaction
product of Example (I) 1 and used to make prepregs.
This mixture has a viscosity at room temperature (25
C) of 11.6 Pa s and is workable even after 10 hours.
The prepregs are produced on the laboratory scale
by spreading khe reaction mixture onto a glass-filament
fabric in a satin weave, measuring about 0.1 m2, which
after impregnation is lined on both sides with release
sheets and then stored at room temperature.
Aftar 24-hour storage at room temperature, the
material has aged sufficiently to be processed as a
slightly tacky prepreg in several layers by the
hot-press molding method at 0.1 bar and temperatures of
from 100 to 120 C, in from 30 minutes to 1 hour, into
high-strength molded articles. The finished product,
fully cured in this manner, exhibits no flaws of any
kind with respect to adhesion of the individual prepreg
layers.
The storage-stability values given in Table 1 are
determined on the basis o~ conditions duplicating those
used in actual practice. The impregnated fabric is
~tored between two polyethylene sheets at 23 C under
standard climatic conditions. A layer of a specimen is
molded at 24-hour intervals under conditions
duplicating those used in actual practice (1 hr, 120
C, 0.1 bar). The storage-stability value indicated is
based on the last day on which the resin is fluid under
hot-press molding conditions. The other
~torage-stability values given in Table 1 are
determined in the same way as the one for Example 1.
J~K-SCHE~ING:2505.APP
22
Table 1
Storage stability of solventless prepregs
_ -~
Example Curing Curing Epoxy Storage
agent, agent, resin, stability,
Example g 100 g days
I .... _ ... _
1 (I) 1 14 Bisphenol ~ 6
value
_
2 (I) 2 5 id. )25
3 (I~ 3 5 id. ~ 6
4 (I) 4 14 id. ~ 6
_ _ _
(I) 5 14 id. ~ 6
(III) DETERMINATION OF INFLUENCE OF CURING AGENT
To determine the properties of the curing agent as
a function of structure, the mixtures, composed only of
epoxy resin and curing agent so as to eliminate any
distorting influences of reinforcements and additives,
are fully cured and tested.
In the examples listed in Table 2, a glycidyl
ether based on bisphenol A and having an epoxy value of
0O53 is used as epoxy resin.
~ o produce the test specimens, 100 g of epoxy
r sin is mixed in each case at room temperature with
the amount of curing agent indicated in Table l and
, completely cured in a steel mold for 2 hr at ~20 C to
give flat molded parts 4 mm thick. From these molded
parts, test specimens are then taken by sawing or
milling. On these specimens, the properties specified
in Table 2 are determined in conformity with the test
standards listed below.
JM~C--SCHERING: 2505 .APP
Test-specimen dimensions
~ . . _ .. . _.
Flexural DIN 53,452 80 x 10 x 4 mm
strength
i - ---__
Deflection DIN 53,452 80 x 10 x 4 mm
Impact strength DIN 53,453 50 x 6 x 4 mm
Tensile strength DIN 53,455 Dumbbell No. 3
=~ .__ _
Elongation DIN 53,455 Dumbbell No. 3
_ ~ _ ._ .__ ~T
Modulus of DIN 53,457 Dumbbell No. 3
elasticity
Heat-distortion DIN 53,461 120 x 10 x 4 mm
temperature
Glass-transition DIN 53,445 80 x 10 x 1 mm
temperature
. _
Table 2
Thermal and mechanical properties
.
IExample _ (unit? 1 2 3 4 5
¦Flexural N/mm2 93 45 26 82 78
¦strength
I ~ _
¦Deflection mm 13.74.3 4.8 8.3 7.8 ¦
¦Impact kJ/m2 12.5 3.03.1 8.55.5 ¦
Istrength
I . _
¦Tensile N/mm2 50 31 34 41 43
strength
_ . I
Elongation ~ 2.21.2 1.2 1.7 1.8
_
l Modulus of N/mm2 28002850 2970 2850 2740
l elasticity
__ , _ _
Heat- C 127 127127 127 122
distortion
temperature
_ ~
3G Glass C153 163151 153149
transition
temperature _
JMK-SCHERING: 2 505 . APP