Note: Descriptions are shown in the official language in which they were submitted.
7 9 3
! O.Z. 0050/41598
Cyclohexenone oxime ether~, their pre~ara~ion and thei.r
use a~ herbicides
The present invention relates to novel herbicidal
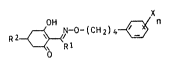
cyclohexenone oxime ethers of the formula I
~ ,-o-(cH2)4 ~ xn (I)
whe~e Rl i5 Cl-C6-
X is halogen,
n is from 1 to 5,
R2 is Cl~C~-alkoxy-Cl-C6-alkyl or Cl-C4 alkyl~hio-Cl-C~-
alkyl,
C3-C7-cycloalkyl or C5-C7-cycloalkenyl, and these groups
may additionally carry from one to three radicAls
selected from the group con~isting of Cl-C~-alkyl, Cl-C4~
alkoxy, Cl-C4-alkylthio, Cl-C4-haloalkyl, hydroxyl and
halogen,
a 5-membered saturated heterocyclic structure which
contains one sr two hetero atoms ~elected from the group
consisting of oxygen and sulfur and may additionally
carry from one to three radicals selected from the group
consisting C1-C4-alkyl, Cl-C4-alkoxy, Cl-C4-alkylthio and
Cl-C4-haloalkyl,
a 6-membered or 7-me~bered saturated or mono- or
diunsaturated hoterocyclic structure containing one or
two~.hetero atom~ selected from the group con~isting of
oxygen and sulfur, and the heterocyclic ~tructure may
additionally carry ~rom one to three radicals selected
from the group con~i~tinq of hydroxyl, halogen, C~-C4-
alkyl, Cl-C4-alkoxy, C~-C4-al~ylthio and C1-C4-haloalkyl,
30 a 5-membered heteroaromatic ~tructure containing one to
three hetero atoms selected from the group consisting of
two nitrogen atom~ and one oxygen or ~ulfur atom, and
this ring may additionally carry from one to three
radicals ~elected from the group consi~ting of halogen,
cyano, Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-alkylthio,
: .
`` 2~79~3
- 2 - O. Z . 0~50/41598
Cl-C4-haloalkyl, C2-C6-alkenyl, C2-Ca-aIkenyloxy, CZ-C6-
haloalkenyl and Cl-C4-alkoxy- C1-C4-alkyl,
phenyl or pyridyl ~ and the~e group~ may additionally
carry from one to three radicals selected from the group
consisting of halogen, nitro, cyano, Cl-C4-alkyl, Cl-C4-
alkoxy, Cl-C4-alkylthio, Cl-C4-haloalkyl, C3-C6 alkenyloxy,
C3-C6-alXynyloxy and -NR3R4, where
R3 is hydrogen, C1-C4-alkyl, C3-C6-alk~nyl or C3-C6-alkynyl
and
R4 is hydrogen, C1 C4-alkyl, C3-C6-alkenyl, C3-C6-alkynyl,
C1 C6-acyl or benzoyl, and ~he aromatic ring may addition-
ally carry from one to three radical~ selected from the
group consisting of nitro, cyano, halogen, Cl-C4-alkyl,
Cl-Cb-alkoxy, Cl-C4-alkylthio and C1-C4-haloalkyl,
and ~heir agriculturally usable salts and esters of
Cl-C1O-carboxylic acid and inorganic acid~.
The present invention furthermora relates to a
process for their preparation and their use as crop
protection a~ents.
The novel cyclohexenone~ I obviously have acidic
character, ie. they can form simple reaction products,
such a~ salts of alkali metal or alkaline earth metal
compounds or enol e~ters.
Tha compounds of the formula I may occur in a
plurality of tautomeric forms, all of ~hich are embraced
by the claim.
DE-A 38 38 309 describes herbicidal cyclohexenone
oxi~ne ethers whoRe general formula cover~ the axyl-
butyleneoximinocyclohexanediones I defined at tha outset.
Compound of the formula I'
X
2~4 ~ I'
o R
where X is p-trifluoromethyl or p-tert-butyl, n i 0 or
1 and R1 and R2 have the meanings stated under I, are
2 ~ 3
I - 3 - O.Z. 0050/41598
mentioned in particular.
It i~ an object of the present invention to
synthesize cyclohexenon~ oxime ether~ which have high
sel~ctivity compared with the known members of thi~ class
of substances in controlling weeds in gramineous crop~,
such as rice.
We have found that thi~ object is achieved by the
novel cyclohexenone oxime ether of the formula I which
are defined at the outset and which have a good herbicid-
al action, preferably against specie~ from the gra sfamily (Gramineae). They are tolerated and hence selec-
tive in broad-leaved crops and in monocotyledon culture~
which do not belong to the Gramineae. They also include
compounds which exhibit selectivity also in gramineous
crops and at ~he same time centrol undesirable gras~e~.
The cyclohexenone~ of the formula I can be
prepared in a conventional manner from derivatives of the
formula II (EP-A 80 301, EP-A-125 094, EP-A-142 741, US-
A 4 249 937, EP-A 137 174 a~d EP-A 177 913) and the
corresponding hydro~ylamines of the formula III (EP-A 169
521).
R2 ~ ~ ~ H2NO(CH2)4 ~ ~ R2 ~ ~o(CH2)4 ~ xn
II III I
The reaction is advantageously carried out in the
heterogeneou~ pha~a in a solvent, at an adequate tempera-
ture below about 80C, in the pre~ence of a base, and the
hydroxylamine III i~ used in the form of it~ ammonium
salt.
Examples of ~uitabla ba~es are carbonates,
bicarbonates, acetat0~, alcoholates or oxide~ of alkali
or alkaline ear~h metals, in particular ~odium hydroxide,
potas~ium hydroxide, magnesium oxide and calcium oxide.
Organic ba~es, ~uch a~ pyridine or tertiary amine~, can
. ,: , . , . ,:
,. . . , :
2~1 7~3
I ~ 4 - U.Z. 0050/41598
also be used~ The ba~e i~ added, for example, in an
amount of from 0.5 to 2 mole equivalents, based on the
ammonium compound.
Examples of suitable solv2nt~ are dLmethyl
S sulfoxide, alcohol~, such a methanol, ethanol and
isopropanol, aromatic hydrocarbons, such as benzene and
toluene, chlorohydrocarbons, ~uch as chloroform and
dichloroethane, aliphatic hydrocarbons, such as hexane
and cyclohexane, esters, such as ethyl acetate, and
ethers, such as diethyl ether, dioxane and tetrahydro~
furan. The reaction is preferably carried out in
methanol using sodium bicarbonate as the base.
The reaction is complete af~er a few hours. The
desired compound can be isolated, for example by evapora-
ting down the mixture, distributing the re~idue in
methylene chloride/water and di tilling off the solvent
under reduced pressure.
However, the free hydroxylamine base, for example
in the form of an aqueou~ solu~ion, can also be used
directly for this reaction; depending on the solvent u~ed
for the compound II, a single-phase or two-phase reaction
mixture i5 obtained.
Examples of suitable ~olvents for this varian~
are alcohol~, such a methanol, ethanol, isopropanol and
cyclohexanol, aliphatio and aroma~ic hydrocarbon3 and
chlorohydrocarbon~, such a~ hexane, cyclohexane, meth-
ylene chloride, toluene and dichloroethane, esters, such
a~ ethyl acetate, nitrile3, such a~ acetonitrile, and
cyclic ethQrs, such a~ dioxane and tetrahydrofuran.
Alkali metal ~alts of the compounds I can be
obtained by treating the 3-hydroxy compound~ with sodium
hydroxide, potas~ium hydroxide or a ~odium or pota~ium
alcoholate in aqueous solution or in an organic solvent,
such as methanol, ethanol, acetone or toluene.
Other metal salt~, such a~ mangan2se, coppPr,
zinc, iron, calcium, magnesium and barium salt3, can be
prepared from the ~odium salt~ in a conventional manner,
!
2~ 7~3
- 5 - O.Z. 0050/4159~
aR can ammonium and pho~phonium salts using ammonia or
phosphonium, sulfonium or sulfoxonium hydroxide.
The compounds of type II can be prepared, for
example, from the corresponding cyclohexane-1,3-dione~ of
the formula IV
OH
R2 ~ IV,
Y o
where Y is hydrogen or methoxycarbonyl, by known methods,
for example as described in Tetrahadron Lett. (1975),
2491.
It is also possible to prepare the compounds of
the formula II by the enol e~ter intermediate~ V which
are obtained in the reaction of compounds of the formula
IV with acyl chloride3 VI in the presence of a basa and
are then subjec~ed to a rearrangemen~ reaction with
certain Lmidazole or pyridine derivatives (Japanese
Preliminary Published ~pplication 79/063 052).
R2~ + R~CI - ~ R2~ ~ a
IV VI V II
The compounds of tha formula IV are obtained by
a num~er of known process s~eps, starting from known
intermediates.
The synthesis of the hydroxylamines I~I is
carried out according to the reaction ~cheme below, for
example by
A) alkylation of N-hydrox~phthalimide VII with
suitabla phenylbutyl halide~ YIII and sub~equ~nt elLmina-
tion of the protective group, for example with hydrazine
or ethanolamine, sLmilarly to Examples in EP-A-244 786 or
Houben-Weyl, Methodan der organischen Chemie, Volume X/l,
page 1152 et seq. or
,
.
2~7~
i - 6 ~ O.Z. 0050/41598
B) hydrogenation of N-4-phenylbutenylo~yphthal-
imides Xa, b, whose preparation is descri~ed in DE-A 38
38 310, by means of suitable catalysts, eg. palladium on
active carbon, in suitable inert solvent~, such as
methanol, tetrahydrofuran or dioxane, and subsequent
elimination of the protective group as described above.
The hydrogenation is advantageously carried out
at from 20C to the boiling point of the solvent, in
particular at room temperature, by a conventional method,
under atmospheric, superatmospheric or reduced pressureO
A pressure rAnge of from 1 to lO, in particulax 1 to 2,
bar ig preferred.
Reaction scheme:
RouteA)
O N~H + Ha 1~0 --~ D N ~ ~( X ) n
IX
O O H 2N~OH
VII YIII ~a~. 1 ,
R out e B )
D N-O~l ~ ( X ) n
or III
(X)n _
o
Xb
In the cyclic hydroximide~ VII, D is, for ex-
I, `'; ' ' '
~ .
2~ 7~
7 _ o . z . 0050/41598
ample, C2-C3-alkylene, C2-alkenylene or a 5-membered or 6-
membered ring which contains not more than 3 double bonds
and may contain 1 nitrogen atom, for example phenylene,
pyridinylene, cyclopentylene, cyclohexylene or
cyclohexenylene. Examples of suitable substances a-e the
following:
o o o
N -OH ¦¢N~ N--OH
O O O
O¢ N-OH ¢N{)H (;~N-OH
O O O
C~N_OH
The cleavage of the cyclic imide ethers VIII is
carried out similarly to a proce~s de~cribed in EP-A 244
786, using alkanolamine~. After thi~ proce~ , hydroxyl-
amines III can be i~olated a3 free bases or, after
precipitation with acid~, a~ salt~. Readily crystalliz-
ing salt~ are obtained by reacting the bases with oxalic
acid.
In view of the biological activity, preferred
cyclohexenone~ of the formula I are those in which
Rl is alkyl, such as methyl, ethyl, propyl or n-bu~yl, in
particular ethyl or propyl,
X is halogen, such a~ fluorinel chlorine, bromine or
iodine, in particular fluorine or chlorine,
n is from 1 to 5, in particular 1, 2 or 3, the sub-
stituent3 being identical or different where there i~ a
.
'
7 ~ ~
I - 8 - o.Z~ 0050/41598
plurality of radicals X,
R2 is alkyl a~ st~ted under Rl, which may carry an alko~y
or alkylthio group stated below, preferably in the 1-, 2--
or 3-position, in particular 2-ethylthiopxopyl, or
S cyclohexyl which may carry from 1 ~o 3 methyl or hydroxyl
groups, in particular 4-methylcyclohexyl or 3,4-di-
hydroxycyclohexyl,
5-membered hetaryl~ such as pyrazolyl or isoxazolyl,
a 6-membered heterocyclic struc~ure, such as tetrahydro-
pyran-3-yl, tetrahydropyran-4-yl or tetrahydrothiopyran-
3-yl,
phenyl or pyridyl,
where the cyclic radicals may carry from one to three
alkyl, alkoxy, alkylthio and/or haloalkyl group~ and, in
the case of 6-membered radical~, al~o halogen as stated
under X or hydroxyl, for example
alkyl, such as methyl ethyl, propyl, 1-methylethyl,
butyl, l-methylpropyl, 2-meth~lpropyl or 1,1-dLmethyl-
ethyl, in particular methyl or l,l-dimethyle~hyl,
alkoxy, such as methoxy, ethoxy, propoxy, l-methylethoxy,
butoxy, l-methylpropoxy, 2-me~hylpropoxy or 1,1-dimethyl-
ethoxy, in particular methoxy, ethoxy, l-methylethoxy or
l,l-dimethylethoxy,
alkylthio, such a~ methylthio, ethyl~hio, propylthio, 1-
methylethylthio, butylthio, 1-methylpropylthio,2-methyl-
propylthio or 1,1-dimethylethylthio, in particular
methylthio, or
haloalkyl ~uch as fluoromethyl, difluorome~hyl, tri-
fluoromethyl, chlorodifluoromethyl, dichloro~luoromethyl
or trichloromethyl, in p3rticular difluoromethyl or
trifluoromethyl.
2,4,6-Trimethylphenyl is particularly preferred.
The 5-membered heteroaromatic radical~ R2 may
carry ths following radicals a3 substituent~:
halogen a~ ~tated under X, in particular fluorine or
chlorine.
In the case of the phenyl and pyridyl radicals,
- 2~7~
_ 9 _ o.z. 0050/41598
suitable substituents in addition to the abovementioned
group~ are the following radicals:
alkynyloxy, such as 2-propynyloxy, in particular proparg-
yloxyphenyl or
amino which may carry one or two acyl radicals, such as
acetyl or benzoyl.
Particularly preferred cyclohexenone oxime ethers
of the formula I are summarized in the Table below:
11 , ~ ' '
. , ;
9 ~
- 10 - O.Z. 0050/~1598
,_ ~
-- E E
._ _ _ _ ~ _ _ _, U o
` I :~ T r ~ ~ I S ~ ~t
. o ~
~, _ _ _; - ~ ~ ~ ~J
, ~ t r --
E ~` o o o o o o o o ` o o a-
tO _ I ~- S
L ~ ~
_ ? ~ V ~ ~ V ~ D ~ --
t aE _ _, _ _ _ _ _ _ _
Z ~ ~
OOOOOOOOOOOO
L L L L t L h h h h F~l h L L
O O O O O O O O C:l O O O C~
C~ -- S -- -~
_ _ _ _ _ _
~., _ C C ~
~ ~I L L ~ * ~ ~ ~ ~ t L L L
' C O ~b e ~ C C ' ' 0~ 0 90~ 0
L L ~-- ~-- L L L L L L ~
r- L L L L L L L L L L L L L
0~0 ~ ~ S ~ ,~ L L
~11 ~ ~ ~ +~ ~ ~ .~
~ C~~ ~ ~ L ~ L ~ L
_. ~ cL ~ ~ w ~ L~ 1 ~ w ~:L w ~
C~ -- o o O C:~
- 11 - O.Z. 0050/~1598
T .~ T _ ~T ~ r
~_ I N -r N I--'~ ~ ` `
N ~ ._ ~V~:1
E --~ E ` E~ ~ EiE e ~
-- E -- E -- E ---- -- ~ ~ ,~, --
a~ ~ o o
o oo _~ ~
O
^ I ~ I I T
` E ` ~. E ~ ~ ~ ~ E
E -- ~ E ~ E ~ -- E -- E Ea -- --
_ ~ -- E
E~ ~ o 0- 0 Cr~ ~ ~1 0 -- O --
N t~l N
:r: I r r ^ ~ r ^ T I ~: ~ I S I T -- ~ -- I --'
~o ~o ~ ~ ~ ~ r ~ T
_~ E ~ E ` ~ ' ~ ~ E ~ E ~ ~ ~ E ~ ~ ~ '~
ui ~ - - -
s z _ o ~ o ~ o o _ -- ~ -- -- o
.~
h
O O O
O O O O O O C~ O O O i J ,~
~ h ~ h
O O OO O O O ~ri'~i ~ri
s ~ a
I ~ ~ ~
,_ ~
I I I Ii ~ I I I I i ~ '
L ~7 ~ ~ ~ ~ ~ L ~t
L L L L ~ L L. L L ~
O O O C~ O O O O O O ~-- O O O
L L L L L rl r~ L
s s c '' ~ r s s c s :-~ c s
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ C ~ ~ ~
S_ ~ L L L L L L L L ~ L L L
a~
E~
O ~ ~. ~ L ~ ~ ~ L ~ L L ~ L
~i ~i
~ ~ ~ ~ ~_ ~ i i i i i i i i U;
X _ _ _ _ ~
t! I . . `
. ~ ,
2 ~ 3
12 - o . z . 0050/41598
s ~ ~ 2 ~ ~ r ~: ~
v ~ E ~ ~ E ~ E
7 ~ _ ~ u~ In In
o o ~ ~o u o _
I ~ T
~ T I
E
_ U~
E
~0 '-- ^ I ^ ~ 1 ^ I ^ ^ V ^ --
. * ~` V ~ E V V ~ E ~ ~ ~ V _ E -- I~ -- -- --
,_ z ~ ~ ~ - -- O ~~ CO cr o _ o o- _ _ ~ _ ~ o~
~_ o 1~ o r~ 1~ o 1~ o 1~ _ _ _ 1~ o 1~ _
c c ~ .c o ~ o o
. i C~u O. r
I S E X o 1~ L
C CL ~ O ~ ~. C~, ~
L e ~ L
~ X ~ L' C
a. ~. e
L I-- ~ L ~ 0 ~) O O O
~ I I :,. ~ e o o ~ v v
CI i O S ~ ::1 ~ ,:~
C C ci o
~ L L $ ~ O O ~L ~ ~d ~
r~ ~ I I (O J ~V
ffi
E~
O O ~ g c O ~:: ~ o r
V _. ~ t ~ ~ L ~ ( ~ ~L. ~ ~
~ CL ~ I~J ~i ~ hi CLl~i ~Li ~ ~i ~i
_l
,
~ ~ . . ' .
I) , :
,
- 13 - O. z . 0050/41598
8`
U~
_.
T
_~
E
C~
,~
:~ r
_ _
~ ~ u~ In
s 2
o r.
.. O
~:
X ~
.;~r
L
C~
. . V
t
~ I_
2 .~
g - CL
U
~ $
E~ Z -~ *
,~
I, ' ' ' ',;
2~7~
i - 14 - O.Z. 0050J41598
The cyclohexenone oxime ethex~ I are suitable as
herbicides, in particular for con~rolling plant species
from among the Gramineae (grasses).
The cyclohexenone derivatives I or the herbicides
containing them can be used, for example, in the form of
directly sprayable solution~, powders, suspensions,
including concentrat0d aqueous, oily or other suspensions
or dispersions, emulsions, oil dispersions, pas~es,
dusting agents, broadcasting a~en~s or granules, by
spraying, atomizing, du~ing, broadca ting or pouring.
The application forms depend on the intended uses; they
should in any case ensure very fine distribution of the
novel active ingredients.
Compounds I are suitable in general for the
preparation of directly sprayable ~olution~, emulsions,
pastes or oil disper~ions. Suitable inext additives are
mineral oil fractions having a medium to high boiling
point, such as kerosene or diesel oil, as well as coal
tar oils and oils of vegetable or animal origin, aliphat-
ic, cyclic and aromatic hydrocarbons, eg. toluene,xylene, paraffin, tetrahydronaphthalene, alkylated
naphthalenes and derivatives thereof, methancl, ethanol,
propanol, butanol, cyclohe~anol, cyclohexanone, chloro-
benzene, isophorone or strongly polar solvents, such as
N,N-dLmethylformamide, dLmethyl sulfoxide, N-methylpyr-
rolidone or water.
Aqueous application forms can be prepared from
emulsion concentrates, dispersions, pastes, w ttable
powder~ or water-dispersible granules by adding water.
For th~ prepaxation of emulsion~, pa tes or oil disper-
sion~, the sub~trates, as such or in ~olution in oil or
solvent, can be homogenized in water by means of wetting
agents, adhesive~, di~persant~ or emulsifiersO However,
it is also po~sible for concentrates which consi~t of
active substances, wetting agent~, adhesives, disper~ants
or emulsifier~ and may consi t of solvents or oil and
which are suitable for dilution wi~h water to be
2~7~3
- 15 - o. z . 0050/41598
prepared.
Suitable surfactants are the alkali metal,
alkaline earth metal and ammonium salts o~ aromatic
sulfonic acids, fox example lignin-, phenol-, naph-
thalene- and dibutylnaphthalenesulfonic acid, and of
fatty acids, alkyl- and alkylarylsulfonates, alkyl-
sulfates, lauryl ether sulfates and fatty alcohol ~ul-
fates, and salts of sulfated hexa-, hepta- and octa-
decanols, and of fatty alcohol glycol ethers, condensates
of sulfonated naphthalene and it~ derivatives with
formaldehyde, condensate~ of naphthalene or of naph-
thalenesulfonic acids with phenol and formaldehyde,
polyoxyethyleneoctylphenolethers,ethoxylated isocotyl-,
octyl-or nonylphenol, alkylphenol polyglycol e~hers,
tributylphenyl polyglycol ether~, alkylaryl polyether
alcohols, isotrideoyl alcohol, fatty alcohol/ethylene
oxide condensates, ethoxylated ca~tor oil, polyoxy-
ethylene alkyl ethers or polyoxypropylene~ lauryl alcohol
polyglycol ether aceta~e, ~orbitol e ters, ligninsulfite
waste liquor~ or methylcellulose.
Powder~, broadcasting agent~ and duating agents
can be prepared by mixing or milling the active sub-
stances together with a solid carrier.
Granule~, for example, coated, impregnated and
homogeneous granules, can be prapared by binding the
active ingredients to solid carriers. Solid carriers are
mineral earths, such as ~ilica , ~ilica gel~, silicates,
talc, kaolin, limestone, lime, chalk, bole, loess, clay,
dolomite, diatomaceou~ earth, calcium ~ulfate, magnesium
sulfate, magne ium oxide, milled pla~tic~, fertilizers,
such a~ ammonium sulfate, ammonium phosphate, ammonium
nitrate, ureas and vegetable product~, ~uch as cereal
meal, ground bark, woodmeal and nu~shell meal, cellulo~e
powder and other ~olid carriers.
The formulation~ contain from 0.02 to 95, prefer-
ably from 0.5 to 90, ~ by weight of active ingredient.
The active ingredients ar~ used in a puri~y of from 90 to
I~ : : ,
.
7 9 3
I - 16 - O.Z. 0050/~159~
100%, preferably from 95 to 100% (according to ~he NMR
spectrum).
The novel compounds I can be formulated, fox
example, as follows:
I. 90 parts by weight of compound No. 1.05 are mixed
with 10 parts by weight of N-msthyl-~-pyrrolid-
one, and a solution suitable for use in the form
of very small drops i~ obtained.
II. 20 parts by weight of compound No. 1.03 are
dissolved in a mixture which consists of sn part~
by weight of ~ylene, 10 parts by weight of the
adduct of rom 8 to 10 moles o~ ethylene oxide
with 1 mole of oleic acid N-monoethanolamide, 5
parts by weight of the calcium salt of dodecyl-
benzenesulfonic acid and 5 parts by weight o an
adduct of 40 moles of ethylene oxide with 1 mole
of castor oil. By pouring the ~olution into
100,000 part~ by weight of water and finely
distributing it therein, an aqueous di~persion
which contain~ 0.02% by weight of the active
ingredient is obtained.
III. 20 parts by weight of compound No. 1.11 are
dis~olved in a mixture which con~i~ts of 40 part~
by weight of cyclohQxanone, 30 part~ by weight of
isobutanol, 20 part~ by weight of ~he adduct of
7 moles of ethylene oxid~ with 1 mole of iso-
octylphenol and 10 parts by weight of the adduct
of 40 mole~ of ethylene oxide with 1 mole of
castor oil. By pouring the ~olution into 100,000
part~ by weight of water and finely distributing
it therein, an aqueous disper~ion which contain~
0.02% by weight of th~ active ingredienk is
obtained.
IV. 20 part~ by w~ight of ac~ive ingredient No. 1.11
are dissolved in a mixture which consist~ of 25
part~ by weight of cyclohe~anone, 65 part~ by
wei~ht of a mineral oil fraction boiling within
! ~ .
,
7 ~ 3
I - 17 - O.Z. 0050/41598
a range from 210 to 280C and 10 parts by weight
of ~he adduct of 40 mole~ of ethylene oxide with
1 mole of castor oil. By pouring the solution
into 100,000 parts by weight of water and finely
S distributing it ~herein, an aqueous dispersion
which contain~ 0.02~ by weight of the active
ingredient is obta ned.
V. 20 parts by weight of active ingredient No. 1.05
ar~ thoroughly mixed with 3 par~s by weight of
the sodium ~alt of diisobutylnaphthalene-~-
sulfonic acid, 17 parts by weight of the sodium
salt of ligninsulfonic acid obtained fro~ a
sulfite waste liquor and 60 part~ by weight of
silica gel powder, ~nd the mixture is milled in-
a hammer mill. ~y finely distributing the
mixture in 20,000 parts by weight of water, a
spray liquor which contain~ 0.1~ by weight of ~he
active ingredient is obtainad.
VI. 3 parts by welght of active ingredien~ No. 1.03
ara mixed with 97 part~ by weigh~ of finely
divided kaolin. A dusting agent which contain~
3~ by weight of ~he active ingredient i~ obtained
in this manner.
VII. 30 part~ by weight of active ingredient No. 1.05
are thoroughly mixed with a mixture of 92 parts
by weight of ~ilica gel powder and 8 parts by
weight of liquid paraffin which ha~ been sprayed
onto the ~urface of thi~ ~ilica gel. a formula-
tion of the active ingredient ha~ing good ad-
hesion i3 obtained in this manner.
VIII. 20 part~ by weight of active ingredient NoO 1~03
are thoroughly mixed with 2 parts by weight of
the ~alcium salt of dode~ylbenzene~ulfonic acid,
8 parts by weight of a fatty alcohol polyglycol
ether, 2 parts by weight of the sodium salt of a
phenol/urea/formal~ehyde conden~ate and 68 parts
by weight o~ a paraffinic mineral oil. A ~table
,
, .
- :
,
2,'~7~3
I - 18 - O.Z. 0050/41598
oily dispersion is obtained.
Th0 agent~ can be applied by the preemergence or
postemergence method. If the active ingredients are less
well tolerated by certain crops, it is also possible to
use application methods in which the herbicides are
sprayed with the aid of the sprayers in such a way that
there is as far as possible no contact with the leav~s of
the sen~itive crops while the active ingredient~ reach
the leaves of undesirable plants growing underneath or
reach the uncovered soil area (post-directed, lay-by~.
The application rates of active ingredient are
from 0.001 to 3, preferably from 0.01 to 2.0, kg/ha,
depending on the season, the target plants and the stage
of growth.
In view of the prac~icable ac~ion spec~rum for
weed control, the toleration by crops or the desired
effect on the growth ~hereof and because of the wide
range of application metho~s, the novel compounds can be
used in a large number of cxops. Example of suitable
crops are the following:
Botanical name Common name
Allium cepa onions
Anana~ comosu~ pineapples
Arachis hypogaea peanut~ (groundnuts)
Asparagus off icinali8 asparagus
Beta vulgari~ spp. alti sima ~ugarbeets
Beta ~ulgari~ pp. rapa fodder beets
Brassica napu~ var. napus rapeseed
30 BrasYica napus var. napobra~ica swedes
Brassica rapa var. silve~tris beets
Camellia sinensi~ tea plants
Carthamus tinctoriu~ ~afflower
Carya illinoinensi~ pecan tree~
Citrus limon lemon trces
Citru~ ~inensi4 orang~ tree~
Coffea arabica (Coffea canephora,
Coffea liberica) coffee plants
Cucumis sativus cucumbers
2 ~ 3
I - 19 - O.Z. 0050/41598
Botanical name Common name
Cynodon dactylon sermudagrass in turf
and lawn~
Daucus carota carrots
Elaeis guineensis oil palm~
Fragaria vesca ~trawberrie3
Glycine max soybean~
Gossypium hirsutum
(Gossypium arboreum cotton
Gossypium herbaceum
Gossypium viti~olium)
Helianthus annuus sunflowers
Helianthus tuberosus Jerusalem artichoke
Hevea brasiliensis rubber plants
Hordeum vulgare baxley
Humulus lupulus hops
Ipomoea batatas sweet potatoes
Juglans regia walnut trees
Lens culinari~ lentils
Linum usitatissimum flax
Lycopersicon lycoper~icum tomatoes
Malus spp. apple tree~
Manihot esculenta cas~ava
Medicago ~ativa alfalfa (lucerne)
Musa spp. banana plants
Nicotiana tabacum tobacco
(N. rustica)
Olaa europaea olive trees
Oryza ~ativa rice
Pha~eolu~ lunatu~ limabeans
Phaseolus vulgari~ 3napbeans, green
bean~, dry beans
Picea abiQs Norway spruce
Pinus spp. pine trees
Pisum ~ativum English pea~
Prunus avium cherry tree~
~,, .
., `
,
7 ~ 3
I - 20 O.Z. 0050/4159
Botanical name Common name
Prunus dulci~ almond trees
Prunus per~ica peach trees
Pyrus communis pear trees
Ri~es sylve~tre redcurrants
Ricinus communis castor-oil plants
Saccharum afficinarum sugar cane
Secale cereale rye
Solanum tuberosum Irish potatoes
Sorghum bicolor (s. vulgare) sorghum
Theobroma cacao cacao plants
Trifolium pratense red clover
Triticum aestivum wheat
Triticum durum durum wheat
Vicia faba tick beans
Vitis vinifera grapes
Zea may3 Indian corn, sweet
corn, maize
To broaden the action spec~rum and to achieve
synergi~tic efects, the cyclohexenone derivative~ of the
formula I can be mixed both with one another and with
member~ of other groups of herbicidal or growth-
regulating active ingredient~ and applied together.
Example~ of suitable component~ for the mixture are
diazine~, 4H-3,1-ben~oxazine derivatives, benzothia-
diazinone~, 2,6-dinitroaniline~, N-phenylcarbamates,
thiocarbamates, halocarboxylic acid~, triazine~, amides,
ureas, diphenyl ether~, triazinone~, uracils, benzofuran
derivative~, quinolinecarboxylic acids, sulfonylurea
derivatives, cyclohexenones, (hetero)aryloxyphenoxypropionic
acids, their ~alts, e~ters and amide~ and others.
It may also be useful to apply the cyclohexenone
derivative~ o~ the formula I, or herbicides containing
them, alone or together in combination with other herl-
icide~ or mixed with further crop protection agent~, for
example with pesticidea, agents for controlling
.. ,
!l :
~ ..
.
2 V ~ 3
I - 21 - O.Z. 0050/41598
phytopathogenic fungi or bactericides. The miscibility
with mineral ~alt solution~ which are used for eliminat
ing nutrient and trace elemen~ deficiencies is also of
interest. It is also possible to add nonphytotoxic oils
and oil concentrates.
EXAMPLES
4-Phenylbutoxyamine
~ONH 2
Method A:
167.S g (1.21 mol) of potas~ium carbonate and 2 g
of potassium iodide were added to 302 g (1.85 mol) of
N-hydroxyphthalimide in 1.9 1 of anhydrous N-methyl-
pyrrolidone. After the reaction mixture had been heated
to 50C, 395.2 g tl.85 mol) of 4-phenylbutyl bromide were
added and this tPmperature was maintained for a further
6 hours. After cooling, the mixture was poured onto 6 l
of ice wa~er and taken up with dichloroethane, and the
organic phase wa~ washed with dilute sodium hydroxide
solution, dried and evapora~ed dow~ under reduced pres-
sure. Yield: 379 g of N-~4~phenylbu~o~y)-phthalLmide
(73%).
250-MHz lH-NNR (DMSO-d~)
(ppm) = 1.65-1.85 (m, 4H); 2.66 (t, 2H); 4.18 (t, 2H)
7.1-7.4 (m, 5H); 7.86 (~, 4H)
81,8 g (1.34 mol) of e~hanolamine were added to
396 g (1.34 mol) of the phthalLmidoether obtained above
in 1,300 ml of ethyl acetate. The reaction mixture was
heated at 60C for 5 hours. Thereafter~ stirring was
carried out for 24 hour~ at room temperature, the
precipitated cry~tal~ were filtered off under suction and
washed with a little ethyl acetate and a vigor~us ~tre~m
of hydrogen chloride gas was pa~ed through the combined
mother liquors ~or 15 minute~, during which tha intarnal
tempera~ure should not exc0ed 40C. ~he precipitated
- 22 - O.Z. 0050/41598
crystals were filtered off under suction, washed with a
little ethyl acetate and dried under reduced pressure.
Yield : 148.5 g of 4-phenylbutoxyamine hydrochloride
(55~). Mp.: g3 94C.
250-MHz lH-NMR (DMSO-d~)
~ppm) - 1.5-1.7 (m, 4H); 2.58 (t, 2H); 4.04 (t, 2H)
7.1-7.3 ~m, 5H); 11.1 (broad s, 3H)
4-(4-Fluorophenyl)-butoxyamine
F~3(C~12) 4--ONH2
Method B: Hydrogenation
N-(4-(4-Fluorophenyl)-butoxy)-phthalimide
2 g of palladium on ac~ive carbon (10% ~trength)
were added to a solution of 71.5 g (0.23 mol) of N-(4-(4-
fluorophenyl~-3-butenyloxy~-phthalLmide(preparedaccord-
ing to German ~aid~Open Application DOS 3,838,310) in
300 ml of tetrahydrofuran. Hydrogenation was carried ou~
under slightly superatmospheric pre~sure until 1.2 times
the theoretical amount of hydrogen had been consumed. The
mixture was fil~ered under ~uction over kie~elguhr, the
filtrate wa~ evaporated down and the crude product was
recrystallized from isopropanol.
Yield: 62.8 g 187~); mp.: 67-68C
2S0-MHz lH-NNR (DMSO-d~)
(ppm) = 1.8-1.9 (m, 4H); 2.65 (t, 2H); 4.15 (t, 2H)
7.0-7.35 (m, 4H); 7.86 (s, 4H)
61.8 g (0.197 mol) of the phthalimidoether
previously prepared were introduced a little at a tLme
into 92 mol of ethanolamine. The mixture was heated at
60C for 3 hour~, after which it wa~ allowed to cool and
was then poured into 400 ml of ice water and extracted
with dichloroethane. The combined organic phase~ were
wa~hed with saturated ~odium chloride ~olution, dried and
evaporated down under reduced pre~sure. ~he 4-~4-fluoro-
phenyl)-butoxyamine was obtained as an oil.
., .
7 ~ 3
- 23 - o.z. 0050/ds15g8
250-MHz lH-PIMR (CDCl3)
~ppm) = 1.5--1.74 (m/ 4~; 2.61 (t, 2H); 3.68 (t, 2H)
5.4 (broadL s, 2E~); 6.9-7.2 (m, 4H)
4- ( 4-Chlorophenyl ) -butoxyamine
Ct~
~(CH2) 4{~NH2
4-(4-Chlorophenyl)-buto~yamine wa~ obtained as an
oil in a total yield of 60% similarly ~o the Example
described above, starting from N-(4 (4-chlorophenyl)-
butenyloxyphthalLmide (German Laid-Open Applica~ion DOS
3,838,310).
250-MHz lH-NNR (CDCl3)
(ppm) = 1.65-1.85 (m, 4H); 2~66 (t, 2H); 4.lB (t, 2H)
7.1-7.4 (m~ 5H); 7.86 (~, 4H)
N-(4-(4-Chlorophenyl~-butoxyphthalimide
C ~ cH 2 ) 4~N~3
Mp.: 72~73C (isopropanol)
250-MHz lH-NMR (DMSO-d6)
(ppm) = 106-1.85 (m, 4H); 2.6a (t~ 2H~; 4.17 (t, 2H)
7.2-7.4 (m, 4H); 7.88 (s, 4H)
2-{1-[4-(4-Fluorophenyl)-butoximino]-butyl}-5-tetrahydro-
thiopyran-3-yl-3-hydroxycyclohex-2~enone (compound 1.04)
2.2 g (12 mmol) of 4-(4~fluorophe~yl)-butoxyamine
were added to a solution of 3 g tll mol) of 2 butyryl-3-
hydroxy-5-tetrahydrothiopyran-3-ylcyclohex-2-enone in
100 ml of dry methanol. ~he mixture was stirred for
16 hours at room temperature and was then evaporat*d to
drynes~ under reduced pres~ure. The residue wa~ taken up
in diethyl ether and the ~olution was chromatographed
over silica gel. Yield . 3.6 g (80% o~ theory).
The cyclohexenone compounds shown in Table 1 can
be obtained similarly to thi~ method.
. .
.. . .
.
- ~ . .
7 ~ 3
- 24 - O.Z. 0050/41598
Use Examples
The herbicidal action of the cyclohexenone oxime
ethers of the formula I could be demonstrated by yreen-
house experiment~:
The culture ve ~els used were plas~ic flowerpots
containing loamy sand with about 3% of humus as a sub-
strate. The seeds of the test plan~s were sown separate-
ly according to species.
In the cAse of preemergence trea~ment, the active
ingredients ~uspended or emul~ified in water were applied
directly after sowing, by means of nozzles providing a
fine distribution. The vessel~ were lightly watered in
order to promote germination and growth and then covered
with transparent plastic covers un~il the plants had
begun to grow. This covering ensures uniform germination
of the ~est plant~, unles~ this i~ adversely affected by
the active ingredients.
For the purpo~e of postemergence treatment, the
test plant~ wers treated with the active ingredients
suspended or emul~ified in water, bu~ only after reaching
a height of growth of from 3 to 15 cm, depending on the
growth form. The application rate for the postemergence
treatment was 0.06 kg/ha of active substance.
The plants were kept at 10-25C or 20-35C,
depending on the specie~. The te~t period extended over
2-4 weeks. During this time, the plants were tended and
their reaction to the individual treatments ~as
e~aluated.
Evaluation was based on a cale from 0 to 100.
100 means no emergence of the plant~ or complete destruc-
tion of at lea~t the above-ground parts and 0 means no
damage or normal growth.
The plants used in the greenhouse experiments
consisted of the following 3pecies:
2~793
I - 25 - O.z. 0050/41598
Latin name _ Commen name
Oryza sativa rice
Echinochloa crus-galli barnyardgra~s
Setaria italica foxtail millet
When 0~06 kg/ha of active substance is used in
the postemergence method, undesirable gramineous plants
can be very readily controlled using Example 1.11, 1.03
and 1.05, with simultaneous toleration by the example
crop rice.
,
.