Note: Descriptions are shown in the official language in which they were submitted.
- ~44236
Our Ref.:TS-284 (200-2146)
-- 1 --
METHOD FOFc PREVENTING COAGULATION OF POWDER
The present invention relates to a method for
preventing coagulatlon of powder which is likely to be
coagulated. More particularly, it relates to a method
for preventing coagulation of powder by using a water-
soluble cellulose ester as an anticoagulant~
A powder of an amine such as piperazine or
triethylenediamine (hereinafter referred to simply as
TEDA) is a compound which usually has coherence and
adherence and thus is likely to be coagulated. Not to
mention such a specific powder, a highly hydroscopic
powder or a highly sublimable po~der in general usuall~
readily undergoes coagulation due to an inclusion of a
small amount of moisture or due to an increase of the
temperature. Therefore, such a coagulable powder is
required to be handled with due care, and once such a
powder has coagulated, the handllng tends to be extremely
difficult. As measures to prevent coagulation of such a
coagulable powder, it is common to employ a method of
removing impurities contained in the powder and enlarging
2~4~23~
-- 2 --
the particle size of the powder itself, a method of
adding an anticoagulant to the powder or a method of
storing the powder by means of a closed vessel. However,
among coagulable powders, there is one which undergoes
coagulation even when stored in a closed vessel, such as
piperazine, or a highly sublimable substance such as TEDA
which tends more likely to be coagulated when the purity
is increased. Therefore, there has been no appropriate
method for preventing coagulation of such a powder.
Further, such a powder has extremely high coagulability,
and it is usually difficult to prevent the coagulation by
enlarging the particle size. As a method for preventing
coagulation of such a highly coagulable powder, it is
usually believed to be effective to incorporate a
suitable anticoagulant.
For the selection of such an anticoagulant, it is
desired to select an anticoagulant which is capable of
effectively accomplishing the object in an amount as
small as possibl2 and which does not impart an odor or a
color to the powder by the addition. Further, it is
desired to select an anticoagulant which presents no
adverse effects to the physical properties of the powder
in connection with the purpose of the powder and which is
inexpensive. As conventional anticoagulants, silica
powder (Japanese Unexamined Patent Publication No.
203039/1982) and polyethylene glycols (Japanese Examined
Patent Publication No. 46758/1988) are known. However,
.
20~236
-- 3 --
the silica powder is effective only to temporarily avoid
the contact of crystals to one another and its anti-
coagulating action is very weak. On the other hand,
liquid anticoagulants such as polyethylene glycols may
simply be mixed with TEDA powder. ~s a consequence,
however, the TEDA powder tends to be wet, and in a long
range storage, the liquid tends to flow to the bottom of
the container and tends to be non-uniform in the
container r whereby the anti-coagulating action tends to
be low. Further, in either case, the anticoagulant is
required to be added at a relatively high concentration,
whereby the purity of ~EDA will be low.
Whereas, Japanese Examined Patent Publications No.
62241/1988 and No. 3142/1989 disclose that by an addition
of a TEDA polymer (ethylene-piperazine copolymer) as an
additive during a prècipitation step, it is possible to
simplify the process of the addition so that the process
control can be easy, and the TEDA polymer exhibits a high
- level of anti-coagulating action, ~hereby adequate
effects can be obtained by an addition of a very small
amount of the polymer. However, this TEDA polymer is
insoluble in most organic solvents.
Powders usually have coherence and adherence in many
cases. It is common to employ an operation such as
granulation or classification to reduce such nature.
However, in a case of a coagulable powder such as a
highly sublimable powder of e.g. TEDA, sublimation and
2~2~6
-- 4 --
condensation are repeated due to a change of e.g. the
external temperature, whereby a strong bridge will be
formed between powder particles (crystals). Thus, TEDA
tends to be coagulated entirely in the container and
tends to be hardly disintegrated.
TEDA i5 usually synthesized or produced f~om e.g. N-
aminoethylpipera~ine or hydroxyethylpiperazine. By such
a method, TEDA is obtainable as slightly yellow white
crystals. As a by-product, an alkylpiperazine or the
like is contained. This by-product has an anti-
coagulating action to some extent. However, TEDA
crystals of high purity have been desired in recent
years, and consequently, TEDA crystals having a purity of
at least 99.9% are now produced as a result of an
improvement in the purification technique. Accordingly,
the coagulability of TEDA has been thereby sharply
increased, and there has been a problem from the
viewpoint of the production process or the storage.
It is an object of the present invention to provide a
method for preventing coagulation of powder having
coagulability, whereby prevention of coagulation can
effectively be conducted by adding a small amount of an
anticoagulant which is excellent in the solubility to
various solvents and which is inexpensive and has no
adverse effect to the physical properties of the powder,
as compared with the conventional methods.
As a result of an extensive study in view of the
~0~23~
- 5
above-mentioned circumstances, the present inventors have
found it possible to effectively control coagulation of
powder by using a water-soluble cellulose ester as an
anticoagulant, and have arrived at the present invention
on the basis of this discovery.
Thus, the present invention provides a method for
preventing coagulation of coagulable powder, which
comprises incorporating to the powder a water-soluble
cellulose ester alone or in combination with other
anticoagulant, to control the coagulability.
Now, the present invention will be described in
detail with reference to the preferred embodiments.
In the present invention, the coagulable powder means
a highly hygroscopic and sublimable powder of e.g.
piperazine, TEDA, ammonium sulfate, ammonium chloride or
sodium chloride.
Two types of causes are conceivable as the main
causes for coagulation of powder i.e. coagulation due to
absorption of moisture and coagulation due to bridginy of
powder particles (crystals) by sublimation and
condensation. The former can be avoided by packayiny.
Otherwise it can be avoided by improving the quality
control o~ the product. With respect to the latter,
there has been no effective method discovered which
presents no adverse effects to the physical properties of
the powder and which fully satisfies other conditions.
The present invention presents a very effective
2 3 ~
-- 6 --
coagulation-preventing method by incorporating an
anticoagulant which prevents absorption of moisture and
the sublimation and condensation action.
The mechanism for preventing coagulation in the
present invention is considered to be as follows. Water-
soluble cellulose esters exhibit excellent solubility to
various solvents and they are capable of forming
transparent strong films from their solutions in water or
in organic solvents. A solution of such a water-soluble
cellulose ester in water or in an organic solvent is
mixed to the powderr followed by drying to form a film on
the surface of the powder and thereby to microcapsulate
the crystals, so that the contact of the crystal-forming
component with outer atmosphere or the contact of
crystals to one another is prevented, whereby the
absorption of moisture and the sublimation and
condensation, are suppressed. Thus, coagulation of
crystals to one another is suppressed, and coagulation-
preventing effects can be obtained.
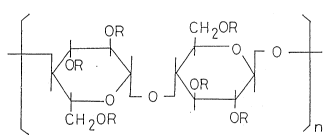
The water-soluble cellulose ester in the present
invention may, for example, be a compound of the
following formula (1):
OR C H 20R
~ jO (~)
CH 20R OR
n
204~2~6
- 7
wherein n is an integer of at least 1, and R is ~ or a
branched or linear Cl-C10 alkyl group or ~-CH2-C~(OH)CH3-
O~mH, wherein m is an integer of at least 1.
Specifically, methyl cellulose, ethyl cellulose,
hydroxypropylmethyl cellulose or propyl cellulose may be
mentioned. Among them, particularly preferred is
hydroxypropylmethyl cellulose and propyl cellulose. A
product having the hydroxyl group in the cellulose
residue substituted by a methoxy group, a hydroxylpropoxy
group or both of them, is commercially available as
Metlose (manufactured by Shinetsu Chemical Co., Ltd.) or
Nisso HPC (manufactured by Nippon Soda Co., Ltd.).
Such water-soluble cellulose esters are available
with a wide range of molecular weights ranging from a low
molecular weight of 10,000 to an average molecular weight
exceeding lOOrOOOr and the viscosities of their solutions
are proportional to the average molecular weights.
Selection of a particular ~7ater-soluble cellulose ester
among such various water-soluble cellulose esters depends
on the properties required. ~owever, hydroxypropyl
cellulose or hydroxypropylmethyl cellulose is preferred
in a case where it is used as dissolved in a wide range
of organic solvents when the powder is to be used. The
average molecular weight of the water-soluble cellulose
ester to be used may be at any level. However, if the
molecular weight ls too high, the viscosity of the
2~236
-- 8 --
solution tends to be high, whereby the dissolving me-thod
and handling tend to be dif~icult. Preferably, the
water-soluble cellulose ester has an average molecular
weight of from 10,000 to 100,000.
According to the present invention, there is no
particular restriction as to the method for adding a
water-soluble cellulose ester to the powder. For
example, it is common to employ a method wherein after
the preparation of powder, the powder and a solution of
the water-soluble cellulose ester in water or in an
organic solvent are thoroughly mixed by means of a mixing
apparatus such as a ribbon blender or a V-type mixer.
However, to employ such a mixing apparatus, the process
tends to be complex, and the cost is expected to be
substantial. Whereas, if a solution of the water-soluble
cellulose ester in water or in an organic solvent is
sprayed or otherwise added during the liquid removal step
immediately after precipitation i.e. to a TEDA crystal
cake in a centrifugal separator, followed by drying, it
is possible to effectively and uniformly accomplish the
coating on the TEDA crystal surface without requiring any
mixing apparatus. Otherwise, it may be added during the
precipitation step, as disclosed in Japanese Examined
Patent Publication No. 62241/1988.
Anticoagulants presently used are silica powders and
polyethylene glycols. The anticoagulating ability of
such conventional additives is relatively low, and when
2~4~3~
g
the coagulable powder is TEDA, such conventional
additives are required in an amount of about 1 part by
weight per lO0 parts by weight of TE~A. Whereas,
according to the present invention, the water-soluble
cellulose ester is capable of forming a thin strong film,
and a sufficient anti-coagulating action can be obtained
when the water-soluble cellulose ester is contained in an
amount of at least 0.001 part by weight relative to lO0
parts by weight of TEDA. The larger the amount, the
higher the anti-coagulating effects. However, the purity
of the powder decreases as the amount of the additive to
the powder increases. Therefore, the amount of addition
should preferably be as small as possible. According to
the present invention, the amount of the water-soluble
cellulose ester added to powder is preferably from 0.001
to 2 parts by weight, more preferably from 0.01 to 0.1
part by weight, per 100 parts by weight of the powder.
This amount corresponds to from 1/10 to l/lO0 of the
amount of khe conventional additives.
Further, the water-soluble cellulose esters are
colorless and transparent and chemically very stable, and
they are not substantially decomposed by acids or
alkalis. Therefore~ they do not adversely affect the
physical properties of the powder, and they show
excellent solubility to various solvents and thus have
excellent properties as additives or coating agents.
AS described in the foregoing, the present invention
2~4236
-- 10 --
provides an epoch-making coagulation-preventing technique
in which a very small amount of a water-soluble cellulose
ester is added to powder to coat it on the powder surface
to impart excellent coagulation-preventing effects by
suppressing moisture absorption and sublimation of the
powder and preventing the contact of the powder particles
; to one another.
Now, the present invention will be described in
further detall with reference to Examples. However, it
should be understood that the present invention is by no
means restricted by such specific Examples.
EXAMPLE 1
For the test to study coagulability, TED~ powder
having a purity of at least 99.95% was used, and as an
anticoagulant, methyl cellulose was used.
500 g of a TEDA cake was prepared on a Buechner
funnel, and 200 ml of a 0.5% alcohol solution o the
above anticoagulant was added thereto. The mixture was
thoroughly mixed to contact the solution with TEDA
crystals, followed by filtration to collect the TEDA
crystals. The crystals were thoroughly dried under
vacuum to obtain 350 g of a sample. The quantitative
analysis of the coated water-soluble cellulose ester was
conduced in accordance with Japanese Pharmacopea. As a
result, the coated amount was 0.05 g.
The measurement of the coagulation degree and the
evaluation standards were as follows. Namely, the
2~23~
obtained sample was packed in a container having a size
of 5 cm x 5 cm and a height of 2 cm, and a plastic plate
of 5 cm x 5 cm was placed thereon. A weight of 300 g was
place~ thereon, and the container was stored in a
desiccater having a humidity or not higher than 1%.
During the storage, the pressure exerted to the crystals
was 12 g/cm2. After the storage in the desiccater for
one month, the weight and the container were removed, and
a pressure was exerted to the center portion of the
crystal block having the plastlc plate located beneath,
by a Kiya-type hardness meter, whereby the pressure at
breakage was read. The values thus obtained were
classified into the following three rankings, which were
used as indices for evaluation of the coagulation deyree.
In the following Examples and Comparative Examples, the
coagulation degree was evaluated in the same manner. The
results are shown in Table 1.
As is evident from Table 1, TEDA treated in this
Example belongs to A rank, thus indicating excellent
anticoagulating ef:fects.
A rank: Crystal block which can readily be broken
with a slight impact with a breaking
pressure of not higher than 1.0 kg/cm~ and
in which no substantial progress of
coagulation was observed.
B rank; Crystal block with a breaking pressure
of not higher than 10.0 kg/cm2 which can not
20~423~
- 12 -
be broken by a low level of impact and in
which coagulation was found progressed
entlrely.
C rank: Crystal block which requires a considerably
strong impact for breakage with a breaking
pressure of at least 10.0 kg/cm2 and in
which coagulation was found completely
progressed~.
EXAMPLE 2
10The operation was conducted in the same manner as in
Example 1 except that as the anticoagulant,
hydroxypropylmethyl cellulose was used instead of methyl
cellulose. The cvated amount was 0.05 g/340 g.
The results are shown in Table 1.
EX~MPLE 3
The operation was conducted in the same manner as in
Example 1 except that as the anticoagulant, hydroxypropyl
cellulose was used instead o methyl cellulose. The
coated amount was 0.06 g/350 g.
20The results are shown in Table 1.
COMPARATIVE EXAMPLE 1 ~
The operation was conducted in the same manner as in
Example 1 except that no anticoagulant was used.
The results are shown in Table 1.
.
COMPARATIVE EXAMPLE 2
3,00~0 ml of ~a TEDA methanol solution having a
composition comprising 50 parts by weight of TEDA and 50
20~4236
- 13 ~
parts by weight of methanol, was introduced into a flask
having an internal capacity of 5,000 ml, and 0.15 g of
TEDA polymer (ethylene-piperazine copolymer prepared by
the synthesis disclosed in Japanese Unexamined Patent
Publication No. 62241/1988), was added thereto. The
mixture was subjected to methanol removal by an
evaporator, whereby 1,100 ml of methanol was distilled.
The residual liquid was left to stand still at a room
temperature and then cooled to a liquid temperature of
20C. Precipitated TE~A crystals were collected by
filtration under suction with a filter paper of No. 5C
and then dried under vacuum to obtain 450 g of TEDA
crystals. The TEDA polymer contained in the TEDA
crystals was 0.05 g. With respect to this sample, the
coagulation degree was evaluated in the same manner as in
Example 1.
The results are shown in Table 1. As is evident from
Table 1, excellent coagulation-preventing effects were
exhibited, but when made into a 33 wto6 dipropylene glycol
solution, a certain level of turbidi-ty was observed.
COMPARATIVE EXAMPLE 3
500 g of TEDA and 2 g of silica gel (manufactured by
Nippon Sil1ca Gel Kogyo K.K., bulk density: 4og/e~
average particle size: 2 ym) were thoroughly mixed by a
V-mixer, and the obtained mixture was used as a sample.
Otherwise, the operation was conducted in the same manner
as in Example 1. The results are as shown in Table 1,
-` 20~23~
- 14 -
and a certain degree of coagulation was observed.
COMPARATIVE EXAMPLE 4
500 9 of TEDA and polyethylene glycol #200
(manu~actured by Kanto Kagaku) were thoroughly mixed by a
V-type mixer, and the mixture was used a sample.
Otherwise, the operation was conducted in the same manner
as in Example 1. The results are shown in Table l.
EXAMP~E 4
Using hydroxypropylmethyl cellulose as the
anticoagulant, 500 g of TEDA and 50 ml of a 0.5% methanol
solution of hydroxymethyl cellulose were thoroughly mixed
by a V-type mixer and then dried in a vacuum drier. The
added amount was 0.1 g. The results are shown in Table
1.
- 15- 2~423~
_
.v~ ~n
rl) ~
3 Q
. ~
v ~ v rl~ ru rn
r~ rl) Q ~ ~1
rn ~ C~ v ~ a
O ~
a) cr id L~ rn rn
g u ~ o 3 c~ 3
. _ _
l l
ra ~
~ ~ "~ o ~ c~ ~ m m
~ ~ ,
o,O 1) ~ ~
c ~ v p~ ru
~ . _
~ m ~ Ln
o o o ~ I o ~ u~
. . . .
~ ^ oo o o o
~d -- . _ . .
O O O
v v v
ra ~ ~a
,. ~ ~ ~ ~ .,~ ._1 ~
Q ~ ~ .~ .P~ .~ .Q,
rd 1) v ~d ra rd X rl) rll rl)
~ a) o o o ,~
E~ ~ . ~ ~
r. 1:~ o o o o o o o o
~ Ln ~r Ln o ~ u~ o o
O _~~ r~ ~ Ln r~ n Ln
~0~ _
_ ~ ~0
~ .C ;~ n
~ ~ ~ ~ ~ ~a
,~ O O O ~1 3
E~ O
t) P~
~> ~1 0 ~ O ~ O h .C
,~ ~1 X ~ X ~I X ~ a
~ ~ O ~ O ~ o :: ,~ U
~1.C ~1 ~J1~ ~ h ~1 :~ ~1 >
~ V ~ ,-1~ ~1 ~ ,_1 ~1 ~1 ~ r-S
raa) ~ ~ ~rl O ~ O
~ ~U ~ O Z; ~ U~ ~
. _ _ . ~
~ ~ r
a~ aJ ~ d ~ ~d a) a ~ ~a
~1 ,~ ~ ~ ~ ~ ~ ,
~a ~ ~a ~ ~a
a ~ ,a ~ ~ ~a
X X X X O .rl X O rl X O r1 X O ~ ~(
~ ~, W ~_. ~ v ,t~ W C.) ~ ~
20~423~
- 16 -
EXAMPLE 5
Using hydroxypropyl cellulose (Nisso HPC), the coated
amount and the coagulation-preventing effects were
compared. The operation was conducted in the same manner
as in Example l except that instead of the alcohol
solution of methyl cellulose, (l) 2%, (2) 1%, (3) 0.5%,
; (4) 0.1~ or (5) 0.05~ alcohol solution of hydroxypropyl
cellulose, or (6) pure alcohol was used.
The amount of T~DA, the coated amount of
hydroxypropyl cellulose and the coagulation preventing
action are shown~in Table 2~ As is evident from Table 2,
a certain degree of coagulation preventing action was
observed even at a coated amount of lO ppm, and excellent
coagulation-preventing action was observed at a
concentration of 100 ppm or higher. Further, no
formation of turbidity or flowing substance was observed
in solutions of various solvents such as alcohols, water
and glycols.
204~236
- 17 -
. ~__
o
.,, ~:
~I C ~ ¢ ¢ ~ m m ~)
o
.~,
~a a) JJ
o ~ C~
o
.. ,,__ ~
_ In ~ ~D ~ o
.IJ N ~1 0 0 o o
a) ~ o o o o O
~ O
O
C~ ~
.-., _ .
~0 ^
O O O O O O
~ -- ~ ~r Lr)
a
E~
, ._
O ::
.,~~ O
V~ rl ~n ~ ~
J ~1 ~ ~ . . o
O O
~ O C~
W rC ~ Ul
O
O
o O
._
,~
~ ~ ~ ~ In ~
_____._
X
~q
:_ ,, , _ ,
2~236
- 18 -
EXAMPLE 6 AND COMPARATIVE EXAMPLE 5
Into a flask having an internal capacity of 2,000 ml,
1,000 g of a methanol solution of piperazine having a
composition comprising 40 parts by weight of piperazine
and 60 parts by weight of methanol, was introduced, and
0.5 g of hydroxypropylmethyl cellulose was added thereto.
The mixture was heated by a mantle heater, and heating
was stopped when 300 ~1 of methanol was distilled off.
The flask was immersed in a water bath and cooled until
the liquid temperature became 20C. Precipitated
piperazine was collected by filtration under suction with
a filter paper of No. 5C and washed with a small amount
of methanol. The product was dried under vacuum to
obtain 90 g of piperazine. Here, hydroxypropylmethyl
cellulose contained in the piperazine was 0.02 g. This
sa~lple was subjected to e~7aluation of coagulation degree
in the same manner as in ~xample 1. As a result, the
coagulation degree was found to be A rank.
Whereas, ln a case where the same operation was
conducted without addition of hdyro~ypropylmethyl
cellulose, coagulation was found progressed to the
interior, and the coagulation degree was found to be C
rank.
EXAMPLE 7 AND COMPARATIVE EXAMPLE 6
Into a flask having an internal capacity of 2,000 ml,
1,000 g of an aqueous ammonium chloride solution having a
composition comprising 35 parts by weight of ammonium
2~4236
- 19
chloride and 65 parts by weight of water, was introduced,
and 0.5 g of hydroxypropyl cellulose was added thereto.
The mixture was heated by a mantle heater, and heating
was stopped when 300 ml of water was distilled off. The
flask was immersed in a water bath and cooled until the
liquid temperature became 20C. Precipitated ammonium
chloride was collected by filtration under suction with a
filter paper of ~o. 5C and washed with a small amount of
pure water. The product was dried under vacuum to obtain
95 g of ammonium chloride. Here, hydroxypropyl cellulose
contained in the crystals was 0.025 g. ~his sample was
subjected to evaluation of the coagulation degree in the
same manner as in Example 1. As a result, the
coagulation degree was found to be A rank.
Whereas, in a case where the same operation was
conducted without adding hydroxypropyl cellulose,
coagulation was found progressed to the interior, and the
coagulation degree was found to be C rank.
EX~MPLE 8 ~ND COMPARATIVE EXAMPLE 7
Into a flask having an internal capacity of 2,000 ml,
1,000 g of an aqueous ammonium sulfate solution having a
composition comprising 40 parts by weight of ammonium
sulfate and 60 parts by weight of water, was introduced,
and 0.5 g of hydroxypropylmethyl cellulose was added
thereto. The mixture was heated by a mantle heater, and
heating was stopped when 400 ml of water was distilled
off. The flask was immersed in a water bath and cooled
204~23$
- 20 -
until the liquid temperature became 20C. Precipitated
ammonium sulfate was collected by filtration under
suction with a filter paper of No. 5C and washed with a
small amount of pure water. The product was dried under
vacuum to obtain ~40 g of ammonium sulfate. Here,
hydroxypropylmethyl cellulose contained in the ammonium
sulfate was 0.07 g. This sample was subjected to
evaluation of coagulation degree in the same manner as in
Example 1. As a result, the coagulation degree was found
to be A rank.
Whereas, in a case where the same operation was
conducted without adding hydroxypropylmethyl cellulose,
the coaguiation was found progressed to the interior, and
the coagulation degree was found to be C rank.