Note: Descriptions are shown in the official language in which they were submitted.
f
--1--
TITLE
IMPROVED PROCESS FOR RECOVE~ING
ORGANIC VAPORS FROM AIR
BACKGROUND OF THE INVENTION
1~ Field of the Invention
The present invention relates to an improved
process for recovering organic vapors from an organic
vapor/air mixture wherein organic vapors are present in
the so-called ~window concentratiQn~ range, being too
high for conventional carbon adsorption and too low for
efficient compression/condensation recovery. Msre
specifically, the present invention relates to
separating an organic vapor and air feed stream, by use
of a semipermeable membrane unit, into a vapor depleted
stream that is then process by carbon adsorption and a
vapor ~nriched strQam that is then processed by
compression and condensation.
2. Description of Related Art Including
Information Disclosed under 1.97-1.99
It is well known and a common commercial
practice to employ organic solvents such as hydrocar- ::
bons, halogenated hydrocarbons, oxygenated hydrocarbons
and the like in chemical processes, in the production
of articles of manufacture, in the cleaning industry as
well as in many other application~ wherein an organic
vapor/air mixture is produced as a by-product or waste
stream. Historically such spent organic vapor/air
30 streams would either be vented or flared to the
atmosphere. However, contemporary emission standards,
~timulated by such considerations as the ngrePnhous~"
effect, potential depletion of ætratospheric ozone as
well as general health considerations and conservation
35 of resource~ and basic economic considerations
CH-1595-~ associated with wasting c05tly raw material, manda~e
--1~
- , . . ~ -,
: - , - ,.. ..
- ! ' : , :
'
., , " .,
', ' ' ' .",
.
r~
--2--
that the organic solvent from the spent vapor/air
mixtures be reclaimed.
Generally, the particular solvent and the
concentration of its vapor in the air is dependent upon
the particular process or application generating the
spent stream. When the organic solYent i5 a high
boiling liquid, its recovery can usually be carried out
effectively by cooling to condense it to a liquid.
However, when the organic solvent is a low boiling
liquid there are two traditional methods for its
recovery. Thus it is generally known that at high
loading of organic vapor in air the organic solvent can
be economically reclaimed by compression and
condensation and at low loading of organic vapor in the
air the organic solvent can be economically reclaimed
by carbon adsorption.
The difficulty with the traditional methods
is that there is a so-called "window concentration"
range (typically from about 6 volume percent to 30
volume percent organic vapor) wherein neither method is
practical. At too low of a concentration compression
and condensation is inefficient in that greatly
increased cost of compression together with r~duced
amount o~ solvent recovery and increased emission o~
uncondensed vapor make the process prohibitively
expensive. On the other hand, at too high of a concen-
tration the exotherm associated with carbon adsorption
raises the temperature of the adsorbent bed thus
reducing the adsorption ~f~iciency and in some cases
leading to the possibility of spontaneous combustion~
Furthermore, high vapor concentration requires more
frequent regeneration of the carbQn adsorption tower
thus again addiny to the cost. At present, if a low
boiling organic vapor and air mixture in the ~window
concentration" range is produced, one must further
--3--
dilute the stream and then recover the organic phase by
adsorption.
In U.S. Patent 4,553,983 a process for
recovery of organic vapor from a feed stream of air
having an organic vapor content of no more than 20,000
ppm by volume (2 volume percent) is disclosed wherein a
thin sPmipermeable membrane with a permeation
selectively of at least 50 favoring the organic vapor
and a permeability of at least 3 x 10-7
cm3(STP) cm/cm2-sec-cmHg is used in combination with a
partial vacuum on the permeate side to enrich the
permeate with oxganic vapors. Similarly, in an article
by D. L. Roberts et al. entitled nRecovery of Freon
Gases with Silicone Rubber Membrane", Inq. Eng. Chem.
Process Desi~n. Dev., 1986, 25, pp 971-973, the
recovery of fluorocarbons and chlorofluorocarbons from
air mixtures using a semipermeable membrane is
disclosed. In U.S. Patents 4,316,364 and 4,417,451 a
refrigerant monitor system employing a membrane with a
permeation selectivity favoring air relative to the
refrigerant is disclosed.
SUMMARY OF T~E INVENTION
ThP present invention provides an improved
process for recovering organic vapors from organic
vapor/air mixtures, particularly mixtures wherein the
concentration of the organic vapor to be recovered is
in the so-called "window concentration~ range (i.e.,
too high for efficient recovery by the carbon
adsorption method and too low for fficient recovery by
the compression/condensation method). ThP improved
method according to the present invention involves the
imultaneous use of a membrane separation ~ystem in
combination with a conventional carbon a~sorption
system and a conventional compression/condensation
system, thus producing a hybrid recovery unit.
-3-
- ............................ : .
: . ,
, . . .
~J S , ~
--4--
According to one embodiment of th~ present invention, a
two-stage membrane separation system is used to produce
a feed side effluent at the first stage that is
sufficiently depleted of organic vapors that it can be
treated by conventional carbon adsorption~ The
permeate from the first stage is then introduced to the
feed side of the second stage of the membrane
separation system to produce further organic vapor
enriching of the second stage permeate that is then
treated by conventional compression and condensation.
The feed side effluent of the second stage is recycled
to the feed side of the first stage. Such a process is
particularly useful in recovering chlorofluorocarbon,
hydrochlorofluorocarbon, and hydrofluorocarbon vapors
emitted to the air during the manufacture of various
foamed plastic products and the like. In another
embodiment of the present invention, a feed side
effluent at the first stage of a two~stage membrane
separation system is organic vapor enriched and sent to
the compression/condensation separator while the
permeate from the second stage is organic vapor
depleted and, therefore, processed by carbon
adsorption. FurthPr according to the present
invention, a regeneration/recycle procedure employing
multiple carbon adsorption bed and the return of
effluent from an adsorption bed being regenerated with
an in situ stream is provided to further reduce
emission of the halocarbon.
Thus, the present invention provides a
process for separating and recovering organic vapors
from a ~eed str am of organic vapor and air wherein
or~anic vapors are present in the ~window
concentration~ range, being too high for conventional
carbon adsorption and too low for efficient
compression/condensation recovery, comprising the steps
of:
--4--
~, Ø~ . . J
-5-
(a) providing a semipermeable membrane means
for separating organic vapors from air having
a feed side and a permeate side wherein said
semipermeable membrane means is characterized
as having a selectivity for allowing the
passage of organic vapor relativs to air or
for allowing the passage of air relative to
organic vapor of at least 10 and permeability
for the permeate gas of greater than 1 x 10-7
cm3(STP) cm cm~2 cmHg~1 sec~1;
(b) passing a feed stream of oryanic vapor and
air, wherein said organic vapor is present in
the ~window concentrationn range, across the
feed side of the semipermeable membrane such
that organic vapor or air, but not both,
: passes preferentially through the membrane to
form an organic vapor depleted ~ir stream
characterized by an organic vapor
concentration below the nwindow
concentration'J range and an organic vapor
enriched stream characterized by an organic
vapor concentration above the ~window
concentrationN range;
(c) subj~cting said or~anic vapor depleted air
stream produced in step (b) to carbon
adsorption, thus separating and recovering
organic vapor therefrom; and
(d~ subjecting ~aid enrichPd stream produced in
step (b) to compression and condensation,
thus separating and recovering organic vapor
therefrom.
It is an object of the preæent invention to
provide an improved proc~s for the efficient and
economic recovery of low boiling or~anic solvent vapors
- 35 from an organic vapor and air mixturs, wherein the
organic vapor to air mixture is at a concentration such
--5--
, .. . . .
:: :
,
S" '' !'~ ' ' "' ~ ',;S
-6-
that the organic vapor concentration i6 too low for
efficient recovery by compression/condensation methods
and too high for efficient recovery by carbon
adsorption methods. It is an additional object of the
present invention to provide an efficient method of
recovering low boiling organic solvent vapors from the
above-described vapor/air mixtures by use of a semiper-
meable membrane separation stage that works in combina-
tion, simultaneously, with a conventional carbon
adsorption system and a conventional compression/con-
densation system. It is still a further ob~ect of the
present invention to provide a hybrid membrane-carbon-
adsorption-compreRsion/condensation vapor recovery
system that will recover chlorofluorocarbons from a
chlorofluorocarbon and air mixture particularly those
associated with the commercial use of blowing and
foaming agents in the manufacture of foamed plastics,
fibers, films and the like. Fulfillment of these
objects and the presence and fulfillment of other
objects will be apparent upon complete reading of th~
specification and claims taken in conjunction with the
attached drawing.
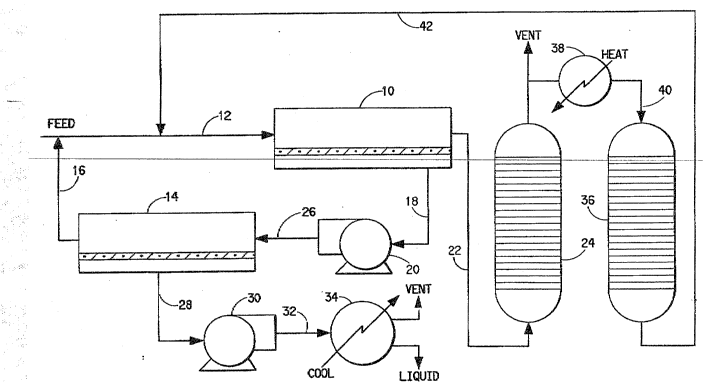
BRIEF DESCRIPTION OF THE DRAWING
'rhe Figure represents a schematic illustra-
tion of a typical improved process ~or recovery of
organic vapors according to one embodiment o~ the
present invention.
~ESCRIPTION OF THE PREFERRED EMBODIMENTS
The improved process for recovering organic
vapors from an organic vapor/air mixture according to
the present invention, how it differs from yet is
related to previously known methods of recov~ring
organic vapors, what advantages there are associated
with the use of the improved method relative to these
--6--
,, . . : ,
-, , '
--7--
prior art methods, and under what circumstances the
present invention provides these advantages can be best
explained and understood by reference to the drawing.
The schematic flow diagram of the Figure
illustrates the basic components and stages of one
particular embodiment of the present invention. As
illustrated in the Figure, a feed stream (i.e., a
halocarbon/air mixture in the ~window concentrationn
range) is continuously introduced into the first stage
10 of the two-stage semipermeable membrane units via
line 12. This gaseous feed stream entering the feed
side of first stage membrane separator 10 is
continuously commingled with the recycle gas~ous
effluent from the feed side of the second membrane
separator 14, via line 16, prior to the combined
mixture being sent to membrane separator 10. Within
separator 10 (in this particular illustrated
embodiment) an organic vapor enriched permeate is
continuously withdrawn via line 18 by virtue of vacuum
blower 20 while an organic vapor depleted stream is
withdrawn from the feed side via line 22 and sent to
(again in this particular illustrated embodiment) one
of two essentially equivalent carbon adsorber units,
24, for recovery of the organic vapors. The permeate
from the first stage membrane separator 10 i5 then
delivered to the feed side of the second stage
separator 14 via line 26. Within separator 14 further
organic vapor enriching of the permeate occurs with the
permeate being withdrawn via line 28 by virtue of
vacuum blower/compressor 30 and sent, via line 32, ~o
condenser/separator 34. The effluent from the feed
side of membrane separator 14, having an organic vapor
concentration substantially the same as the original
feed, is continuously returned and commingled with the
feed as previously mentioned.
,
.
,
,
f~ r , 7 , ~ ~
-8-
During operation of the embodiment of the
Figure, liquid halocarbon is continuously removed from
the bottom of the condenser/separator 34 while
halocarbon is simultaneously being deposited on the
carbon bed within adsorber 24. Halocarbon depleted air
may be vented from both the adsorber 24 and
condenser/separator 34. Because of the finite capacity
of the carbon adsorption bed, an additional adsorption
unit 36 is provided for continuous operation. While
bed 24 is actively adsorbing halocarbon the other
adsorption unit 36 can be regenerating~ The Figure
illustrates regeneration in that a portion of the air
effluent from adsorber 24 is passed through heater 38
and introduced into adsorber 36 via line 40. The
halocarbon thus removed from the regenerating adsor~er
36 is then sent back to an appropriate location in the
overall process for ~urther isolation and recovery of
halocarbon. As illustrated in the Figure the recycle
of halocarbon enriched regeneration gas is being
directed via line 42to the feed stream entering the
first membrane separation unit lO. It should be
appreciated that depending on the level or
concentration of halocarbon in this recycle loop, the
regeneration stream can be reintroduced advantageously
to other locations in the overall process, including
the permeate side of the first membrane unit 10 or
even directly to the compression/conden~ation unit if
the halocarbon concentration is sufficiently high. It
should also be appreciated that various additional
valves, conduits, and heat exchangers and the like
(not shown in the Figure) can be incorporated into the
process, as generally known in the art, to accomplish
the switching of adsorber units and regeneration with
recycle features of the present invention.
3~ It should be appreciated that the particular
two stage embodiment illustrated in the Figure is most
--8--.
.
" ' ', . . ~"
'
,
'
useful when the feed stream is relatively dilute with
respect to the so-called ~window concentration" range
or, stated in other terms, when the feed stream is
su~ficiently dilute to be further depleted of organic
vapors in a single membrane stage such as to be
amenable to carbon adsorption. Thus, in principle, the
process according to the present invention is
contemplated as being use~ul in embodiments that
involve a single staye membrane unit, a series or
lo sequence of membrane units wherein the final pair of
stags invslve a recycle loop. The basic point of
interest in each of these embodiments is that the use
of the semipermeable membrane unit, although not
achieving isolation and recovery of the organic vapor
per se, does produce simultaneously an organic vapor
enriched and an an organic vapor depleted gaseous
stream without external dilution which can then be
further treated by conventional carbon adsorption and
compression/condensation to recover the organic phase.
It should be further ~ppreciated that the specific
embodim2nt illustrated in the Figure is to be employed
with a semipermeable membrane that is selective with
respect to the preferential passage of organic vapor
relative to air. When a æemipermeable membrane
selective to the passage of air relative to organic
vapor is employed, the role of the carbon adsorption
unit 24 and the compression/condensation unit, 30 and
34, are interchanged.
The advantages of the improved process for
recovering organic solvent vapors according to the
present invention are significant and numerous. First
and foremost, the improved method affords an efficient
and technically convenient method of recovering solvent
vapors from feed streams that are in the J'window
concentration~' range, without resorting to excessive
dilution, oversized adsorption towers, frequent
_g_
-
:, . ,, - : "
.
,
_ ~ - r~
--10--
regeneration of columns or taxing the capacity of
existing adsorption tower to its limit or,
alternatively, forcing the compression/condensation
equipment to operate under impractical and inefficient
conditions including possible excessive emission
losses. By eliminating the need for further dilution
yet producing a vapor depleted effluent stream for
conventional carbon adsorption, the overall capacity of
a given adsorption tower is optimized and the risks
associated with relatively high conc~ntration of vapors
(excessive exotherms, hot spots and/or spontaneous
combustion) is significantly reduced. Simultaneously,
the production of an effluent stream enriched in
organic vapors further optimiz~s the compressionlcon-
densation step used to isolate and recover the organicsolvent.
The particular feed streams that are amenable
to the aforementioned advantages and benefits of the
present invention are generally any low-boiling organic
solvent vapor mixed with air or similar carrier gas
wherein the concentration of the organic vapor phase is
within the "window conc~ntration~ range. For purposes
of this invention, the organic vapor would include, by
way of example, but not limited thereto, low boiling
hydrocarbons, halogenated hydrocarbons, oxygenated
hydrocarbons and the like. The present invention is
viewed as bein~ particularly useful in the recovery of
halogenated hydrocarbons fr~quently employed in
commercial cleaning processes and as solvent or
blowing/foaming agents in many plastic ~abrication and
article manufacturing processes. In particular, thi~
would include the chloro- and/or ~luorocarbons, the
chlorofluorocarbons and the hydrochloro~luorocarbons as
commonly known and used in industry.
Generally when the organic solvent is a
liquid with relatively high boiling point, its recovery
--10--
..
-~
.' '
-11~
is relatively simple (i.e., cooling to bring about
condensation provides efficient recovery). In
contrast, when the organic solvent is a liquid with
relatively low boiling point, such as a boiling point
below about 40C wherein a large portion of the solvent
is in the vapor state at ordlnary temperatures, simple
condensation is not sufficient. Currently, there are
two traditional methods available ~or vapor recovery
for low boiling organic solvents, i.e., eithPr by
compression/condensation or by use of an adsorbent.
Unfortunately, for many organic vapor/air mixtures
th~re is an upper limit to that concentration of
organic vapors that can be processed by say caxbon
adsorption and a minimum concentration required to
efficiently recover vapor by compression/condensation.
In such ca es a "window concentration~ range may exist
wherein the concentration of organic vapor present in
the air or carrier gas is too high for conventional
carbon adsorption and too low ~or efficient
compression/condensation recovery. It should be
appreciated that the specific concentration associated
with this ~o-called ~window concentration~ range may
vary according to the ~pecific organic vapor and the
carrier gas as well as the operating conditions being
employed. Thus, generally and for purposes of this
invention, the "window concentrationn range can be
defined by viewing or considering what concentration
cannot be e~ficiently processed by conventional means.
However, as an al~ernative to this defini-
tion, the "window concentration~ range can be inter-
preted from an affirmative viewpoint by considering
the concentration ranges that can be advantageously
processed by the improved process according to the
present invention. To a great extent these viewpoints
arè synonymous but the latter de~inition may tend to
broaden the specific quantitative concGntration range.
: , - : ~
; ~ ' ' ',
-12-
Thus, for example, when the concentrations of
solvent vapor are sufficiently high in solventlair
mixture, say about 30 volume percent, efficient
recovery is possible by the compression/condensation
method wherein the solvent/air mixture is compressed
and cooled to condense the solvent vapor into liquid
solvent. The liquefied solvent can then b~ separat~d
and stored for further use. When the concentration of
solvent vapor in solvent/air mixture is low, for
lo example about 6 volume percent or lower, efficient
recovery is possible by the carbon adsorption method
wherein such solvent air mixture is contacted with
activated carbon. Typically this is performed in an
adsorption tower, wherein the solvent is preferentially
adsorbed on the carbon and air devoid of solvent can be
relea~d into the atmosphere. When the carbon in the
adsorption tower is saturated with the solvent, the
solvent/air stream is directed to another adsorption
tower. Solvent adsorbed on carbon can then be
o recovered by a number of ways such by raising the
temperature or passing steam through the tower.
However, when the concentration of low
boiling solvent in a solvent/air mixture is between
from about 6 volume percent and about 30 volume
percent, the above-described solvent recovery processes
- become inefficient and uneconomical. The present
invention provides an improvement in solvent recovery
process wherein low boiling solvent in a solvent/air
mixture is present in a concentration range which makes
recovery by either compr~ssion/condensation or carbon
adsorption method impractical and uneconomical. In
principle, the present invention provides a process for
treating a solvent/air mixture in the above-described
nwindow concentration~ range to obtain two different
solvent/air mixtures, i.e., one in which the solvent
concentration is sufficiently high for efficient
,, . ,~,.. . .. . ..
: :........... . . .
~ : . ~ : ,.
-13
recovery by the compression/condensation method and the
other in which the concentration of the solvent is
sufficiently low ~or efficient recovery by the carbon
adsorption method.
The semipermeable membrane unit useful in the
present invention can be generally any such device as
well known in the art, including by way of example, but
not limited thereto, semipermeable membrane thin layer
of film, spiral wound membrane, hollow fiber
semipormeable membrane or the like. For the
semipermeable membrane to eeparate the organic
vapor/air mixture into a vapor enriched component and a
vapor deplete component, there must be a difference in
the permeation rates for the organic vapor and air
(i.e., 2 and N2). For purpos~s of this invention the
ratio of permeation rate for the organic vapor through
the barrier membrane to the permeation rate ~or air
(usually measured with respect to nitrogen) should be
at least 10. Preferably this selectivity (or
separation factor) should be lQ0 or even up to 10,000
with the actual absolute permeation rate of organic
vapor being typically at least 1 x 10-7
cm3(STP)-cm-cm~2-cmHg~1-sec~1 or greater. Typically,
the barrier membrane is an elastomeric polymer film
made from natural rubbers, polyiosprenes, polybutenes,
polybutadienes, silicone rubbers, neoprene or the like
as generally known in the art. Preferably, for the
separation of ¢hlorofluorocarbons and hydrochloro-
fluorocarbons ~rom air, wherein the permeation
selectivity is to favor passage oP the halocarbon, a
barrier membrane of dimethyl sllicone rubber is
employed. When the permeation selectivity i~ to favor
the passage of air/ potential harrier membrane
materials include glasses, ceramics, polymeric
plastics, films and elastomers, natural products such
as ~ellulose and rubber as w211 as porous metals or
-13-
.. ..
,,, , , :,
':, ~ , ' ', ' ' ' ' , ~
r
--14 ~
metal films, such as stainless steel, palladium,
platlnum and cold rolled steel, as generally known in
the art.
The organic vapor depleted effluent stream
5 from the feed side of the membrane separation unit can
be processed for recovery of organic solvent by any of
the adsorption tower techniques as generally known in
the art. Preferably a carbon adsorption is used, but
~or purposes of this invention other convent1onal
adsorption units based on molecular sieve or adsorption
media should be considered equivalent to the pref~rred
carbon adsorption. Typically such systems involve a
plurality of individual adsorption towers wherein one
unit is selectively adsorbing organic vapor as the
effluent stream from the membrane section passes
through while the other units are being stripped of the
organic solvent and regenerated for further vapor
adsorption, again as generally known in the art.
The organic vapor enriched permeate stream
from the membrane separation unit can ~e processed by
any of the conventional compression and conden~ation
techniques as generally known in the art. Typically
the process involves a compression followed by a
condenser/heat exchange which is adapted to recover
liquid condensate. The liquid phase organic solvent
recovered as well as that recovered ~rom the carbon
adsorption process can either be sent to stor3ge or
recycled to the particular manu~acturing or process
system that generated the organic vaporJair mixture
being processed.
An example of a process which produces
organic vapor/air ~ixtures wherein the concentration of
the organic solvent in the vapor/air mixture is from
about 10 to 25 volume percent, i.e., in the ~window
concentrationn range, is in the manu~acturing of
continuous-fiber, spunbonded polyethylen2 fabric sheets
-14-
. " . . , ~ , ,
,
,
~J J, ~
-15-
which ars used, for example, in the housing industry.
In this flash-extrusion process a chloroforocarbon,
namely, monofluorotrichloromethane (CFC13, CFC-11; b.p.
23.8C) is used as the processing solvent. This
solvent is particularly useful because it provides
certain processing advantages which lead to superior
properties in the finishPd product. However, this
solvent is comparatively expensive and is considered to
be a contributor to the ~tratospheric ozone layer
depletion process. Thus, there is a two fold incentive
~environmental and economic) to recover as much of this
solvent as possible and in a cost effective way.
In this process, polyethylene is intimately
mixed and heated with mono~luorotrichloromethane in a
mixing tank to form a solution of polyethylene in
CFC-11. This solution is flash-extruded onto a moving
continuous belt in a chamber whereby the CFC-11 is
rapidly vaporized. The continuos-fiber polyethylene
mat is then bonded by heat and calender pressure to
provide the desired spunbonded sheet The vaporized
CFC-ll, now admixed with air present in the chamber,
has a concentration in the range of from about 10
volume percent to about 25 volume percent. Since this
concentration of CFC-ll in the CFC-ll/air mixture is
too low for efficient recovery by the compression/con-
densation method and too high for efficient recovery by
the car~on adsorption method, it was necessary to add
additional air to this mixture ~uch that the
concentration of CFC-ll was raduced to below 7 volume
percent 60 that recovery can be made by the carbon
ad~orption method. The step o~ adding additional air
so the solvent recovery by carbon adsorption method can
be used provided problems such as (13 considerably
greater volume of solvent/air mixture must now be
treated, (2~ if lesser amount of air is used to reduce
problem (1) above, the efficiency of the adsorption
-15-
C? r~
-16-
tower is reduced due to temperature rise in the
adsorption tower from the heat of adsorption, and, in
~act, in some situations, temperature may rise so high
that fire may cccur in the tower; and (3) since all of
the solvent is recovered via the carbon adsorption
tower, there is a constant need to switch adsorption
towers when it is saturated with CFC-11, and with the
attendant need to desorb CFC-11 from carbon and
reqeneration of the carbon adsorbent, certain loss of
CFC-11 into the atmosphere was unavoidable. By the use
of the present invention process, there is almost
complete recovery of CFC-11 for reuse and the
atmospheric release of CFC-ll has been greatly reduced.
The following examples are presented to
further illustrate specific embodimen~s of the
invention. In presenting these examples all ref~rences
to percentages of components in the gaseous phase are
by volume percent unless otherwise indicated.
EXAMPLE 1
The improved method according to the present
invention was carried out during a commercial process
for making polyethylene sheet~ wherein the process
comprised the steps of intimately mi~ing poly~thylene
beads with a chlorofluorocarbon (CFC-11), hea~ing and
mixing the mixture under pressure and then
flash-extruding the solution o~ polyethylene in CFC-11
onto a continuous moving belt in a chamber at
atmospheric pressure containing air. Under these
conditions CFC-11 which boils at 23.8C vaporizes very
rapidly leaving the desired polyethylene sheet on the
moving belt. The CFC-ll/air mixture exiting the
chamber measured during the 51 runs carried out
averaged 17.9 volume percent (range of from 10 volume
percent to 29 volume percent). Previously this
CFC-11/air mixture was diluted with additional air so
-16-
,
.-: . , : -
, : . , . , ,.. :
.,
:, .:-, . ,
--17 ~
that CFC concentration was rsducPd to a few volume
percent so that CFC-11 could be recovered by the carbon
adsorption method. In carrying out the process o~ the
invention, the CFC-11/air mixture was contacted with
semipermeable membrane unit. The semipermeable
membrane utilized was an elastomeric dimethyl silicone
wherein the membrane is incorporated into spiral wound
membrane module. The unit is operated by maintaining
the vapor pressure on the permeate side lower than the
vapor pressure on the feed side. This is done by
operating a vacuum pump on the permeate side. In the
51 runs, the feed to the permeation unit which averaged
17.9 volume percent o~ CFC-11 was separated into CFC-11
enriched component which averaged 73.82 volume percent
CFC-11 (range of 50 volume percent to approaching 100
volume percent) and CFC-ll depleted component which
averaged a 6.8 volume percent CFC-ll (range of 3.3
volume percent to 12.0 volume percent). With the
average concentration of CFC-ll of 73.8 volume percent
which on the average represents enrichment o~ 4.1 fold,
CFC-11 from such a mixture was recovered efficiently by
the usual compression/condensation method. The CFC-11
depleted component with an average concentration of
CFC-11 of 6.8 volume percent was readily treated in the
carbon adsorption tower.
E~A~
A continuously feed str~am o~ CFC-11 and air
withdrawn from a chamber containing a moving belt upon
which a spunbonded, random weave, polyethylene fabric,
is being cbntinuously manufactured is fed to a hybrid
membrane-carbon-adsorption-c:ompression/condensation
~ystem as illustrated in the Figure~ This feed stream
is delivered at a rate of 1000 scfm and contains an
average of 12 volume percent CFC-ll. Prior to
introduction of the feed stream to the first stage
-17-
" , , ' ~ ~ . . : '
.
.~ f . . .
: '
~ -
~s
-18-
~piral wound membrane unit hav~ng a silicon polymer
semipermeable membrane, it is combined with a 12 volume
percent CFC-ll recycle effluent at a rate o~ 71.3 scfm
from the feed side of the second ~tage membrane unit.
A vacuum blower delivers a 60 volume percent CFC-11
permeate stream from the first ~tage membrane unit to
the ~econd stage membrane unit at a flow rate of 169.1
scfm. The second stage silicone polymer spiral wound
membrane unit is used in combination with a ~econd
lo vacuum blower to withdraw a 95 volume percent CFC-11
permeate stream at 97.8 scfm, while the previously
mentioned 71.3 scfm, 12 volume percent CFC-ll feed side
effluent is recycled to the inlet of the first membrane
separator. The 9S volume percent CFC-11 permeate
stream at 97.8 scfm is directed to a conventional
compressor/condenser stage for final removal and
recovery of CFC-11 while an 3 volume percent CFC-11
effluent stream from the feed side of the first
membrane separator at a rate of 9C2.2 scfm is directed
to a conventional carbon adsorption unit for separation
and recovery of CFC-11.
Having thus described and exemplified the
invention with a certain degree of particularity, it
should be appreciated that the following claims are not
to be so limited but are to be aforded a scope
commensurate with the wording of each element of the
claim and equivalents thereof.
-18-