Note: Descriptions are shown in the official language in which they were submitted.
CULTURE ~G
BACKGROUND OF THE INVENTION
1. Field of the Invention:
The present inventlon relates to a culture bag for
culturing cells such as animal tissue. More particularly,
it is concerned with a culture bag made of plastic sheet
having good flexibility, clarity, and gas permeability.
2. Description of the Prior Art:
The culture of cells (such as animal tissue) in open-
type containers such as Petri dish and flask has a disad-
vantage that the culture medium is liable to contamination
with airborne infectious microbes which enter the incuba-
tor when it is opened. To cope with this problem, there
have been proposed a variety of closed-type containers as
mentioned below.
"Biotechnology Series, Technique of Cell Culture"
pp. 137-141, issued by Kodansha Scientific (1985),
describes using plastic bags for the culture of wall-
sticking cells.
Japanese Patent Laid-open No. 214178/1988 discloses a
cell culture container consisting of a bag-like container
proper (formed by bonding two flexible plastic sheets to
each other) and an inlet tube and an outlet tube each pro-
vided with a coupler at its end. According to this dis-
~ ~ ` ' ! ., , . `,
closure, the container should be made of a different mate-
rial depending on its use. For aerobic culture, the pre-
ferred material includes ethylene-vinyl acetate copolymer,
polyethylene, po]ypropylene, polybutadiene, Teflon~, and
silicone rubber, which have good gas permeability. For
anaerobic culture, the preferred material includes polyvi-
nylidene chloride, polyvinyl alcohol, polyethylene -tere-
phthalate, and copolymer of acrylonitrile-butadiene rubber
grafted with acrylonitrile and methyl methacrylate, which
have good gas barrier properties. In addition, these
materials may be combined with one another to form a lami-
nate, if necessary.
Japanese Patent Laid-open No. 202378/1988 (USP Serial
Number 008,213) discloses a technique of culturing spe-
cific cells in an airtight con-tainer provided with an
access tube. According to this disclosure, the airtight
container is made of a copolymer film about 0.09-0.23 mm
thick, having an oxygen permeability not smaller than
about 1.8 x 105 ~m3 (STP) per m2-sec-Pa. Ihe copolymer
fi]m is one which is formed by lamination or coextrusion
from any of (a) ethylene--~-olefin (C410) copolymer having a
density of about 0.915-0.925 g/cm3~ (b) ethylene-
methacrylic acid copolymer, (c) an ionomer, and (d) ionom-
er/polyester elastomer and linear low-density polyethylene
elastomer.
The contalners proposed in the above-mentioned dis-
closures have a disadvantage in clarity and gas permeabil-
ity and hence they cannot be used as cell culture bags.
Japanese Patent Publication No. 50063/1980 (USP
3,780,140) discloses a resin composition from which to
produce flexible plastic containers. This resin composi-
tion is composed of a copolymer consisting essentially of
(a) 40-80 wt~ ethylene, (b) 3-30 wt% carbon monoxide, and
(c) 5-60 wt~ comonomer (preferably vinyl acetate) and
polyvinyl chloride or any other polymer in an amount low
enough for it to be compatible with said copolymer.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a
culture bag which has an adequate degree of gas permeabil-
ity to oxygen and carbon dioxide and permits one to freely
control the area of gas permeation per unit volume, and
which is superior in clarity.
The present invention was completed to achieve the
above-mentioned object.
The first aspect of the present invention is embodied
in a culture bag which comprises a culture room formed by
fusion-bonding a transparent sheet for the culture room
(this sheet will be referred to as the "sheet for the
culture room" in the specification and claims) and at
least one opening formed therein, said sheet being made of
-- 3
a resin composition composed of 100 parts by weight of
vinyl chloride homopolymer or copolymer and lS0-260 parts
by weight of ethylene-n-butyl acrylate-carbon monoxide
copolymer, and having a gas permeability of 600-3000
ml/m2-24hr-atm to oxygen and a gas permeabili-ty of
1000-30,000 ml/m2-24hr-atm to carbon dioxide.
The second aspect of the present invention is
embodied in a culture bag which comprises an lntegrally
formed culture room and medium storage room, and at least
one each of cell injection opening and medium discharge
opening formed in the culture room and a medium injection
opening formed in the medium storage room, with the
culture~room and medium storage room communicating with
each other through a passage, said culture bag being
formed by fusion-bonding the peripheries of two sheets for
the storage room (this sheet will be referred to as the
"sheet for the storage room" in the specification and
claims) and one sheet for the culture room in such a
manner that the two sheets for the storage room face to
each other to form the medium storage room and one of the
two sheets for the storage room and the one sheet for the
culture room face to each other to form the culture room,
said sheet for the storage room being made of a resin com--
position composed of 100 parts by weight of vinyl chloride
homopolymer or copolymer and 30-150 parts by weight of
~ ~ J
ethylene-vinyl acetate-carbon monoxide copolymer, and
having a gas permeability of 100-350 ml/m2 24hr atm to
oxygen and a gas permeability of 200-lO00 ml/m2-24hr-atm to
carbon dioxide, said sheet for the culture room being
defined as above.
The third aspect of the present inventlon is embodied
in a culture bag which comprises a culture room formed by
fusion-bonding the sheet for the culture room (defined
above) and a medium storage room formed by fusion-bonding
the sheet for the storage room (defined above), said two
rooms communicating with each other through a passage con-
nected to at least one opening formed in the culture room
and at least one opening formed in the medium storage
room.
The culture bags defined in the first, second, and
third aspects of the present invention may be constructed
such that the two sheets constituting the culture room are
partially fusion-bonded to form a partition which sepa-
rates the culture room into upper and lower compartments,
said partial fusion-bonding being accomplished in such a
manner that at least one unsealed part forms a cell
passage and the upper side of at least one of the sealed
parts functions as the guide which leads cells to the
unsealed part. In addition, the sheet for the culture
room constituting the culture bags may be a three-layer
laminate sheet, with the outer layers being made of a
resin composition composed of 100 parts by weight of vinyl
chloride homopolymer or copolymer and 150-260 parts by
weight of ethylene-n-butyl acrylate-carbon monoxide
copolymer, the core being made of ethylene-n-butyl
acrylate-carbon monoxide copolymer.
In the present invention the gas permeability to
oxygen and carbon monoxlde was measured according to ASTM
D1434, the melt flow rate was measured according to ASTM
D1238, and the total light transmittance was measured
according to JIS K7105.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a partly cutaway plan view showing the
culture bags in Examples 1 to 4.
Fig. 2 is a partly cutaway plan view showing the
culture bag in Example 5.
Fig. 3 is a longitudinal sectional view showing the
culture bag in Example 5.
Fig. 4 is a sectional view showing the stopper which
is not yet cut.
Fig. 5 is a sectional view showing the stopper which
has been cut.
Fig. 6 is a partly cutaway plan view showing the
culture bag in Example 6.
Figs. 7 to 9 are partly cutaway plan views showing
the culture bags in Example 7.
Fig~ 10 is a sectional view showing the sheet for the
culture room in Example 10.
DETAILED DESCRIPTION OF THE INVENTION
The culture bag pertaining to the first aspect of the
present invention comprises an airtight culture room and
at least one opening formed therein, said culture room
being formed hy fusion-bonding a transparent sheet for the
culture room having specific properties.
Oxygen is necessary for cells to grow in a medium and
carbon dioxide is necessary for a medium to be in the pH
range 7.4-7.6. At a pH higher than 7.8, cell growth does
not proceed smoothly. Therefore, it is necessary that the
culture bag be made of a material having a good gas perme-
ability to oxygen and carbon dioxide. In addition, the
material for culture bags should have flexibility, heat-
sealability that ensures airtightness, good clarity that
permits the microscopic examination of cell growth, and
freedom from plasticizers (such as dioctyl phthalate and
diisodecyl phthalate) which are toxic to cells. The sheet
for the culture room to meet these requirements is made of
a resin composition composed of 100 parts by weight of
vinyl chloride homopolymer or copolymer and 150-260 parts
by weight, preferably 170-230 parts by weight, of
c ` ~
ethylene-n-butyl acrylate-carbon monoxide copolymer. It
has a gas permeability of 600-3000 ml/m2-24hr atm, prefer-
ably 800-2600 ml/m2-24hr-atm, to oxygen and a gas perme-
ability of 1000-30,000 ml/m2-24hr-atm, preferably
1500-25,000 ml/m2-24hr-atm, to carbon dioxlde. If the
amount of ethylene-n-butyl acrylate-carbon monoxide
copolymer is less than specified above, the resulting
sheet is poor in clarity and flexibility; in the opposite
case, the resulting sheet is poor in strength and liable
to blocking. The sheet for the culture room should have a
total light transmittance of 80-100%.
The vinyl chloride homopolymer or copolymer mentioned
above should be one which has an average degree of polym-
erization of 300--5000, preferably 800-2000, as measured
according to JIS K6721. The vinyl chloride copolymer may
contain as one or more comonomers a-olefins (such as ethy-
lene and propylene), vinyl esters (such as vinyl acetate
and vinyl stearate), vinyl ethers ~such as methyl vinyl
ether and cetyl vinyl ether), vinyl halides (such as vinyl
bromide and vinyl fluoride), unsaturated acids (such as
maleic acid, maleic anhydride, and fumaric acid) and
esters thereof, and other vinyl and vinylidene compounds
than mentioned above ~such as styrene, acrylonitrile, and
vinylidene chloride). The conten-t of the comonomer should
be 1-10 wt%, preferably 2-8 wt%. The preferred vinyl
chloride copolymer is a vinyl chloride-ethylene copolymer
containing 2-8 wt% ethylene and having an average degree
of polymerization of 800-2000.
The ethylene-n-butyl acrylate-carbon monoxide copoly-
mer should be one which is composed of 40-80 wt%, prefer-
ably 60-70 wt%, ethylene, 15-60 wt%, preferably 20-35 wt%,
n-butyl acrylate, and 5-30 wt%, preferably 5-15 wt%,
carbon monoxide. This copolymer may be produced by
copolymerization of the comonomers by vigorous stirring at
a high temperature (preferably 160-230C) under a high
pressure (preferably 24,000-27,000 psi) in a reactor in
the presence of a polymerization catalyst such as t-butyl
peroxyisobutyrate and azodiisobutyronitrile. If neces-
sary, this copolymer may be further copolymerized with an
additional comonomer. This copolymer should have a melt
flow rate of 1-500 g/10 min, preferably 5-100 g/10 min.
The resin composition for the sheet for the culture
room may be prepared by melt-blending the vinyl chloride
homopolymer or copolymer and the ethylene-n-butyl
acrylate-carbon monoxide copolymer, together with optional
additives including a stabilizer (such as calcium stearate
and zinc stearate), a lubricant (such as polyethylene wax
and stearic acid), and the like. The blending may be
accomplished by using a batchwise mixer (such as roll and
mixer) or a continuous mixer (such as twin-screw
i:' ', " . ': i. . .',j
extruder).
The sheet for the culture room may be produced by
extruding the resin composition in pellet form. The sheet
should be 0.1-0.5 mm thick, preferably 0.15-0.~ mm thick.
With a thickness smaller than 0.1 mm, the culture bag
would be broken durlng filling. With a thickness larger
than 0.5 mm, the sheet would be poor in clarity and gas
permeability.
The culture room should have at least one opening
which is formed preferably at one end thereof. This
opening is used to inject and discharge a medium and
washing liquid such as saline solution and phosphate
buffer saline solution, to inject cells, to recover cul-
tured cells, and to sample cells during culture. This
opening is formed by attaching a tube (preferably 3-5 mm
in diameter) to the culture room. This tube should pre-
ferably be made of the same material as that from which
the culture room is made. This tube may be produced by
extruding the resin composition in pellet form.
The culture bag of the present invention may be
formed from two pieces of the sheet for the culture room
cut in approximate rectangle of desired si~e from -the web.
The two sheets are placed one over the other, with a tube
for the opening held between them at one end thereof, and
- 10 -
the peripheries of the sheets are fusion~bonded together
with the tube by high-frequency sealing or heat sealing
which makes the culture bag completely airtight.
The culture bag of the present invention is used in
the following manner. First, the culture bag is steril-
ized by ethylene oxlde. The sterilized culture bag (with
or without a sterilized bag holding it) is brought into a
clean bench. The culture bag (which constitutes the
culture room) is supplied with a culture medium and cells
through the opening. The opening is closed with a pinch-
cock or a sterilized cap or rubber stopper to keep the
culture bag airtight. The culture bag is allowed to stand
for the static culture of cells in a desired gas atmo-
sphere. After culture is completed, the culture bag is
emptied of the culture medium and cultured cells through
the opening. During culture, sampling may be facilitated
if the culture room is provided with a sampling tube
having a rubber cap inserted therein.
Since the sheet from which the culture bag is made
has good gas permeability and clarity, the culture bag
mentioned above offers an advantage that it permits the
permeation of as much gas as necessary for cell culture
and it also permits the content to be observed from
outside. Therefore, the culture bag permits the culture
in an airtight system and hence prevents the entrance of
infectious microbes into the culture medium. In addition,
the culture bag can be easily made in any desired shape
and size by fuslon-bonding. The sheet for the culture
room has a good gas permeabillty which can be controlled
as desired by selecting a proper sheet thickness and
blending ratio for the resin composition.
The culture bag pertaining to the second aspect of
the present invention comprises an integrally formed
culture room and medium storage room, and at least one
each of cell injection opening and medium discharge
opening formed in the culture room and a medium injection
opening formed in the medium storage room, with the
culture room and medium storage room communicating with
each other through a passage, said culture bag being
formed by fusion-bonding the peripheries of two sheets for
the storage room and one sheet for the culture room in
such a manner that the two sheets for the storage room
face to each other to form the medium storage room and one
of the two sheets for the storage room and the sheet for
the culture room face to each other to form the culture
room.
This culture bag employs the same sheet for the
culture room as used for the culture bag pertaining to the
first aspect of the present invention. The medium storage
room is formed from the sheet for the storage room which
- 12 -
has a low gas permeability so that the medium storage room
protects the medium from deterioration by oxidation. In
addition, the storage room should be made of a sheet
capable of fusion-bonding so that the culture bag is air-
tight. To meet these requirements, the sheet for the
storage room is formed from a resin composition composed
of 100 parts by weight of vinyl chloride homopolymer or
copolymer and 30-150 parts by weight, preferably 40-120
parts by weight, of ethylene-vinyl acetate-carbon monoxide
copolymer, and having a gas permeability of 100-350
ml/m2-24hr-atm to oxygen and a gas permeability of 200-1000
ml/m2-24hr-atm, preferably 200-900 ml/m2 24hr atm, to
carbon dioxide.
The vinyl chloride homopolymer or copolymer from
which the sheet for the storage room is made is the same
one as that from which the sheet for the culture room is
made. The ethylene-vinyl acetate-carbon monoxide copoly-
mer as one component of the above-mentioned resin composi-
tion should preferably be one which is produced by
copolymerization of 100 parts by weight of ethylene, 10-90
parts by weight of vinyl acetate, and 3-50 parts by weight
of carbon monoxide. This copolymer may be produced by
copolymerization of the comonomers by vigorous stirring at
a high temperature (preferably 155-230C) under a high
pressure (preferably 20,000-35,000 psi) in a reactor in
- 13 -
S~
the presence of a polymerization catalyst such as t-butyl
peroxyisobutyrate and azodilsobutyronitrile. This copoly-
mer should have a melt flow rate of 0.1-500 g/10 min, pre-
ferably 0.5-100 g/10 min.
The sheet for the storage room may be produced in the
same manner as the sheet for the culture room. It should
be 0.2-0.5 mm thick, preferably 0.3-0.4 mm thick. With a
thickness smaller than 0.2 mm, the storage room would
burst when it is filled and be poor in gas barrier proper-
ties. With a thickness larger than 0~5 mm, the storage
room does not permit the smooth discharging of its con-
tents.
The culture room is usually provided with at least
one opening for cell injection and at least one opening
for medium discharge, which are formed at one end thereof.
The storage room is provided with an opening for medium
injection. These two rooms communicate with each other
through a passage. The openings and a passage may be
formed in the same manner as mentioned above. They should
preferably be formed from a tube, 3-5 mm in inside diame-
ter, made by extrusion molding of the same material as
that of which the rooms are made.
The culture bag pertaining to the second aspect of
the present invention may be formed from two pieces of the
sheet for the storage room and one piece of the sheet for
- 14 -
the culture room, both cut in approximate rectangle of
desired size from the web. The three sheets are placed
one over the other, with tubes for the openings and the
passage held between them at desired positions, in such a
manner that the former two sheets face to each other to
form the medium storage room and one of the former two
sheets and the latter sheet face to each other to form the
culture room. The peripheries of the three sheets are
fusion-bonded together with the tubes by high-frequency
sealing or heat sealing which ensures complete airtight-
ness.
The thus obtained culture bag consists of the culture
room and the medium storage room which are integrally
formed. It is used in the following manner. First, the
medium storage room is filled with a culture medium, with
the passage closed. On the occasion of starting the
culture, the passage is opened and the medium is trans-
ferred from the storage room to the culture room, and
cells are injected into the culture room through the
opening for cell injection. The culture bag is placed in
an incubator of desired gas atmosphere for static culture.
Finally, the medium is discharged through the medium dis-
charge opening, and the discharged medium is tested for
grown cells. The medium storage room keeps the medium in
good conditions, because it is constructed of two sheets
- 15 -
of vinyl chloride type resin having good oxygen permeabil-
ity and carbon dioxide permeability. The culture room
permits gas permeation through the outside sheet and also
permits the observation of its contents through the
outside sheet, which is made of a transparent vinyl chlo-
ride type resin having good oxygen permeability and carbon
dioxide permeability.
As mentioned above, the culture bag pertaining to the
second aspect of the present invention has integrally
formed two rooms, one being the medium storage room to
store the medium in good conditions, the other being the
culture room which permits the permeation of gas necessary
for culture and also permits the observation of its con-
tents from outside. Moreover, the two rooms communicate
with each other through a passage, so that the medium can
be transferred from the medium storage room to the culture
room. An advantage of this constitution is that the
medium can be injected into the culture room in a very
short time without contamination. An additional advantage
is that the integrally formed storage room and culture
room are easy to handle.
The culture bag pertaining to the third aspect of the
present invention is made up of one culture room and one
medium storage room, each having at least one opening,
which rooms communicate with each other through a passage.
- 16 -
? ~
The culture room is made of sheets for the culture room,
and the medium storage room is made of sheets for the
storage room. The sheet for the culture room is the same
as that in the case of the culture bag pertaining to the
second aspect of the present invention. Also, the sheet
for the culture room is the same as that in the case of
the culture bag pertaining to the second aspect of the
present invention.
According to a preferred embodiment, the culture room
is provided with an opening for cell injection and an
opening for medium discharge, and the medium storage room
is provided with an opening for medium injection. The
culture room may be formed from two pieces of the sheet
for the culture room cut in approximate rectangle of
desired size from the web. The two sheets are placed one
over the other, with tubes held between them at desired
positions, and the peripheries of the sheets are fusion-
bonded together with the tubes by high-frequency sealing
or heat sealing. The medium storage room may also be
formed from two pieces of the sheet for the storage room
in the same manner as mentioned above.
The culture bag pertaining to the third aspect of the
present invention may be used basically in the same manner
as the culture bag pertaining to the second aspect of the
present invention.
- 17 -
~ J
The above-mentioned three types of culture bags may
be produced by an alternative method. For example, the
culture room or medium storage room may be formed from a
folded sheet in place of two flat sheets. In the case of
the first and third types of the culture bag, the culture
room may be formed from one piece of the sheet for the
culture room and one piece of a sheet which is not origi-
nally intended for the culture room. In other words,
there is no problem if one of the two sheets constituting
the culture room is the sheet for the culture room. The
sheet which is not originally intended for the culture
room should be one which is capable of fusion-bonding with
the sheet for the culture room and has no adverse effect
on culture. In the case of the second and third types of
the culture bag, the medium storage room may be formed
from one piece of the sheet for the storage room and one
piece of a sheet which is not originally intended for the
storage room. The sheet which is not originally intended
for the culture room and the sheet which is not originally
intended for the storage room may be produced from polyvi-
nyl chloride, ethylene-vinyl acetate-carbon monoxide
copolymer, or ethylene-vinyl acetate copolymer (containing
40-50 wt% vinyl acetate), or a mixture thereof. These
sheets should have a degree of gas permeability equal to
or lower than that of the sheets for the culture room and
- 18 -
storage room. In the case of the second and third types
of the culture bags, the tube as the passage should pre-
ferably be provided with a stopper which can be opened at
an appropriate time. This stopper should preferably be
fusion-bonded to the tube.
The first, second, and third types of the culture
bags may be modified such that the culture room is divided
into upper and lower two compartments by a partition. The
partition may be formed zigzag or aslant. The zigzag par-
tition may have a gap at the lower corner through which
cells can pass. The aslant partition consists of two
parts, one extending aslant downward from the left side of
the culture room and the other extending aslant downward
from the right side of the culture room, which do not join
each other. The partition may be curved.
The partition facilitates the separation of grown
cells after static culture simply by suspending the
culture bag, with the tubes upward. In the suspended
culture bag the grown cells move downward along the aslant
partition and enter the lower compartment of the room
through the gap. After cell separation, the culture bag
is turned upside down and the culture medium is discharged
through the tube. The cell separation in this manner
eliminates the necessity of using mechanical separation
(such as centrifugation and filtration) which is liable to
-- 19 --
cause damage to the grown cells. Moreover, the cell sepa-
ration in this manner makes it possible to continue cell
culture without any fear of contamination simply by reple-
nishing a new culture medium, because the grown cells
remain in the bag.
The sheet for the culture room used to constitute the
culture room of the first, second, and third types of the
culture bag may be a three-layer laminate sheet, with the
outer layers being made of a resin composition composed of
100 parts by weight of vinyl chloride homopolymer or
copolymer and 150-260 parts by weight of ethylene-n-butyl
acrylate-carbon monoxide copolymer, the core being made of
ethylene-n-butyl acrylate-carbon monoxide copolymer. The
outer layers of the laminated sheet should have a gas per-
meability of 600-3000 ml/m2 24hr atm to oxygen and a gas
permeability of 1000-30,000 ml/m2-24hr-atm to carbon
dioxide. The outer layers give the laminated sheet high
mechanical strength and good heat resistance. On the
other hand, the core layer has good gas permeability. The
thickness of the core layer should preferably be 150-300
~m. With a core thinner than 150 ~m, the laminated sheet
needs thicker outer layers, which lowers the gas perme-
ability. With a core thicker than 300 ~m, the laminated
sheet is poor in heat resistance and mechanical strength
- 20 -
and liable to wrinkling. The total thickenss of the lami-
nated sheet should preferably be 200-400 ~m. The lami-
nated sheet thicker than 400 ~m is poor in clarity and
flexibility. The laminated sheet thinner than 200 ~m is
poor in mechanical strength and liable to wrinkling.
Basically, this laminated sheet may be prepared in the
same manner as used for the above-mentioned sheet for the
culture room.
The laminated sheet may be prepared by one of the
following four methods. (a) Coating both sides of a core
sheet with a solution of resin composition which forms a
film after drying. Or, dry bonding of a core sheet with
two sheets as the outer layers. (b) Thermolamination of
a core sheet with two sheets as the outer layers. (c)
Coating both sides of a core sheet with an extruded molten
resin which forms the outer layers. (d) Lamination of a
core sheet and outer layers by coextrusion.
The thus obtained laminated sheet is superior in gas
permeability and free of plasticizer such as dioctyl
phthalate. In addition, it is characterized by flexibil-
ity, high mechanical strength, and good fabricability such
as fusion-bonding.
The invention will be described in more detail with
reference to the following examples, which are not
intended to restrict the scope of the invention.
- 21 -
Referential Example 1
An ethylene-n-butyl acrylate-carbon monoxide copoly-
mer was prepared by vigorously stirring a mixture composed
of 57 wt% ethylene, 33 wt% n-butyl acrylate, and 10 wt%
carbon monoxide, together with t-butyl peroxyisobutyrate,
in a reactor at 180C and 27,000 psi. This copolymer has
a melt flow rate of 12 g/10 min (as measured according to
ASTM D1238).
Referential Example 2
An ethylene-vinyl acetate-carbon monoxide copolymer
was prepared by vigorously stirring a mixture composed of
66 wt% ethylene, 24 wt% vinyl acetate, and 10 wt% carbon
monoxide, together wi~h t-butyl peroxyisobutyrate, in a
reactor at 179-C and 27,000 psi. This copolymer has a
melt flow rate of 35 g/10 min (as measured according to
ASTM D1238).
Example 1
A resin composition was prepared by melt-mixing at
180-C using an extruder 100 parts by weight of vinyl
chloride-ethylene copolymer (having an average degree of
polymerization of 1300 and containing 9 wt% ethylene), 160
parts by weight of ethylene-n-butyl acrylate-carbon monox-
ide copolymer (containing 57 wt% ethylene, 33 wt% n-butyl
acrylate, and 10 wt% carbon monoxide) prepared in Referen-
tial Example 1, 1.0 part by weight of Ca-Zn type stabi-
lizer ("Mark 37" made by Adeka Argus Co., Ltd.), 15 partsby weight of epoxidized soybean oil ("0-130P" made by
Adeka Argus Co., Ltd.), and 1 part by weight of polyethyl-
ene wax ("Hiwax 4202E" made by Mitsui Petrochemical Co.,
Ltd.). The resin composition was pelletized.
The pellets were formed into a sheet (0.15 mm thick)
by extrusion at 180 C through a 60-mm single-screw
extruder equipped with a 400-mm wide T-die. The pellets
were also formed into a tube (4 mm in inside diameter and
5 mm in outside diameter) by extrusion at 180-C through
the same extruder as above equipped with a tube die.
The thus obtained sheet (for the culture room) was
cut into two approximate rectangles measuring 20 cm by 27
cm. The two rectangular sheets were placed one over the
other, with three pieces of the tube held between them at
one end thereof. The peripheries of the sheets were
fusion-bonded, together with the tubes, by high-frequency
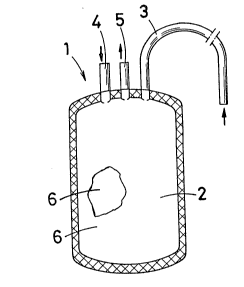
sealing. Thus there was obtained a culture bag (1) con-
sisting of a 200-ml culture room (2), a medium injection
tube (3), a cell injection tube (4), and a sampling tube
(5), as shown in Fig. 1.
Examples 2 to 4
Culture bags were prepared in the same manner as in
Example 1 except that the amount of ethylene-n-butyl
- 23 -
acrylate-carbon monoxide copolymer in the resin composi-
tion was changed to 240 parts by weight (in Example 2),
the sheet thickness was changed to 0.4 mm (in Example 3),
and the amount of the copolymer in the resin composition
was changed to 240 parts by weight and the sheet thickness
was changed to 0.4 mm (in Example 4).
Tests for physical properties
The sheets used for culture bags in Examples 1 to 4
were tested for total light transmittance and permeability
to oxygen and carbon dioxide. The results are shown in
Table 1.
Experiment 1
The culture bags obtained in Examples 1 to 4 were
used for experiments on cell culture in the following
manner. First, the culture bag was sterilized with ethy-
lene oxide. The culture room was filled with 200 ml of
serum-free medium (made by Kyokuto Pharmaceutical Indus-
trial Co., Ltd.) through the tube (3). Then, the culture
medium was inoculated with as many cells as 5 x 104
cells/ml, which were injected through the tube (4). These
operations were carried out in a clean bench. The cells
are the hybridomas obtained by cell fusion between
P3/NS1/1-Ag4-1 cells (ATCC No. TIB-18, referred to as "es-
tablished cell line NS-1" hereinafter) and mouse spleen
cells. With all the tubes closed by pinchcocks, the
- 24 -
culture bag was placed in an incubator at 37C for five
days for static culture in an atmosphere of 5% carbon
dioxide. On the fifth day of culture, the culture medium
was sampled through the tube (5) to count the number of
cells.
For comparison, static culture was carried out for
five days in a carbon dioxide incubator under the same
conditions as mentioned above, using a 150-cm3 polystyrene
culture flask (made by Iwaki Glass Co., Ltd.) containing
30 ml of serum-free medium and hybridomas (5 x 104
cells/ml). During incubation, the stopper of the culture
flask was kept slightly open. On the fifth day of
culture, the number of cells was counted.
The results are shown in Table 1.
Experiment 2
Cell culture was carried out in the same manner as in
Experiment 1, except that the culture medium (140 ml) was
serum-free medium RPMI 1640 (purchased from Dainippon
Pharmaceutical Co., Ltd.) incorporated with 10 vol% fetal
calf serum under sterile conditions. The cells were NS-1
as many as 5 x 104 cells/ml. At the end of cell culture,
the number of cells was counted.
For comparison, the same procedure as above was
repeated using a culture flask as in Experiment 1 that
- 25 -
employed 30 ml of culture medium. The cells were NS-1 as
many as 5 x 104 cells/ml. At the end of cell culture, the
number of cells was counted.
The results are shown in Table 1.
It is noted from Table 1 that the culture bags in
Examples 1 to 4 are superior in gas permeability and
clarity and they permit a high growth rate of cells.
- 26 -
a e E x x o x x
3 ~ ~ b b b b b
-o= X X X X X
Oo~ In U~ U~ U~ U~
= ~ ~
~ ~ ~ O æ ~ ,
=~ E--~
~~C ~ o~ ~i
= ~ o o o o l
~e e ~ 0 D o _
E ~ c~l ~ _ ~ ~
Example 5
A resin composition was prepared in the same manner
as in Example 1 except that the ethylene-n-butyl acrylate-
carbon monoxide copolymer was replaced by 100 parts by
weight of ethylene-vinyl acetate-carbon monoxide copolymer
(obtained in Referential Example 2) and the amount of
epoxidized soybean oil was changed to lO parts by weight.
This resin composition was made into a 0.4-mm thick sheet
for the storage room. This sheet was cut into two approx-
imate rectangles. This sheet has an oxygen permeability
of 170 ml/m2 24hr atm and a carbon dioxide permeability of
890 ml/m2-24hr-atm. This sheet is the under-mentioned
sheet (12) for the storage room.
The resin composition prepared in Example 1 was
formed by extrusion into a 0.2-mm thick sheet for the
culture room. This sheet was cut into approximate rectan-
gles as mentioned above. This sheet has an oxygen perme-
ability of 1420 ml/m2-24hr-atm and a carbon dioxide perme-
ability of 9210 ml/m2-24hr-atm. This sheet is the under-
mentioned sheet (13) for the culture room.
The thus obtained two kinds of sheets were formed
into a culture bag which is constructed as schematically
shown in Figs. 2 and 3. The culture bag (11) is composed
of the medium storage room (15) and the culture room (14).
The medium storage room (15) is formed by two sheets (12
- 28 -
and 12) and the culture room is formed by one each of
sheet (12) and sheet (13). These three sheets are joined
together by fusion-bonding their peripheries. The culture
room (14) is provided with the cell injection tube (16)
and medium discharge tube (17), and the medium storage
room is provided with the medium injection tube (18). The
culture room is connected to the medium storage room by
the connecting tube (19) through which the medium is
transferred from the former to the latter.
The connecting tube (19) is provided with the stopper
(20) which prevents the medium-flow from the medium
storage room (15) into the culture room (14) until it is
required for culture. This stopper is fusion-bonded to
the inside of the connecting tube (19). This stopper has
the blind hole (21) and the narrow part (22) at its
center, as shown in Fig. 4 and Fig. 5. For the medium to
be transferred, the stopper (20) is broken at its narrow
part (22) by bending the connecting tube so that the blind
hole (21) becomes open, as shown in Fig. 5. The chip (23)
formed by breaking the stopper (20) prevents the backflow
of the medium
The thus obtained culture bag is used in the follow-
ing manner. First, the medium storage room (15) is filled
with a culture medium through the medium injection tube
(18), with the connecting tube (19) closed by the stopper
- 29 -
(20). The culture medium is stored in the storage room
(15) until it is used for culture. Then, the stopper (20)
is broken to open the connecting tube (19), and the
culture medium is transferred from the storage room (15)
to the culture room (14). Immediately after that, cells
are injected into the culture room (14) through the cell
injection tube (16). The medium transfer may be accom-
plished by forcing a gas into the storage room (15)
through the medium injection tube (18), or by suspending
the culture bag (11), with the connecting tube (19) down-
ward, so that the medium siphons from the storage room
(15) into the culture room (14).
Experiment 3
The culture bags obtained in Example 5 was used for
experiments on cell culture in the following manner.
First, the culture room was filled with 140 ml of serum-
free medium (RPMI 1640, the same one as used in Experiment
2) through the medium injection tube. With the medium
injection tube closed by a pinchcock at its end, the
culture bag was kept in an incubator of 5% carbon dioxide
atmosphere at 37C for 16 hours. Then, the connecting
tube was opened to transfer the culture medium into the
culture room, and as many cells as 5 x 104 cells/ml were
injected into the culture room through the cell injection
- 30 -
tube. Cell culture was carried out under the same condi-
tions as in Experiment 1, and the number of cells was
counted on the fifth day of culture. It was found that
the number of cells increased from 5 x 104 cells/ml to 7.8
x 10S cells/ml after culture for five days.
Example 6
A resin composition was prepared in the same manner
as in Example 1 except that the ethylene-n-butyl acrylate-
carbon monoxide copolymer wàs replaced by 80 parts by
weight of ethylene-vinyl acetate-carbon monoxide copolymer
(obtained in Referential Example 2) and the amount of
epoxidized soybean oil was changed to 10 parts by weight.
This resin composition was made into a 0.35-mm thick sheet
for the storage room. This sheet was cut into two approx-
imate rectangles. This sheet has an oxygen permeability
of 180 ml/m2 24hr atm and a carbon dioxide permeability of
780 ml/m2-24hr-atm.
The resin composition prepared in Example 1 was
formed by extrusion into a 0.15-mm thick sheet and a
0.3-mm thick sheet for the culture room. This sheet was
cut into approximate rectangles as mentioned above. The
0.3-mm thick sheet has an oxygen permeability of 1080
ml/m2-24hr atm and a carbon dioxide permeability of 6810
ml/m2-24hr-atm. This resin composition was formed into
tubes.
The thus obtained sheets were formed into a culture
bag which is constructed as schematically shown in Fig. 6.
The culture room (39) is composed of the two kinds of
sheets (32 and 33), which are placed one over the other,
with their peripheries fusion-bonded by high-frequency
sealing, together with the cell injection tube (36), the
medium discharge tube ~37), and the connecting tube (38)
inserted at the ends of the culture room. The medium
storage room (40) is composed of the two sheets (39 and
34), which are placed one over the other, with their
peripheries fusion-bonded by high-frequency sealing,
together with the medium injection tube (35) and the con-
necting tube (38) inserted at the ends of the medium
storage room. Thus, the culture bag (31) is composed of
the culture room (39), 200 ml in volume, and the medium
storage room (90), 200 ml in volume, which are connected
to each other through the connecting tube (38).
The connecting tube (3R) is provided with the stopper
(20) of the same s~ructure as used in Example 5. Basi-
cally, the culture bag is used in the same manner as that
in Example 5. The transfer of the culture medium into the
culture room (39) can be accomplished by positioning the
medium storage room (40) upside down or by pressing the
medium storage room (40) with hands.
- 32 -
~xperiment 4
The culture bags obtained in Example 6 was used for
experiments on cell culture in the following manner.
First, the culture room was filled with 140 ml of serum-
free medium (RPMI 1640, the same one as used in Experiment
2) through the medium injection tube. With the medium
injection tube closed by a pinchcock at its end, the
culture bag was kept in an incubator of 5% carbon dioxide
atmosphere at 37C for 16 hours. Then, the connecting
tube was opened to transfer the culture medium into the
culture room, and as many cells as 5 x 104 cells/ml were
immediately injected into the culture room through the
cell injection tube. Cell culture was carried out under
the same conditions as in Experiment 1, and the number of
cells was counted on the fourth day of culture. It was
found that the number of cells increased from 5 x 104
cells/ml to 8.0 x 105 cells/ml after culture for four days.
Example 7
Cell culture was carried out using the culture bag
(41) as shown in Fig. 7. This culture bag is constructed
in the same manner as that in Example 1, except that it
has the zigzag partition (94). The zigzag partition has
the qaps (45 and 46) at its lower corners, so that cells
can pass through the gaps. The aslant parts of the parti-
tion function as the guide for cells.
- 33 -
A modified culture bag (51) is shown in Fig. 8. This
culture bag has the partition (55) composed of two parts
(52 and 53), one extending aslant downward from the left
side and the other extending aslant downward from the
right side, forming the gap (54) through which cells pass.
Another modified culture bag (61) is shown in Fig. 9.
This culture bag has the partition composed of two parts
(62 and 63), one extending aslant downward from the left
side and the other extending aslant downward from the
right side beyond the end of the first part, forming the
gap (64) through which cells pass.
The partitions shown in Figs. 7 to 9 are formed by
partially fusion-bonding the two sheets (43 and 43) which
form the culture room (42), so that the gaps are left
unsealed.
The culture room (42) is provided with the medium
injection tube (3), the cell injection tube (4), and the
sampling tube (5) at its upper end.
The culture bags shown in Figs. 7 to 9 permit grown
cells to be introduced into the lower compartment of the
bag through the gap of the partition, when it is sus-
pended, with the tubes upward. Therefore, the culture bag
permits the culture medium to be discharged when it is
positioned upside down.
- 34 -
t ' . ~
Example 8
A three-layer laminated sheet (74) as shown in Fig.
10 was prepared from a core (71) and two outer layers (72
and 73) by thermolamination. The outer layers (0.05 mm
thick) were prepared from a vinyl chloride -type resin com-
position which has the same composition as in Example 1,
except that the amount of the ethylene-n-butyl acrylate-
carbon monoxide copolymer is 160 parts by weight and the
amount of the epoxidized soybean oil is 10 parts by
weight. The core (0.25 mm thick) was prepared from the
same ethylene-n-butyl acrylate-carbon monoxide copolymer
as mentioned above. This laminated sheet was cut into two
rectangular sheets, which were then formed into the
culture bag in the same manner as in Example 1.
Example 9
The same procedure as in Example 8 was repeated
except the following changes. The first outer layer (72),
0.08 mm thick, was prepared from a vinyl chloride type
resin composition containing 80 parts by weight of
ethylene-n-butyl acrylate-carbon monoxide copolymer. The
second outer layer, 0.04 mm thick, was prepared from a
vinyl chloride type resin composition containing 160 parts
by weight of ethylene-n-butyl acrylate-carbon monoxide
copolymer.
~ J
Comparative Example 1
Culture bags were prepared in the same manner as in
Example 1 except that the amount of ethylene-n-butyl
acrylate-carbon monoxide copolymer in the resin composi-
tion was changed to 100 parts by weight and the sheet
thickness was changed to 0.36 mm.
Tests for physical properties
The sheets used for culture bags in Examples 8 and 9
and Comparative Example 1 were tested for total light
transmittance and permeability to oxygen and carbon
dioxide. The results are shown in Table 2.
Experiment 5
The culture bags obtained in Examples 8 and 9 and
Comparative Example 1 were used for experiments on cell
culture in the same manner as in Experiment 1. The number
of cells was counted on the fourth day of culture. The
results are shown in Table 2.
It is noted from Table 2 that the culture bags used
in Examples are superior in gas permeability and clarity
and they permit a high growth rate of cells.
- 36 -
c E E b o b ~ ., c `~
~ X X X X
8a~ X X X X
.~i N O O
~I ~ ~
E ~ ~D ~ v~ _
c ~ E u~ I~ c~
E o o o --