Note: Descriptions are shown in the official language in which they were submitted.
6-Alkylpyridazine derivatives, m~thod o~
preparation and pharmaceutical compositiuns
in which they are pre~ent:
Pyridazine derivatives have been proposed as
drugs for many years, especially those which are active
on the cardiova~cular system or the central nerv~us
sys~em.
05 In particular, French patent 2 510 998 and
European patent 72 726 disclo,e pyridazine derivative~
which are variously substituted on the pyridazine ring
and which all carry, in the 3-position, an amine sub-
stituent of the type
-NH-alkylene-N \
in which X and Y are independently hy~rogen or an alkyl
group or form, with the nitrogen atom to which they are
bonded, a heterocycle such as morpholine.
All these compounds are ackive on the central
nervous system as antidepressants.
According to the present invention, it has now
been found that by modifying the substituents on the
pyridazine ring, compounds are ob~ained which have lost
their antid~pressant activity and acquired a valuable
activity as Ml-t~pe cholinergic receptor ligands.
The presen~ inven~ion relate~ to novel pyrida-
zine derivatives of the ~ormula
~ (I)
R3~ NH-R4
N~J
in whi~h
- Ar is a phe:nyl group o~ the formula
3S
.
., , , , :
.:
: : .
- ' . : ' "
'. '' , '. .: ' '.. ~' ' " ', ' . ~ ' '
2 ~
Rl
~,
R2~
05 a pyridyl group or a thienyl group;
~ ~1 and ~ are each indep~nderltly hydrogen, a halogen
atom, a hydroxyl group, a C~-C4 alkyl group, a C1-C4
alkoxy or the trifluoromethyl gxoup;
- R3 is a linear or bxanched C1-C5 alkyl, a group -CH2
~ ~ or a group -CH2-CH~C~5; and
- R is the group
-X-N
in which
X is a linear or branched CrC6 alkylene and
~pecially
either a group -(CH~)n~, in which n is an integer
from 1 to 3;
or a group
Xl
-CH2~C~(CH2)nl~
~1
in which n is 0 or 1 and Xl is a CrC3 alkyl
group; and
~ R5 and R6 are each independ~ntly hydrogen or a
: linear or branched C1-C4 alkyl or form, with the
nitrogen ato~ to which they are bonded, a hetero
cycle selected from pyrrolidine, morpholine or
pipe~idine; or
35 ` - R4 is a group elected Prom
, .
:
. .
,~ , .
2~ 2
-- 3 --
~(CH2)n~
05 ~7
in which n is as defined above and R7 is hydrogen or
a C1-c~, pref erably C ~C2, alkyl group ;
~tCH2)n ~
N
in which n is as def ined above,
-CH2 _ N
\J
~0
{~N-C2H5
or : (~,N-(CH2~2-
~:,
and to their ~;alts with mineral or organic acids.
The salts of the compounds of formula ( I )
according to the pre~ent invention include both those
with mlneral or organic acids which permit appxopriate
: ~ 35 separation ox cry~tallizatiorl of the compounds of :
~ : ,
:: : '
..... , .. ~., . ~,. .. ..
. . ,
~ ' ' , . ' ' " ' ~ ' ' ' ' ' . ' ,' '
' ' ' ' ', ~ ' . ' " '"' ' ' '
81~2
formula (I), such as picric acid or oxalic acid, and
those with mineral or organic acids which form pharma-
ceutically acceptable salts such as khe hydrochloride,
hydrobromide, sulfate, hydrogensulfate, dihydrogen-
05 phosphate, methanesul~onate, methylsulfate, maleate,
~umarate or naphthale~e-2-sul~onate.
According to another feature, the pre~enk
invention relates to a methoal of preparing the co~-
pounds oE formula (I), which comprises reacting a 3w
chloropyridazine of the formula
Ar
~ (II)
R~ Cl
N_N
in which Ar and R3 are as defined above, with an amine
o~ the ~ormula
~NH2
in which R4 is as defined above, to yive tha compounds
(I) according to the invention and, if desired, conver-
ting the resulting compound ~o a salt with a mineral or
organic acid.
The substitution reaction of the 3-chloropyri-
dazine (II) with the amine ~NH2 is carried out at a
temperature of between 100 and 150C, in the absence of
a solvent or in the presence o~ an inert solven~ such
as n-butanol.
The resulting product of formula (I) is isola
ted, in the form of the ~ree base or a salt, by the
conventional techni~ues.
when the compound of formula (I) is obtained in
the ~orm of the free base, conversion to a salt is
.
. , .
'. '` ' ' ` `' `
.
5~ 2~48h62
e~fected hy treatment with the chosen acid in an orga-
nic solvent. Treatment of the free base, for example
dissolved in an alcohol ~uch as i~opropanol, with a
solution of the chosen acid in the sam~ solvent gives
05 the corresponding salt, which is isolated by the con-
ventional techniques. The hydrochlori.de, hydrobromide,
sul~ate, hydrogensulfate/ dihydrogenphosphate, mekhane-
sulfonate, methylsulfate, oxalate, maleate, fumarate or
naphthalene-2 sul~onate, for example, is prepared in
this way.
When the reaction is complete, the compound of
formula (I) can be isolated in the ~orm of one of its
salts, for example the hydrochloride, oxalate or
fumarata. In this case, if necessary, the free ba~e
can be prepared by neutralization of sai.d salt with a
mineral or organic base such as sodium hydroxide or
triethylamine, or with an alkali metal carbonate or
bicarbonate such as sodium or potassium carbonate or
bicarbonate~
When Rl and/or R2 are a hydroxyl group, the
compound according to the inv~ntion is obtained ~rom
the compound (I) in which Rl and/or R2 are an alkoxy,
all the other substituents being as de~ined above, by
dealkylation using known methods.
The 3-chloropyrida~ines (II) used as starting
materials are prepared from the 2H-pyridazin-3-ones
(III) of the formula
Ar
R3~O ( III )
N.rN~
N
by reaction with exaess phosphorus oxychloride under
,
~ .
~ 6 - 2~
the action of heat, without a solvent or in the pre-
~ence of an inert solvent such as acetonitrile.
The 2H-pyridazin-3-one:s ~ L3 above are obtai~
ned by dehydrogenation of th4 compounds o~ ~ormula
05 tIV):
Ar
R3~=0
N_
~or example by reaction with bromine in acetic acid.
The 2,3,4,5-tetrahydropyridazin 3-ones o~ the
formula
Ar
R3 ~ - (IV)
N_
H
and the 2,3-dihydropyridazin-3-one~ of ~ormula (III)
which are novel and form part o~ the invention, ar~
prepared by the methods described by SUT~ et al., J.
Am. Chem. Soc., 1942, 64, 533-536, MUKHIRJI D., Science
and Culture, 1948, 13, 426, and PINNA G. et al., Il
Farmaco, Ed. Sci., 1988, ~, 539-549.
The intermediates o~ the ~ormulae
: ,
,~, .
` .
',
2~4~
-- 7 ~
Ar Ar
R3 _~= 0 R3 ~ ~0
N_N~ N_N
05 }I ~H
(IV) (III)
in which Ar and R3 are a~ de~ined above, which are use-
~ul ~or the preparation o~ (I), are novel and form part
of the invention with the exception oP the compounds in
which Ar is an un~ubstituted phenyl and R3 is a methyl
group. By way o~ example, compounds of formulae (IV)
and (III) are described in rrables 1 and 2 below.
The chlorinated compounds of ~ormula (II):
Ar
~ (II)
R3 ~\ ~ 51
~N
which are novel and form part of the invention, are
prepared by the customary methods. By way of example,
compounds of formula (II) are de~cri~ed in Table 3
below.
Substitution of these chlorinated compounds
(II) with an amine makes it possible to v~tain the
compounds (I), ~he preparation o~ which is illustrated
by the reaction scheme below.
: 30
:
.
:! :
,
8 --
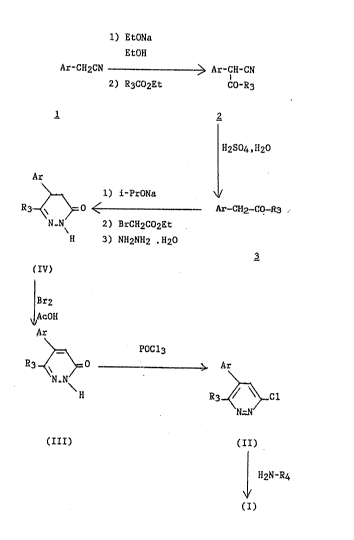
SC~ME 1
1) EtONa
05
EtOH
Ar-CH2CN ~-~ Ar-CH-CN
2) R3Co2Et Co-R3
H2S04, H20
Ar
1) i-PrONa ~ /
R3~ ~ o ~ CH2-CO-R3
N N 2~ BrCH~C02Et
H 3) NH2N~l2 . H20
~IV~
~r2
AcOI{
~9r
~ POC13
--<N~N>= ---~ Ar
H R3~ Cl
N~N
(III) (II)
H2N~R4
~ /
(I)
,:
.
20~62
Heating a nitrile 1 in the presence of a base
and a carboxylic acid ester derivative R3CO2Et gives
the ~-ketonitrile 2, which is ~onver~ed to the corre~-
ponding ketone derivative 3 in an acidic medium.
05 Cyclization to the 2,3,4,5-tetrahydropyridazin-
3-one is effected by reaction with ethyl bromoacetate
in an alcoholic solvent at room temperature, followed
by reaction with hydrazine hyd:rate under the action of
heat.
Dehydrogenation of the compound (IV) by reac-
tion with bromine in acetic ac:id makes it possible to
prepare the 2,3-dihydropyridazin-3-one (III).
In the case where n1 = I the amines
~ R5
H2N-X-N ~
~6
in which X is a group
~1
CH2 (~
in which Xl is a~ defined above, are prepared ~rom a
cyano derivative of the formula
CN
(X1)2 ~ OH
by reaction with an amine o~ the formula HNR5R6, if
appropriate in the presence of a salt of a strong acid,
such as sodium or magnesium suIfat~, at a temperature
of between 40 and 80 C, to give the aminonitrile of the
formula
:
, .: : '
. ~ , . ~ .
-- 10 --
CN ~ Rs
( Xl ) 2~C~M~R
05 which is hy~rated under the customary conditions, in an
acidic medium, to give the corresponding amide of the
formula
CO~H2 / R5
( ~1 ) 2 - ~ - N \
R~
which is then reduced by reaction with a metal hydride,
such as lithium aluminum hydride or boron hydride, to
give the expected amine
H 2N-CH2~-N
\R6
The following Examples illustrate the invention
without howeYer implying a limitation.
EXAMPLE 1
25 3-(2-Diethylamino-2-methylprop-1-yl) amino-5- ( 4 -f luoro-
phenyl)-6-propylpyridazine
A) ~-Butyryl-4-fluorophenylacetonitrile
10.9 g of sodium are added to 150 ml of abso-
lute ethanol. While maintaining re~lux, a solution Q~
30 49.5 g of 4--fluoroph*nylacetoni~rile in 65.2 g of e~hyl
butyrate is adde~. The reaction mixture is re~luxed
Por ~ hours and then left to ætand overnight at room
temperature. The solvents are concentrated under
vacuum and the residue is taken up in water and washed
with ether. The aqueous ph~se is cooled in an ice bath
.
..
,
6$~,
~- 11 n~
and acidi~ied to p~ 5 6 by the addition of concentrated
acetic acid to give crystals, which are filtered off,
washed with water and dissolved in methylene chloride.
The organic phase is decanted, dried over
05 ~gS04~ filtered and concentxated under vacuum.
m = 50 g
M.p. = 53-54 C
B) 1-(4 Fluorophenyl)pentan-2-one
50 g of the product obtained above are treated
10with 36 ml of water and 148 ml of concentrated sulfuric
acid, which is added dropwise at 4 C, with stirring.
The mixture is then heated at 80C for ~ hour and
cooled, 534 ml of water are then added and the mixture
is refluxed overnight.
15The mixture is extrac-ted with methylene chlo-
ride and the organic phase is decanted, dried over
Na250~, ~iltered and concentrate~ under vacuum.
The residue is chromatographed on silica gel
using 50/50 cyclohexane/methylene chloride aæ the
eluent.
m = 39.2 g
C) 5-(4-Fluorophenyl)-6-propyl-2,3,4,5-tetrahydro-
pyridazin-3-one
2O76 g o~ sodium are dissolved in 115 ml o~
isopropanol. A solution of 21.56 g o~ the prcduct
obtained above in 30 ml of isopropanol is added, with
cooling in an ice bath. The mixture is st.irred ~or one
hour at room temperature, a ~olution o~ 1~ ml of ethyl
~romoacetate in 20 ml of isopropanol is then added
dropwise and the mixture is le~t to stand at roam tem-
perature ~or 24 hours.
The residue is taken up in acidi~ied water
(HCl - pH = 2) and extracted with methylene chloride~
The organic phase is decanted and dri~d over ~gS04. It
is filtered and concentrated under vacuum~ The residue
,
-~
' ' '
~:,
.
2 ~ 2
- 12 -
is taken up in 75 ml of butanol, 11.25 ml o~ hydrazine
hydrate are added and the reaction mixture is refluxed
for 4 hours.
The mix~ure is concentra~ed under vacuum and
05 the residue is taken up in wiater ancl extracted with
methylene chloride. The organic phase is decanted,
dried over MgS04, filtered and concentrated under
vacuum.
m = 13~7 g
M.p. = 78-79 C
D) 2,3-Dihydro-5-( 4-fluorophenyl)-6~propylpyridazin-3-
one
9.37 g of the product obtained above are di~-
solved in 40 ml of acetic acid and heate~ to 80 C.
solution o~ 7. 6 g of bromine in 70 ml o~ acetic acid
is then added dropwise and the reaction mixture is
re~luxed for 4 hours. It is cooled to 15C and a pre-
cipitate is filtered o~fO
m = 6.6 g
M.p. = 193-194 C
E) 3 Chloro-5-(4-~luorophenyl)-6-propylpyridazine
4.64 g of the product obtained above are dis-
solved in 28 ml of acetonitrile and g.6 ml of phos-
phorus oxychloride and ~he reaction mix~ure is re~luxed
for 4 hours and then concentrated under vacuum. ~he
residue i~ taken up in iced water, aqueou~ ammonia i~
added and the mi~ture is then extracted with methylene
chloride. The organic phases are decanted~ dried over
MgS04, filtered and concentrated under vacuum. The
residue iB chromatographed on silica gel using 95/5
methylene chloride~ethyl ~cetate as the eluent.
m = 4.35 g
M.p. - 64 C
~~ 13 --
F) Compound l
1 g of the product obtained above and 3 g of 1-
amino-2-diethylamino-2-methylpropane are heated at
120 C, under nitrogen, for 48 hours.
05 The mixture is concent:rated under vacuum and
the residue is taken up in a 5% solution o~ sodium
carbonate and extrac~d with methylene chloride. The
organic phase is decant~d, dried over MgS0~, ~iltered
and concentrated under vacuum. The residue is chroma
tographed on Merck H silica gel uslng 96/4 methylene
chloride/methallol as the eluent.
The pure product ~ractions are concantrated
under vacuum, the residue is taken up in ether and the
addition of a solution of hydrogen chlori~e in ether
makes it possible to prepare the hydrochloride, which
is filtered of~.
m = 1.4 g
M.p. = 100 115~C
EXP~PLES 2 T0 19
A) The 2,3,4,5~tetrahydropyridazin-3-ones lis~ed in
Table 1 below are obtainPd by following the procedure
described in Example 1, steps A~ B and C, except that
the starting nitrile is replaced with another nitrile
derivative, where appropriate, and the carboxylie acid
ester is varied~
, : .
TABLE
Rl
05
(rv
R3 ~ -O
N \
}I
._ _ ~_~
1 _ ~ 3 _. M.p.; C Recrystallization sol~ent
IEI ~C~C~3 131-132 hexane
n-C~7 104-105 hexane
lEI i-~3~7 137-138 heptane
H i-C4~19 ~2-93 he~ane
neopentyl 155-156 hexane
-C6H5 114-115 hexane
-CH2-~2-C6~5 1~2-143 ~t20
~ n-C3~7 78_7g heptane
J~i-C4E[9 106.5-107.5 heptane
Cl CE13 160-161 Et20
Cl-C~2CH3 121-122 hexane .
ClIl-C,I(, ~
.
: B) The 2,3-dihydropyridazin-3 ones listed in Table 2
below are obtained ~rom the derivatives in Table 1 i~
~:25 ths manner described in Example 1, step D, by r~aGtiOn
with bromine followed ~y dehydrohalogenation. These
compounds were all :recrystallized ~rom a methylene
chloride~hexane mixture.
: : :
; ~ 3
~ ::
.
,~ , - . .
,.
15- 20~
TABL~3 2
R
05 ,~
\~< (~II)
R3 ~\~0
\H
Rl R3 lI.p., C
-C~2C~3 123-124
H n-C3~ 162-163
~1 i-'C3E17 1'78-17g
H i~C~,~g 154-155
~neopentyl 181-182
-CH -C ~ 140-141
-C~2-C}I2~C~H5 169-170
F n~c3~7 1~3~194
~ i~ g 194-195
Cl -C~3 212-214
Cl -C~2CH3 206~207
Cl "-C,~7 187-1B8
C) The chlorinated derivatives ara prepared from the
derivatives in: Table 2 by reaction with phosphorus oxy-
25 chloride in the manner described in Example 1, step E.These chlorine derivatives, which are listed in Table 3
:; below, were all ~ccrys~allized ~rom me~hylene chloride.
~,: :: :
:
'' '
,
8 ~
-- 16 --
~ABLE 3
R
05 ~
~< (II)
R3~_ Cl
N_
__ _ _ _.
Rl R3 M.p.; C
__
-C~2C~3 49
~1 n-C3~7 58
H i-C31I7 86-87
i-C4~9 ï0-71
neopentyl 99~100
-~ -C ~ 99
H-C~2-~2-C6H5 ~4-~5
F n-C3~7 64
F i-Ca~g oil
Cl -C~3 127-ï28
Cl -C~2C~3 84-85
C~ Il-C,I~, 63
1)~ Substi~ution o~ the chlorinated deriYatiVeS with an
amine, acc::ording to Bxample 1, step F, makes it pOB-
~5 sible to prepare thP compounds ( I ) according to t}leinvention, which are listed in Table~s 4, 5 and 6 below.
~.
- . -
,
: :
2 ~
-- 17 -
R~
OS ~ /~
R5 ( I )
R3~ NH- (CH ) N /
N_N ~ 6
. ., _ . _ ~ U.p.; 'C
Exa~ple Rl R3 n -~R6 Salt
.. _ ___ .. ~
1 5 2 ~-C~ 2 -N-(C~ 5) 2 88 base
3 ~n-c3~ 7 2 -N-(~n5)2 Eton fu~arate
4 H -CH 2 -N ~ 241 2~Cl
2 0 3 ~ EtO~
R -CR3 2 -N O 93 base
hexane
B Cl ~ B7 3-I-(Cz ~ )z ~ ~s~
_.
:
: :
:. : ~ ' : ~ ' : :
:
~ 18 - 2~4~ 2
~5
R~
05
k~
R3~ NH R4 (I)
.. _ - . ~
Exa~ple Rl R2 R4 Recrystalllzation sol~ent
_. _ __ _- ~ . .... ~__
7 ~ -cl,~3 ~1 154 sesquifunarate
~ a~r acetone
. CH~-CH3
~ 3 n I ~N2-cs3
2~ 9 n ~ n-c~n7 nt
~ 5 Etz,O 2~Cl
~13
: .
:
:: :
.
:-, .
34g~J
TABLE_ 5 ( Cont ' d )
R~:~
05 ~
R3~ ,~N~-( R4) (I)
__ _, . _ _ . _~_
EYa~ple Rl R2 R4 Recrystalilziat;on solvent
_. ___ _ __. __
~ ¦ C ~ ~ ¦ Et
¦ 12 ~ b ¦ ~ -C~ 1i690~ tri~u arate
13 1~ i-C4~g M 99 difu~arate
¦ ~ t,o~i oC beoil Idrate
1~ ~ i~c4~ 9 ~ 151 difu~arate
30 ~L~ t~o
_ _ .... . . .
`,
. ,' ;
'" ' ' ' ' . ' ,:
J
-- 20 --
TA~,E 6
R~
~ C~13 ~5
05 ~3~ NH-CH2-C-N~
N_N dH3 R6
.. _ __ .~ -~ p.; C
~xa~ple R1 R3 ~R Recrystalllzation solvent
. __ 6 _
~o 15 11 -c~3 -N-(C2~;)2 13~-134 fu~arate ~120
acetone
16 11 -C2~5 -N-(C2~,j)2 95 diPu~arate
17 H n-C3117 ~N-tC2~5)2 121 difumarate
18 ~ i-C3~7 -~1(C2ll5)2 164rlH5 SesquifuDarat;e
19 ~ c-Hc2~, -N(C2~5)2 148-149 difuDarate
~ ~ (c2lls)2 1 5 21 Cl
21 H -C~2 -N(C2~5)2 114-115 2~Cl
~BIl Et20
22 ~ (~B~)2 -N(c2~)2 ~t20
23 F i-C4~!~ -N~C2~5)2 161-162 sesquifu~arat~
.
' '
.
-
. . .
- 21 ~ 2~ 2
24 Cl -CH3 -N-(C2~5)~ 100-l05 2~Cl
Cl ~C2~s -N~lC2~l532 95-100 2~Cl
05 26 Cl n-C,R7 -N-(C,Rs), _ _._ _ _
The compounds according to the invention were
studied for their pharmacological pxoperties and in
particular for their affinity for the Ml-type and M~-
type muscarinic cholinergic receptors.
In vitro, the compounds (I) were tested accor-
ding to the technique described by L. POTTER et al., J.
Pharmacol. Exp. Ther., 1989, 28~, 974-97~, ~or their
af~inity for the M1-type receptors, and according to
the technique described by HAMMER R. et al., Life
Science, 1986, 38, 1653-1662, for their af~inity for
the M2-typ~ receptors.
~O The compounds according to the invention have ~
good affinity for the M~-type receptors and a mar]ced
specificity ~or the ML-type central receptors compared
with the ~a-type receptors.
By way of example, compound 6 showed 50% inhi-
bitory conGentrations~ expressed in micromol, of 0.16and 1.5 on the Mi and M2 receptors respectively.
Likewis~, compound 25 showed 50% inhibitory
concentrations of 0.04 and 0.9 on the ~ and M2 recep-
tors respectively.
In vivo, the compounds according to the inven-
tion were su~jected to the test for the rotations
induced by .intrastriatal pirenzepine, described by
Worms P. et al., P~ychopharmacology, 1987, 93, 489-493.
At a dose of 3 mg per kg of body weight, admi-
nistered orally, the products according to the inven-
:
" .
" , . . .
.. .
: ~ ~ : ';.' . ' ''
' ~ : ' '. '
.. . . .
2 ~
- 22 -
tion strongly inhibit the number of rotations induced
by pirenzepine. Thu~" by way of example, compound 6
causes a 71~ inhibition of the rotations induced by
pirenzepine.
05 Furthermore, the compounds according to the
invention have proved to be active on the memory de~i~
ciency induced by scopolamine and pentetrazole in the
passive avoidance test on rats, described by WORMS P.
et al., Psychopharmacol., 1989, 98, 286-288.
Thus the results of this test show that com-
pound 6 according to the invention opposes the amnesia
induced by pirenzepine administered intraperitoneally
at a dose o~ 75 mg~kg, with an EDso of 0.47 mg/kg,
administered orally.
F'inally, the compou~ds according to the invan-
tion showed no signs of toxicity at the doses at which
they are active.
Consequently, the compounds (I) can be used as
drugs.
The results indicated show that the compounds
accordin~ to the invention have a good affinity for the
muscarinic receptors and a good activity in the tests
for amnesia induced by scopolamine or pirenzepine.
They make it possible to consider using the products
according to the invention in all cases where a choli-
nergic deficiency is evident, especially ~or the treat-
ment o~ cognitive memvry disorders and degenerative
syndromss a~sociated with senescence and with senile
dementia~
According to another feature, the present
patent application therefore relates to the pharmaceu-
tical compositions in ~hich at least one of the com-
pounds of formula (I) or one of their salts is present
as tke active principle.
In the pharmaceutical compositions of the
;. . : ,. - . :. . - , , . ~
:, . , , ~. . ~ .: .
:' -, , ~
.. ~ ,
s~48~2
~ 23 ~
present invsntion for oral~ sublingual, transdermal or
rectal administration, the active principles of formula
( I ) above can be administered to humans in unit ~orms
of administration, mixed with the conventional pharma-
05 ceutical carriers, especially for the treatment ofcognitive memory disorders or degenerative syndromes.
Appropriate unit Porms of administration include ~orms
for oral administration, such as tablets, gelatin
capsules, powders, granules and solutions or suspen-
sions to be taken orally, ~orms ~or ~ublingu~l andbuccal administration, ~orms for subcutaneous, intra-
muscular or intravenous administration and ~orms ~or
rectal administration.
To obtain the desired effect, the dose of
active principle can vary between 5 and 1000 mg per
day.
Each unit dose can contain from 5 to 200 mg of
active ingredient in combinatlon with a pharmaceutical
carrier~ This unit dose can be administered 1 to 5
times a day.
If a solid composition in the ~orm of tablets
i5 prepared, the main active principle is mixed with a
pharmaceutical vehicle such as gelatin t starch, lac-
tose, magnesium stearate, talc, gum arabic or the like.
The tablets can be coated with sucrose or other appro-
priate substances or they can be treated so as to have
a prolong~d or delayed activity and so as to release a
predetermined amount of active principle continuously.
A preparation in the form of gelatin capsules
is obtained by mixing the active principle with a
diluent and pouring the resulting mixture into soft or
hard gelatin capsules.
Water~dispersible granules or powders can con-
tain the activ~ principle mixed with dispersants or
wetting agents or with suspendinq agents such as poly-
~8:~ ~2
- 2~ -
vinylpyrrolidone, as well as with sweeteners or taste
~orrectors.
Rectal adminiætration is e~fected using 8Up-
positories which are prepared with binder~ melting at
05 the rectal tPmperature, for example cacao butter or
polyethylene glycols.
Parenteral administration is ef~ected using
aqueous suspensions, isotonic saline solutions or
sterile and injectable solutions which contain pharma
ceutically compatible dispersants and/or wetting
agents, for example propylene glycol and butylene
~lycol.
The active principle can also be ~ormulated as
microcapsules, if appropriate with one or more carriers
or additives.
As a pharmaceutical preparation, it is possible
to prepare gelatin capsules containing
Compound 6 0.010 ~
Lactose 0.050 g
Magne~ium stearate 0.005 g
by intimately mixing the above ingredients and pouri~g
the mixture into hard gelatin capsules~
- ~, .
,. , ~ . :