Note: Descriptions are shown in the official language in which they were submitted.
7 ~
O.Z. 0050/41834
Synerqistic aqents for requla~in~ ~lant qrowth
The present invention relates to agent~ for
regulating plant growth, containing a synergistic mixture
of one or more growth-ragulating qua~ernaxy ammonium
salts from the group consis~ing o~ the compounds I,
comprising N,N-dLmethylazacycloheptani~m salt~, N,N
dimethylpipexidinium salts, N,N~dimethyltetrahydro-
pyridazinium salts, N,N,N-trimethyl-N-~-chloroethyl-
ammonium salts~ N-methylpyridinium salts and N,N-dLme-
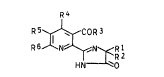
thylpyrrolidinium salts, and an imiclazolinyl derivativeof ~he ~eneral formula II
R4
R 5~COR 3 I I
R 6 N~R 1
HN O
wh~re R1 i~ Cl-C4-alkyl, R2 i~ Cl-C4-alkyl or C3-C~ CYC1G_
alkyl, R3 iS ~ydrogen, hydro~yl, Cl-Cl2-alkyl, Cl-Cl2-
alkoxy, C1-C1~-alkylamino, di-Cl-Cl2-alXylamino or is
phenyl t phenoxy or ph~nylamino, where the aromatic
radicals may carry from one ~o five halogen atoms and/vr
from one to three of the following group~: Cl-C4-alkyl ~
Cl-C4-haloalkyl, Cl-C4-alkoxy, C~-C4-haloalko~r, C~ 4-
alXylthio or C1-C4-haloalkylthio, R4 is hydrogen, halogen?
Cl-C4-alkyl, Cl-C4-haloallcyl, Cl-C,,-alkoxy, Cl-C4-
haloalkoxy, Cl~C4-alkylthio or C1-C4-haloalkylthio and ~5
and R~ lndepend~ntly of one another are each one of the
group~ ~tated for R4 or together form a 1,3-butadiene-1,4-
diyl group which may carry from one to four halogen atomsand/or from one to three of the following groups: C1-C4-
alkyl r C1-C4-haloalkyl, Cl-C4-alkoxy, Cl~C4 haloalko:~y, Cl-
C4-alkylthio or Cl-C4-haloal~ylthio, and inert additives.
The present invention furthermore relates to
methods ~or regulating plant growth with these agents.
~ uaternary ammonium compound~ are disclosed in
the literature as plant growth regulators
(DE-A 22 07 575). The li~erature alco describes
'j
,~ ~" ~
- Z - O.Z. 0050~41834
imidazole deriYatives as herbicides ~DE-A 31 21 736).
Mixtures of N,N,N-trimethyl-N ~-chloreethylammonium s~lts
and 2-(4-isopropyl-4-methyl-5-oxo-2-Lmidazolin-2-yl)-3-
quinolinecarboxylic acid having growth-regulat.ing
properties are ~lso known (EP-A 149 083~.
I~ is an ob~ect of the present in~ention to
provide novel effective ~ynergistic mixtures for regula~-
ing plant growth.
~e have found that this ob~ect is achieved by ~he
synergistic mixtures defined at the outset. Method~ ~or
u~ing these mixtures as plant growth regulators were also
found.
In view of the intended use in the novel syner-
~istic growth-regulating agents, suitable substituents of
the c~mpound~ II are the following radicals~
R1 is Cl-C4-alkyl, such as methyl, ethyl, propyl, 1-
methyleth~l, butyl/ l-methylpropyl, 2-methylpropyl or
dimethylethyl, preferably methyl, ethyl or 1-methyl
ethyl, in particular methyl or l-methylethyl;
R~ is Cl-C4-alkyl, such as me~hyl, ethyl, propyl, 1-
methylethyl, butyl, l-methylpropyl, ~-methylpropyl o~
1/l-~imethylethyl, preferably methyl or ethyl, in par-
ticular meth~1,
or C3-C6-cycloal~yl, ~uch a~ cyclopropyl, cyclobutyl,
cyclopentyl or cyclohexyl, preferably cyclopropyl or
cyclopentyl, in particular cyclopropyl;
: R3 iY hydrogen;
C1-C12-alkyl, prefsrably branched or straight-chain Cl-C6-
alkyl, such as methyl, ethyl, pxopyl, l-methyllethyl,
3~ butyl, l-mathylpropyl, 2-methylpropyl, 1,1-dLmethyllethyl,
pentyl., 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 2,2
dimethylpropyl, l-ethylpropyl~ hexyl, 1,1-dimethylpropyl,
1,2-di~ethylpropyl, 1-m~thylpentyl, 2-mekhylpentyl, 3-
methylpentyl, 4~methylpentyl, 1,1-dimethylbutyl, 1,2-
dlmethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-
d:Lmethylbutyl~ 3,3-dimethylbutyl, l-ethylbu~yl, 2-e~hyl-
butyl, l,1,2-trimethylpropyl, 1,2,2-trimekhylpropyl,
- 3 - O.~. 0050~41834
1-e~hyl-1-methylpropyl and l~ethyl-2-methylpropyl, in
particular br~nched or straight-chain Cl-C4-alkyl, such as
methyl, ethyl, propyl, l-methylethyl, butyl, l-methyl
propyl, 2-methylpropyl or 1,l~dLmethylethyl;
C~-C12-alkoxy, preferably branched or straight chain C~-
C6-alkoxy, such as methoxy, ethoxyl propoxy, 1-methyl-
ethoxy; butoxy, l-methylpropoxy, 2-me~hylpropoxy, 1,1-
dLmethylethoxy, pentyloxy, 1-methylbutoxy, 2-methyl~
butoxy~ 3-methylbutoxy, 2,2-dLmethylpropoxy, 1-ethylprop-
oxy, he~yloxy, l,l-dLmethylpropoxy, 1.,2-dLmethylpropoxy,
l-me~hylpentyloxy, 2-methylpentylo~y/ 3-mathylpentyloxy,
4~Nethylpentyloxy, 1,l-dimethylbutoxy, 1~2-dimethyl-
butoxy, 1~3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-
dimethylbutoxy, 3,3-dimethylbutoxy, 1-ethylbuto~y, 2-
lS ethylbuto~y, 1,1,2-trimethylpropo~y, 1,2,2-trimethyl-
propo~y, l-ethyl-1-met~ylpropoxy or 1-ethyl-2-methyl-
propoxy/ in particular branched or straight-chain Cl-C4-
alkoxy, such as methoxy, etho~y, propoxy, l-methylethoxy,
: ~uto~y, l-me~hylpropoxy, ~-methylpropo~y or 1,1-dLmethyl-
ethoxy;
Cl-Cl2-alkylamino, in particular C1-C6-alkylamino, such as
methylamino, ethylamino, propylamino, l-methylethylamino,
butylamino, 1-methylpropy~lamino, 2-methylpropylamino,
1,~ dimethyle~hylamino, pentylamino, 1 methylbutylamino,
2-methylbutylamino~3-methylbutylamino,2,2-dLmethylprop-
ylamino, l~ethylpropylamino, hexy~lamino, 1,1-d~nethyl-
propylamino, 1,2-dimethylpropyl2mino, l-methylp~antyl-
amino, 2-m~thylp~antylamino, 3-methylpentylamino, 4-
methylpen~ylhmino, ~ dimethylbutylamino, 1,2-dlm~athyl-
butylamino, 1,3-dimethylbutylamino, 2,2-dimethylbutyl-
amino, 2,3-dimethyl~u~ylamino, 3,3-dimeth~lbutylamino, 1-
ethylbutylamino~ 2 ethylbutylamino; 1,1,2-trimethylprop-
yl~nino, 1,2,~-trimethylpropylamino, 1 ethyl-l-methyl-
~,, propylamino and 1-ethyl-2-methylpropylamino;
di-C1-C12-alkylamino, in particular di-C~-C~-alkylamino,
preferably di-Cl-Cb-alkylamino, ~uch a~ N,N~dimethyl~mino,
diethylamino, N,~-dipropyl~mino, ~,N-di-(1-methyl-
.,
;~
, .,
,, .
- ~ - O.Z. 0050/41~34
ethyl)amino, N,N-dibu~ylamino, N,N-di~(1 methylpropyl)~
amino, N,N-di-~2-methylpropyl)-amino, N,N-di-(1,1-
dLmethylethyl)-amino, N-ethyl-N-methylamino, N-methyl-N
propylamino, N-methyl-N-(l-methylethyl)-amino, N-butyl-
N-methylamino, N-methyl-N~ methylpropyl)-amino, N-
methyl-N-(2-methylpropyl)-amino, N-(1,1-d~nethylethyl)-
N-methylamino, N-ethyl-N-propylamino, N-ethyl-N-(l-
methylethyl)~amino, N-butyl-N~ethylamino, N-ethyl-N-(l-
methylpropyl)-amino,N ethyl-N-(2-methylpropyl)-amino,N-
ethyl-N~ d~nethylethyl)-~mino, N~ methylethyl)-N-
propylamino, N-butyl N-propylamino, N-(1-methylpropyl)-
N propylamino, N~(2-methylpropyl)- N-propylamino,:N-(l,l-
dLmethylethyl)-~-propylamino, N-butyl-N-tl-methylethyl)
amino, N~ methylethyl)-N-(1-methylpropyl)-amino, N~
methylethyl)-N (2-methylpropyl)-aminol N-(1,l-dLmethyl-
ethyl)-N-Il-methylethyl) amino, N-butyl-N~ methyl-
propyl)-amino,N-butyl-N~(2-methylpropyl)-amino,N-butyl-
N-(l,l-dimethylethyl)-amino, N~ methylpropyl)-N-(2-
methylpropyl)-amino, N-(l,l-dLmethylethyl)-N (1 methyl-
propyl)-amine and N-(l,l-dLmethylethyl)-N~(2-methyL-
propyl)-amino;
phenyl, phe~o~y or phenylamino, where the aromatic
radical3 may carry from one to five halogen atoms, such
a~ fluorine, chlorine, bromine or iodine, preferably
fluorina, chlorin* or bromine, in particular f1UOI ine or
chlorine,
and/or from one to threa of the following group~:
Cl-C4-alkyl, such as methyl, ethyl, propyl, l-methylethyl,
butyl, l~methylpropyl, 2-methylpropyl or 1,l-dimethyleth-
yl~ praferably methyl, ethyl or 1-methylethyl, in par-
ticular methyl;
Cl-C4-haloalkyl, in particular Cl- or C2 haloalkyl, such a~
chloromethyl, dichloromethyl, trichloromethyl, fluoro-
methyl, difluoromethyl, trifl.uoromethyl, chloro1uoro-
methyl, dichloro1uoromethyl, chlorodifluoromethyl, 1-
fluoroethyl, 2-fluoroethyl, 2l2-difluoroathyl, 2,2,2-
tri~ oroethyl, 2-chloro-2-fluoroethyl,
~ 5 - O.Z. 0050/41834
2-c~loro-2,2-difluoroethyl, 2,2-dichloro~2-fluoroethyl,
2,2,2-trichloroethyl or pentafluoroethyl, preferably
difluoromethyl or trifluoromethyl, in particular tri-
fluoromethyl;
Cl-C4-alkoxy, such as methoxy, ethoxy, propoxy, 1 methyl-
ethoxy, butoxy, l-methylpropoxy, ~methylpropoxy or 1,1-
dLmethylethoxy, preferably methoxy, ethoxy, 1-methyl-
ethoxy or 1,1-dLmethyletho~y, in particular metho.~y or 1-
methylethoxy;
Cl-C4-haloalkoxy, in particular Cl- o:r C2-haloalkoxy, ~uch
as chloromethoxy, dichloromethoxy, trichloromethoxy,
fluoromethoxy, difluoromethoxy, trifluorometho~y, chloro~
fluoromethoxy,dichlorofluoromethoxy,chlorodi1uorometh-
oxy, 1 fluoroethoxy, 2-fluoroethoxy, 2,2-difluoroe~hoxy,
lS 2,2,2-trifluoroe~ho~y,2-chloro-2-fluoroethoxy,2-chloxo-
2,2 difluoroethoxy, 2,2-di~hloro-2-fluoroethoxy, 2,2,2-
trichloroethoxy or pentafluoroetho~y, preferably di-
fluorometho~y or trifluorome~ho~y;
Cl-C4-alkylthio, such as methylthio~ ethylthio, propyl-
~0 thio, l-methylethylthio, butylthio, l-methylprop~lthio,
2-methylpropylthio or l,l--dLmethylethylthio, preferably
methylthio,
or C1-C4-haloalkylthio, in particular C~ or C2 haloalkyl-
thio, ~uch as chloromethylthio, dichlorome~hylthio,
txichloromethylthio, fluoromethylthio, difluoromethyl-
thio, trif luoromethylthio, chlorofluoromethylthio~
dichlorofluoromethy~thio, chlorodifluoromethylthio, 1-
fluoroethylthio, 2 fluoroethyl~hio, ~,2-difluoroethyl-
thio, 2,2, 2-trif luoroethylthio, 2-chloro-2-fluoroethyl
thio, 2-chloro-~,2-difluoroethyl~hio, 2~2-dich:Loro-2-
fluoroethylthio, 2,2,2-trichloroQthylthio or penta-
fluoroethylthio, preferably difluoromethylthio;
R4 is hydroyen;
halogen, such as fluorine, chlorine, bromine or iodine,
; 35 pre~erably ~luorine, chlorine or brominet in paxticular
~luorine or chlorine;
Cl-C4-alkyl/ such as methyl, ethyl, propyl, 1-methyle~hyl~
~ '
- 6 - O.Z. 0050/~1834
butyl~ l-methylpropyl, 2-methylpropyl or lrl-dlmethyleth-
yl, preferably m~thyl, ethyl or l-methylethyl or
1,1 dimethylethyl;
C1-C4-haloalkyl, in particular Cl~ or C2-haloalkyl, such as
chloromethyl, dichloromethyl, trichloromethyl, fluoro-
methyl, difluoromethyl, trifluoromethyl, chlorofluoro-
methyl, dichlorofluoromethyl, chlorodifluoromethyl, 1~
fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-
trifluoroethyl, 2-chloro-2-fluoroethyl, 2-chloro-2,2
difluoroe~hyl, 2,2-dichloro-2-fluoroethyl, 2,2,2 tri-
chloroethyl or pent~fluoroethyl, preferably difluorometh-
yl or trifluoromethyl,
Cl-C4-alkoxy, such as metho~y, ethoxy, propoxy, 1 methyl-
ethoxy, buto~y, l-methylpropoxy, 2-methylpropoxy or 1,1-
dLme~hyletho~y, preferably metho~y, ethoxyl 1-methyl-
ethoxy or 1,1-dLmethyletho~y, in particular methoxy or
l,1-dLmethylethoxy;
Cl-C4~haloalkoxy, in particular C~- or C2-haloalkoxy, such
as chloromethoxy, dichloromethoxy, tr.ichloromethoxy,
; 20 fluoromethoxy, difluorometho~y, trifluoromethoxy, chloro-
fluoromethoxy r dichlorofluorometho~y,chlorodifluorom2th-
oxy, 1 fluorosthoxy, 2-fluoroethoxy, 2,2-difluoroethoxy,
2/2,2-trifluoroe~hoxy,2-chloro~-fluoroethoxy,2-chloro-
2,2-difluoroetho~y, 2,2-dichloro-2-fluoroethoxy, 2,2,2-
trichloroethoxy or pentafluoxoethoxy, preferably di-
fluoromethoxy or ~rifluorome~hoxy;
Cl-C4-alkylthio, such as methylthio, ethylthio, propyl-
thio, l-methylethylthio, butylthio, l-methylpropylthio,
2-methylpropylthio or l,l-dLmethylethylthio, preferably
methylthio or ethylthiof
or C1-C4-haloalkylthio, in particular Cl- or C2-haloalkyl-
thio, such a3 chloromethylthio, dichloromethylthio,
tr~chloromethylthio, fluoromethylthio, difluoromethyl-
thio, triEluoromethylthio, chlorofluoromethylthio,
dichlorofluoromethylthio, chlorodifluoromethylthio, 1-
fluoroethylthio, 2-fluoroethylthio, 2,2-difluoroethyl-
thio, 2,2,2~rifluoroethylthio; 2-chloro-2-fluoxoethyl-
- 7 - o.Z. 0050/41834
thi~, 2-chloro-2,2-difluoroethylthio, 2,2-dichloro-2-
fluoroe~hylthio, 2,2,2-trichloroethylthio or
pentafluoroethylthio, preferably difluorome~hylthio, and
R5 and R6 independnetly of one another are each one of the
groups stated in general and in particular for R4 or
together form a 1,3-butadi0ne-1,4-diyl group which may
carry from one to four halogen atom~3, such as fluorine,
chlorine, bromine or iodi~e, preferably fluorina, chlor-
ine or bromi~e, in particular fluorine or chlorine,
and/or from one to three of the following groups:
Cl-C4-alkyl, such as methyl, ethyl, propyl, l-methylethyl,
butyl, l-methylpropyl, 2-meth~lpropyl or l,l-dLmethyleth-
yl, preferably methyl, ethyl or l-msthylethyl;
Cl C4-ha}oalkyl, in particular Cl- or C2-haloall~yl,~3uch as
chloromethyl, dichloromethyl, trichloromethyl, fluoro~
methyl, difluoromethyl, trifluoromethyl, chlorofluoro-
methyl, dichlorofluoromethyl, chlorodifluoromethyl, 1-
fluoroethyl, 2-fluoroethyl, 2~2-difluoroethyl, 2,2,2-
trifluoroethyl, 2-chloro-~-fluoroethyl, 2-chloro~2,2-
difluoroethyl, 2,2-dichloro-2-flu~roethyl, 2,2,2-tri-
chloroethyl or pentafluoroethyl, preferably ~i~luorometh-
yl or trifluoromethyl;
Cl-C4--alkoxy, ~uch a~ methoxy, etho~y, propoxy, l-methyl-
ethoxy, butoxy, 1-methylpropoxy, 2-methylpropo~y or 1,1-
dLmethylethoxy, preferably methoxy, ethoxy, l-methyl-
athoxy or l,l-dLmethylethoxy;
C1-C4-haloalko~y, in particular Cl- or C2-haloalkoxy, such
: as chloromethoxy, dichloromethoxy, trlchloromathoxy,
fluoromethoxy, difluoromethoxy, trifluoromethoxy, chloro-
fluoromethoxy,dichlorofluoromethoxy,chlorodifluorometh-
oxy, 1-fluoxoethoxy, 2-~luoroethoxy, 2,2-difluoroethoxy,
2,2,2 trifluoroetho~y,2-chloro-2-fluoroethoxy,2~chloro~
2,2-difluoroethoxy, 2,2-dichloro-2-~luoroethoxy, 2,2,2-
trichloroethoxy or pentafluoroethoxy, pref~rably di-
fluoromethoxy or trifluoromethoxy;
C1-C4-alkyl~hio, ~uch a~ methylthio, et.hylthio, propyl-
thio, l-methylethylthio, butylthio, l-methylpropylthlo,
, ,
,
- 8 - O.Z. 0050/4183~
2-methylpropylthio or 1,1-d~nethylethylthio, preferably
methylthio or Pthylthio,
or Cl-C4-haloalkylthio, in particular Cl- or C2-haloalkyl-
thio, such as chloromethylthio, dichloromethylthio,
trichloromethylth.io, fluoromethylthio, difluoromethyl-
thio, trifluoromethylthio, chloro~luoromethyl~hio,
dichlorofluoromethylthio, chlorodifluoromethylthio, l-
fluoroethylthio, 2-fluoroethylthio/ 2,2-difluoroethyl-
thio, 2,~,2-trifluoroethylthio~ 2-chloro-2-fluoroethyl
thio, 2-chloro-2,2-difluoroethylthio, 2,2 dichloro-2-
fluoroethylthio, 2,2,2-trichloro~thylthio or penta-
fluoroethylthio, preferably difluoromethylthio.
In view of the intended use in the synergis~ic
agents, particularly preferred compounds II are those in
which ~he substituents have the following meanings-
R1 is C1 C~-alkyl as stated above, in particular methyl;
R2 is Cl-C4-alkyl as sta~ed above, in particular methyl or
1 methylethyl,
or C3-C6-cycloalkyl as stated above, in particular cyclo-
propyl;R3 i~ hydrogen; hydxoxyl;
Cl-C8-alkyl a~ stated abo~e, in particular branched or
straight-chai~ Cl-C6-alkyl a~ stated above;
Cl-Ca-alkoxy a~ ~tated above, in particular branched or
straigh~-chain Cl C~-alkoxy a~ s~ated above;
C1-C~-alkylamino as stated above, in particular Cl-C6-
alkylamino a~ ~tated above;
di-Cl-C8 alkyl~nino as stated a~ove, in particular di-Cl-
C4-alkylamino as stated a~ove;
phenyl, phsnoxy or phenylamino, where the aromatic
radicals may carry from one to ~ive halogan atoms as
stated above, ln particular fluorine or chlorine,
and/or from one to thres of the following groups:
Cl-C4-alkyl as stated above~ in particular methyl;
C1-C~,-haloalkyl as sta~ed above, in particular difluoro-
methyl or ~rifluoromethyl;
C1-C4-alko~y as ~tated above~ in particular methoxy
.
.' .
.
. ~ ; . ,.
g ~ O.Z. 0050/~1834
ethoxy, 1-methylethoxy or l,1-dLmethylethoxy,
Cl C4-haloalkoxy as stated above, in particular
difluoromethoxy or trifluoromethoxy;
Cl C4-alkylthio as stated above, in particular methylthio
or ethyl~hio,
and C~-C4-haloalkylthio as stated above, in particular
difluoromekhylthio;
R4 is hydrogen;
halogen as stated above~ in particular fluorine or
chlorine;
Cl-C4-al3~yl as ~tated above, in particular methyl or 1,1-
dLmethylethyl,
Cl-C4-haloalXyl as stated above, in particular difluoro-
- methyl or trifluoromethyl;
C1-C4-alkoxy as stated above, in particular methoxy or
l,l-dimethylethoxy;
C~-C4-haloalkoxy a~ ~tated above, in particular difluoro-
; methoxy or trifluoromethoxy;
C~-C4-al~ylthio as ~tatad above, in particular methylthio
or ethylthio,
or C1-C4-haloalkylthio as stated above, in particular
di~luoromethylthio, and
R5 and R~ indepedently of one another are each one of ~ha
groups ~tated in general and in particular for R4 or
togethar fonm a 1,3-butadiene-1,4-diyl group which may
carry from one to four halogen atom~ as stated above, in
~rticular fluorine or chlorine,
and/or from one to three of the following groups:
C~-C~-alkyl a~ stated above, in pa.rticular meth~l;
C'1~C4-haloalkyl as ~tated above, in particular trifluoro-
methyl;
Cl-C4-~al3coxy a~ s~ated above, in particular methoxy;
Cl-C4-haloalkoxy a~ stated above, in particular difluoro-
methoxy or trifluorome~ho~y;
C1-C4-al3~ylthio as ~tated above, in paxkicular met.hylthio,
and C~-C4-haloalkylthio as stated above, in particular
difluoromethylkhio.
- 10 - O.Z. 0050/41~34
Particularly preferred synergistic agen s for
regulating plant growth are those which contain an
N,N-d ~ ethylpiperidinium salt or an N,N,N trimethyl-N 2-
chloroethylammonium salt as the growth-regulating quater-
nary ammonium salt.
Particularly prefarred ~nergistic agents of thi~
type are ~hose which contain, in addition ~o the N,N-
dime~hylpiperidinium salt~ or N,N;N-trimethyl-N 2-chloro~
ethylammonium salts, an Lmidazolinyl derivative of the
general formula II, where
R1 is C1-C4-alkyl as stated ahove, in particular methyl;
R2 i~ C1-C4-al ~ 1 as stated above, in particular methyl or
l-methylethyl,
or C3-C6-cycloal ~ 1 a stated above, in particular cyclo-
p~pyl;
R3 is hydrogen; hydroxyl;
Cl-C~-alkyl as stated above, in particular branched or
straight-chain C1-C:~-alkyl a~ stated above;
C1 Ct~-alkoxy as stated above, in pa.rticular branched or
2 0 straight-chain C1-C6-alkoxy as st~te~ abo~e,
C1-CB-a~ nO a~ stated above, in particular C1-C6-
alkylamino as stated above;
di-Cl-Ca-alkylamino a~ stated above, in particular di-C1-
C4-alkylami~o as ~tatsd above~ or
phenyl, phenoxy or phenylamino, where the aromatic
radicals may carry from one to five halogen atoms as
~tated above r in particular f luorine or chlc~rine,
_.,
and/or from one to three of the following groups:
C1-C4-alkyl a~ ~tated above, in particular methyl,
Cl-C4-haloalkyl a~ stated above, in particular difluoro-
methyl or trifluorome~hyl;
Cl~C4-alko~y a~ statad above, in particular metho~y or
ethox~;
C1-C~-haloalko~y a~ stated above, in particular difluoro-
methoxy or ~rifluorometho~y;
C1-C4-alkylthio a stated above, in paxticular methylthio
or ethylthio,
?
~ O.Z. 0050/41~34
and C,-C4-haloalkylthio as stated above, in particular
difluoromethylthio;
R4 is hydrogen
or halogen as stated above, in particular fluorine or
chlorine, and
Rs and R6 independently of one another are each
hydrogen;
halogen as stated above, in part:icular ~luorine or
chlorine;
Cl-C4-alkyl as stated above, in particular methyl;
Cl-C4 haloal~yl a ~tated above, in particular trifluoro-
methyl;
Cl C4 alkoxy a3 stated above, in particular methoxy or
I t l-dimethyletho~y;
Cl-C4-haloalkoxy as stated above, in particular difluoro-
methoxy or trifluoromethoxy;
Cl-C~-alkylthio a~ ~tated abo~e, in particular methylthiot
:~ or Cl-C4-haloalkylthio as stated above, in particular
difluoromethyl~hio,
or tog0~her form a 1,3-butadiene-1,4-diyl gxoup which may
carry from on~ to four halogen atoms as stated above, in
: particular fluorine or chlorine,
and/or one of the following groups.
Cl-C4 alkyl a~ st ted above, in particular methyl;
: 25 Cl~C4-haloalkyl a~ ~ated above, in particular trifluoro-
methyl;
Cl-C4-alkoxy as stated abo~e, in particular methoxy;
C1-C4-haloalkoxy as ~tated above, in particular difluoxo
methoxy or trifluorometho~y;
Cl~C4-alkylthio as stated above, in particular methylthio,
or Cl-C4~haloalkylthi.o as stated above, in particulax
difluoromethylthio.
~'he active ingredients can be applied together or
separately, at the ~ame time or in succession~ before,
; 35 duxing or after ~mergence o~ the plan-ts.
In the syner~istic agent~, the weight ratio of
~mmonium salt I to imidazoli.ne derivative II is from
- 12 - O.Z. 0050/41834
0.1 . l to 1000 : 1, preferably from 1 : 1 to 950 , 1, in
particular from 1.5 : 1 to 920 : 1.
The novel agents can be used, for example, in ~he
form of directly sprayable solutions, powders, suspen-
sions, including concentra~ed Aqueous, oily or other
suspensions or dispersions, emulsions, oil dispersion~,
pastes~ dusting agents, broadcasting agents or granules,
by spraying, atomi ing~ dusting, broadcasting or pouring.
The application forms depend on the intended uses. Th~y
should in any case ensure a very fine distribution of the
novel active ingredien~s.
The compounds I and II axe suitable in general
for the preparation of directly sprayable solution~,
emulsions, pastes or oil dispersions. Suitable inert
additive~ are mineral oil fractions ha~ing a medium ~o
high boiling point, such a~ kero~ene or diesel oil, and
coal tar oil~ and oils of vegetable or animal origin,
aliphatic, cyclic and aromatic hydrocarbon~, eg. toluene,
xylene, paraf~inr tetrahydronaphthalene, alkylated
naph~halenes or derivative3 thereof~ methanol, ethanol,
propanol, butanol/ cyclohexanol, cyclohexanone, chloro-
benzene, i~ophorone or strongly polar solvents, such as
~,N-dimethylformamide, dimethyl sulfoxide, N-methyl-
pyrrolidone or water.
Aqueous application forms can be prepared from
emulsion concenkra~, dispersion~, pastes, wettable
powders or water-disper~ible granules by adding water.
For the preparation o emulsion~ t paste~ or oil dispar-
sion~, the ~ubstrates, a~ such or dissolved in an oil or
sol~ent, can be homogenized in water by means o wekting
agent~, adherents, disper~ant~ or emul~ifier~. Howev9r,
it i3 also possible to prepare concentrates which con~ist
o~ activ~ ~ubstance, wetting agent~, adherents, di~per-
san-t~ or emulsl~ier~ and possibly 301~ent or oil and
which are suitable for dilution with water.
Suitable surfactant~ are the alkali metal,
alkaline earth metal and ammonium salts o~ aromatic
- 13 - O.Z. 0050/41834
sulfonic acids, for example lignin-, phenol-,
naphthalene- and dibutylnaphthalenesulfonic acid, and of
fatty acids, alkylsulfonate~ and alkylarylsulfonates,
alkylsulfate~, lauryl ether sulfates and fatty alcohol
sulfates, and ~alts of sulfated hexa-, hepta- and octa-
decanols, and of fatty alcohol glycol ethers, condensate~
of sulfonated naphthalene and its derivatives with
formaldehyde~ condensates of naphthalene or of naph-
thalenesulfonic acids with phenol and formaldehyde~
polyoxyethylene oc~ylphenol ethers, ethoxylated
isooctyl,- octyl- or nonylphenol~ alkylphenol polyglycol
ether~, tributylphenyl polyglycol ethers, alkylaryl
polyether alcohols, isotridecyl alcohol, fatty
alcohol/ethylene oxide condensates, ethoxylated cas~or
oil, polyoxyethylene alkyl ethers or polyoxypropylene,
lauryl alcohol polyglycol ether acetate, sorbitol esters,
ligninsulfite waste liquors or methylcellulose.
Powders, broadcasting agents and dusting agents
can be prepared by mixing or milling the active sub-
stances together with a solid carrier.
Granules, for example coa~ed, Lmpregnated and
homogeneous granules, can be prepared by binding the
active ingredients to solid carriers. Solid carriers are
mineral earth~, such as silica gel, silicas, silicates,
talc, kaolin, limestone, lime, chalk, bole, loess, clay,
dolomite, kie~elguhr, calcium sulfate, magnesium sulfate,
magnesium oxide, milled plastics, fertilizers, such as
ammonium sulfate, ammonium phosphate, ammonium nitrate
and urea3, and vegetable products, such as grain flours,
bark meal, wood meal and nutshell meal, cellulosic
powders and other solid carrier~.
~he formulations contain from 0.1 to 95, prefer-
ably from 0.5 to 90, % by weight of the active in-
gredients. The active ingredients are used in a purity
of from 90 to lO0~, preferably from 95 to 100% (according
to the NMR spectrum).
~he growth regulators or the active ingredients
O . Z . 0050/41834
can be applied by the preemergence or postemergence
method. If the active ingredients are less well
tolerated by certain crops, it i~ pos~ible to use
application methods in which the herb.icides are sprayed
5 with the aid of sprayers in such a way that as far as
poss.ible they do not come into contact with the leaves of
the sensitive crops while the active ingredients reach
the leaves of undesirable plant~ g:rowing underneath or
the uncovered soil surface ~post-directed, lay by).
The application rates of active ingredient
mixture are from 0.01 ~ 0.01 to 2 ~ 0.001, preferably
front 0 . 03 + O . 02 to 1 + O . 001, kq/ha of active substance
(a.s.), depending on the s~a~on, target plants and stage
of grow~h.
The synergistic mixtures can influence virtually
all ~tage3 of development of a plant in di~ferent ways
and are therefoxe used a~ growth regulators. The range
of activity of the plant growth regulators depend~ in
particular
a) c7}1 the plant ~pecies and variety,
b) on the time of application~ ba~ed on the stage of
development o~ tha plant, and on the ~ea~on,
r) on the place and method of application (for example
s~ed dre~ing, ~oil treatment, foliage application
or trunk in~ection in .he case of trees),
d) on climatic ~actors, eg. temperature, amount of
_ precipitation and also length of day and light
inten~ity,
e) on the soil quality ~including fertilizer applica-
tion),
f) on the formulation or application form of the active
ingredient and finally
g) on the ~oncen~rations in which ~he active substance
is used.
From ~he various possibl.a applications of the
novel agents in cultivation, in agriculture and in
hortictllture, ~ome are mentioned below.
- 15 - o.Z. 0050/~1834
A. The vegetative growth of the plant~ can be
greatly inhibited with the compounds which can be used
according to the invention, this being manifested in
paxkicular in a reduction in the growth in length. The
treated plants accordingly h ve a stunked growth; more-
ov~r, a darker leaf coloration is ob~erved.
Reduce~ intensity of growth of yrasses along road
edges, hedges and canal banks and on lawn area~, such as
parks, sports grounds, orchards, ornamenkal lawn~ and
airfield~, proves advantageous in practice, enabling the
labor-inten~ive and expensive cutting of lawns to be
rsduced.
The increase in the strength of crop~ su~c~ptible
to lodging, such a~ cereals, corn, sunflowers and soy-
bean, is also of economic interest. The re~;ultingshortening and strengthening of the stem reduce or
slLminate the danger of lodging (of bending) of plants
- under unfavorable weather conditions prior to harvesting.
The u~e of growth regulator~ for inhibiting the
growkh in length and for changing $he tLme of ripening of
ootton iB also importan~. This permit~ completely
mechanized harvesting of thi~ important crop.
In the ca3a of fruit tree~ and other trees, the
growth r~gulators permit a reduction in cutting cost~.
~oreover, the alternance of fruit trees can be broken by
growth ragulators.
The lateral branching of the plants can also be
increased or inhibited by the u~e of grow~h regulators.
Thi~ is of interest if, for example, in the case of
tobacco plants, it i~ intended to inhibit the formation
of ~ide ~hoot~ in favor of leaf growth.
The ~rost rasi~tanco can also be considerably
lncrea ed with growth regulator3, for example in wintar
rape. On the one hand, the growth in length and the
development of a leaf or plant mas~ which i3 too luxuri-
ant (and hence particularly su~ceptible to fro3t) are
inhibited. On the other hand, the young rape plants are
16 - O.Z. OOS0/41834
held back in the vegetative stage of development after
sowing and before the onset of the winter frosts~ in
spite of favorable growth conditionst This also elLmina
tes the danger of fro~t to plants which tend to ~xhibit
a premature decline in ~he inhibition of blooming and to
go over into the genPrative phase. In other crops too,
for sxamplP winter cereals, it is advantageous if the
stocks are well tillered in the fall as a result of
treatment ~ith novel compounds bul: do no~ en~er thP
winter with too luxllriant a growt.h. This makes it
possible to avoid high sensitiYity to frost and, owing
to the relatively small leaf or plant mass, attack by
various diseasP~ ~ fox example fungal disease). Inhibi
tion of the vegetative growth furthermore permits denser
planting of the soil in the case of many cxop~, making it
possible to achieve a greater yiel , ba~ed on soi:L area.
. The growth regulators make it possible to achieve
greater yield both of plant part~ and of plant in-
gredient~. For example, it i~ po ~ible to induce the
grow~h of laxger amounts o bud~, blooms, leaYes, fruits,
seeds, roots and tuber~, to increase the content of sugar
in sugar beet~, sugar cane and citru~ fruits, to increase
. the protein content of cereal~ or soybean or to stLmulate
greater lat~x flux in rubbsr tree~.
By intervening in the plant metaboli~m or by
prcmoting or inhibiting vegetative and/or generative
growth, -the synergistic agents can result in increased
yield~,
C. Finally, both shortening or lengthening of the
8taga9 of development and acceleration or re~ardation o~
the ripening of the haxvested plant parts bsfore or after
harve~ing can be achLeved with plant growth regula~or~.
For example, it is of economic interest to
facilita~e harve~ting, thi~ being pe~litted by con-
centrated dropping or reduction in ~the adhesion to the
tree in the ca~e of citrus fruit~, olive~ or other
specie~ and varieties ~f pomes, drupes and hardshall
;
- 17 ~ O.Z. 0050/~1834
fruit. The same m~chanism, ie. promotion of the forma-
tion of absci~sion tissue between the fruit or leaf part
and the sprout part of the plant is also essential for
readily controllable defoliation of crops such as for
example cotton.
D. Furthermore, the water consumption of plants c~n
be reduced with growth regulators. This is particularly
important for agricultural areas which have to be artifi-
cially irrigated at great expense, for example in arid or
semiarid regions. By using the novel ~ubstances, it is
po~sible to reduce the intensity of irriga~ion and hence
to carry out more economical farming. Under the influ-
ence of growth regulators, the available water is bet~er
utilized becau~e, in~er alia,
15 - ~he extent of opening of the stomata is re~uced,
- a thicker epidermi~ and cuticula are formed,
- root penetration o~ the soil i~ improved and
- the microclLmate in the crop is advantageou~ly
affected by more compacted growth.
The novel synergis~ic agents c~n be fed to the
crops both via the seed ~as seed dre~sings) and via the
soil, ie. through the root and, particularly preferably,
via the foliage by spraying.
Because of the good toleration by plants, the
application rate can ba greatly varied.
In view of the wide range of applica~ion methods,
the novel agents can be used in cere 1 crops and cotton.
The synergi~tic effect can be determined by the
formula of S.R. Colby (Wa~ds 15 (1967), 20~2)
E = X ~ Y - X Y / 100
where the variables have th~ following meanings:
X 18 the ~ea~urable effect of the active ingredient I at
an applica~ion .ra~e ~a],
; Y is the mea8urable efEect of the active ingredient II at
an appl~cation rate ~b] and
is the expected measurable efect of a mixture of
active ingredient I at the application rate ~a] and of
j;, , ! , ~;, . ..
- 18 ~- O.Z. 0050/41834
active ingredient II at the application rate ~b].
From the difference between the expected v~lue ~
according to Colby and the measured value, it is possible
~o determine whether there is a synergistic efect
(reinforcement of the effect ox increase of activity) or
an antagonistic effect (weakening of the effect or
reduction in activity), or if there are merely additive
effec~s on the activity, this being the case if the two
values correspond.
The Lmproved biological action of the mixtures
compared with the individual substances in cereal crops
and cotton can be demonstrated in field trials.
Various mix~ures of th~ following compouncls were
inves~igat~d:
A
N~N,N~Trimethyl-N-~-chloroethylammoniumchloride(chloro-
mequat chloride)
N,N-DLmethylpiperidinium chloride ~mepiquat chloride)
C
2-~4-Isopropyl-4-methyl-5-oxo-2-imidazolin 2-yl)-3-
quinolinecarbo~ylic acid (imazaquin)
D
I~opropylammonium 2-(4-isopropyl-4-methyl-S-oxo-2-
imidazolin-2~yl)~3-nicotinecarboxylate (imaæapyr)
: E
5-Ethyl 2-(4-isopropyl-4-methyl 5-oxo-2-imidazolin 2 yl)-
3 nicotinecarbo~ylic acid (imazethapyr)
~he trial~ were carried out on plots, each
measuring 12.5 m2 (cereals) or 50 m2 (cotton), with 4
replicates in each ca~e. Cultivation, ~ertilizer ap-
plication and the use of crop protection agent~ again~t
growth of weeds and attack by fungi and by in~ect pes~s
were carried out in a manner conventional ~or t~le
location.
The novel agent~ were applied by the
post-emergence method (3.5 bar, 400 l/ha of water).
~ '` l"`1
r r
- 19 - - Z ~ 0(~50/41~33
EXAMPLES 1 TO 5
The ~bovemPntioned cexeal trial~ show that the
novel mixtures are clearly superior to the known mixture
A ~ C (EP-A-149 083). Furthenmore, Ta~le 2 shows that a
mixture consisting of two diferent quaternary ammonium
compounds with a triazole has an action superior to that
of ~he known combination of A + C.
TABL~ 1
Crop: Winter barley (variety: Marinka)
Type of soil: Clayey loam
Sowing: Saptember 28, 1988
Treatment: March 28, 1989
Evaluation: Plant damage Nay 12, 1989
Height of growth May 17, 1989
Sub~tance g/ha of Plant Height of grow~h
active damage cmDif~erence rom
ingredient % control
~ (cm)_
Control - 0 102.1
A 690 0 101.60.5
D 10 5 72.5- 29.6
A + D 6gO ~ 5 6 71.9 - 30.2
A + D 690 ~ 10 6 57 4 6 - 44.5
A + D 690 ~ 20 11 41.4- 60.7
A ~ C 759 ~ 16.5 3 84.0- 18.1
~ ~ C 1150 + 25 10 78.9- 23.2
- 20 - OOZ~ 0050/4183
TABLE 2
Crop: Winter wheat (variety Baroudeur)
Type of soil: Clayey sand
Sowing: October 2, 1989
Txeatm~nt: March 28, 1990
Evaluation: Plant damage May 17, 1990
Height of growth June 5 t 1990
Substance g/ha of Plant Height of growth
active damage cm Difference from
ingredient % control
~ . (cm)
Control 0 0 108.0
A + E 920 + 1 0 100.8 - 7.2
A + E 920 t 2 0 99O2 - 8.8
A + E 920 ~ 4 0 99.8 - 8.2 ?
A ~ B ~ E 690 + 230 ~ 20 100.5 - 7.5
A + C 920 + 2 0 101.0 7.0
T~BLE 3
Crop: Winter wheat (varietyO ~axoudeur)
~ype of ~oil: Sandy loam
Sowing: October 12, 1989
Treatment: March 5, 1990
Evaluation: Plant da~age June 6, 1990
: Internodal length Ju~e 6, 1990
Ear length June 6, 1990
Substance g/ha o Plant Internodal length Ear length
active damage cm Dif~erence cm Difference
ingredient ~ fxom con~rol From control
cm cm
~
Control 0 a 5.2 8.4
A ~ C 920 -~ 2 0 3.B - 1.4 8.3 ~.l
A -~ E 920 ~ 2 0 3.5 - 1.7 8.5 +0,1
. . :
:., -. ,
, ~ . ''
:` :
- 21 - O.Z. 0050/41834
TABLE 4
Crop: Winter wheat (variety: Baroudeur3
Type of soil: Sandy loam
Sowing: October ~, 1989
Treatments March 1, 1990
Evaluatîons Plant damage May 10, 1990
Height of yrowth May 10, 1990
Substance g/ha of Plant Height of growth
active damage cm Difference from
13 ingredient ~ con~rol
~cm)
Control 0 0 96.7
A ~ C 920 + 2 0 ~7.6 ~ 9.1
: 15 A + E 920 ~ 1 0 86O2 ~ 10.5
A + E 920 ~ 4 0 86.9 - 9.8
_ ,
- 22 - O. 2 . 0050/4183
TABLE 5
Crop . Winter wheat ( vari ty: Rraka
Type of soil: Loamy sand
Sowing : November 7, 1 9 8 9
Treatment: April 2, 1990
~valuation: Plant damage June 6, 1990
Height of growth June 6, 1990
Substance g/ha of Plant Height of growth
active damage cm Diffç?rence from
ingredien~ % control
( cm
Control 0 0 117 . 0
B 690 0 107 . 0 - 10 . 0
1~ C 5 0 11~.5 ~ 1.5
B ~ C 690 + 2 . 5 0 lG6 .1 -10 . 9
A + C 690 ~ 15 0 106 . 9 - 10 .1
EXANPI.ES 6 AND 7
The ~ynergistic e~fect of ~he mixture A + D is
demon~trated using barley. It is noted that the addition
oiE > 10 g/ha of active ingredient o~ ~ubstance D can lead
to plant damage.
.
'
'
,
- 23 - O.Z. 0050/41834
TABLE 6
Crop: Su~mer barley (variety: Gimpel)
Type of ~oil: Clay
Sowing: March 20, 1989
Treatment: May 18, 1989
Evaluation: Plant damage June 2, 1989
Height of growth Juns 12~ 1989
Substance g/ha of Plant Height of growth
active damage cm Difference from
ingredient % control
~cm)
Control 0 0 101.2
A 690 0 9fi.9 ~ 4,3
D 10 5 55.6 - 45.6
A ~ D 6~0 ~ 5 7 44.6 ~ 56.6
A ~ D690 ^~ 10 8 42.9 - 58.3
A + D690 t 20 8 42.5 - 58.7
TABL~ 7
: 20 Crops Winter barley (variety: Igri)
Type of soil: Very clayey loam
Sowingo September 22, 1988
Trea~ment: Rpril 21, 1990
Evalllation: Plant dama~e May 15, 1989
: 25 Height of g~owth MAY 22, 1989
Substance g/ha of Plant Height of growth
_ active damage cm Difference from
ingredien~ % control
(cm)
.... ~
Control 0 0 111O2
~90 0 111.2 0
D 10 12 82.0 - 29.2
A + D 690 ~ 10 21 71.2 - 40.0
A ~ D 690 -~ 20 54 63.8 - 47.4
_ ~4 ~ 0.2. 0050/41~34
~XAMPLES 8 TO 10
In the case of wheat and barley, the mixture A -~
E also leads to synergistic shortening of the stem, im-
proved strength and correspondingly greater yields.
TABLE 8
Crop: Summer wheat (variety: Ralle)
T~pe of soil: Clay
Sowing: March 20, 1989
Tre~tmen~. May 18, 1989
Evaluation: Plan~ damage June ~, 1989
Height of growth June 130 1989
Substance g/ha of Plant Height of growth
active damage cm Differ~:nce from
ingredient ~ control
(cm)
Control 0 0 7g.8
A 690 0 63.0 - 16.8
E 10 0 7~.8 - 1.0
A + E 690 ~ 5 0 50~1 - 19.7
A + E69~ ~ 10 0 57.8 - 22.0
E690 + 20 4 56.0 - 23.8
TABL~ 9
Crop: Summer wheat (variety$ Ralle)
Type of soil: Loam~ ~and
Sowing~ February 21, 1989
Tre~ment: ~ay 12, 1989
Evaluation: Plant damage Jun~ 21, 1989
Height of growth Augu~t 3, 1989
Substanca g/ha of Plant Height o~ gxowth
active damage cm Differerlce from
ingred.ient % control
~cm)
Control0 0 36.2
6~ 0 36.8 ~ 0.~
E 10 0 3~.5 - 1.7
'' ' .
.
- 25 - O.Z. 0050/41834
A + E 690 ~ 10 0 39O1 + 2.9
TABLE 10
Crop: Winter barley (varietyo Igri~
Type of soil: Very clayey loam
Sowing: September 22, 1989
Treatment/ April 21, 1989
Evaluation: Plant damage May 15, 1989
Lodging July 5, 1989
Grain yield 3uly 11, 198g
10 Substance g/ha of Plant Lodging/ Grai~ yield/
active damage difference ~iff~rence
ingreclient from control frc~ con~l
% ~%] [%]
. . .
Control0 0 46 75.5
690 0 48 + 2 69.7 5.8
0 ~ + ~ 69.3 - 6.2
A + E690 + 10 0 34- 12 76.9 ~ 1.4
A + E690 + 20 0 3610 76.2 ~ 0.7
EXAMPLES 11 TO 15
Mixkures consisting of compounds B + C lead to a
synergistic inhibition of growth in barley, whsat and
cotton. The resulting improved strength is illu~trated
by Exampl~ 13.
- 26 - 0.~. OGSO/41834
TABLE ll
Crop: Summer wheat (variety Ralle)
Type of ~oil: Clay
Sowing: March 20, 1989
Treatment: May 19, 1989
Evaluation: Plant damage June 22, 1989
Height of growth June 13, 1989
Substance g/ha ofPlant Height of growth
activedamage cm Difference from
ingredient ~ co~trol
(cm)
.. _ . . ~
ControlO 0 80.6
B 690 0 66.0 ~ 14.6
C 10 0 ~l.O + 0.4
: ~ ~ C690 + lO O 61.5 - 19.1
B + C690 + 20 7 49~8 - 30.8
~ABL~ 12
Crops Winter wheat (variety: Kraka)
Type of soil: Loamy sand
Sowing: November 7, 1989
Tr~atment: April 30, 1990
. Evaluation- PLant damage June 5, 1990
Heigh~ of growth June 5, 1990
Substance g~ha ofPlant Haight of growth
active damage cm Diff~rence from
_ ingredient ~ control
(cm)
Control O 0 9S.1
B 690 0 86.0 - 9.}
C 5 0 95.4 ~ 0.3
B + C 690 ~ 1.25 0 80.3 - 14.8
B + C 6gO ~ 2.5 0 84.0 - 11.1
B + C 690 + 5.0 0 84.5 - 10.6
,, .
- 27 - O.Z. 005~/41834
TABLE 13
Crop: Summer barley ( varie-ty O Gimpel )
Type of soil: Clay
Sowing O Marc h 2 0, 1 9 8 9
Treatment: May 18, 19 8 9
Evalua~ion: Plant damage June 2, 198
Height of growth :rune 12, 198
Lodging July 2 4, 19 8 9
Sub~tance g/ha of Pla.nt Height o:E Lodging~
active damage growth~ dif ference
ingredient ~ difference from control
from control [cm]
[cm]
Control 0 0 95 . 4 38
B 690 0 94 . 4 -1 G 0 4g + 11
C 10 0 91 O 0 -4 . 4 ~0 ~ 2
B + C 690 + 5 0 82 . 2 -13 . 2 0 - 38
B ~ C 690 + 10 14 61. 6 -33 . 8 0 - 38
B + C 690 ~ ~0 14 S4 ~ 8 -40 . 6 0 - 38
TABLE 1 4
Crop: Cotton (variety: SJ - 2 )
Type o~ soll s Sandy loa~
Sowing: ~ebruary 24, 1989
25 Treatmen$ - June 2~, 198g
Evaluations Plant damagl3 July 6, 1989
Heiçrht of growth October 5, 1989
Sub~tance ~/ha of Plant Height of growkh
active damage cm Difference from
ingredient ~ control ~cm]
Control 0 0 125 . 0
B 30 0 116 . 2 -8 . 8
C 10 0 12~.2 ~1.2
B+ C 30 ~ 5 0 ill . 2 -13 . 8
B~ C 30 ~10 0 110 . 5 -14 . 5
13+ (~ 30 ~~0 0 107 . 5 -17 . 5
,.i j .,,, ,1,.
- 28 - O.Z. 0050/~1834
TABLE 15
CropO Cotton (variety- Stoneville sa5 )
Type of soil: Sandy loam
Sowing: May 26, 1989
Treatment: July 24, 1989
Evaluation: Plant damage July 31, 1989
Substance g~ha of Plant Height of growth
active damage cm Differ~nce from
ingredient % control [cm]
1 0
Control 0 0 121.2
B 60 0 111.0 - 10.2
~ 10 0 122.8 + 1.6
B ~ C 60 + 5 0 112.5 - 8.7
B ~ C 6Q + 10 0 lOg.8 - 11.4
B ~ C 60 + 20 0 107~5 - 13.7
EXAMPLES 16 TO 18
Mixture~ o substances B + ~ also lead to a
æmaller plant height and grea~er yield~ in the case of
barley, wheat and cotton.
TABLE 16
~ Crop: Summer wheat (variety: Ralle)
.~ Type of soil 5 Loamy sand
Sowing: Augus~ 15, 1989
Z5 ~reatment: August 15, 1989
Evaluations Plant damage June 2, 1989
~Ieight of growth A~gust 3, 1989
Sub~tance g/ha of Plant Hsiyht of growth
active dama~e cm Dif~erence from
ingredient % control [cm]
Control 0 0 38.0
B 690 0 40.5 -~ 2.5
D 10 0 38.4 + 0.4
B -~ D690 + 5 0 44.6 + 6.6
B -~ D690 -~ ln 0 40.1 + 2.1
B + D690 t 20 5 31.0 - 7.0
I `' ` ~ "' . ' ! .
- 29 - O.Z. 0050/41834
TABLE 17
Crop: Summer barley (~ariety: GLmpel)
~ype of soil: Clay
Sowing: March 20, 1989
Treatment May 18, 19 8 9
Evaluation: Plant damag~ June 2, 1989
Heigh~ of grow~h June 12, 1989
Substance g/ha of Plant Height of growth
active dam~ge cmDifference from
ingredient % control ~cm]
Control 0 0 g5.4
B 690 0 94.4 - 1.0
D 10 5 51.8 - 43.6
B + D 690 -~ 10 0 43.9 - 51.5
B + D 690 + 20 6 43.5 51.9
TABL~ 18
Crop: Cotton (variety~ ~C - 510)
Type of soil: Loam
Sowing^ ~ebruary 28, 1989
Treatment: June 27, 1989
Evaluation: Plant damage July 6, 1989
Height of growth July 26, 1989
Substance g/ha of Plant Height o~ grow~h
ac~ivQ damage cmDifference from
ingredient % control [cm]
Control 0 0 g6.6
B 30 0 88.7 - 7.g
D 10 6 96.6 0
B ~ D 30 ~ 5 3 a4 . 1- 12 ~ 5
B ~ D 30 ~ 10 6 83.4 - 13.2
B -~ D 30 ~ 20 8 80.8 - 15.8
EXAML~LES 19 TO 25
In tha ca~e of baxlsy, oat~, wheat and cotton, it
wa~ al~o possible to reduce the height of growth, reduca
lodging and increa3e the yield by mixturQs of substances
:~ Y'1
- 30 - O.Z. 0050/41834
B ~ E.
TABLE lg
Crop~ Summer wheat (variety: Ralle)
Type of soil: Clay
Sowing: March 20, 1989
Treatment: May 19, 1989
Evaluation: Plant damage June 2, 1989
Height of growth June 13, 1989
Sub~tance g/ha of Plant Height of grow*h
active damage cmDiffer~nce from
ingredient % control [cm]
Contxol 0 0 80.6
B 690 0 66.0- 14.5
E 10 a 8~oO+ 1.4
B ~ E690 ~ 5 9 60.0- 20.6
; B + E690 * 10 0 57.023.6
690 + ~0 5 54.0~ 26.6
TA~LE 20
-~ 20 Crop: Winter wheat ~variQtys Xanzler)
~ype of soil: Clay
Sowing: ~ctober 4, 1989
: Treatment: April 5, 1990
E~aluation: Plant damage June 5, 1990
Height of growth June 5; 1990
Sub~tanc~ g/ha of Plant Haight of gr~wt;h
active damage cm Di~ference frvm
inyredient % control [cm]
Control 0 0 122.9
B 690 0 117.2 - 5.7
E 5 0 122.8 - 0.1
B + ~ 690 + 1.25 0 113.2 - 9.7
B + ~ 690 t 2.5 0 114.8 ~ 8.1
B ~ 90 t 5 0 113.4 - 9.5
, - '' ~ ' :
~ ~ .
- 31 - O.Z. 005~/4183
TABLE 21
Crop: Winter wheat (variety: Kraka)
Type of soil: Loamy sand
50wing: November 7, 1989
Treatment: May 2, 1990
Evaluation: Plant damage June 5, 1990
Height of growth June 5, 1990
Substa~ce g/ha o~ Plant Height of grow*h
active damage cm Difference fro~
lD ingredient % con~rol ~cm]
Control a 0 97.9
B 690 0 88.7 - 9.2
E 5 0 ~9.1 ~ 1.2
B ~ E 690 ~ 2.5 0 86.0 - 11,.9
B ~ E 690 -t 5 0 85.4 12.5
TAB~E 22
Crop: Oats (variety. Fl~mingsnova)
Type of soil: Sandy lo~m
Sowing. March R, 1990
Trea~ment: May 131 1990
: Evaluation: Plant damage June 8, 1990
. Height of growth June 8~ 1990
; Sub~tance g/ha of Plant Height of growth
active damage cm DifferQnce from
ingredient % control [cm]
Control0 0 97.5
B 690 0 91.9 - 5.6
E 5 0 95.8 ~ 1.7
B + E690 + 5 0 89.1 - 8.4
- 32 - O. Z . 0050/41834
TABLE 2 3
Crop: Summer barley ( variety: Gimpel )
I~ype of soil: Clay
Sowing: March 20~ 1989
Treatment: May 18, 19 8 9
Evall~ation~ Plant damage June 2, 1989
Height of growth June 12, 1989
Lodging J uly 2 4, 1 9 8 9
Substance g/ha of Plant Height of Lodging/
active damage growt:h/ dif feren~e
ingredient % difference from con
f rom control trol [ % ]
[cm]
Control 0 0 95 . 4 38
B 690 0 94 . 4 - 1. 0 49 ~ 11
E 10 0 93.1 - 2.3 42 +4
E~ ~ E 690 ~ 10 0 91. 6 -3 . 8 36 - 2
B ~ E ~9û ~ 20 6 67 . 5 -27 . 9 0 - 38
2 û TAB~l~ 2 4
Crop s Cottoll ( variety: S~ - 2 )
Type of ~oil: Sandy loam
Sowing : Fe~ruary 2 4, 1 9 8 9
I'reatment o June 2 7, 19 8 9
. ~ 25 Evaluation: Plant dsmage July 6, 198~
HQight of growth October 5, 1989
Sub~tance g/ha of Plant Height of grow~h
ac:tive damage cm Di~erence from
ing-redient % control ~cm]
3 0 ~
Control 0 0 125 . 0
B 30 0 116.2 8.8
2 130 . 0 + 5 . 0
B -1 E 30 + 5 0 111. 2 13 . 8
3S B ~ E 30 ~ 10 ~ 111. 2 -13 . 8
~ 1
- 33 -O.Z. 0050/41~34
TABLE 25
Crop~ Cot~on (variety: GC - 510)
Type of soil: Loam
Sowing~ February 28, l9B9
Treatment: June 27, 1989
Evaluation: Plant damage June 6~ 1990
Yield of seed cotton June 6, 1990
Sub~tance g/ha of PlantYield of seed co~ton
active damagekg/ha Difference ~rom
ingredient ~ control
(kg/ha)
:
Con~rol 0 0 3233
B 30 0 3375 + 142
E 10 1 2857 - 376
30 + 5 ~ 3~53 + 22~
B + E 30 ~ 10 4 4003 ~ 770
B ~ E 30 + 20 3 2693 ~ 540