Note: Descriptions are shown in the official language in which they were submitted.
2~859
PRODUCTION OF Low-c~TnRIDE AL~TT M~TAL PHOSPHATES
The present invention is directed to the production
of alkali metal phosphates, in particular, potas~ium
phosphates with low residual chloride content and useful
as a fertilizer.
Chemical fertilizers originally were produced as
substitutes for manure. The nature of fertilizer
products and their production methods, however, have
changed drastically with the development of modern
agricultural practices. The goal for modern farmers is
to obtain maximum yields of plant produce per unit area
of ground. Included in the trend toward maximum yields
are the planting of the same crops year after year on
the same ground, growing a larger number of individual
plants per unit area and the use of new plant varieties
bred specifically for crowded planting for maximum
yield. For this intensive type of agriculture, chemical
fertilizer has to be applied at very high rates and in
such a scheme so as to supply all plant nutrients in a
form which is readily available to the plants.
Moreover, when chemical fertilizer is applied at high
rates, it is important to prevent accumulation in the
soil of undesirable ingredients, such as chloride ion
and sulphate ion. A low-chloride chemical fertilizer
also is necessary for application to chloride-sensitive
crops, such as tobacco, potatoes and grapes, as well as
for plant germination or foliage spraying.
Potassium phosphates have been long recognized as a
potentially attractive fertilizer product because of
their high nutrient content, their chloride-free
advantage over conventional NPK fertilizers, their slow
release characteristics and excellent agronomic
properties in general. Shipping and storage costs for
high nutrient (P2O5, K2O) potassium phosphates (80-85%
nutrient value for monopotassium phosphate and 90-100%
nutrient value for potassium polyphosphates) would be
significantly lower than for their lower nutrient value
20~98~9
-
competitive products. In addition, being a chloride-
free fertilizer, potassium phosphates may replace
fertilizer in areas where chloride build-up in the soil
or plant sensitivity to chlorides is a concern.
Agronomic studies have confirmed that potassium
phosphates are high quality, slow release fertilizers.
While technologies for the production of potassium
phosphate do exist, (including neutralization of H3PO4,
acidulation of KCl with excess H3PO4, high temperature
and pressure fusion of KCl and H3PO4 and ion-exchange
and/or solvent extraction of KCl and H3PO4), the
commercial manufacture of this valuable fertilizer in
large quantities has not yet been realized, chiefly
because of economic reasons. Limited quantities have
been produced by direct neutralization of phosphoric
acid with caustic potash for specific applications where
a high sale price justified the high cost of production,
such as in the greenhouse and hydroponics industry, seed
germination and plant foliage spray. The high cost of
caustic potash coupled with its corrosive nature and
difficulty in storage have prompted many researchers and
chemists to look for a process to replace potassium
hydroxide with a much cheaper source of potassium, such
as potassium chloride.
Monopotassium phosphate can be produced by the
reaction of phosphoric acid with potassium chloride,
according to the well known reaction:
H3PO4 + KCl KH2PO4 + HCl
In practice, the removal of hydrogen chloride from the
reaction mixture is difficult to achieve, unless a high
temperature and/or a large excess of phosphoric acid are
employed. In the first case, the water-insoluble
potassium metaphosphate (KPO3)n is preferentially formed
and, in the second case, separation of pure potassium
2~98S~
-
salt from the excess acid is an impractical as well as
expensive unit operation.
A number of workers have described specific
methods for the removal of chloride from the reaction
product of potassium chloride with phosphoric acid.
Ross and Hazen in U.S. Patent 1,456,831 teach the
reaction of potassium chloride with phosphoric acid at
temperatures of 250C and above and describe the use of
air to increase the rate of HCl evolution. Askenasy et
al, Zeit. Anorg. u. Allgem. Chemie, 189,305-28 (1930),
discuss the use of steam in place of air for increasing
the rate of reaction of potassium chloride with
phosphoric acid and indicate that, even at 200C, a
residual chloride content of 4% Cl is obtained in the
product. Thompson, U.S. Patent No. 4,158,558 is a
process for producing potassium polyphosphate uses
temperatures from 200 to 300C to remove residual
chloride from a product produced from a mole ratio of
potassium-to-phosphorus of 1:1 to 1.25:1.
Britzke et al., J. Chem. Ind. (Moscow), 7, 4-11
(1930), describe experiments similar to those of
Askenasy et al. as well as the use of reduced pressure
in accelerating the reaction of potassium chloride with
phosphoric acid. The use of reduced pressure also is
mentioned by Kaselitz, U.S. Patent 1,805,873. More
recently Provoost, French Patent 1,395,837 has described
the preparation of a specific fertilizer having a P2Os
to K2O weight ratio of 1:1 by addition of one mole of
sulfuric acid for each two moles of phosphoric acid used
in the reaction. Provoost also describes the use of air
to increase the rate of reaction.
In Curless, U.S. Patent No. 3,554,729, there is
described a procedure for the production of a potassium
phosphate containing less than 2 wt.% Cl, as required in
the present invention. However, this result is achieved
in this reference by a combination of the utilization of
2 0 ~
reduced pressure for the process, steam stripping of the
material and utilization of the presence of at least
0.02 mole of sulfuric acid per mole of potassium
chloride. The addition of the sulfuric acid results in
the presence of potassium sulfate in the product.
Potassium sulfate is substantially less soluble in water
than potassium phosphate, so that additional processing
of the liquid fertilizer would be necessary prior to its
use.
The prior art, therefore, has disclosed that the
reaction of potassium chloride with phosphoric acid
should be carried out at temperatures higher than about
200C and/or in the presence of excess phosphoric acid
and/or the use of additives. Even under such stringent
operating conditions, the chloride content in the
resulting reaction product could not be reduced further
than 4 wt.% chloride. However, to be universally
acceptable as a low-chloride fertilizer, the finished
product should contain 2 wt.% or less of chloride, as
required for certain crops or applications. Moreover,
to be economical, the process should be carried out at a
temperature below 200C for three reasons. First, at
temperatures near or above 200C, the cost of suitable
materials for construction increases to such an extent
that the process cannot be operated economically.
Secondly, impurities normally associated with
fertilizer-grade phosphoric acid, particularly iron and
magnesium, react with the phosphorus and potassium
components of the system above 200C to form complex
materials which may be unavailable for utilization by
plants. Thirdly, above about 200C, organic materials
associated with the commonly-used phosphoric acid is
evolved, contaminating the by-product HCl and rendered
it unsuitable for sale.
Other forms of potassium phosphate which are
commercially useful include tetrapotassium pyrophosphate
204~5~
-
(TKPP), K4P2O7.3H2O, which is used in liquid detergents
and cleaners as well as in water treatment, for example,
scale prevention in high pressure boilers, cleaners for
electroplating operations, where TKPP acts as a
sequestrant, in drilling muds for oil wells, where it
function as a thinner for clays. Based on P2O5 content,
TKPP accounts for approximately 79% of total phosphate
production.
Currently, TKPP is produced by neutralization of
furnace-grade phosphoric acid with caustic potash (KOH),
according to the two-step reaction depicted by the
following equations:
2KOH + H3PO4 ---~ K2HPO4 + 2H2
2K2HPO4 + Heat K4p2O7 + H2O
Overall: 2H3PO4 + 4KOH ---_ K4P2O7 + 5H2O.
As may be seen from these equations, in the first stage
of the reaction, caustic potash (KOH) reacts with the
furnace-grade phosphoric acid to dipotassium hydrogen
phosphate (K2HPO4). This reaction is controlled by
monitoring the pH of the solution. In the second stage
of the reaction, K2HPO4 is fed into a rotary kiln, where
it is heated to approximately 400C. Water evolves and
TKPP is formed in solid form. The procedure is simple
but energy intensive, since, as may be seen from the
overall equation, five moles of water are required to be
evaporated to produce one mole of TKPP.
We have now surprisingly found that it is possible
to form potassium phosphate from potassium chloride and
phosphoric acid with a residual chloride content below
about 2 wt.%, preferably below about 1 wt.%, utilizing a
reaction temperature below about 200C. This result is
achieved by employing a specific molar ratio of
phosphorus to potassium in the reaction and a specific
two-step procedure to remove by-product HCl.
The present invention is particularly concerned
with the manufacture of potassium phosphate useful as a
6 2049859
fertilizer from fertilizer grade potassium chloride and
techn;cal grade phosphoric acid. However, the invention
~ is broadly directed to the production of potassium
phosphate for a variety of purposes, including
conversion into other useful chemical products. In the
product produced by the process of the invention, the
available amount of P2O5 is highly water-soluble and
hence the product is useful for the production of
various types of fertilizer, such as liquid, suspension
or solid forms.
Since the K2O-P2O5-H2O (potassium phosphate) system
parallels the sodium system in many respects, the
reaction of phosphoric acid and sodium chloride produces
the equivalent monosodium phosphate (NaH2P04).
Accordingly, the present invention includes such
reaction. As in the potassium phosphate system, higher
molar Na:P ratios produce different phosphate salts,
namely disodium monohydrogen phosphate (Na2HPO4-
Na:P=2) and trisodium phosphate (Na3PO4 - Na:P=3).
Accordingly, in one aspect of the present
invention, there is provided a process for the
production of a mono alkali metal phosphate selected
from potassium phosphate and sodium phosphate, which
comprises (a) reacting potassium chloride or sodium
chloride with phosphoric acid in a molar feed ratio of
phosphorus to potassium in the range of at least about
1.3:1 at a temperature of about 130 to about 200C to
form potassium phosphate from the potassium chloride or
sodium phosphate from the sodium chloride and by-
product hydrogen chloride; (b) removing the hydrogen
chloride from the reaction mixture by passing air
through the reaction mixture during a first portion of
the period for the reaction of the alkali metal chloride
and by passing steam through the reaction mixture during
the remainder of the period of the reaction of the
alkali metal chloride to result in a potassium phosphate
~A
204 9~9
product from potassium chloride or a sodium phosphate
product from the sodium chloride having a residual
chloride content less than about 2 wt.%; and (c)
recovering the stripped hydrogen chloride in the form of
hydrochloric acid having a concentration of at least
about 20 wt.% HCl.
The invention is particularly described hereinafter
with specific reference to the formulation of potassium
phosphate from potassium chloride. However, as noted
above, the invention includes the production of sodium
phosphate from sodium chloride or sodium carbonate by
equivalent procedures.
In another aspect, the present invention provides a
method for producing potassium pyrophosphate, which
comprises steps (a), (b) and (c) from the first aspect
of the invention to form potassium phosphate; (d)
reacting the potassium phosphate with potassium
hydroxide under pH conditions sufficient to neutralize
the potassium phosphate; and (e) calcining the
neutralized potassium phosphate at a temperature of
about 200C to about 400C to form potassium
pyrophosphate.
According to the present invention, a process
is provided for the manufacture of potassium phosphates
and liquid fertilizer by reacting potassium chloride
with phosphoric acid at a temperature below 200C at a
specific mole ratio of phosphorus to potassium. The
solid potassium phosphate product contains less than
about 2 wt.% chloride and the liquid fertilizer product
contains preferably less than about 0.5 wt.% chloride.
A novel air-steam combination ter-hn;que is employed
to efficiently remove almost all the by-product hydrogen
chloride from the reaction mixture. We have found by
experimentation that, while air or steam alone may be
used to facilitate the removal of hydrochloric acid,
such use at this low temperature for the prolonged
, ,.
7a 204985'~
period necessary to remove almost all the chloride is
detrimental to the outcome of the process. First, at a
~ .
~ O ~ ~ S ~ 9
reaction temperature below 200C, the reaction between
potassium chloride and phosphoric acid proceeds rather
slowly, such that the blowing of air through the
reaction mixture for a prolonged period of time leads to
dehydration, condensation and probably transformation of
phosphoric acid from the reactive form (ortho) to an
unreactive form (meta). Consequently, a product that
contains a chloride level higher than about 2% Cl is
produced. On the other hand, the use of steam as the
sole stripping means results in a very dilute by-product
hydrochloric acid which requires costly treatment to
either concentrate the acid for sale or for disposal.
We have found, in accordance with this invention,
that air stripping followed by a short steam strip,
preferably in combination with continued air stripping,
succeeded in removing almost all chloride at a low
temperature of under 200C and within a specific ratio
of P:K, as well as producing a high strength by-
product hydrochloric acid suitable for commercial use.
The role of the steam as practiced in our invention is
to effect the extent of reaction as well as rate,
causing resumption of liberation of hydrogen chloride
after equilibrium conditions have been obtained and the
reaction has ceased at the end of the air strip step.
In other words, the reaction is enhanced when steam is
utilized following the air strip step due to its
hydrolysis effect on the dehydrated phosphate and
phosphoric acid.
It is usually preferred to effect a high
proportion of the reaction during the air stripping step
to minimize the diluting effect of the steam on the
hydrochloric acid strength attainable. Generally, air
stripping is utilized to an extent which permits the
recovery of a hydrochloric acid having a strength of at
least about 20 wt.% HCl, up to about 33 wt.% HCl. In
order to produce the required hydrochloric acid strength
20498~9
and produce a product having a residual chloride content
below about 2 wt.%, it is generally necessary to effect
at least 90% of the reaction of the potassium chloride,
and preferably about 90 to 95 % of the reaction of the
potassium chloride, while air stripping is effected,
with the balance of the reaction being effected while
steam stripping is effected. Preferably, air stripping
is continued during the steam stripping step.
The process of the present invention is carried out
by reacting potassium chloride with phosphoric acid.
The potassium chloride is employed in solid particulate
form and preferably is fertilizer grade (i.e. Muriate of
Potash, 60 to 63% K2O), where the potassium phosphate
product is to be used as a fertilizer. The phosphoric
acid may be any commercial phosphoric acid containing
from about 30 to about 75 wt.% P205. Preferably,
particularly when the product is to be used as a low-
chloride-content fertilizer, the phosphoric acid is
technical grade phosphoric acid containing a least about
50 wt.% up to about 75 wt.% P205.
The process is carried out at a temperature below
about 200C, generally from about 130 to about 200C,
preferably about 150 to about 190C. Below about
130C, the reaction becomes impractically slow while
above 200C, undesirable products form.
The potassium chloride and phosphoric acid are
reacted in a mole ratio of phosphorus to potassium of at
least about 1.3:1, preferably about 1.5:1 to about
2.0:1. A molar ratio of phosphoric acid to potassium
chloride up to about 4:1 generally can be employed.
Higher molar ratios are possible but the process is
generally uneconomic, in view of the large amount of
unreacted phosphoric acid which is present in the
product, if carried out in a batch process, and the
large volume of recycle required, if carried out in a
continuous process. At a mole ratio below 1.3:1, it is
20~9g~9
-
not possible to achieve a residual chloride
concentration below 2 wt.% even when employing the
combined air/steam process steps of the invention.
The process is sufficiently flexible to permit the
preparation of a wide variety of fertilizer
compositions by appropriate treatment of the potassium
phosphate product. In one particular embodiment of the
process according to the invention, the reaction product
is recovered as pure potassium dihydrogen phosphate with
a high analysis of plant nutrient. In another
embodiment, the reaction product is dissolved in water
to yield a low-chloride (less than 1 wt.%) liquid
fertilizer of analysis such as 0-20-10 (N-K-P). The
resulting reaction product also may be ground up to
yield a solid fertilizer material of high analysis, such
as 0-52-28.
Alternatively, the product may be ammoniated to
make a near neutral pH (pH 6 to 8), low-chloride (less
than 1 wt.%) liquid fertilizer with a desired nutrient
analysis, such as 3-18-9. Further, the product may be
neutralized with caustic potash solution, followed by
nitration with ammonia and urea, to produce a low-
chloride liquid fertilizer having a desired analysis,
such as 5-15-10, 10-15-10, 10-10-10, 12-12-12;
suspension fertilizer, such as 15-15-15, or high
analysis low-chloride solid fertilizer, such as 18-18-
18.
The process of this invention may be operated on a
continuous basis as well as a batch operation, as
described below with reference to the Figures of
drawings.
The potassium phosphate product also may be
employed to form potassium pyrophosphate. This
procedure involves a two-step operation, in which the
potassium phosphate first is neutralized with potassium
hydroxide and then the neutralized potassium phosphate
204~
-
11
is dehydrated to form the potassium pyrophosphate, in
accordance with the equations:
KH2PO4 + KOH , K2HPO4 + H2O
2K2HP04 + Heat ~ K4P207 + H20
for a batch process employing a molar ratio of H3PO4/KCl
of about 1.5, the overall reaction to produce one mole
of K4P2O7 can be represented by the equation:
1.33KCl + 2H3PO4 + 2.67KOH ~ K4P2O7 +
3.67 H2O + 1.3HCl
The neutralization step is effected under pH
conditions sufficient to obtain the desired
neutralization to dipotassium hydrogen phosphate,
generally from about 8 to about 10, preferably about
pHs.
The dehydration step is effected by calcining the
neutralized potassium phosphate at a suitable
temperature, generally about 200 to about 400C. The
potassium pyrophosphate is recovered as a white powder
which is highly water-soluble (187g/lOOg of water at
25C) but is insoluble in ethanol.
It is preferred to effect the production of
potassium pyrophosphate employing the continuous
production of potassium phosphate, since this enables
less phosphoric acid to be used and consequently less
potassium hydroxide to be employed to produce one mole
of TKPP, as seen from the following overall equation:
2KCl + 2H3PO4 + 2KOH --~ K4P207 +
3H2O + 2HCl
As mentioned above, the process of the invention
may be used to form sodium phosphate from sodium
chloride and phosphoric acid. The sodium chloride may
be replaced in this reaction by sodium carbonate.
The sodium phosphate may be recovered in the form
of pure monobasic or dibasic sodium orthophosphates,
depending on the reaction condition.
20~g85~
-
12
The sodium phosphate product may be neutralized
with caustic soda to produce trisodium phosphate, which
is used in many heavy-duty cleaning processes as well as
disinfectant cleaners, scouring powders and automatic
dishwasher formulations.
A mixture of the monobasic and dibasic sodium
orthophosphate, in the molar ratio of about one mole of
monobasic to about two moles of dibasic, may be calcined
to form sodium tripolyphosphate, which is widely used as
a builder for heavy-duty washing powders.
The invention is described further, by way of
illustration, with reference to the accompanying
drawings, wherein:
Figure 1 is a flow sheet of a continuous process
for the production of low-chloride potassium phosphate
from fertilizer grade potassium chloride and technical
grade phosphoric acid, according to one embodiment of
this invention; and
Figure 2 is a corresponding flow sheet for a batch
process for the production of low-chloride potassium
phosphate, according to another embodiment of this
invention.
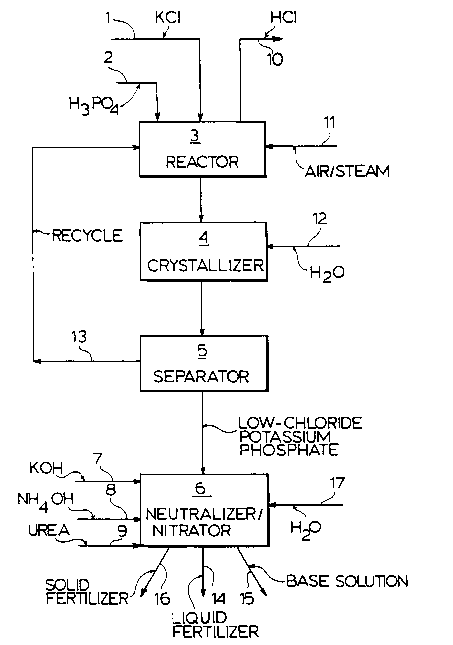
In Figure 1, which is a flow sheet of a continuous
process, solid particulate potassium chloride from a
conduit 1 and phosphoric acid from conduit 2 are fed
continuously to an acidulation reactor 3 maintained at
the desired reaction temperature in desired manner. In
the reactor 3, the feeds along with recycle by-product
provide the desired mole ratio of phosphorus to
potassium. Air and steam are injected sequentially to
the reactor 3 by line 11. Hydrochloric acid, together
with some water vapor, is removed from the reactor 3 by
line 10. The product formed in reactor 3 is sent to a
cooler/crystallizer 4 in continuous manner. Cold water
is introduced into the crystallizer from conduit 12.
Pure solid crystalline potassium phosphate is separated
20~8~J~
13
from unreacted excess phosphoric acid and soluble
phosphate salt in separator 5. The recycle stream 13
containing the unreacted phosphoric acid and soluble
phosphate salts is recycled to reactor 3 in continuous
manner.
The resulting low-chloride potassium phosphate
crystals may be processed further. For this purpose,
the crystals may be fed to a neutralizer/nitrator unit 6
into which also are introduced caustic potash by line 7,
ammonia and/or urea by lines 8/9 and water by line 17.
Depending on the operating conditions in the
neutralizer/nitrator 6, a solid product may be removed
by line 16, or a base solution may be removed by line 14
or a liquid product may be removed by line 15.
Referring now to Figure 2, which is a flow sheet of
a batch process, solid particulate potassium chloride
from storage 1 and phosphoric acid from storage 2 are
fed to the acidulation reactor 3 to provide the desired
phosphorus to potassium mole ratio therein. The
reactor 3 is maintained at the desired reaction
temperature in any convenient manner. Air from conduit
4 and later steam from conduit 5 are injected into
reactor 3. Hydrochloric acid and some water vapor are
removed from the reactor 3 by line 16. The product,
low-chloride potassium phosphate from reactor 3 may be
fed to the nitrator 7 into which also are introduced
ammonia from conduit 8 and/or urea from conduit 9 to
produce low-chloride liquid fertilizer having a neutral
pH of 6 to 8 and thence to storage by line 13.
Alternatively, the potassium phosphate-containing
product from reactor 3 may be fed to a hydrator lo into
which cold water is injected by line 11 to yield a low-
chloride, acidic base solution to be stored by line 14.
The low-chloride potassium phosphate-containing
product from reactor 3 also may be fed to a
cooler/crusher 12 from which a low-chloride, high
~ ~ 4 3 ~ ~ ~
_
14
analysis solid fertilizer is passed to storage by line
15 .
The invention is illustrated by the following
Examples:
EXAMPLE I
Several experiments were run in which solid,
particulate fertilizer-grade potassium chloride (Muriate
of Potash, 62% K20) was fed into a glass stirred reactor
containing hot technical grade phosphoric acid. The
range of feed molar ratio of H3P04 to KCl in the reactor
was 1:1, 1.3:1, 1.5:1, 1.8:1 and 2:1. The temperature
ranged from 154C to 180C. Various stripping means were
employed, including hot air and a two-step air/steam
stripping operation. When the air/steam stripping
operation was effected, steam was introduced 90 minutes
after the start of the reaction and air stripping
continued to be applied.
The resulting products were analyzed for chloride
contents as well as P205 and K20. The hydrochloric acid
evolved was collected in a condenser and analyzed for
the acid content. All the experiments, except
experiments 4 and 5 (see Table I below) were conducted
batchwise, while experiments 4 and 5 were conducted in a
continuous manner as illustrated in Figure 1.
The results of these experiments are summarized in
the following Table I:
~O~g~5~
oooooo~roooooo
O ~,D ~ ~ O O C~ O ~ r~ O O
o o ~~ ~
~ o ~ ~~ ~ ,.,
v
u
-- ~ U~ U~ o ,~~ ~ U7
O ~ ~ ~ ~ O o ~ er _I ~ ~r
o o ~ o
r~
o o o o ~ o o r~ ~1 o
a~ ~ ~nw w a~ I` co a~ ~ ~ w
o
t~
_ ~ ,L ~ ._
~n :~ ~ t ,~ t tn ~ ~: tn ,~ t ,~
Q). OOOOOOOOOOOOO
r~ r~ r~ r~ r~ _~
~ _
oooo~roooooooo
o w ~ o~ w u~ oo c~ oo o~ w oo co w
r~l r ~ r~ r~ r ~ _~ r ~ rt
o o o w a~ ~ In ~ ~ ~ o o o
............
.
o ~
20'1!38~3
16
It is apparent from the results set forth in Table
I that the chloride removal efficiency is closely
associated with the feed molar ratio of H3P04 to KCl.
For a feed molar ratio higher than 1.5, the chloride
removal was satisfactory (i.e. less than about 2 wt.%
Cl- in product) at the reaction temperature of 154C as
well as at 180C. Under these circumstances, steam
sparging or air sparging followed by a steam injection
may be used as the stripping vehicle to facilitate the
removal of hydrogen chloride. However, when steam
stripping alone is used, the product hydrochloric acid
is very dilute and hence is unsatisfactory. However, at
a molar feed ratio of H3P04 to KCl equal to 1.5, only
air stripping followed by steam sparging succeeded in
reducing the chloride level in the product to a desired
content (less than 2 wt.%) and, at the same time,
produced a high concentration hydrochloric acid (at
least 20 wt.% HCl). At a molar feed ratio below 1.3,
even with air stripping followed by steam sparging, it
was not possible to obtain satisfactory levels for
residual chloride.
EXAMPLE II
A study on the optimum conditions in the
acidulation reaction in batch processing was carried
out. Three experiments were conducted in which solid,
particulate fertilizer-grade potassium chloride (Muriate
of Potash, 62% K20) was reacted with technical grade
phosphoric acid in a 2.5L constant stirring glass
reactor. The molar ratio of H3P04 to KCl in the reactor
in all three experiments was 1.5. The reaction was
carried out at 180C using the air/steam stripping
technique as described in Example I. The steam
stripping was conducted for 60 minutes in all three
experiments, but the air stripping duration was 60, 180,
140 minutes respectively. Air was sparged into the
reactor at 50 SCFH for all three experiments.
2 0 ~ 9
_.
17
Analysis of the chloride content in the product for
all three experiments was 0.78, 0.72, 0.40 wt.% Cl-
respectively.
EXAMPLE III
Similar experiments to those conducted in Example
II were carried out. While all the operating conditions
as well as feed molar ratio of H3PO4 and KCl were
similar to Example II, the air flow rate range varied
from 50 to 30 and 10 SCFH. The duration of air sparging
was 60 minutes for all three tests. Corresponding
chloride analysis showed a level of 0.40, 0.51, 1.70
wt.% Cl- for the three products obtained from the
acidulation reactor.
EXAMPLE IV
The viscous mixture of potassium phosphate and
excess phosphoric acid as obtained from Experiment 5,
Table I, was treated by two techniques to produce a
liquid fertilizer. In a first technique, the hot melt
product at 180C was poured into a glass beaker
containing cold water at 22C. The soluble liquid
fertilizer reached a maximum temperature of 51C with a
solubility at room temperature of 56 g/100 g cold water.
Analysis of the resulting acidic base fertilizer
solution showed a N-P2O5-K2O value of 0-19.8-9.8 with a
low chloride content of 0.12% Cl. The liquid fertilizer
was able to be stored at temperatures of 22C or 5C
without crystallization.
In a second technique, the resulting hot melt
product at 180C was poured into a dilute ammonia
solution (1 to 5 wt.%). The maximum temperature of the
liquid fertilizer was recorded at 70C. The solution,
having a neutral pH of 7.0, analyzed 3.1-18.4-9.1 with a
low chloride of 0.10 wt.% Cl- and had good storage
quality.
2 il ~
-
18
EXAMPLE V
The procedure of Example 1 was repeated for various
mole ratio of H3PO4 to KCl, namely 1.5, 2.0 and 2.65 at
a temperature of about 180 + 5C. The reaction was
carried out for two hours during which preheated air was
used as a sparging means. The by-product HCl evolved
during this time was absorbed in an HCl absorber. The
product KH2PO4, containing some excess H3PO4 was cooled
to room temperature.
A known quality of this product was neutralized to
a pH of 8.8 to 9.0 using a 45 wt.~ KOH solution.
Calcination of the neutralized solution was conducted at
200C and 400C for various calcination time in a Mellen
tube furnace.
The products of the calcination were analyzed and
the results are set forth in the following Table II:
o
:~ u . . . . .
oVVVV
~ ~ ~ ~ ~ ~ a~ ~ I`
r ~ a~ O ~ ~1 ~ ~1 0
0 r- OD O ~ r- a~
H
H ~;
O U~ O O O O O O
-
.C O O O O O O O O O
~ OOOOOOOOO
. _
~ ~ U ~ O O O O O U~
5 ~P o v v v v
O O O O O ~ ~ 00
~, ........
~ .
2 ~ ~ t, S ~ 9
_
In summary of this disclosure, the present
invention provides a novel procedure for forming low-
chloride potassium phosphate useful as a fertilizer or
convertible into a variety of fertilizer materials or
other useful products by employing specific process
conditions and utilizing a unique two-step stripping
procedure using air and steam to remove hydrogen
chloride and recover a high strength hydrochloric acid
by-product. The procedure also may be used to form
sodium phosphate. The potassium phosphate may be
converted to potassium pyrophosphate. Modifications are
possible within the scope of this invention.