Note: Descriptions are shown in the official language in which they were submitted.
P~1/5-18413/A/~AG 2076
Heterocyclic compounds
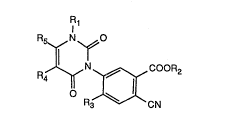
The present invention relates to heterocyclic compounds, that is to 3-aryluracils of the
general forrnula
~,N~COOR2
R CN
.
wherem
Rl is hydrogen, Cl-C4aL~cyl, C3-C4aLIcenyl, C3-C4aLlcynyl, C2-C6aL~coxyaLkyl or
Cl-C4haloalkyl,
R2 is hydrogen, Cl-C6alkyl, C2-C6alkoxyaL~cyl, C4-C7cycloaLkenyl-Cl-C4aLlcyl that is
unsubstituted or ring-substituted by from 1 to 3 Cl-C3alkyl groups, C3-C4aL~cenyl,
C4-C7cycloaLtcenyl-C3-CsaL~cenyl that is unsubstituted or ring- substituted by from 1 to 3
Cl-C3aL~cyl groups, aryl-C3-C5aLkenyl, C3-C4aL~cynyl, C4-C7cycloaLtcenyl-C3 CsaLlcynyl
that is unsubstituted or ring-substituted by from 1 to 3 Cl-C3aLkyl groups, or aryl-C3-Cs-
aLIcynyl,
R3 is hydrogen or halogen,
R4 is hydrogen, halogen or Cl-C4a1kyl and
Rs is Cl-C4aL~yl or Cl-C4haloaLkyl, or
R4 and Rs together are tri- or tetra-methylene, with the proviso that, when Rs is
Cl-C4haloaLIcyl, Rl is other than Cl-C4haloalkyl and R2 is other than hydrogen~
and the corresponding enol ethers of those compounds of ~ormula I wherein Rl is other
than hydrogen or Cl-C4haloaLIcyl, and salts of those compounds of forrnula I wherein R
and/or R2 are (is) hydrogen.
There are thus to be understood by the above-mentioned enol ethers the compounds of the
97
- 2 -
formula
R5~ N~ORl
~ COOR2 Ia
R3~ CN
wherein Rl' is Cl-C4alkyl, C3-C4alkenyl, C3-C4alkynyl or C2-C6alkoxyalkyl.
The compounds of the invention, that is to say the compounds of -formula I and the enol
ethers and salts thereof, are herbicidally active and are suitable as active ingredients of
weed control compositions. The invention thus also includes weed control compositions
that contain compounds of the invendon as active ingredients, processes for the
preparation of those-compounds and the use of the compounds and compositions forcontrolling weeds.
In the above formula I, "halogen" includes fluorine, chlorine, bromine and iodine. The
alkyl, alkenyl and alkynyl radicals may be straight-chain or branched, this applying also to
the or each aL~cyl, alkenyl or alkynyl moiety of the vaIious alkyl-, alkenyl- oraLtcynyl-containing groups, respectively, such as, for exarnple, "C4-C7cycloalkenyl-C3-Cs-
alkenyl that is unsubstitueed or ring-substituted by from 1 to 3 Cl-C3alkyl groups". A halo-
alkyl group may contain one or more identical or different halogen atoms. Aryl (of the
aryl-C3-Csalkenyl and aryl-C3-Csalkynyl groups) is especially phenyl, it being possible
for such groups to be substituted by from 1 to 3 halogen atoms, Cl-C3alkyl, Cl-C3halo-
alkyl, Cl-C3aLIcoxy, Cl-C3alkylthio, C2-C4alkanoyl, C2-C4alkoxycarbonyl, Cl-C3aLIcyl-
sulfonyl, nitro and/or cyano. The fused rings forrned by R4 and R~ (R4 and Rs together are
tri- or tetra-methylene) are represented by the following par~al structures:
The salts of the compounds of folmula I are especially aLIcali metal salts, e.g. sodium and
potassium salts; aLkaline earth metal salts, e.g. calcium and magnesium salts; ammonium
salts, i.e. unsubstituted ammonium salts and mono- or poly-substituted ~mmonium salts,
e.g. triethylammonium and methylammonium salts, and also salts with other organic
bases, e.g. with pyridine.
The possible presence of at least one asymmetric carbon atom in the compounds offormula I means that the compounds may occur in optically isomeric forms. As a result o~
the possible presence of an aliphatic C=C double bond, geometric isomerism may also
occur. Formula I shall include all those possible isomeric forms and also mixtures thereof.
If Rl or R2 is alkenyl or ,llkynyl, then that group is preferably allyl or 3-buten-2-yl, or
propargyl or 3-butyn-2-yl, respectively. In general, a halogen atom that may be present is
preferably fluorine.
Independently of one another, Rl is preferably Cl-C4alkyl, especially methyl; R2 is
preferably C1-C6aLkyl, C~-C6alkoxyalkyl, C3-C4all~enyl or C3-~4alkynyl; R3 is preferably
hydrogen or fluorine; R4 is preferably hydrogen, methyl or fluorine; and Rs is preferably
Cl-C4alkyl or trifluoromethyl. Likewise, R4 und Rs together are preferably tri- or tetra-
methylene.
Especially preferred compounds of formula I are:
2-cyano-5-(1 ,2,4,5,6,7-hexahydro- 1 -methyl-2,4-dioxo-3H-cyclopenta[d]pyrimidin-
3-yl)-benzoic acid isopropyl ester,
2-cyano-4-fluoro-5- ( 1,2,4,5 ,6,7-hexahydro- 1 -methyl-2,4-dioxo-3H-cyclopenta[d] -
pyrimidin-3-yl)-benzoic acid isopropyl ester,
2-cyano-5-[i,4,5,6,7,8-hexahydro-1-methyl-2,4-dioxo-3(2H)-quinazolinyl]-benzoic acid
isopropyl ester,
2-cyano-4-fluoro-5-rl ,4,5,6,7,8-hexahydro-1-methyl-2,4-dioxo-3(2H)-quinazolinyl]-
benzoic acid isopropyl çster,
2-cyano-5- ~3,~-dihydro-3,4-dimethyl-2,6-dioxo- 1 (2~I)-pyr~midinyl]-4-fluoroben~oic acid
isopropyl ester,
2-cyano-5-[3,6-dihydro-3-methyl-4-trifluoromethyl-2,6-dioxo-(1(2H)-pyrimidinyl]-benzoic acid isopropyl ester and
2-cyano-5-[3,6-dihydro-3-methyl-4-trifluoromethyl-2,6-dioxo-1 (2~I)-
pyrimidinyl]-4-fluorobenzoic acid isopropyl ester,
and the corresponding methyl, ethyl, n-propyl, n-butyl, sec-butyl, isobutyl, tert-butyl,
methoxymethyl, 1-methoxyethyl, 2-methoxyethyl, 1-methoxypropyl, 1-ethoxyethyl,
.
2~ 7
2-methoxy-1-rnethyiethyl, allyl, 1-methyl-2-propenyl, 1-ethyl-2-propenyl, 2-butenyl,
propargyl, 2-butynyl, 1-methyl-2-propynyl- and 1-ethyl-2-propynyl esters of the above
compounds.
Further examples of compounds of formula I are:
2-cyano-4-fluoro-S-[S-chloro-3,6-dihydIo-4-methyl-2,6-dioxo-1(2~I)-pyrimidinyl]-benzoic
acid isopropyl ester,
2-cyano-4-fluoro-5- [5-chloro-3,6-dihydro-3,4-dimethyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluo,ro-5-~3,6-dihydro-4-trifluoromethyl-2,6-dioxo- 1 (2H)-pyrimidinyl~-benzoic
acid isopropyl ester,
2-cyano-4-fluoro-5- [4-ethyl-3,6-dihydro-2,6-dioxo- 1 (2H)-pyrimidinyl]-benzoic acid iso
propyl ester,
2-cyano-4-fluoro-5-E4-ethyl-3,6-dihydro-3-methyl-2,6-dioxo-1 (2H)-pyrimidinyl]-benzoic
acid isopropyl ester;
2-cyano-4-fluoro-5-[3,4-diethyl-3,6-dihydro-2,6-dioxo-1(2H~-pyrimidinyl]-benzoic acid
isopropyl ester,
2-cyano-4-fluoro-5-~4-ethyl-3,6-dihydro-5-methyl-2,6-dioxo-1(2H~-pyrin~.idinyl]-benzoic
acid isopropyl ester,
2-cyano-4-fluoro-5-~4-ethyl-3,6-dihydro-3,5-dimethyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5-[3,4-diethyl-3,6-dihydro-5-methyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5-~3,6-dihydro-4-isopropyl-2,6-dioxo-1(2H)-pyrimidinyl]-benzoic acid
isopropylester,
2-cyano-4-fluoro-5-[3,6-dihydro-4-isopropyl-3-methyl-2,6-dioxo-1(2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5-[3-ethyl-3,6-dihydro-4-isopropyl-2,6-dioxo-1(2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5-[3,6-dihydro-4-isopropyl-5-methyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5-[3,6-dihydro-3,5-dimethyl-4-isopropyl-2,6-dioxo- 1 (2~)-pyrimidinyl]-
benzoic acid isopropyl ester,
2-cyano-4-fluoro-5- [3-ethyl-3,6-dihydro-4-isopropyl-5-methyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-benzoic acid isopropyl ester,
37
4-chloro-2-cyano-5-(1 ,2,4,5,6,7-hexahydro-1-methyl-2,4-dioxo-3H-cyclopenta[d]-
pyrimidin-3-yl)-benzoic acid isopropyl ester,
4-bromo-2-cyano-5-(1,2,4,5,6,7-hexahydro-1-methyl-2,4-dioxo-3H-cyclopenta[d]-
pyrimidin-3-yl~-benzoic acid isopropyl ester,
2-cyano-5-[3,6-dihydro-3-methyl-4-trifluoromethyl-2,6-dioxo-1(2H)-pylimidinyl]-benzoic
acid methyl ester and the coIresponding ethyl, n-propyl, n-butyl, sec-butylj isobutyl,
tert-butyl, methoxymethyl, l-methoxyethyl, 2-methoxyethyl, l-methoxypropyl, l-ethoxy-
ethyl, 2-methoxy-1-methyletllyl, allyl, 1-methyl-2-propenyl, 1-ethyl-2-propenyl,2-butenyl, propargyl, 2-butynyl, 1-methyl-2-propynyl and 1-ethyl-2-propynyl esters of that
compound,
2-cyano-4-fluoro-5-[3,6-dihydro-3-methyl-4-trifluoromethyl-2,6-dioxo- 1 (2H)-
pyrimidinyl]-benzoic acid methyl ester and the corresponding ethyl, n-propyl, n-butyl,
sec-butyl, isobutyl, tert-butyl, methoxymethyl, l-methoxyethyl, 2-methoxyethyl,
l-methoxypropyl, l:ethoxyethyl, 2-methoxy-1-methylethyl, allyl, 1-methyl-2-propenyl,
l-ethyl-2-propenyl, 2-butenyl, propargyl, 2-butynyl, 1-methyl-2-propynyl and 1-ethyl-2-
propynyl esters of that compound.
The process according to the invention for the preparation of compounds of formula I and
their enol ethers and salts comprises
a) for the preparation of those compounds of formula I wherein Rl is hydrogen and R2 is
other than hydrogen and R4 is other than chlorine, bromine or iodine and, if desired, metal
salts of those compounds, subjecting to cyclisation, under basic conditions, a compound of
the general formula
H
Rs~ N ~0
,J, H-N II
R4~ C-OR6 \
o \~, COOR2
R3~CN
- 6-
wherein R3 and Rs are as defined above, R2' has the meaning given above for R2 with the
exception of hydrogen, R4' iS hydrogen, fluorine or Cl-C4alkyl or, together with R5, is tri-
or tetra-methylene, and R6 is lower alkyl, preferably Cl-C4alkyl, and, if desired,
converting a possibly resulting metal salt forrn of the uracil derivative into the corres-
ponding acid form (Rl = hydrogen) by treatment with an acid,
b) for the preparation of those compounds of formula I wherein R1 is hydrogen and R2 is
other than hydrogen, R4 is other than chlorine, bromine or iodine and Rs is other than
Cl-C4haloalkyl and, if desired, metal salts of those compounds, subjecting to cyclisation,
under basic conditions, a compound of the general formula
R5' ~N~ ~;o
J~HN~COOR~' III
R CN
wherein R2' and R3 are as defined above, R4' is hydrogen, fluorine or Cl-C4alkyl or,
together with Rs~, is tri- or tetra-methylene, Rs~ is Cl-C4alkyl or, together with R4', is tri-
or tetra-methylene, and R7 is lower alkyl, preferably Cl-C4alkyl,
and, if desired, converting a possibly resulting metal salt of the uracil derivative of
formula I into the acid form (R1 = hydrogen) by treatment with an acid,
c) for the preparation of those compounds of formula I wherein Rl is C1-C4alkyl,C3-C4aL~cenyl, C3-C4alkynyl, C2-C~alkoxyalkyl or Cl-C4haloalkyl, subjecting a uracil
derivative of the general formula
H
R5 N ~O
COOR2 1'
R CN
7 2~
wherein R2, R3, R4 and Rs are as defined above,
to alkylation with a corresponding alkylating agent comprising a Cl-C4alkyl, C3-C4-
alkenyl, C3-C4alkynyl, C2-C6aLkoxyalkyl or Cl-C4haloalkyl group,
d) for the preparation of all compounds of forrnula I and the enol ethers, treating a uracil
derivative of the general formula
~R1
R~COORz IV
wherein Hall is halogen, preferably chlorine or bromine, and Rl, R2, R3, R4 und Rs are as
defined above,
or the corresponding enol ether, with a metal cyanide,
e) for the preparation of those compounds of formula I wherein R2 is hydrogen and Rs is
other than Cl-C4haloalkyl, and the enol ethers thereof, hydrolysing a benzoic acid ester of
the general formula
R1
R5~ N ~;0
COOR2' 1"
R CN
wherein Rl, R2', R3, R4 and Rs' are as defined above,
to form the corresponding benzoic acid,
f) for the preparation of those compounds of formula I wherein Rl and R2 are each other
than hydrogen and Rs is other than Cl-C4haloalkyl, and the corresponding enol ethers,
8 2~
esterifying a benzoic acid of the general forrnula
"
R5' N ~O
~3~COOH 1"'
wherein Rl" is Cl-C4alkyl, C3-C4alkenyl, C3-C4alkynyl, C2-C6alkoxyalkyl or Cl-C4halo-
alkyl and R3, R4 and Rs' are as defined above,
or the corresponding enol ether, it being possible for the benzoic acid or its enol ether to
be in the form of a reactive derivative, with a hydroxy compound of the general formula
HO-R2' V
wherein R2' is as defined above,
or with a reactive derivative of that hydroxy compound,
g) for the preparation of those compounds of formula I wherein Rl and R2 are each other
than hydrogen, and the enol ethers thereof, subjecting a benzoic acid ester of the general
formula
Rl"
~a~COOR2" 1""
wherein Rl", R3, R4 and R5 are as defined above and R2" is Cl-C6aLkyl, C3-Cqalkenyl,
C3-C4alkynyl or C2-C6alkoxyaLkyl,
or the corresponding enol ether, to a transesterification reaction with a hydroxy compound
- 9 -
of the above formula V, the reagent V having a higher boiling point than the respective
alkanol, alkenol or alkynol R2"0H,
h) for the preparation of those compounds of formula I wherein R4 is chlorine, bromine or
iodine, and the corresponding enol ethers, chlorinating, brominating or iodinating a uracil
derivative of the general forrnula
Rl
R5~ N ~oO
J~N~}~COOR2 I""'
R CN
wherein Rl, R2, R3 and Rs are as defined above, or the corresponding enol ether,
i) for the preparation of the enol ethers of the compounds of formula I, treating a uracil
derivative of the general formula
R~ N~ Hal2
COOR2 VI
R3~CN
wherein R2, R3, R4 and Rs are as defined above, and Hal2 is chlorine or bromine,
with an alkanol, alkenol or alkynol Rl'OH in the presence of an organic base, or widl the
corresponding alcoholate, alkenolate or alkynolate, respectively, of the general formula
Rl'o~)M~3 VII
wherein Rl' is as defined above and M~33 is an equivalent of a metal ion
and, if desired, converting a resulting compound of formula I wherein Rl and/or R2 are (is)
- - 10-
hydrogen into a salt.
The cyclisation according to process variant a) or b) can advantageously be carried out by
treating the compound of formula II or III in an inert protic organic solvent, such as an
alcohol, e.g. methanol, ethanol or isopropanol; in an inert aprotic organic solvent, such as
an aliphatic or cyclic ether, e.g. 1,2-dimethoxyethane, tetrahydrofuran or dioxane, or an
aromatic compound, e.g. benzene or toluene; in an inert aprotic polar organic solvent, e.g.
dimethylformarnide or dimethyl sulfoxide, it being possible, if desired, for such solvents
to be used in a two-phase mixture with a hydrocarbon, e~g. n-hexane or toluene; or in
water, with a base, at temperatures from -78C to the reflux temperature of the reaction
mixture. Suitable bases are preferably sodium alcoholates, alkali metal hydraxides,
especially sodium hydroxide and potassium hydroxide, alkali metal carbonates9 especially
sodium carbonate and potassium carbonate, and sodium hydride. If an alkanol is used as
solvent, then that solvent advantageously corresponds to the respective hydroxy compound
E~2'-OH; by this means undesired competing trans-esterification reactions are avoided. If
sodium hydride is used as base, th~ solvent is preferably an aliphatic or cyclic ether,
dimethylfonnamide or dimethyl sulfoxide, it being possible for any of those solvents to be
used in admixture with toluene.
When the cyclisation is complete, if one of the above-mentioned bases or the like is used,
the product is in the form of the corresponding alkali metal salt. This can be isolated and
purified in a manner known E~r se, or the mixture can be acidified in order to isolate the
respective cornpound of formula I itself. For that purpose preferably a minera~ acid, such
as hydrochloric acid, or a strong organic acid, such as acetic acid ~r p-toluenesul~onic
acid, is used.
In process variant c), the tenn "aLcylation" denotes the substitution of the hydrogen atom
of the Nl atom of ~he uracil nucleus by a Cl-C4aLcyl, C3-C4alkenyl, C3-C4aLlcynyl,
C2-C6alkoxyaLcyl or Cl-C4haloaLcyl group. There is advantageously used as aLkylating
agent a Cl-C4aLcyl, C3-C4alkenyl, C3-C4aL~ynyl or C2-C6aL'coxyaLcyl halide, especially
the appropriate chloride or bromide, or sulfate, or a polyhalogenated Cl-C4alkane, such as,
e.g., chlorodifluoromethane, or a mono- or poly-halogenated alkene, such as, e.g., tetra-
fluoroethane.
The aL~cylation is advantageously carried out in the presence of an inert protic Grganic
solvent, such as a lower aL~anol, for example ethanol, i~ desired in adrnixture with water;
3 7
an inert aprotic orgaDic solvent, such as an aliphatic or cyclic ether, e.g. 1,2-dimethoxy-
ethane, tetrahydrofuran or dioxane; a ketone, e.g. acetone or butan-2-one; or an inert
aprotic polar organic solvent, e.g. dimethylformamide, dimethyl sulfoxide or acetonitrile,
and also in the presence of a base, such as sodium hydride, an alkali metal hyd}oxide,
especially sodium or potassium hydroxide, an alkali metal alcoholate, especially sodium
alcoholate, or an aL~ali metal carbonate or hydrogen carbonate, especially sodium
carbonate, potassium carbonate, sodium hydrogen carbonate or potassium hydrogen
carbonate, at temperatures from OC to the reflux temperature of the reaction mixture,
preferably at room temperature or, in the case of substitution of the hydrogen atom of the
Nl atom with a Cl-C4haloalkyl group, preferably at temperatur~s from 50C to 100C. In
a preferred embodiment, the uracil derivative of formula I' is ~lrst of all treated with the
base, such as sodium hydride, ethanolate or carbonate, in the solvent and, after a short
reaction time, the halide is added in the same solvent. In a further embodiment, the uracil
derivative I', together with a dialkyl sulfate, is reacted at reflux temperature in the
presence of an alkali metal hydrogen carbonate, especially sodium or potassium hydrogen
carbonate, in the solvent, e.g. acetone. The reaction is usually complete within a relatively
short time or after a few hours, depending on the solvent used.
Process variant d) is an exchange reaction of the halogen substituent of the benzene
nucleus. That halogen atom is thus replaced by the cyano group by means of the metal
cyanide. The latter is especially a transition metal cyanide, preferably copper(I) cyanide.
The reaction is advantageously carried out in the presence of an aprodc polar solvent, such
as an aL~ylnitrile, e.g. acetonitrile, propionitrile or butyronitrile; an aL~cylurea, e.g. tetra-
methylurea; a dialkylarnide, e.g. dimethylformarnide; a dialkyl sulfoxide, e.g. dimethyl
sulfoxide; N-methyl-2-pyrrolidone; 1,3-dirnethyl-imidazolidin-2-one; 1,3-dirnethyl-
3,4,5,6-tetrahydro-2(1H)-pyrimidinone; or hexamethylphosphoric acid triamide, atelevated temperatures, that is from 80C to 200C, preferably from 150C to 200C. In the
starting material I", R4 is preferably hydrogen or fluorine.
The hydrolysis of the benzoic acid ester I" or the enol ether thereof according to process
variant e,~ can be ca~ied out according to methods known ~ se, especially using an
organic solvent in aqueous solution, such as an aqueous alkanol, e.g. ethanol, or an
aliphatic or cyclic ether, e.g. 1,2-dimethoxyethane, tetrahydrofuran or dioxane, in aqueous
solution, and an inorganic base, such as lithium, sodium or potassium hydroxide, ~r an
alkaline earth metal hydroxide, e.g. magnesium or calcium hydroxide, at temperatures
from 0C to 60C, preferably at room temperature.
.
- ~ 20
- 12-
.
Process variant f) is an esteriFlcation of benzoic acid or the enol ether or a reactive
derivative thereof, which can be carried out according to methods known ~ se. For
example, a salt of a benzoic acid of formula I"' or of the corresponding enol ether is
reacted with a halide, especially the chloride, bromide or iodide, or the sulfate? mesylate or
tosylate of the hydroxy compound V in an inert diluent at temperatures from roomtemperature to 100C, e.g. at the reflux temperature of the reaction mixture, preferably in
a temperature range from 40C to 70C. Suitable salts of the benzoic acid of formula I"' or
of the corresponding enol ether are especia~ly alkali metal salts, e.g. the sodium,
potassium or lithium salt, alkaline earth metal salts, e.g. the magnesium, calcium or
barium salt, and salts with organic bases, such as tertiary amines, e.g. triethylamine, 1,5-
diaza-bicyclo[4.3.0]non-5-ene, 1,~-diaza-bicyclo[5.4.0]undec-7-ene.and 1,4-diaza-
bicyclo[2.2.2]octane, with the alkali metal salts, especially the sodium salt and the
potassium salt, being preferred. The diluents that can be used are preferably inert organic
solvents, such as lower alkanols, e.g. ethanol, aliphatic and cyclic ethers, e.g. diethyl ether,
tetrahydrofuran and dioxane, ketones, e.g. acetone and 2-butanone, dimethylforrnarnide,
dimethyl sulfoxide, acetoni~ile and hexamethylphosphoric acid triamide. The salt can be
produced in situ by reacting the acid with a suitable inorganic base, e.g. an alkali metal or
alkaline earth metal carbonate, hydrogen carbonate, hydroxide or hydride, or organic base,
to -form the salt, which can then be reacted with the second reactant in the same reaction
medium.
When an acid halide of the benzoic acid of formula I"' or of the correspondin~ enol ether
is used as reactive derivative, it is advantageously reacted with the hydroxy compound of
forrnula V in an inert organic solvent, such as an aliphatic or cyclic ether, e.g. diethyl
et'ner, 1,2-dimethoxyethane, tetrahydrofuran or dioxane, an aliphatic or aromatic hydro-
carbon, e.g. n-hexane, benzene or toluene, or a halogenated, especially chlorinated,
hydrocarbon, e.g. methylene chloride, chloroforrn or carbon tetrachloride, at temperatures
of approximately from -20C to 100C, preferably from 0C to 50C. ~n addition, the
reaction is advantageously carried out in the presence of an acid-binding agent, such as an
organic base, e.g. triethylamine, pyridine, 4-dimethylaminopyridine, 1,5-diaza-bicyclo-
[4.3.0]non 5-ene, 1,~-diaza-bicyclo[5.4.0]undec-7-ene or 1,4-diaza-bicyclo[2.2.2]octane.
The acid halide is preferably the acid chloride.
Other suitable reactive derivatives of the benzoic acid of formula I"' vr of thecorresponding enol ether are the corresponding O-acyl-1,3-dicyclohexylisourea and the
13 -
corresponding N-acylimidazole or acid anhydride. Such derivatives can, like the acid
halide, be reacted with the hydroxy compounds of forrnula V in order to obtain the desired
benzoic acid esters. In those cases, however, the use of an acid-binding agent is not
necessary.
The reaction according to process variant g) can advantageously be carried out by heating
the benzoic acid ester of formula I"" or its enol ether in excess hydroxy compound of
formula V in the presence of a weakly basic catalyst, such as sodium cyanide or preferably
tetraisopropyl or tetrapropyl orthotitanate, preferably at the reflux temperature of the
reaction mixture. In the course of the reaction the radical R2" of the benzoic acid ester I""
is replaced by the group R2' of the hydroxy compound V, the lower boiling alkanol,
alkenol or alkynol R2"OH being freed and removed from the reaction mixture.
The chlorination or bromination according to process variant h) is advantageously carried
out using elemental chlorine or sulfuryl chloride, or elemental bromine or sulfuryl
bromide, respectiveiy, in the presence of an inert organic solvent, such as acetic acid or a
chlorinated aliphatic hydrocarbon, e.g. methylene chloride, chloroform or carbon tetra-
chloride, and in a temperature range from 0C to 60C, preferably at room temperature. In
addition, the reaction can be carried out with the assistance of an acid-binding agent,
sodium acetate and tertiary amines, such as triethylamine, dimethylaniline and pyridine,
being especially preferred acid-binding agents for that purpose.
The iodination according to that process variant is advantageously carried out using
elemental iodine as iodinating agent and of a low-boiling aliphatic carboxylic acid, such as
acetic acid, as solvent, at temperatures from approximately 0C to approximately 110~,
preferably at room temperature. In addition, it has proved advantageous to caIry out the
reaction in the presence of an acid, such as fuming nitric acid. When the reaction is
complete, saturated aqueous sodium hydrogen sulfite solution can be added in order to
remove excess lodlne.
In process variant i), the term "metal ion" indicates especially an aL~cali metal ion, e.g. the
sodium or potassium ion, or an alkaline earth metal ion, e.g. the calcium or magnesium
ion. ~he sodium ion is the preferred metal ion. If the aL~anol, alkenol or aL~cynol Rl'OH is
used, then the suitable organic base is especially pyridine.
The reaction is advantageously carried out in an excess of the corresponding alcohol
- ,
2~ 7
- 14-
R~'OH as diluent, and at temperatures from 0C to 50C, preferably at room temperature.
If they cannot be prepared directly by the above-described cyclisation canied out under
basic conditions, the desired salts of the compounds of formula I in which Rl is hydrogen
can alte~natively be prepared from those compounds I in a manner known ~ se, forexample by dissolving the compound of formula I in a solution of an appropriate organic
or inorganic base. The salt formation is usually effected within a short time at room
temperature. In one embodiment, the sodium salt is prepared by dissolving the uracil
derivative I in aqueous sodium hydroxide solution at room temperature, using equivalent
amounts of the uracil derivative and of sodium hydroxide. The solid salt can then be
isolated by precipitation with a suitable inert solvent or by evaporating off the solvent.
Another embodiment comprises introducing an aqueous solution of an alkali metal salt of
the uracil derivative I into an aqueous solution of salt that has a metal ion other than an
alkali metal ion, the second metal salt of ~he uracil derivative being produced. This
embodiment is generally used to prepare uracil metal salts that are insoluble in water.
The compounds of formula I, enol ethers and salts obtained can be isolated and purified
according to methods known ~ se. Also familiar to the person skilled in the art is the
sequence in which possible combinations of process variants c) to h) are advantageously
to be carried out so as to avoid possible undesired competing reactions.
If no specific synthesis for the isolation of pure isomers is carried out, the product may be
obtained in the form of a mixture of two or more isomers. The isomers can be separated
according to methods known ~ se. If desired, for example, pure optically active isomers
can also be produced by synthesis from corresponding optically active starting materials.
The starting materials of formula II, which are novel, can be prepaled in a manner known
~ se, e.g. in accordance with the following Reaction Schemes 1 ~Methods aa), bb) and
cc)]:
Reaction Schemes 1
z~
15 -
R5 ~0 H2N ~0
6 ~COOR2
VIII IX
R5 ~N~O
R4 ICI _OHR6N X COOR2
O R3 CN
R4 XGOR6 ~XCOOR2
X XI
R5~N~O
R C OR ~XCOOR2
II
R5~ ~R8 H2N ~O
CC) R ~ C OR HN~COOR2'
R3 CN
XII ~
R5' 1~O
R4~ ~C-OR6 COOR2'
O R3~ CN
II"
In the above reaction schemes, R2~, R3, R4~, Rs, Rs~ and R6 have the meanings given
hereinbefore; R4~ is hydrogen or Cl-C4alkyl or, together with Rs~, is tri- or
tetra-methylene; and R8 is lower aL~cyl, preferably Cl-C4alkyl.
Method aa) is advantageously caIIied out by reacting the compounds of fonnulae VIII and
IX with each other in a substantially anhydrous diluent in the presence of an acid catalyst
at elevated tenmperature. Suitable diluents are especially organic solvents that form
azeotropes with water, such as aromatic compounds, e.g. benzene, toluene and xylenes;
halogena~ed hydrocarbons, such as methylene chloride, chloroform, carbon tetrachloride
and chlorobenzene; and aliphat;c and cyclic ethers, such as 1,2-dimethoxyethane,tetrahydrofuran and dioxane, and suitable acidic catalysts are especially strong mineral
acids, such as sulfuric acid and hydrochloric acid; organic acids, such as p-toluenesulfonic
acid; phosphorus-containing acids, such as orthophosphoric acid and polyphosphoric acid;
and acidic cation exchangers, such as "Amberlyst 15" (Fluka). The reaction is generally
~ ; ~
- 17-
carried out in a temperature range from approximately 70C to 120C, preferably at the
reflux temperature of the reaction mixture. Under those reaction conditions the desired
rapid removal of the water forrned during the reaction is achieved.
The reaction according to Method bb) is advantageously carried out in the presence of a
substantially anhydrous aprotic organic solvent, such as an aliphatic or cyclic ether, e.g.
diethyl ether, 1,2-dimethoxyethane, tetrahydrofuran or dioxane, an aliphatic or aromatic
hydrocarbon, e.g. n-hexane, benzene, toluene or a xylene; or a halogenated, aliphatic
hydrocarbon, e.g. methylene chloride, chloroform, carbon tetrachloride or
1,2-dichloroethane; and also where appropriate in the presence of a base, especially an
organic tertiary base, such as triethylamine or pyridine, it being possible for the latter to be
used both as solvent and base, or a metal hydride, such as sodium or potassium hydride.
The reaction temperatures are preferably in the range of approximately from -80C to
50C, temperatures of from -30C to room temperature being especially preferred.
The reaction according to Method cc) is advantageously carried out in an inert
water-miscible organic solvent, such as an aliphatic or cyclic ether, e.g.
1,2-dimethoxyethane, tetrahydrofuran or dioxane, or a lower alkanol, such as ethanol, at
temperatures from 50C to 100C, preferably at the reflux temperature of the reaction
mixture, or in an aromatic solvent, such as benzene, toluene or a xylene, in the presence of
an acidic catalyst, such as hydrochloric acid or p-toluenesulfonic acid, at temperatures
from 50C to 100C, preferably from 60C to 80C.
The starting materials of formula III are also novel and can be prepared in a manner
known ~ se, e.g. in accordance with the following ~eaction Schemes 2 ~Iethods dd),
ee) and ff)]:
Reaction Schemes 2
Rg-CI I = C _ o H2N ~\~ GOOR2'
dd) R4-"CH- C=O R3 J~CN
XIII XIV
- 18-
R9-CH2 ~ ~0
R"'lH N COOR2'
CN
XV
o~o/l' R3 COOR2'
CH3
~VI XIV
R" O
2 ~C/ COOR2'
o R3 )~CN
XVII
R' H2N COOR2'
R4' C--6 ~ CN
o
VIII XIV
., ~ j.
- 19-
~C~
H
4 ~ C/ \~ COOR2'
R3~ ~CN
XVIII
XV/XVIVXVIII + H2NCOOR7 1~ m
XIX
In the above Reaction Schemes, R2~, R3, R4~, Rs~, R6 and X have the meanings givenhereinbefore; R4~ is hydrogen or Cl-C4alkyl; R5~ is Cl-C4aL~cyl; and R~ is hydrogen or
Cl-C3alkyl.
The reaction of the amine of forrnula XIV with the diketene of formula XIII according to
Method dd) is advantageously carried out in an anhydrous inert aprotic solvent, such as a
halogenated hydrocarbon, e.g. methylene chloride, chloroforrn, carbon tetrachloride or
chlorobenzene, an aromatic hydrocarbon, e.g. benzene, toluene or a xylene, or an aliphatic
or cyclic ether, e.g. diethyl ether, 1,2-dimethoxyethane, tetrahydrofuran or dioxane, in the
presence of a basic catalyst, such as 4-pyrrolidinopyridine~ 4-dimethylaminopyridine, 1,4-
diazabicyclo[2.2.2]octane, 1,5-diazabicyclo[4.3.0]non-5-ene,
1,8-diazabicyclo[5.4.0]undec-7-ene or diethylarnine. Since the reaction is exotherrnic, it is
generally carried out in a temperature range from -10C to 50C, preferably at room
temperature.
The reaction of the compounds of formulae XVI and XIV with each other in accordance
with Method ee) is advantageously carried out in an anhydrous inert aprotic solvent at
temperatures from approximately 70C to l40C, preferably from 100C to 120C. There
are sui~able as such solvents especially aromatic compounds, e.g. benzene, toluene and
xylenes; halogenated hydrocarbons, e.g. carbon tetrachloride, trichloroethane,
tetrachloroethane and chlorobenzene; and aliphatic and cyclic etheTs, e.g. dibutyl ether,
: .
- 20 -
.
1,2-dimethoxyethane, tetrahydrofuran and dioxane.
The reaction according to Method ff) is an aminolysis, which is advan~ageously carried
out in an anhydrous solvent, or without solvent [see, for example, ~. Soc. Dyes Col. 42,
81 (1926), Ber. 64, 970 (1931) and J.A.C.S. 70, 2402 (1948)] at elevated temperature.
Suitable solvents are especially inert aprotic solvents, such as unsubstituted or halogenated
aromatic compounds, e.g. toluene, xylenes and chlorobenzenes. The reaction is generally
carried out in a temperature range from approximately 13()C to 160C. Where appropriate
the reaction is in addition carried out in the presence of a basic catalyst, e.g. a
higher-boiling amine ~see, for example, Helv. Chim. Acta 11, 779 (1928) and US Patent
Specification No. 2 416 738] or pyridine.
The subsequent reaction of the compound of formula XV, XVII or XVIII prepared in that
manner with the carbamic acid lower alkyl ester of formula XIX is advantageously carried
out in a substantially anhydrous diluent and in the presence of an acidic catalyst at
elevated temperature. Suitable diluents are especially organic solvents that form
azeotropes with water, such as aromatic compounds, e.g. benzene, toluene and xylenes;
cyclic aliphatic compounds, such as cyclohexane; and halogenated hydrocarbons, such as
carbon tetrachloride and chlorobenzene, and suitable acidic catalysts are especially strong
mineral acids, such as sulfuric acid; organic acids, such as orthophosphoric acid and
polyphosphoric acid; and acidic cation exchangers, such as "Amberlyst 15" (Fluka). The
reaction is generally carried out in a temperature range from approximately 70C to
150C, preferably at the reflux temperature of the reaction mixture. Under those reaction
conditions the desired rapid removal of the water formed during the reaction is achieved.
The starting marterials of formula IV, the corresponding enol ethers and their preparation
are for the most part described in European Patent Publications Nos. 195 346 and 260 621.
Those starting materials IV and enol ethers for which the preparation is not described may
be prepared analogously to the known starting materials.
The starting matenals of formula VI used in process variant i) can be prepared by
halogenating the corresponding uracil derivative of the above formula I'. The halogenating
agent used for the halogenation is especially thionyl chloride, phosphorus pentachloride or
phosphorus oxychloride, or phosphorus pentabromide or phosphoryl bromide. If desired a
mixture of phosphorus pentachloride and phosphorus oxychloride, or of phosphoruspenta~romide and phosphoryl bromide, may be used, it being possible for excess
2~ d
phosphorus oxychloride or phosphoryl bromide, respectively, to act as diluent. The
chlorination or bromination can be carried out in the presence of an inert diluent,
especially an aprotic organic solvent, such as an aliphatic or aromatic hydrocarbon, e.g.
n-hexane, benzene, toluene or a xylene; a halogenated aliphatic hydrocarbon, e.g.
methylene chloride, chloroform or 1,2-dichloroethane; a halogenated aromatic
hydrocarbon, e.g. chlorobenzene, or a tertiary amine, e.g. N,N-dimethylaniline, but this is
not necessary if phosphorus oxychloride or phosphoryl bromide is used as halogenating
agent. If the halogenating agent used is thionyl chloride it has proved advantageous to add
a catalytic amount of dimethylformamide. The reaction temperatures generally range
from 0C to the reflux temperature of the reaction mixture, preferably from 80C to
120C.
The uracil derivatives of formulae I', I", I" ', I" " and I" " ' and enol ethers used as starting
materials in process variants c), e), f), g) and h) are sub-groups of compounds of formula I
and the enol ethers thereof. The remaining starting mate~ials and reagents involved in the
process variants and in the reaction schemes are either known or can be prepared by
methods that are known ~ se.
The compounds of formula I and their enol ethers and salts (referred to in the following
collectively as "compounds of the invention" or "active ingredients") are suitable for
controlling, preventing or eliminating plant growth, especially undesired plant growth.
The compounds of the invention possess especially herbicidal propeIties and are suitable
for controlling weeds, including grass weeds, for example Abutilon theophrasti,
Amaranthus retroflexus, Agropyron repens, Alopecurus myosuroides, Avena fatua,
Bromus inermis, Cyperus esculentus, Ipomoea purpurea, Poa annua, Sorghum halepense,
Stellaria media, Cassia obtusifolia, Chenopodium album, Chrysanthemum segetum,
Datura strarnonium, Digitana sanguinalis, Echinochloa crus-galli, Galium apanne,Matricaria chamomilla, Setaria faberii, Sinapis arvensis and Xanthium pennsylvanicum, in
diverse crops of useful plants, for example rape, soybean, Gotton, rice, wheat and maize
crops, but especially in cotton crops. In addition, the compounds are both preemergence
and postemergence herbicides. Furtherrnore, the compounds of the invention can be used
to control undesired plant growthf e.g. in potatoes, cotton plants, sunflowers~ seed
vegetables and water weeds. They may be used, for example, as burning-off agents to
facilitate the harvesting of potatoes and cotton.
In practice, usually a concentration of 0.5 to 6.0 kg of the compound of the invenLion/ha,
- 22 - ~35~
preferably 1 g to 200 g of the compound of the invention/ha, is sufficient to achieve the
desired herbicidal effect.
The concentration required for the desired effect can be determined by tests. It is
dependent on the nature of the action, the stage of development of the cultivated plant and
of the weed and on the application (place, time, method) and can vary widely as a function
of those parameters.
The weed control composition according to the invention is characterised in that it
comprises an effective amount of at least one compound of formula I, as defined above, or
of an enol ether or salt thereof, together with formulation adjuvants. The composition
advantageously comprises at least one of the following formulation adjuvants: solid
carriers; solvents or dispersants; surfactants (wetting agents and emulsifiers3; dispersants
(without surface-active action); and stabilisers. Using those and other adjuvants, those
compounds, that is to say the herbicidal active ingredients, can be converted into
customary formulations, such as dusts, powders, granules, solutions, emulsions,
suspensions, emulsifiable concentrates, pastes and the like.
The compounds of formula I and their enol ethers are in general water-insoluble, whereas
the salts, especially the aL~cali metal salts and arnmonium salts, are generally water-
soluble; they can be formulated according to the methods customary for water-insoluble
and water-soluble compounds using the appropriate formulation adjuvants. The
preparation of the compositions can be carried out in a manner known E~ se, e.g. by
mixing the active ingredient in question with solid carriers, by dissolving or suspending in
suitable solvents or dispersants, optionally using surfactants as wetting agents or
emulsifiers and/or dispersants, by diluting ready-prepared emulsi~lable concentrates with
solvents or dispersants etc..
Suitable solid carriers are essentially natural mineral substances, such as chalk, dolomite,
limestone, argillaceous earths and silicic acid and salts thereof (for example diatomaceous
earth, kaolin, bentonite, talcum, attapulgite and montmorillonite); synthetic mineral
substances, such as highly dispersed silicic acid, aluminium oxide and silicates; organic
materials, such as cellulose, starch, urea and synthetic resins; and fertilisers, such as
phosphates and nitrates, it being possible for such caIriers to be, ~or exarnple, in the form
of po~vders or granules.
- 23 ~ 9 9J
Suitable solvents and dispersants are essentially aromatic compounds, such as benzene,
toluene, xylenes and alkylnaphthalenes; chlorinated aromatic compounds and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloroethylenes and methylene chloride;
aliphatic hydrocarbons, such as cyclohexane and paraffins, e.g. petroleum fractions;
alcohols, such as butanol and glycol, and the* ethers and esters; ketones, such as acetone,
methyl ethyl ketone, methyl isobutyl ketone and cyclohexanone; and strongly polar
solvents or dispersants, such as dimethylformamide, N-methylpyrrolidone and dimethyl
sulfoxide, such solvents preferably having flash points of at least 30C and boiling points
of at least 50C, and water. Also suitable as solvents and dispersants are so-called
liquefied gaseous extenders or carriers, which are products that are gaseous at room
temperature under normal pressure. Examples of such products are especially aerosol
propellant gascs, such as hydrocarbons, e.g. propane and isobutane, and halogenated
hydrocarbons, e.g. dichlorodifluoromethane. If the weed control composition of the
invention is in the form of a pressurised gas pack, then advantageously a solvent is used in
addition to the propellant gas.
The surfactants (wetting agents and emulsifiers) may be non-ionic compounds, such as
condensation products of fatty acids, fatty alcohols or fat-substituted phenols with
ethylene oxide; fatty acid esters and ethers of sugars or of polyhydnc alcohols; the
products obtained from sugars or polyhydric alcohols by condensation with ethylene
oxide; block polymers of ethylene oxide and propylene oxide; or alkyldimethylamine
oxides.
The surfactants may also be anionic compounds, such as soaps; fatty sulfate esters, e.g.
dodecyl sodium sulfate, octadecyl sodium sulfate and cetyl sodium sulfate; alkyl-
sulfonates, arylsulfonates and fatty aromatic sulfonates, such as alkylbenzene sulfonates,
e.g. calcium dodecylbenzene sulfonate, and butylnaph~halene sulfonates; and morecomplex fatty sulfonates, e.g. the amide condensation products of oleic acid andN-methyltaurin, and the sodium sulfonate of dioctyl succinate.
Finally, the surfactants may be cationic compounds~ such as aL~cyldimethylbenzyl-
ammonium chlorides, diaL~yldimethylammonium chlorides, aL~cyltrimethylammonium
chlorides and ethoxylated quaternary ammonium chlorides.
Suitable dispersants (without surface-active action) are essentially lignin, sodium and
ammonium salts of lignosulfonic acids, sodium salts of maleic acid anhydride/di-
- 24 -
isobutylene copolymers, sodium and ammonium salts of sulfonated polycondensationproducts of naphthalene and formaldehyde, and sulfite waste liquors.
As dispersants, which are suitable especially as thickeners and anti-settling agents, there
may be used, e.g., methylcellulose, carboxymethylcellulose, hydroxyethylcellulose,
polyvinyl alcohol, alginates, caseinates and blood albumin.
Examples of suitable stabilisers are acid-binding agents, e.g. epichlorohydrin,
phenylglycidyl ethers and soya epoxides; antioxidants, e.~. gallic acid esters and bu~yl-
hydroxytoluene; UV absorbers, e.g. substituted benzophenones, diphenylacrylonitrile acid
esters and cinnamic acid esters; and deactivators, e.g. salts of ethylenediaminetetraacetic
acid and polyglycols.
The weed control compositions according to the invention may comprise, in addition to
the active ingredients of the invention, synergists and other active ingredients, e.g.
insecticides, acaricides, fungicides, plant-growth regulators and fertilisers. Such
combination compositions are suitable for strengthening the activity or broadening the
activity spectrum.
The weed control compositions accordin~ to the invention generally comprise from 0.01
to 95 % by weight, preferably from 0.5 to 75 % by weight, of one or more compounds
according to the invention as active ingredient(s). They may, for example, be in a form
that is suitable for storage and transport. In such formulations, e.g. emulsifiable
concentrates, the active ingredient concentration is normally in the higher range,
preferably from 1 to 50 % by weight, especially from 10 to 20 % by weight. Thoseformulations can then be diluted, e.g. with identical or different inert substances, to the
active ingredient concentrations suitable for practical use, that is to say preferably
approximately 0.01 to 10 % by weight, especially approximately 0.0()5 to 5 % by weight.
The active ingredient concentrations may, however, also be lower or higher.
As mentioned above, the preparation of the weed control compositions according to the
invention can be carried out in a manner known ~ se.
In order to prepare pulverulene compositions, the active ingredient, that is to say at least
one compound of the invention, may be mixed with a solid carrier, e.g. by grinding
together; or the solid caTrier may be impregnated with a solution or suspension of the
- 25 -
active ingredient and then the solvent or dispersant, as the case may be, removed by
evaporation, heating or filtering with suction under reduced pressure. Such pulverulent
compositions can be made readily wettable with water by adding surfactants or
dispersants, so that they can be converted into aqueous sllspensions that are suitable, e.g.,
as spray compositions.
The active ingredient may also be mixed with a surfactant and a solid carrier to form a
wettable powder that is dispersible in water, or may be mixed with a solid pregranulated
carrier to form a granular product.
If desired, the active ingredient may be dissolved in a water-immiscible solvent, such as,
for example, a high-boiling hydrocarbon, that advantageously comprises dissolvedemulsifier so that the solution is self-emulsifying when water is added. Alternatively, the
active ingredient may be mixed with an emulsifier and the mixture then diluted with water
to the desired concentration. Further, the active ingredient may be dissolved in a solvent
and then mixed with an emulsifier. Such a mixture may also be diluted with water to the
desired concentration. Emulsifiable concentrates and ready-for-use emulsions are obtained
in this manner.
The use of the weed control compositions according to the invention, to which the present
invention also relates, may be in accordance with customary application methods, such as
sprinkling, spraying, dusting, pouring or scattering. The method according to the
invention of controlling weeds comprises treating the substrates to be protected against
weeds and/or the weeds with a compound of the invention or with a weed control
composition of the invention.
The following Exarnples are to illustrate the invention further.
I. Preparation of the compounds of forrnula I:
Example 1: A mixture of 50.0 g of 2-chloro-5-~3,6-dihydro-
3,4-dimethyl-2,6-dioxo-1(2H)-pyIimidinyl]-4-fluorobenzoic acid isopropyl ester and
25.4 g of copper(I) cyanide in 225 ml of dimethyl-3,4,5,6-te~rahydro-2(1H)-pyrimidinone
is heated for 2.5 hours at 195C under nitrogen. The mixture is then cooled to room
temperature and 21 of ethyl acetate are added. The mixture is then poured onto 1.31 of
water and 300 ml of 32% hydrochloric acid, and the whole is sti~d for 45 minutes at
2~
- 26-
room temperature until two distinct layers have formed. The organic phase is removed,
washed twice with 200 ml of water each time and dried over anhydrous sodium sulfate.
Concentration by evaporation of the organic solution yields crystalline
2-cyano-5-[3,6-dihydro-3,4-dimethyl-2,6-dioxo- 1 (2H)-pyrimidinyl]-4-fluorobenzoic acid
isopropyl ester, m.p. 188-200C.
Examples 2-10: The compounds of formula I listed in the following Table 1 are obtained
analogously to the process described in Example 1 by treating the corresponding uracil
derivative of formula IV with copper(I) cyanide:
R1
R5~ N ~O
N ~XCOOR2
o R3 CN
Exa- Rl R2 R3 R4Rs Physical
mple data
2 CH3 CH(CH3)2 H HCF3 m.p. 125-127C
3 CH3 CH(CH3)2 F HCF3 m.p. 130-132C
4 CH3 CH(CH3)2 F HC2Fs m.p. 116-118C
S CH3 CH(C~I3)2 F -(CH2)4- m.p. 170-172C
6 CH3 CH(C~I3)2 F -(CH2)3- m.p. 164-166C
7 CH3 CH(CH3)2 F F¦ CF3 m.p. 133-136C
8 CH3 CH3 H H¦ CF3 m.p. 167-168C
9 CHF2 CH(CH3)2 F -(CH2)4- m.p. 142-145C
CHF2 CH(CH3)2 F H ¦ CH3 m.p. 146- 148C
Example 11 A solution of 30.5 g of 2-cyano-5-[3,6-dihydro-3,4-dimethyl-2,6-
dioxo-1(2H)-pyrimidinyl]-4-fluorobenzoic acid isopropyl ester (see Example 1) in 150 ml
of methanol is maintained at 1-3C for 15 minutes with 4.0 g of sodium hydroxide in 5û
ml of water and then stirred for 23 hours. I'he solution is substantially concentrated by
evaporation under reduced pressure and the residue is adjusted to a pH value of 2 with 2N
- 27 ;~
hydrochloric acid. The resulting precipitate is filtered off with suction and subsequently
washed with n-hexane to yield 2-cyano-5-[3,6-dihydro-3,4-
dimethyl-2,6-dioxo-1(2H)-pyrimidinyl]-4-~luorobenzoic acid, m.p. 232C (with
decomposition).
Example 12 A solution of 2-cyano-5-[3,6-dihydro-3,4-dimethyl-2,6-dioxo-1~2H)-
pyrimidinyl~-4-fluorobenzoic acid ~see Example 9) in 30 ml of absolute
dimethylformamide is stirred for 2 hours at room temperature with 0.29 g of a 55%
sodium hydride dispersion. A solution of 1.1 g of propargyl bromide in 10 ml of absolute
dimethylformamide is then added dropwise over a period of 10 minutes and the reaction
mixture is subsequently stirred for 3 hours.
The mixture is then dissolved in 100 ml of ethyl acetate, the solution is washed thoroughly
with water, and the organic phase is dried over anhydrous sodium sulfate and concentrated
to dryness by evaporation under reduced pressure. The residue is purified by
chromatography on a silica gel column using n-hexane/ethyl acetate (3:7) as eluant to
yield 2-cyano-S - [3 ,6-dihydro-3 ,4-dimethyl-2,6-dioxo- 1 (2H) -pyrimidinyl]-4-fluorobenzoic
acid propargyl ester, m.p. 174-177C.
Examples 13-15 Analogously to the process describèd in Exarnple 12, the compounds of
formula I listed in the following Table 2 are obtained by esterifying 2-cyano-5-[3,6-
dihydro-3,4-dimethyl-2,6-dioxo-1(2~)-pyrimidinyl]-4-fluorobenzoic acid (see
Example 11) with allyl bromide, methyl iodide and 2-methoxyethyl bromide, respectively:
CH3 ~N~O
~ N~ COOR2
2~5F~
.
- 28 -
Table 2
Example R2 Physical
data
13 allyl m.p. 143-148C
14 methyl m.p. 244-246C
2-methoxyethyl m.p. 126-130C
Example 16 A mixture of 3.0 g of finely pulverised 2-cyano-
5-[3,6-dihydro-3,4-dimethyl-2,6-dioxo-1(2H)-pyrimidinyl]-4-fluorobenzoic acid, 30 ml of
freshly distilled thionyl chloride and 3 drops of dimethylfolrnamide is heated at reflux
temperature for 1 hour with stirring, after which a clear solution has fonned. The mixture
is then concentrated to dryness by evaporation to yield 2-cyano-5-[3,6-dihydro-
3,4-dimethyl-2,6-dioxo-1(2H)-pyrimidinyl]-4-fluorobenzoic acid chloride, m.p. 160C,
which is used, unpurified, as the starting material in the next reaction step.
A solution of l.S g of 1,2,3,6-tetrahydrobenzyl alcohol and 0.015 g of
4-dimethylaminopyIidine in 2.5 g of pyridine is added dropwise, at room temperature, to a
solution of 3.8 g of 2-cyano-5-[3,6-dihydro-3,4-dimethyl-2,6-dioxo-1(2H)-pyrimidinyl]-
4-fluorobenzoic acid chloride in 130 ml of tetrahydrofuran. After heating the reaction
mixture for 12 hours at 60C, the resulting suspension is substantially concenlrated by
evaporation under reduced pressure and the residue is dissolved in ethyl acetate.
The organic solution is washed thoroughly with water, the organic phase is dried over
anhydrous sodium sulfate and concentrated to dryness by evaporation under reduced
pressure, and the residue is puri~1ed by chromatography on a silica gel column using
n-hexane/ethyl acetate (3:7) as eluant to yield 2-cyano-5-[3,6-dihydro-3,4-dimethyl-2,6-
dioxo-1(2H)-pyrimidinyll-4-fluorobenzoic acid 1,2,3,6-tetrahydrobenzyl ester, mass
spectrum (m/e): 397(15)M~.
Biolo~ical Fxamples
Example B 1: Preemer~ence herbicidal action
Immediately after sowing the test plants (a number of weeds, both monocotyledonous and
- ' . :
'
:
- 29 -
dicotyledonous) in seed trays in the greenhouse, an aqueous spray mixture, colTesponding
to a rate of application of 3 kg of active ingredient~ectare, is used to treat the soil surface.
The test compounds are preferably formulated as emulsifiable concen~ates (EC) and
diluted with water to the desired concentration immed;ately before application. Insoluble
compounds are forrnulated as wettable powders (WP) using kaolin as inert carrier. The
wettable powder is suspended in water immediately before application.
The concentrations of active ingredient in g/ha refer to the soil surface in the containers
unless indicated otherwise. The spraying volume is lOOO l/ha (coIresponding to
100 mVm2).
The plant seeds are sown in plastics plant pots of various sizes containing heat-sterilised
(steam-treated) soil (agricul~lral soil 2.6% peat,20% clay,30% silt,47% sand). The plants
are kept in the greenhouse at average tenmperature (17 - 25C in winter,18 - 35C in
summer) (humidity 30 - 90%). The length of the photoperiod is 13 to 16 hours/day and i~,
if necessary, supplemented by artificial light (15000 to 180001ux). The artificial lighting
is also automatically activated if the intensity of the daylight is inadequate.
After 3 weeks the herbicidal action is evaluated by cQmparison with an untreated control
group using an eleven-stage linear evaluation scheme (necrosis, chlorosis, reduction,
deformation) (2 = 80-100% damage, l = 30-79% damage,0 - 0-29% damage)
Table Bl: preemer~ence herbicidal action:
Example S E A A C S A D M C
O ~ V L H T B A A A
R H E O E E U T T S
G I N P N L TU R S
-
01 2222 ~ 22222
02 22 ~ 2222222
03 2222222222
04 2222222222
0~ 2222222222
06 2222222222
07 2222222222
08 2222222222
- 30 -
09 2222222222
2222222222
12 2222222222
13 222222 ~ 222
14 2222222222
2222222222
- Key to the Table:
SORG Sorghum halopense
ECHI Echinochloa crus-galli
AVEN Avena fatua
ALOP Alopecurus myosuroides
CHEN Chenopodium album
STEL Stellaria media
ABUT Abutilon theophrasti
MATR MatricaTia chamomilla
CASS Cassia media
Example B2: Postemer ence helbicidal action (contact herbicide)
A number of weeds, both monocotyledonous and dicotyledonous, are treatèd
postemergence (at the 2- to 6-leaf stage) with an aqueous active ingredient dispersion at a
rate of 3 kg of active ingredient per hectare.
The test compounds are preferably formulated as emulsifiable concentrates ~EC) and
diluted with water to the desired concentration immediately before applica~ion. Insoluble
compounds are formulated as wettable powders (~1P) using kaolin as inert calTier. The
wettable powder is suspended in water immediately before application.
The concentrations of active ingredient in g~a refer to the soil surface in the containers
unless indicated otherwise. The spraying volume is 500 l~a.
The plant seeds are sown in plastics plant pots of vaTious sizes containing heat-sterilised
(steam-treated) soil ('Optima' soil 80% peat,20% loess). The plants are kept in the
greenhouse at average temperature (17 - 25C in winter, l8 - 35C in summer)
(atmospheric humidity 30 - 90%). The length of the photoperiod is 13 to 16 hours/day and
is, if neCeSSaTy, supplemented by ar~ficial light (150û0 to 180û0 lux). The artificial
lighting is also automatically activated if the intensity of the daylight is inadequate.
After 3 weeks the herbicidal action is evaluated by compaIison with an untreated control
group using an eleven-stage linear evaluation scheme (necrosis, chlorosis, reduction,
deformation) (2 = 80-100% damage, l = 30-79% damage,0 = 0-29% damage).
31 ~ r.~
Table B2: postemer~ence herbicidal action:
Example S E A A C S A D M C
O C V L H T B A A A
R H E O E E U T T S
G I N P N L T U E~ S
01 2211212222
02 2222222222
03 2222222222
04 2222222222
05 2221222222
06 2221222222
07 222 ~ 222222
08 2222222222
2222222222
13 2221212221
~. .
- 32-
Formulation examples of active substances of the formula I
(% = percent bY wei~ht)
1. Wettablepowder a) b) c)
Active subst. from Exarnples 1-15 20 % 50 % 0.5 %
Naligninsulfonate 5 % 5 % 5 %
Na lauryl sulfate 3 % - -
Na diisobutylnaphthalenesulfonate - 6 % 6 %
Octylphenol polyethylene glycol
ether (7-8 mol of EO) - 2 % 2 %
Highly-disperse silica 5 % 27 % 27 %
Kaolin 67 %
Sodium chloride - - 59.5 %
The active substance is thoroughly mixed with the additives and thoroughly ground in a
suitable mill. This gives wettable powders which can be diluted with water to give
suspensions of any desired concentration.
2.Emulsion concentrates a) b)
Active subst. from Exarnples 1-15 10 % 1 %
Ca dodecylbenzenesulfonate 3 % 3 %
Octylphenol polyethylene glycol ether
(4-5 mol of EO) 3 % 3 %
Castor oil polyethylene glycol ether
(36 mol of EO) 4 % 4 %
Cyclohexanone 30 % 10 %
Xylene mixture 50 % 79 %
Emulsions of any desired concentration can be prepared ~rom such concen~ates by
diluting thern with water.
3.Dusts a) b)
Active subst. from Examples 1-15 0.1 % 1 %
Talc 99-9 %
Kaolin 99 %
- 33 - ;2~
Ready-to-use dusts are obtained by intimately mixing the calTier with the active substance.
4. Extrudergranules a) b)
Active subst. from Examples 1-15 10 % 1 %
Na ligninsulfonate 2 % 2 %
Carboxymethylcellulose 1% 1%
Kaolin 87 % 96 %
The active substance is mixed with the additives, and ehe mixture is ground and moistened
with water. This mixture is extruded and subsequently dried in a stream of air.
5. Coated granules
Active subst. from Examples 1-15 3 %
Polyethylene glycol (MW 200) 3 %
Kaolin 94 %
The kaolin is moistened with polyethylene glycol and the fimely-gTound active substance
is applied uniformly thereto in a mixer. Dust-free coated granules are obtained in this
manner.
6. Suspensionconcentrate a)
Active subst. from Examples 1-15 5 % 40 %
Ethylene glycol 10 % 10 %
Nonylphenol polyethylene
glycol ether (15 mol of EO) 1 % 6 %
Na ligninsulfonate 5 % 10 %
Carboxymethylcellulose 1% 1%
37 % aqueous formaldehyde solution 0.2 % 0.2 %
Silicone oil in the form of a 75% aqueous
emulsion 0.8 % 0.8 %
Water - 77 % 3~%
The ~mely-ground active substance is mixed intimately with the additives. This gives a
suspension concentrate, from which suspensions of any desired concen~ation can be
prepared by dilu~ing it with water.
- 34 ~
.
7. Salt solution
Active subst. from Examples 1-15 5 %
Isopropylamine 1 %
Octylphenol polyethylene glycol ether
(78 mol of EO) 3 %
Water 91%
The compounds of the formula I are employed as such or preferably as compositions
together with the auxiliaries customary in formulation technology, and they are therefore
processed in a known manner to give, for example, emulsion concentrates, directly
sprayable or dilutable solutions, dilute emulsions, sprayable powders, soluble powders,
dusts, granules, and also encapsulations, for example in polymeric substances. The
application methods, such as spraying, atomising, dusting, scattering or pouring, as well as
the type of compositions are selected to suit the intended aims and the preYailing
circumstances.