Note: Descriptions are shown in the official language in which they were submitted.
CA 02050334 1999-06-07
1
The present invention relates to compounds which can be used
as contrast media for radiography.
lodobenzene compounds containing several iodine atoms in
the benzene ring, usually 3 iodine atoms per benzene ring, and various other
substituents have been used for a long time as contrast materials. These
other substituents are pharmacologically acceptable groups which enable the
compounds to be administered to man and animals. Generally speaking,
these substituents are chosen, on the one hand, in order to confer adequate
solubility in water on the compound so that they can be administered in
aqueous solution and, on the other, in order to confer on these compounds
a tolerance sufficient for their use in the human organism.
For this purpose, non-ionic structures have been suggested,
i.e. iodobenzene derivatives possessing non-ionic substituents.
Thus, in the patent FR-A-2 053 037, carbamoyl iodobenzene
compounds containing a total of at least one N-hydroxy alkyl group and at
least two hydroxy groups were proposed.
A compound illustrative of this class is metrizamide which has,
however, proved to be of limited stability.
In the European patent 357467 A1, non-ionic compounds were
suggested which are well tolerated by the human organism and which exhibit
good stability in aqueous solution.
These compounds correspond to the formula:
R3
CON -R2
0
~R~
CO
~H ~ CH20H
CH20H
CA 02050334 1999-06-07
2
in which
R, is a group of formula
-N-CO-RS
Rs
RS being selected from C -C, ,~Ikyl, C -C , hydroxyalkyl or C -C ,
polyhydroxyalkyl,
Rs being selected from hydrogen, C,-C4 alkyl, C,-C4 hydroxyalkyl or C,-C4
polyhydroxyalkyl)
or a group of formula
R8
-CO-N-R~
R, being selected from C,-C4 hydroxyalkyl or C,-C4 polyhydroxyalkyl,
R8 being selected from hydrogen or C,-C4 alkyl,
R2 is selected from hydrogen, C,-C4 hydroxyalkyl or C,-C,, polyhydroxyalkyl,
R3 is selected from hydrogen or C,-C4 alkyl, and
R4 is selected from hydrogen, C,-C4 alkyl, C,-C4 hydroxyalkyl or C,-C4
polyhydroxyalkyl.
The aim of the present invention is to provide novel compounds
belonging to the family of the compounds of formula I, which are
characterized by both good stability and good solubility in aqueous media.
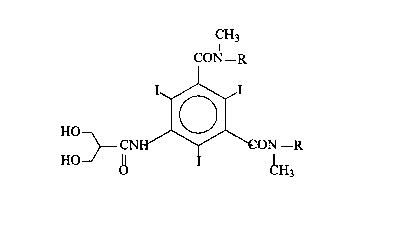
The compounds according to the invention have the following
general formula:
CH3
CON-R
HO
-R
HO~
V c:H3
in which R is selected from 2,3-dihydroxypropyl or 1,3-dihydroxy-2-propyl.
CA 02050334 1999-06-07
3
One of the compounds according to the invention is 5-[3-
hydroxy-2-(hydroxymethyl)-propionamido]-N,N'-dimethyl-N,N'-bis-(2,3-
dihydroxypropyl)-2,4,6-triiodo-isophthalamide) i.e. the compound of formula:
CH3
CON
-,H OH
(A)
HO
~- OH OH
HO~
a c:H3
The other compound according to the invention is 5-[3-hydroxy-
2-(hydroxymethyl)-propionamido]-N,N'-dimethyl-N,N'-bis-(1,3-dihydroxy-2-
propyl)-2,4,6-triiodoisophthalamide corresponding to the formula below:
CH3 OH
CON
OH
(B)
HO OH
C ~ON
HO ~H3 OH
These formula cover not only the racemates but also all of the
stereoisomers associated with the presence of asymmetric carbon and the
prevention of rotation of certain bonds owing to the steric hindrance
contributed by the iodine atoms.
Unexpectedly, the compound of formula A is characterized by
a better solubility in aqueous medium than the compound of example 1 of the
European patent application mentioned above, namely the 5-[3-hydroxy-2-
(hydroxymethyl)-N-(2,3-dihydroxypropyl)propionamido]-N',N"-bis-(2-hydroxy-
ethyl)-2,4,6-triiodo-isophthalamide.
CA 02050334 1999-06-07
4
Furthermore, the compounds according to the invention
possess the advantage, compared with the compound of example 1 of the
patent application mentioned above, of being more easily accessible in that
they do not require a final N-alkylation step which poses problems of
purification at the industrial scale.
The compounds according to the invention may be obtained
according to a procedure consisting of:
a) reacting a diacyl chloride of formula:
COCI
R'-
U
(II)
R' designating a -CH-(CH20H)2 group, the hydroxy groups of which are
p rotected ,
with an amine selected from 1-(N-methylamino) propane-2,3-diol and 2-(N-
methylamino) propane-1,3-diol, so as to produce a compound of formula:
CH3
CON-R
(III)
R'- -R
U C:H3
and b) removing the protecting groups from the R' group.
The compounds of formula II are described in FR-A-2 632 304.
The present invention relates also to contrast media which
contain at least one of the compounds according to the invention.
CA 02050334 1999-06-07
These contrast media are used in man and animals for
radiological purposes.
The preferred pharmaceutical form of the contrast media
according to the invention consists of aqueous solutions of the compounds.
The aqueous solutions usually contain a total of 5 to 100 g of
5 compound according to the invention per 100 ml and the injectable volume
of such solutions usually varies from 1 to 1000 ml.
The aqueous solutions of the compounds according to the
invention may also contain certain additives such as:
- sodium chloride at concentrations between 0.1 and 10 mM
- disodium EDTA at concentrations between 0.1 and 2 mM
- sodium citrate at concentrations between 0.1 and 10 mM
- heparin at doses between 10 and 100 units per 100 ml of
solution.
These compounds may be administered by all routes
conventionally used for iodinated non-ionic contrast materials. Thus they
may be administered by the enteral or parenteral route (intravenous or intra-
arterial route, opacification of the cavities) and in particular into the
subarachnoid space.
An example of the composition according to the present
invention will be given below.
Composition
Compound A according to the invention 65 g
Water for injectable preparation
QS 100 ml
The following examples illustrate the preparation of the
compounds according to the invention.
EXAMPLE 1
Preparation of 5-[3-hydrox<r-2-(hyd_roy methyly-I r~opionamidol~
N.N'-dimethyl-N.N'-bis-( .2 3dihyrdrox~pyly-2.x,6-triiodoisophthalamide.
CA 02050334 1999-06-07
6
a) Preparation of [2-isopropyl-1,3-dioxane-5-carboxamido]-N,N'-dimethyl-
N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide.
74 g (98 mmoles) of 5-[2-isopropyl-1,3-dioxane-5-
carboxamido]-2,4,6-triiodo-isophthaloyl dichloride are suspended in 300 ml
of isopropanol containing 41 ml (294 mmoles) of triethylamine. 31 g (295
mmoles) of N-methyl-aminopropane-2,3-diol are added dropwise. Stirring is
maintained for 12 h at room temperature. The triethylamine hydrochloride
is removed by filtration.
The filtrate is evaporated to dryness, the residue is taken up in
water and eluted from OH resin IRA 67T"".
After evaporation, the product is purified by passage through
silanized silica (KieseIgeIT"' 60 Merck) with water as eluant.
After evaporation to dryness, 60 g of a white powder are
recovered in a yield of 68.5%.
Iodine determination: 96.4%
HPLC Ruritv: 97% HypersilT"" CB 25cm 5 ,um
NaH2P04 0.01 M 60
Methanol 40
TLC Si02, Rf. 0.12
0.25
0.30 0.36
Eluant CHC23 55, MeOH 30, NH3H20 10
NMR (DMSO)'H 200 MHz
0.9 ppm (doublet) CH3 (6H); 2.8 ppm (singlet) N-CH3 (3H); 3 ppm (singlet) N
CH3 (3H); 3.3 ppm (multiplet) N-CHZ and CH (5H); 3.5-3.9 ppm (multiplet)
CHZ and CH (8H); 4.3 ppm (quadruplet) CH (3H); 4.6 ppm (triplet) OH (2H);
4.7 ppm (doublet) OH (2H); 10.2 ppm (enlarged multiplet) NH (1 H).
b) Preparation of 5-[3-hydroxy-2-(hydroxymethyl)-
propionamido]-N,N'-dimethyl-N,N'-bis-(2;3-dihydroxypropyl)-2,4,6-
triiodoisophthalamide.
CA 02050334 1999-06-07
7
45 g (50.6 mmole) of the compound described in a) are
dissolved in 101 ml (10 eq.) of 5 N hydrochloric acid. Stirring is maintained
for 12 h at room temperature. The solution is filtered and evaporated to
dryness. The solid obtained is taken up in 100 ml of ethyl ether, then
filtered
off and eluted from silanized silica (KieseIgeITM 60 Merck) with water. After
evaporation to dryness, 38 g of a white powder are recovered.
Yield = 90%
Iodine determination: 98.3%
HPLC uritv 98% HypersilTM C8 25 cm 5 ,u
NaH2P04 0.01 M 95
MeOH 5
TLC Si02 Rf 0.56
0.63
0.67
Eluant CHCQ3 55, MeOH 30, NH3H20 10
NMR (DMSO)'H 200 MHz
2.7 pm (multiplet) CH (1 H); 2.85 ppm (enlarged singlet) N-CH3 (3H);
3.08 (poorly resolved doublet) N-CH3 (3H); 3.10-3.35 ppm (multiplet) N-CH2;
3.45 ppm (quadruplet) CH2 (4H); 3.6-4 ppm (multiplet) OH (6H); 9.9 ppm
(multiplet) NH (1 H).
EXAMPLE 2
Preaaration of 5-[3-hyrdroxVr-2-(hyrdrox\rmethyrl)-~roaionamido]~
N.N'-dimethyrl-N.N'-bis-(1.3-dihydroxy-2-propyll-2.4.6-triiodois~~hthalamide).
a) Preparation of (2-isopropyl-1,3-dioxane-6-carboxamido)
N,N'-dimethyl-N,N'-bis-(1,3-dihydroxy-2-propyl)-2,4,6-triiodoisophthalamide.
1.46 g (13.9 mmole) of N-methyl-aminopropane-1,3-diol
(synthesized according to the European patent application EP-A-025 083
filed by EPROVA A.G.) is dissolved in a mixture composed of 20 ml of N,N-
dimethylacetamide and 2 ml (114.4 mmole) of triethylamine. 3.5 g
(4.65 mmole of 5-(2-isopropyl-1,3-dioxane-5-carboxamido)-2,4-6-triiodoi-
CA 02050334 1999-06-07
sophthaloyl dichloride are added in portions. Stirring is maintained for
3 hours at 30°C, then for 12 hours at room temperature.
The triethylamine hydrochloride is removed by filtration.
The filtrate is evaporated to dryness and the residue is
crystallized in a mixture composed of 20 ml of isopropanol and 200 ml of
ethyl ether.
After filtration and drying, 3.6 g of a white powder are
recovered in a yield of 87%.
I~GSi02Rf0.16
0.28
Eluant toluene 60 - methyl-ethyl-ketone 35 - formic acid 25.
~ (DMSO)'H : 200 MHz.
0.9 ppm (doublet) CH3 (6H); 1.7 ppm (multiplet) CH (1 H); 2.7 ppm (singlet)
N-CH3 (3H) Z isomer; 2.99 ppm (singlet) N-CH3 (3H) E isomer; 3.4-4 ppm
(multiplet) CH, CH2 (15H); 4.8 ppm (multiplet) CH ~H (4H); 10.2 ppm
(multiplet) NH (1 H).
b) Preparation of
5-[3-hydroxy-2-(hydroxylmethyl-propionamido]N,N'-dimethyl-N,N'-bis-(1,3-
dihydroxy-2-propyl)-2,4,6-triiodoisophthalamide.
3 g (3.4 mmole) of the compound described in a) are dissolved
in 10 ml (15 eq.) of 5N hydrochloric acid. Stirring is maintained for 3 hours
at 45°C, then for 12 hours at room temperature. The solution is
evaporated
to dryness. The residue obtained is taken up in 50 ml of ethyl ether, filtered
off, then dissolved in 50 ml of water before being eluted from a HT"" resin
(IRN 77)TMand a OH resin (IRA 68)T"".
After evaporation to dryness, 1.7 g of a white powder are
recovered in a yield of 60.7%.
Iodine purity: 98.4%
~ Si02 Rf 0.26
0.33
0.45
CA 02050334 1999-06-07
9
Eluant butanol 50, water 25, acetic acid 11.
NMR (DMSO)'H 200 MHz.
2.7 ppm (enlarged singlet) N-CH3 (3H), Z isomer; 3 ppm (enlarged singlet)
N-CH3 (3H), E isomer; 3.3-4.05 ppm (poorly resolved multiplet) CH, C,~-120H
(15H); 4.45 ppm (poorly resolved multiplet) CH20,~ (6H); 9.8 ppm (multiplet)
NH (1 H).
NMR (DMSO)'3 C 200 MHz.
170.6 ppm (2 C); 172 ppm (1 C); 149 ppm (C~-C);
O O O
145 ppm (CA,-NH); 100-98-90 ppm (CAS I); 61-57-52 ppm (C-H); 59.1-59.9
ppm (CH2-OH); 32.8-30.1 ppm (CH3-N).