Note: Descriptions are shown in the official language in which they were submitted.
~051438
Emulgator-free liquid emulsion and method and device for
producing the emulsion
The present invention relates to a preparation method and
device for stably mixing a plurality of substances differing in
the physical properties, especially a liquid and a liquid, and
a mixture emulsion formed thereby.
Various proposals have heretofore been made on the method and
apparatus for mixing a plurality of substances differing in the
physical properties. For example, many methods have been
proposed as the means for obtaining mixtures of a hydrophobic
liquid and water. However, according to these methods, stable
liquid mixtures of water and a hydrophobic liquid are obtained
by using an emulsifier.
Use of an emulsifier results in increase of the cost and when
the water/hydrophobic liquid is used, for examplel for
cosmetics or the like, several limi.tations are imposed in view
of influences on the human body.
It is known that various gases can be dissolved in various
liquids and an optional specific gas is always present in a
unit volume of water in a defined maximum amount, that is, a
saturation amount, under optional predetermined temperature and
pressure conditions. In connection with oxygen, saturation
amounts in octane which is a hydrophobic liquid and methyl
alcohol which is a hydrophilic liquid are shown on page 164 of
Basic Volume 2 of ~Handbook of Chemistry~ (revised 3rd edition,
June 25, 1984) published by Maruzen-Sha.
It is a primary object of the present invention to provide a
mixing method for stably mixing substances differing in the
physical properties, i.e. a hydrophobic liquid and a
hydrophilic liquid.
Another object of the present invention is to provide a mixing
2 2(~15~3~3
device for use in carrying out this mixing method.
Still another object of the present invention ls to provide a
stable mixture emulsion of at least one hydrophobic liquid
phase and at least one hydrophilic liquid phase and prepared or
preparable by this mixing apparatus.
The emulgator-free emulsion, the method and the mixing device
in accordance with the present invention have especially the
features as described in the claims.
In accordance with the present invention, there is provided a
colloidal emulgator-free emulsion comprising a hydrophilic
liquid, especially water, and a hydrophobic liquid, wherein
fine liquid drops of the disperse liquid phase are
homogeneously and stably distributed in the emulsion without
the aid of an emulsifier. In the present invention, the
preferred composition of the water/hydrophobic liquid mixture
is such that the amount of the hydrophobic liquid is up to 20%
by volume based on water.
Furthermore, in accordance with the present invention, there is
provided a mixture comprising water and a hydrophobic liquid,
wherein fine liquid drops of the water are homogeneously and
stably distributed in the hydrophobic liquid without the aid of
an emulsifier. In the present invention, the preferred
composition of the water/hydrophobic liquid mixture is such
that the amount of the water is 5-35% by volume based on the
hydrophobic liquid.
Moreover, in accordance with the present invention, there is
provide a colloidal mixture comprising a hydrophilic liquid and
a hydrophobic liquid, wherein fine liquid drops are
homogeneously and stably distributed without the aid of an
emulsifier. In the present invention, the preferred composition
of the hydrophilic liquid and the hydrophobic liquid is such
that the amount of the hydrophobic liquid is up to 20% by
volume based on the hydrophilic liquid.
~51~3~
The term "colloid" or ~colloidal~ state means a state in which
colloidal particles having a size of about 1,000 nm or less are
contained, the existence of the colloidal particles and the
occurrence of a Brownian movement are confirmed by an
ultramicroscope, and a Tyndall phenomenon is observed.
The present invention will now be described in detail with
reference to preferred embodiments illustrated in the
accompanying drawings.
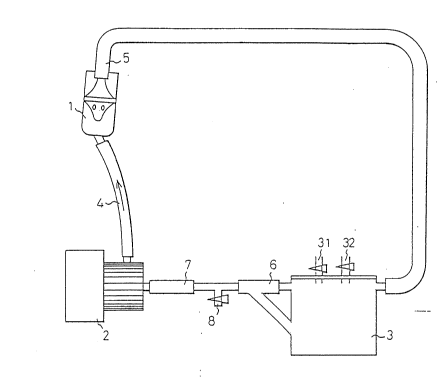
Fig. 1 is a diagram illustrating a general layout of a
preferred first embodiment of the mixing device comprising a
lS mixing apparatus used in the present invention.
Fig. 2 is an elevation sectional view of the mixing apparatus 1
shown in Fig. 1.
Fig. 3 is a cross sectional view of the top portion of the
mixing apparatus 1 shown in Fig. 1.
Fig. 4 is a diagram illustrating a second embodiment of the
mixing device used in the present invention.
Fig. 5 is a diagram illustrating a third embodiment of the
mixing device used in the present invention .
Fig. 6 is a diagram illustrating the structure of an example of
the cylindrical housing defining the hollow interior space of
the jet pump 50 of the mixing device shown in Fig. 5.
Fig. 7 is a diagram illustrating a mixing device resembling the
mixing apparatus shown in Fig. 4.
Fig. 8 is a diagram illustrating an example of the apparatus
used for measuring the stability of a mixture containing a
- saturation amount of a gas in a hydrophobic liquid or
.2(~5~3E3
hydrophilic liquid.
A general device according to the first embodiment of the
device for use in carrying out the present invention is shown
in Fig. l. The elevation of a mixing apparatus 1 is shown in
Fig. 2 and the top section of the mixing apparatus l in the
plane of a hole of the mixing apparatus l is shown in Fig. 3.
At first, the mixing apparatus 1 is described with reference to
Figs. 2 and 3.
The mixing apparatus 1 comprises a cylindrical wall which is
opened at one end 10 and closed at the opposite end 11. Within
this cylinder, there is defined a hollow element 13 comprising
a first portion 14 having a circular rim 12 connected to the
central part thereof and a second portion 17. The first portion
14 of this element 13 has a shape of a substantially hollow
parabolic surface arranged in a closed chamber formed between
the rim 12 and the closed end 11 of the mixing apparatus 1. In
a preferred embodiment, the first portion 14 has a shape of a
completely hollow parabolic surface. A certain number of holes
15 are formed through wall of the element 13 at the height
(about 1/3 in the embodiment) of the upper position of the
first portion 14. In a preferred embodiment, the holes 15 are
formed in the tangential direction. A duct 16 is extended from
the closed end 11 of the mixing apparatus l slightly inclinedly
with respect to the axis of the mixing apparatus. The element
13 includes the second portion 17 communicating with the first
portion 14 at the plane of the rim 12, and this portion has a
substantially tapered shape which is continuous as a short
cylindrical duct 18. In a preferred embodiment, the portion 17
has a shape of a complete hyperbola. The mixing apparatus l is
formed of glass. A fluid component is caused to flow to the
first portion 14 through the inclined duct 16 while being
rotated, and in the first portion 14, the majority of the fluid
component introduced into the holes 15 is caused to flow along
the inner wall of the fist portion 14 toward the top (downward
in Fig. 2) while being further rotated. At the top, the flow is
reflected and the flow speed is gradually increased, and a
.~:~5~
vortex state is formed on the central axis toward the second
portion. Different substances are mixed by this vortex state.
In use a liquid mixture of a hydrophobic liquid phase and an
hydrophilic liquid phase is introduced through the inlet duct
16. Owing to the off-axis and oblique arrangement of the inlet
duct 16 the inflowing fluid will rotate around the central axis
of the mixing apparatus 1 and the rotating fluid enters in
through the tangential openings 15 in the wall 14 of the first
chamber portion. In the first chamber portion which has a
rotational paraboloid shape of nth grade, a focal line is
formed where pressure is at minimum, and along the axis the
pressure decreases in axial direction towards the outlet
opening. This means that pressure is decreasing gradually both
in radial and axial direction, and the fluid rotates around the
axis and flows in axial direction.
In the second chamber portion, in the tapering section 17, the
speed of flow and the velocity of rotation increases gradually
toward the outlet duct portion 13 and in radial direction the
pressure decreases towards the axis and a minimum is
experienced along the axis. It is preferred if the rotational
hyperboloid function has thè same grade as the paraboloid
function of the first chamber portion has.
The flow will have a structure which can be visualized in such
a way as if the fluid mass consisted of an infinitely high
numbers of annular hollow tubes having a form substantially
following that of the tapering section 17, and the speed of
rotation was different in case of each tube so that the
elementary tubes were sliding on each other during their
rotational movements. Moreover, the elementary tubes slide with
respect to each other not only due to their differing speeds of
rotation but they are moving and sliding in axial direction as
well.
From this flow pictu~e it will be clear that the imaginary
contacting surface of phase boundaries will be extremely large
6 2~
and owing to the imaginary shearing effects between the
elementary tubes, very effective contacts will be formed
between differing components of the mixture. While the pressure
minimum lies in the central axis, the components with lower
S specific mass will tend to collect at the axis i.n the vicinity
of which the speed is at maximum. This ensures that tiny
particles cannot escape from getting in the active zones.
The flow rate should be adjusted in such a way that phase
transition (i.e. vaporization of any component) do not take
place, nevertheless the minimum pressure should be just above
the vapour pressure of the liquid mixture. As several liquid
components are present, this condition should relate to the one
which has the highest vapor pressure at the given temperature.
This condition is equivalent to the statement that cavitation
cannot occur in the flow. The mixing apparatus being preferably
of glass, said condition can be adjusted by increasing the flow
velocity up to a measure at which fine gas bubbles appear in
the duct portion 18, and then lowering the flow velocitity by a
small amount just until the gas bubbles dissappear again.
The first embodiment will now be described with reference to
Fig. 1. In the mixing apparatus 1, a closed circulation passage
comprising a pump 2 and a vessel 3, which are connected to each
other through conduits 4, 5, 6 and 7, is formed. Reference
numeral 8 represents a withdrawal opening for the withdrawal of
a mixture, which has a cock. The opening is always closed
except at the time of the withdrawal of the mixture. The vessel
3 has a cock-provided duct 31 and a cock-provided duct 32 for
charging starting materials to be formed into a mixture. The
fluid flows as indicated by arrows.
How a stable water/hydrophobic liquid mixture is prepared by
introducing a hydrophobic liguid into water without the aid of
an emulsifier by using the device shown in Figs. 1 through 3
will now be described.
At first, the cock of the duct 31 is opened and 9 1 of
7 ~s~
distilled water is filled in the vessel 3. Then, the cock of
the duct 3~ is opened and 1 l of a vitamin A oil as the
starting oil is filled in the vessel 3 and the cocks of the
ducts 31 and 32 are closed. Incidentally, the vessel 3 is fully
filled with water and vitamin A oil, or the upper portion of
the vessel 3 may be vacant.
In this arrangement, the pump 2 is started. This pump has a
flow quantity of 25 l/min. The inner diameters of the conduits
10 4, 5, 6 and 7 are equally about 14 mm. The flow direction is
indicated by arrows in Fig. l. Water and vitamin A oil are
introduced into the mixing apparatus 1, and in the mixing
apparatus 1, water and vitamin A oil flow into the interior of
the cylindrical wall from the inclined duct 16 while being
rotated and flow in the first portion 14 through the tangential
holes 15 to form a vortex in the hollow element 13. This will
now be described in detail. The majority of the rotating fluid
component first flows to the closed top of the paraboloid and
is reflected forward therefrom, and because of the
exponentially tapered shape of the second portion 17 of the
hollow element 13, the fluid component is promptly rotated
together with the other component and the fluid component is
advanced in the conduit 5 toward the vessel 3. Thus, the fluid
component is circulated in the closed system until the pump 2
is turned off. After the flow of the mixture of water and
vitamin A oil stops, the cock is opened and the water/vitamin A
oil mixture is withdrawn from the withdrawal opening 8.
An example in which a hydrophilic liquid is mixed with a
hydrophobic liquid by using the mixing device of the embodiment
shown in Fig. 1 will now be described. The cock of the duct 31
is opened, and the vessel 3 is filled with 9 l of ethyl
alcohol, and then the cock of the duct 32 is opened and 1 l of
of a vitamin oil is filled in the vessel 3. The subsequent
procedures are the same as described above. Furthermore, a
mixture of 9 l of a vitamin A oil and 1 l of ethyl alcohol is
similarly prepared according to the above-mentioned method. The
obtained mixture formed by mixing ethyl alcohol and vitamin A
8 2(~5~
oil without the aid of an emulsified according to the
above-mentioned method can be widely used for cosmetic lotions
and cosmetic creams.
An example of the second embodiment of the present invention
will now be described in detail with reference to Fig. 4. The
same members as in the first embodiment are indicated by the
same reference numerals. The second embodiment is different
from the first embodiment mainly in that a vessel 9 for forming
a second vortex is used instead of the mixing apparatus 3. The
vessel 9 has a substantially spherical upper part 91, a lower
part 93 tapered downwardly and an intermediate part 92
connected smoothly to the spherical upper part 91 and the lower
part 93. The upper part 91 and intermediate part 92 have a
convex face and the lower part 93 has a concave face. Thus, an
inflection face is formed between the intermediate part 92 and
the lower part 93. In a preferred embodiment, the vessel 9 is
formed of glass so that the process occurring in the vessel 9
can ~e observed. Three ducts 95, 96 and 97 are formed on the
top wall of the upper part 91 and they are sealed. The vessel 9
is filled with starting substances.
The vessel 9 further has two openings. At a substantial height
where the vessel has a maximum diameter, a duct 98 extends
obliquely from the upper portion of the intermediate 92. The
duct 98 forms an acute angle to each of equator and tangent
planes of the vessel 9 and the axis of the duct 98 is slightly
inclined inwardly and upwardly in the interior direction of the
vessel 9. In general, these angles are smaller than 30. The
second opening is the end of the open bottom of the lower part
93 of the vessel 9. A circulation passage comprising the pump
2, the mixing apparatus 1 and four conduits 4, 5, 6' and 7 is
arranged between the lower part 93 and the inclined duct 98.
The second embodiment will now be described with reference to
Fig. 4. In the mixing apparatus 1, a closed circulation passage
is formed through the pump 2, the vessel 9, withdrawal opening
8 and the conduits 4, 5, 6' and 7.
9 ~5~L~3Z 3
How a water/hydrophobic liquid can be prepared without the aid
of an emulsifier by using the device shown in Fig. 4 is
described. At first, 9.5 1 of distilled water is filled in the
vessel 9 through the duct 97. Then, 0.5 1 of squalane is filled
in the vessel 9 through the duct 95. The ducts 95 and 97 are
sealed. Incidentally, the vessel 9 may be completely filled
with water and squalane, or the upper part of the vessel 9 may
be left vacant. In this arrangement, the pump 2 is started. The
flow direction is indicated by arrows in Fig. 4. When water and
squalane are introduced into the mixing apparatus 1, they flow
through the tangential holes 15 to form a first vortex in the
hollow element 13, and this first vortex is formed in the same
manner as described above. Thus, water and squalane flow into
the vessel 9 in the tangential direction throught the inclined
inlet duct 98.
Water and squalane which have been quiet in the vessel 9 begin
to turn, and a second vortex is formed. A certain time (about 1
to about 2 minutes) is necessary for attaining a stationary
state in the vortex. The rotation number of the vortex at the
topmost part and maximum diameter part is about 50 r.p.m., and
the rotation number increases substantiarly exponentially
toward the lower part. Thus, the mixture of water and squalane
is circulated in the closed system until the pump 2 is turned
off. After the flow of the mixture stops, the cock is opened
and the water/squalene mixture is withdrawn from the withdrawal
opening 8.
An example of the first embodiment of the present invention
will now be described with reference to Figs. 5 and 6. The same
members as in the first embodiment are represented by the same
reference numerals. The third embodiment is different from the
first embodiment mainly in that a jet pump 50 connected to the
mixing apparatus 1 is arranged in the closed system.
In this embodiment, a vessel 30 compxising a cover and ducts 31
and 32 is used. The vessel 30 is filled with starting
2(:115~
substances. The vessel 30 is connected to the pump 2 through
the conduit 6~, withdrawal opening 8 and conduit 7 located at
the lower portion of the vessel 30. The flowout conduit 4 is
connected to the inlet of the jet pump 50. The internal
structure of the jet pump 50 is shown in Fig. 6. An inlet duct
54 of the jet pump 50 communica~es with the vessel 30 through a
duct 51. The jet pump 50 exerts a function of promoting the
mixing of two different liquids. The jet pump 50 has a
substantially cylindrical housing 52 having a hollow internal
space, as shown in Fig. 6. A nozzle 53 is inserted in the
hollow internal space of the housing 52 and the top end of the
no~zle 52 is connected to the duct 4. A cylindrical space is
formed in the vlncinity of the top end of the nozzle 53 and an
inlet duct 54 is inserted in the wall of the housing 52, and as
the result, the hollow internal space of the inlet duct 54
communicates with the cylindrical space in the vincinity of the
top end of the nozzle 53. In this example, the water jet pump
is used, but there may be adoped a method in which a branched
pipe is used instead of the water jet pump and the outlet side
of this tube is connected to the inlet side of the mixing
apparatus 1.
It will now be described how a stable hydrophilic
liquid/hydrophobic mixture is prepared by introducing a
hydrophobic liquid into a hydrophilic liquid without the aid of
emulsifier by using the device shown in Figs. 5 and 6.
At first, the cock of the duct 31 is opened and the vessel 31
is filled with 9.5 1 of ethyl alcohol. Then, the cock of the
30 duct 32 is opened and 0.5 1 of squalane as the starting oil is
filled in the vessel 30. The cock of the ducts 31 and 32 are
closed. When the pump 2 is started, a squalane-rich liquid in
the vessel 30 is sucked into the pump 2 and is caused to flow
in the vessel 30 through the conduit 4, jet pump 50, mixing
apparatus 1 and conduit 5. Thus, a closed system is formed.
Furthermore, an alcohol-rich liquid in the vessel 30 is
injected into the jet pump 50 through the conduit 51. In this
closed system, ethyl alcohol and squalane are mixed by the
11 2~5~
circulation~ In this manner, a mixture of ethyl alcohol and
squalane is formed.
As is apparent from the foregoing description, if the mixing
apparatus 1 is used, a plurality of substances can be mixed
irrespectively of the composition thereof.
Furthermore, if this mixing apparatus l is used, at least two
kinds of liquids can be stably and homogeneously mixed.
Fig. 7 show a device resembling the device shown in Fig. 4. The
pump 2 and mixing apparatus 1 are those used in the first
embodiment, and the vessel 9 is that used in the second
embodiment. An oxygen-supplying gas source 60 and an injector
lS ~with no needle) 61 are used. The gas source 60, injector 61
and duct 97 are connected to conduits 68 (68a, 68b and 68c)
provided with glass cocks 62, 63 and 6~, respectively. The duct
95 is connected to a conduit 65, the end of the conduit 65 is
contained in a trap 66 filled with water, and the trap 66 is
connected to a glass cock 67.
At first, a duct 96 is opened and 10 l of octane as the
hydrophobic liquid is filled in the vessel 9, and the duct 96
is closed.
The glass cock 62 and 64 are opened and a pressing member
(piston) 61a of the injector 61 is taken out from the injector
61. For a while, the glass cock 63 is opened to inject oxygen
into the circulation passage for expelling air present in the
interior. The pressing member is set at the injector 6 so that
the measurement scale is 200 ml. After the glass cock 63 is
closed, the level of water in the trap 66 is made in agreement
with the water level in the conduit 65, and the glass cock 67
is closed. When the pump 2 is started, the pressure in the
vessel 9 is reduced, and a reduced pressure is also maintained
in the conduit 65 and the water level in the conduit becomes
higher than the water level in the trap 66. The pump 2 is
stopped twice a day, and oxygen in the injector 61 is pressed
.2~15~ td~
12
into the circulation passage through the pressing member 61a so
that the water level in the trap 66 is made equal to the water
level in the conduit 65. When oxygen in the injector 61 is
exhausted, in the same manner as described above, the glass
cock 63 is opened and the gas source 60 is connected to the
injector and oxygen is injected into the injector 61. A series
of experiments were carried out in the above-mentioned manner.
The intake of oxygen gas for 28 days' operation was 7440
ml/lO l (21C). This value corresponds to about 2.4 times the
saturation solubility.
After the termination of the experiment, the pump 2 was stopped
and the system was allowed to stand still. After 12 hours'
stoppage of the pump, the water level in the trap 66 was
measured, but no change was observed. Accordingly, it can be
said that octane containing an excessive amount of oxygen was
stable.
Then, the experiment was similarly carried out ,by using 100 ml
of methyl alcohol as the hydrophilic liquid instead of octane.
The intake of oxygen gas for 28 hours' operation was 6640
ml/10 l (21C). This value corresponds to about 2.8 times the
saturation solubility.
After the termination of the experiment, the pump 2 was stopped
and the system was allowed to stand still. After 12 hours'
stoppage of the pump 2, the water level of the trap 66 was
measured. No change was observed. Accordingly, it can be said
that methyl alcohol containing the excessive amount of oxygen
was very small. In the present example, the vessel 9 for
forming the second vortex was used, but any device capable of
supplying a liquid and a gas into the mixing apparatus 1 can be
used.
An embodiment of the apparatus for measuring the stability of a
gas-containing mixture of a hydrophobic liquid or hydrophilic
~(~5~
13
liquid is illustrated in Fig. 8.
An Erlenmeyer flask 76 is charge with 100 ml of octane
containing an excessive amount of oxygen gas, and the upper
space is filled with air. The Erlenmeyer flask 76 is arranged
on a stand 77 and can be heated from below by a burner 78. The
top end portion of the Erlenmeyer flask 76 is connected to a
hose type cooling tube 74 by using a fitting glass. This hose
type cooling tube 74 is perpetually cooled by cooling water not
shown in the drawings. The top end of the hose type cooling
tube 74 is sealed by a plug and is connected to an injector 70
through a glass tube 72 and a silicone tube 71. At first, a
piston is set at a scale of "O". When the concentration of
oxygen in the open air is measured by a gas detector for the
measurement of the oxygen concentration (supplied by Gastec
co.), it is found that the concentration is 20.8%. Then,
octance containing an excessive amount of oxygen gas is charged
in the Erlenmeyer flask 76 and is boiled by the burner 78. By
this boiling, the pressing member of the injector 70 is pressed
back. The injector 70 is taken out, and the oxygen gas
concentration in air in the injector 70 is measured. The oxygen
concentration is 22.1%. The same sample is boiled for 2 hours
and the measurement is conducted in the same manner as
described above. The oxygen concentration i5 22.1%.
Accordingly, it can be said that oxygen contained in octane is
stable in a super-saturated state (about 2.3 times). The
stability measurement test is similarly carried out with
respect to methyl alcohol containing an excessive amount of
oxygen gas by using the same measurement equipment as mentioned
above. The oxygen concentration in the open air is 20.8% and
the oxygen concentration after l hour's boiling is 21.9%.
Furthermore, the oxygen concentration after 2 hours' boiling is
21.9%. Accordingly, it can be said that oxygen contained in
methyl alcohol ln a super-saturated state (about 2.7 times) is
in a stable state.
When the same experiment is carried out with respect to methyl
alcohol by using carbon dioxide instead of oxygen, the volume
14
2~5~3~3
of carbon dioxide contained in methyl alcohol is about 1.8
times the saturation solubility, and at the stability test, a
stable state is observed at a concentration about 1.35 times
the saturation solubility. Accordingly, it is understood that
if the mixing apparatus of the present example is used, a gas
is stably incorporated in other gas in a super-saturation
state.
The physical states of water/hydrophobic liquid mixtures and
hydrophilic liquid/hydrophobic liquid mixtures obtained
according to the above-mentioned first through third
embodiments were tested. One drop of the water/vitamin A oil
. obtained according to the first embodiment was collected from
each of the upper and lower portions of the vessel 30 by a
syringe and dropped on a preparation. The water/vitamin A oil
mixture on the preparation was photographed (600
magnifications) at a photographic sensitivity of ASA1000 by
Nicon F.2 supplied by Nippon Kogakusha, which was attached to
an optical microscope (M-862 supplied by Carton Co.), and it
was confirmed that the vitamin A oil was homogeneously
distributed in the form of droplets having a size of about 500
nm. In order to confirm the stability of the water/vitamin A
oil mixture, the mixture was stored in the sealed state in a
thermostat tank maintained at 50C for 13 days, and the mixture
was observed by a microscope photo in the same manner as
described above. The state was not substantially different from
the state just after the mixing. Thereafter, about 4 ml of the
mixture of water and vitamin A was placed in a cubic cell and,
after setting a slit (width=O.lmm), to a laser beam from a
laser beam source unit ~i.e., GL-803N, manufactured by Nakamura
Rika Ko o X.K.), the cubic cell was irradiated with a laser
beam. As a result, Tyndall phenomenon was confirmed. Then, the
cubic cell was set on a microscope (BH-2 manufactured by
Olympus optical co., Ltd.) and a ultramicroscope was composed,
together with the above-mentioned laser beam source unit. Thus,
the cubic cell was irradiated with a laser beam via the
above-mentioned slit from the laser beam source unit. As a
result, the existence of oil drops was confirmed and the
~:~5~
occurrence of a Brownian movement was also confirmed. As a
comparative sample, a mixture of 10 ml of vitamin A oil and 90
ml of water was placed in a vessel and was stirred for a long
time in a ultrasonic cleaner (i.e., "SONO CLEANER" CA=2480
manufactured by Kaijyo Denki K.K.). The resultant mixture was
allowed to stand at room temperature for one day under a
tightly sealed condition. As a result, it was confirmed that
the water and the vitamin A oil in the vessel were separated
and the underpositioned water was transparent and was not
turbid. On the other hand, when one drop each of the
upperpositioned vitamin A oil and the underpositioned water
placed on a separate preparation was observed by the
above-mentioned optical microscope, it was confirmed that
neither the water drops nor the oil drops were a mixture of
water and oil. Furthermore, neither oil in the water drops nor
water in the oil drops were observed by the above-mentioned
ultramicroscope.
It also was confirmed that as the circulation time in the
embodiment of Fig. l was long, the droplet size became finer.
The water/vitamin A oil mixture on the preparation was warmed
to evaporate water, and adhesion of the oily substance onto the
preparation was confirmed. When the water/squalane mixture was
similarly tested, the same results as described above were
obtained.
Similarly, the physical conditions of mixtures of water and gas
oil obtained from 1 liter of water and 9 liters of gas oil
(i.e., hydrophobic oil) in the above-mentioned first, second
and third embodimants of the present invention were examined.
As a result, it was confirmed by the optical microscope that
the water drops having approximately the same size of about 500
nm were uniformly distributed in the oil liquid. Furthermore,
similarly as mentioned above, the occurrence of the Tyndall
phenomenon, the existence of the water drops by the
ultramicroscope, and the occurrence of the Brownian movement of
the water drops were confirmed. Furthermore, when the mixtures
were allowed to stand for a long time, it was observed that the
~ 5~
16
upper oil-in-water portion and the lower water-in-oil portion
are stably existed as a colloidal condition. In addition, when
the resultant mixtures were centrifugally separated for 5
minutes at 3,000 rpm, it was confirmed by the above-mentioned
optical microscope that the occurrence of the Tyndall
phenomenon was confirmed in the resultant centrifugally
separated portion although the present of the water drops was
not observed. Furthermore, when the centrifugally separated
portion was observed by a ultramicroscope, the existence of the
water drops and the occurrence of the Brownian movement were
confirmed. Accordingly, the size of the water drops was
estimated to be about 100 nm.
Moreover, when the mixtures was prepared by the above-mentioned
lS ultrasonic cleaner, the resultant mixtures were separated after
allowing to stand at room temperature for one hour, into water
and gas oil and the lower water phase was transparent.
When the ethyl alcohol/vitamin A oil mixture prepared according
to the first embodiment as the hydrophilic liquid/hydrophobic
mixture was photographed by the microscope according to the
above-mentioned method, it was confirmed that the droplet size
of the vitamin A oil in ethyl alcohol was about 500 nm. No
substantial change of the state was observed with respect to
the distribution of ethyl alcohol and vitamin A oil between the
mixture just after the mixing and the mixture which had been
stored in a thermostat tank maintained at 50C for 20 days.
When the ethyl alcohol/vitamin A oil mixture on the preparation
was warmed to evaporate water, adhesion of the oily substance
onto the preparation was confirmed.
When the ethyl alcohol/squalane mixture was similarly tested,
the same results as described above were obtained.
In the present embodiment, illustration has also been made with
respect to the combinalion of one liquid and one gas. IIowever,
the present embodiment is not limited to this combination and a
combination of a plurality of liquids and one gas or a
~s~
17
combination of a plurality of liquids and a plurallty of gases
can be used.
A plurality of mixing apparatuss 1 can be arranged in series or
in parallel in one closed flow passage, and other substance can
be supplied linearly or in the reversely rotated state in a
vortex formed within the mixing apparatus. Furthermore, the
mixing method, mixing device and mixture are not limited to
those specifically disclosed in the examples.
As is apparent from the foregoing illustration, by using the
mixing device and mixing method of the present invention, a
plurality of substances different in the physical properties
can be mixed. By the term "hydrophobic liquid'l are meant oily
materials such as carnauba wax and liquid paraffin and fossil
fuels such as benzene, decane, and gas oil, vegetable oils such
as sesame oil, and by the term ~hydrophilic liquid" are meant
various alcohols such as monohydric and dihydric alcohols.
The obtained mixture is advantageous in the cost because an
emulsifier need not be used, and hence, the limitations by the
use of the emulsifier or the like are eliminated and the
mixture can be widely used. Moreover, if a hydrophilic liquid
containing oxygen is used for cosmetic lotions or cosmetic
creams, an effect of activating the skin can be attained.
Furthermore, hydrophobic liquids (e.g., vegetable oil) and
hydrophilic liquids (e.g., ethyl alcohol, propylene glycol)
containing oxygen may be used as a solvent for pharmaceutical
applications such as the production of injections.