Note: Descriptions are shown in the official language in which they were submitted.
A~ 9580
CARBQ~YLTC AClD ESTEI~S OF R~lP~lYCIN
BACKGROUND OF THE INVENTION
This invention relates to novel estcrs of raparnycin and a method for using themin the treatment of transplantation rejection, host vs. graft disease, autoimmune
diseases, diseases of inflammation, and fungal infections.
Rapamycin is a macrocyclic triene antibiotic produced by Streptomvces
hvgrosc~cus, which was found to have antifungal activity, particularly against
Candida albicans, both in vitro and in vivo [C. Vezina et al., J. Antibiot. 28, 721
(1975); S.N. Seghal et al., J. Antibiot. 28, 727 (1975); H. A. Baker et al., J. Antibiot.
31, 539 (1978); U.S. Patent 3,929,992; and U.S. Patent 3,993,749].
Rapamycin alone (U.S. Patent 4,885,171) or in combination with picibanil
(U.S Patent 4,401,653) has been shown to have antitumor activity. R. Martel et al.
[Can. J. Physiol. Pharmacol. 55, 48 (1977)] disclosed that rapamycin is effective in the
experimental allergic encephalomyelitis model, a model for multiple sclerosis; in the
adjuvant arthritis model, a model for rhewmatoid arthritis; and effectively inhibited the
fornnation of IgE-like antibodies.
The immwnosuppressive effects of rapamycin have been disclosed in FASEl~ 3,
3411 (1989), rapamycin has been shown to be effective in inhibiting transplant
rejection (U.S. Patent Application Ser. No. 362,544 filed June 6, 1989). Cyclosporin
A and FK-506, other macrocyclic molecules, also have been shown to be effective as
immunosuppressive agents, therefore useful in preventing transplant rejection [FASEB
3, 3411 (1989); FASEB 3, 5256 (1989); and R. Y. Calne et al., Lancet 1183 (l978).
Mono- and diacylated derivatives of rapamycin (esterified at the 28 and 43
positions) have been shown to be useful as antifungal agents (U.S. Patent 4,316,885)
and used to make water soluble prodrugs of rapamycin (U.S. Patent 4,650,803).
Recently, the numbering convention -for rapamycin has been changed; therefore
according to Chemical Abstracts nomenclature, the esters described above would be at
the 31- and 42- positions.
AHP-9580
- 2- 2~5~7~ :
DESCRIPTION OF THE INVENTION
This invention provides derivatives of rapamycin which are useful as
immunosuppressive, anti-inflammatory, and antifungal agents having the st~ucture
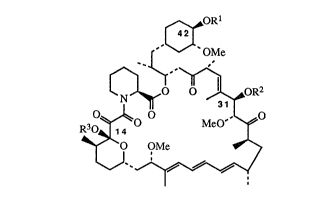
~ORI
' OMe
~0 ~oR2
R3 ~ MeO~O
~0 OMe
o
wherein R1, R~, and R3 are each, independently, hydrogen or - CR4
with the proviso that Rl, R2, and R3 are not all hydrogen;
R4 i8 -(CH2)mX(CH2) nCO2Rs or --~--C02R6
0 RS and R6 are each, independently, alkyl of 1-6 carbon atoms, aralkyl of 7-10 carbon
atoms, or phenyl which is optionally mono-, di-, or tri-substituted with a
substituent selected from alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, hydroxy, cyano, halo, nitro, carbalkoxy of 2-7 carbon atoms,
trifluoromethyl, amino, or a carboxylic acid;
R7
Xis -¢-, O, orS;
R8
R7 and R8 are each, independently, hydrogen or alkyl of 1-6 carbon atoms;
Y is CH or N;
20 misO-4;
nisO-4;
with the proviso that m and n are not both 0 when X is O or S;
or a pharmaceutically acceptable salt thereof.
'
AHP-9580
3 2~5~
Of the compounds, preferred members are those in which
R4 is -(CH2)mX (C~I2) nCO2Rs
The pharmaceutically acceptable salts may be formed from inorganic cations
such as sodium, potassium, and the like; mono-, di-, and triaLlcyl amines of 1-6 carbon
S atoms, per aLkyl group and mono-, di-, and trihydroxyalkyl amines of 1-6 carbon
atoms per alkyl group. Preferred salts are formed from sodium cations and
tris(hydroxymethyl)methylamine.
The compounds of this invention can be prepared by acylating rapamycin with
an acylating agent having the general structure
Il 4
XCR
where X is OH, in the presence of a coupling reagent, such as dicyclohexyl-
carbodimide. The compounds of this invention also can be prepared using an
anhydride of the above described carboxylic acid as the acylating species.
Alternatively, the acylating species can be an acid halide, where X can be Cl, Br, or I.
15 The acylating groups used to prepare the compounds of this invention are commercially
available or can be prepared by methods that are disclosed in the literature.
Immunosuppressive activity was evaluated in an in vitro standard
pharmacological test proçedure to measure Iymphocyte proliferation (LAF) and in two
20 in vivo standard pharmacological test procedures. The first in vivo procedure was a
popliteal Iymph node (PLN) test procedure which measured the effect of compounds of
this invention on a mixed Iymphocyte reaction and the second in yivo procedure
evaluated the survival time of a pinch skin graft.
The comitogen-induced thymocyte proliferation procedure (LAF~ was used as
an m ro measure of the immunosuppressive effects of representative compounds.
Briefly, cells from the thymus of normal BALB/c mice are cultured for 72 hou~rs with
PHA and IL 1 and pulsed with tritiated thymidine during the last six hours. Cells are
cultured with and without various concentrations of rapamycin, cyclosporin A, or test
compound. Cells are harvested and incorporated; radioactivity is determined.
Inhibition of Iymphoproliferation is assessed in percent change in counts per minute
from non-drug treated controls. The results are expressed by the following ratio:
3H-control thymus çells - H3-rapamycin-treated thYmus çells
3H-çontrol thymus cells - H3-test compound-treated cells
AHP-9580
- 4 - Z~L'71
A mixed Iymphocyte reaction (MLR) occurs when lymphoid cells from
genetically distinct animals are combined in tissue culture. Each stimulates the other to
undergo blast transformation which results in increased DNA synthesis that can be
qu~ntified by the incorporation of tritiated thymidine. Since stimulating a MLR is a
function of disparity at Major Histocompatibility antigens, an in vivo popliteal Iymph
node (PLN) test procedure closely colTelates to host vs. graft disease. Briefly,irradiated spleen cells from BALB/c donors are injected into the right hind foot pad of
recipient C3H mice. The drug is given daily, p.o. from Day 0 to Day 4. On Day 3 and
Day 4, tritiated thymidine is given i.p., b.i.d. On Day 5, the hind popliteal Iymph
nodes are removed and dissolved, and radioactivity counted. The corresponding left
RLN serves as the control for the PLN from the injected hind foot. Percent
suppression is calculated using the non-drug treated animals as allogenic control.
Rapamycin at a dose of 6 mg/kg, p.o. gave 86% suppression, whereas cyclosporin Aat the same dose gave q3% suppression. Results are expressed by the ~ollowing ratio:
3H-PLN cells control G3H mouse - 3H-PLN cells rapamycin-treated C3H mouse
3H-PLN cells control C3H mouse - 3H-PLN cells test compound-treated C3H mouse
The second in vivo test procedure is designed to determine the survival time of
pinch skin graft from male DBA/2 donors transplanted to male BALB/c recipients. The
method is adapted from Billingham R.E. and Medawar P.B., J. Exp. Biol. 28:385-
402, (1951). Briefly, a pinch skin graft from the donor is grafted on the dorsum of the
recipient as a homograft, and an aut~ograft is used as control in the same region. The
recipients are tr~ated with either varying concentrations of cyclosporin A as test control
or the test compound, intraperitoneally. Untreated recipients serve as rejection control.
The graft is monitored daily and observations are recorded until the graft becomes dry
and forms a blackened scab. This is considered as the rejection day. The mean graft
survival time (number of days + S.D.) of the drug treatment group is compared with
the control group.
The following table summarizes the results of representative compounds of this
invention in these three standard test procedures.
AHP-9580
2~ 7~
TABLE 1
LAF* PLN* Skin Graft
Compound (ratio) (ratio~~davs + SD
S Example 1 0.37 + 8.2 + 1.2
Example2 0.9 0.69 10.7 + 1.2
Example 3 3.27 1.04~+12.7 + 0.9
Example 4 0.56 1.68++~10.2 + 1.7
Example S 0.02 1 . 1 1++8.0 +1 .7
Example 6 0.01 0.48 8.0 + 0.9
Example7 0.97 0.70 9.3 + 1.6
Example 8 0.22 -1.93 12.0 + 1.7
Exampleg 0.22 0.41 10.2+ 1.2
Example 100.18 0.39 10.8 + 0.8
Example 110.00 0.09 7.8 + 1.7
Rapamycin 1.0 1.0 12.0 + 1.7
* Calculation of ratios was described ~.
+ Notevaluated
++ Results obtained using cremophore/ethanol as a vechicle for administration.
Ratios of 0.20 and 1.08 also were obtained using carboxymethyl cellulose
as a vehicle for administration.
+++ A ratio of 0.42 also was obtained for this compound.
The results of these standard pharmacological test procedures demonstrate
immunosuppressive activity both in vitro and in vivo for the compounds of this
invention. Positive ratios in the LAF and PLN test procedures indicate suppression of
T cell proliferation. As a transplanted pinch skin grafts are typically rejected within ~7
days without the use of an immunosuppressive agent, the increased survival time of the
skin graft when treated with the compounds of this invention further demonstrates their
utility as immunosuppressive agents. While it appears that the compound disclosed by
Example 8 may cause T cell proliferation in the PLN test procedure, it is believed a
negative ratio in this test procedure coupled with an increased survival time observed in
the skin graft test procedure indicates a proliferation of TSuppressor cells, which are
implicated in suppressing the immune response. (see, I. Roitt et al. Immunology,C. V. Moseby Co. 1989, p 12.8-12.11).
AHP-9580
2~!5~7
- 6-
Antifungal activity of the compounds of this invention was measured against 5
strains of Candida albicans using a plate test procedure for measurement of inhibition.
The fol1Owing represents the typical procedure used. Compound to be tested was
placed on sterile dried 114" plate disks, and allowed to dry. Agar plates were seeded
S with fungi and allowed to solidify. The impregnated disks were placed on the seeded
Agar surface and incubated for the time required for the particular culture. Results are
expressed in MIC ( ~g/ml) to inhibit growth. The results of this test procedure showed
that the compounds of this invention have antifungal activity; however, it was
surprising that the compounds of this invention were less active that the parent10 compound, rapamycin.
Table 2*
Strain of Candida albicans
Cornl~oundATCC 10231ATCC 38246ATCC 38247ATCC 38248 3669
Example 1> 0.4 >0.4 >0.4 >0.4 >0.4
Exarnple 2> 0.4 0.4 > 0.4 0.4 0.4
Exarnple3 0.2 0.1 0.4 0.1 0.1
Exarnple 4> 0.4 0.2 > 0.4 0.2 0.4
Exarnple S0.4 > 0.4 > 0.4 >0.4 >0.4
Example 6 0.4 > 0.4 0.4 >0.4 >0.4
Example7 0.1 0.4 0 1 0.1 0.2
Example 8 0.4 > 0.4 0-4 >0-4 >0-4
Example 9 0.2 > 0.4 0.2 0.4 >0.4
Example 100.1 > 0.4 0.2 0.4 >0.4
Example 11> 0.4 > 0.4 >0.4 >0.4 >0.4
Rapamycin0.003 0.025 0.003 0.006 0.025
expressed as MIC (~,lg/ml)
Based on the results of these standard pharmacological test procedures, the
30 compounds are useful in the treatment of transplantation rejection such as, heart,
kidney,1iver, bone marrow, and skin transplants; autoi ~mmune diseases such as, lupus,
rheumatoid arthritis, diabetes mellitus, myasthenia gravis, and multiple sclerosis;
diseases of inflammation such as, psoriasis, dermatitis, eczema, seborrhea, and
inflammatory bowel disease; and fungal infections.
,
. .
: . :
'
'
AHP-9580
7 ~35~ ~7
The compounds may be administered neat or with a pharmaeeutieal earrier to a
mammal in need thereof. The pharmaeeutieal earrier may be solid or liquid.
A solid earrier ean include one or more substances which may also act as
flavoring agents, lubrieants, solubilizers, suspending agents, fillers, glidants,
compression aids, binders or tablet-disintegrating agents; it can also be an encapsulating
material. In powders, the carrier is a finely divided solid which is in admixture with the
finely divided active ingredient. In tablets, the active ingredient is mixed with a carrier
having the necessary compression properties in suitable proportions and eompaeted in
the shape and size desired. The powders and tablets preferably contain up to 99~o of
the aetive ingredient. Suitable solid carriers include, for example, calcium phosphat~e,
magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatin, cellulose, methyl
cellulose, sodium carboxymethyl cellulose, polyvinylpyrrolidine, low melting waxes
and ion exchange resins.
Liquid earriers are used in preparing solutions, suspensions, emulsions,
syrups, elixirs and pressurized eompositions. The aetive ingredient can be dissolved or
suspended in a pharmaeeutieally aeeeptable liquid carrier such as water, an organic
solvent, a mixture of both or pharrnaceutically aeeeptable oils or fats. The liquid earrier
ean eontain other suitable pharmaceutical additives such as solubilizers, emulsifiers,
buffers, preservatives, sweeteners, flavoring agents, suspending agents, thickening
agents, eolors, viseosity regulators, stabilizers or osmo-regulators. Suitable examples
of liquid earriers for oral and parenteral administration inelude water (partially
eontaining additives as above, e.g. eellulose derivatives, preferably sodium
earboxymethyl eellulose solution), aleohols (ineluding monohydrie aleohols and
polyhydrie aleohols, e.g. glyeols) and their derivatives, and oils (e.g. fraetionated
eoeonut oil and araehis oil). For parenteral administration, the carrier can also be an
oily ester such as ethyl oleate and isopropyl myristate. Sterile liquid carriers are useful
in sterile liquid form eompositions for parenteral administration. The liquid earrier for
pressurized compositions can be halogenated hydroearbon or other pharmaeeutieally
aeeeptable propellent.
Liquid pharmaeeutieal eompositions whieh are sterile solutions or suspensions
ean be utilized by, for example, intramuscular, intraperitoneal or subcutaneous
injeetion. Sterile solutions can also be administered intravenously. The compound ean
also be administered orally either in liquid or solid composition form.
Preferably, the pharmaeeutieal eomposition is in unit dosage form, e.g. as
tablets or eapsules. In sueh form, the eomposition is sub-divided in unit dose
eontaining appropriate quantities of the active ingredient; the unit dosage forrns can be
AHP-9580
8 2~5~
packaged compositions, for example, packeted powders, vials, ampoules, prefilledsyringes or sachets containing liquids. The unit dosage form can be, for example, a
capsule or tablet itself, or it can be the appropriate number of any such compositions in
package form. The dosage to be used in the treatment must be subjectively determined
S by the attending physician.
The following examples illustrate the preparation of representative compounds
of this invention.
10 Example 1.
Rapan~vcin- 14!3142-tris(monobenzvlsuccinate!
To a solution of 5.0 g (5.47 mmol) of rapamycin, 3.41 g (16.41 mmol) of
monobenzylsuccinate, and 3.15 g (16.41 mmol) of 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride in 20 mL of dry dichloromethane was added 200 mgof 4-dimethylaminopyridine. The solution was stirred at room temperature for 3 days.
The reaction mixture was poured into 2 N HCl and extracted three times with ethyl
acetate. The organic layers were combined, washed with brine, dried over anhydrous
sodium sulfate, decanted, and concentrated in vacuo to give a light yellow foam. Flash
chromatography on a 60 mm x 150 mm sil;ca gel column eluting with 20 % ethyl
acetate/hexane to 75 % ethyl acetate/hexane gave three fractions. Fraction #1~ upon
concentration, gave 330 mg (4.1 %) of pure rapamycin-14,31,42-tris-
(monobenzylsuccinate).
IH NMR (CDCl3, 400 MHz) ~ 7.353 (bs, 15 H, arom), 5.168 (d~ J = 2.0 Hz,
1 H, CH-O2C), 5.148 (m, 6 H, CH2Pn), 4.672 (m, 1 H, CO2CH-CHOMe), 3.355
(s, 3 H, CH30-), 3.337 (s, 3 H, CH30-), 3.327 (s, 3 H, CH30-), 2.697 ( m, 12 H,
O2CCH2CH2CO2CH2Ph), 1.745 (s, 3 H, CH3C=C), 1.655 (s, 3 H, CH3C=C);
IR (KBr) 3450 (OH), 2950 (CH), 1745 (C=O), 1650, 1460, 1385, 1360, 1160, 1105,
995 cm~l.
AnalysisCalcdforCg4HlogNO2l 3H20 C 66.27; H 7.56; N 0.92
Found C 65.96; H 7.24; N 1.00
The following representative compounds can be prepared from rapamycin and
the appropriate half acid-ester by employing the method used to prepare the title
compound in Example 1.
AHP-9580
2~53 '~
Rapamycin- 14,31,42-tris (monom.-thylsuccinate)
Rapamycin-14,31,42-tris (monophenyl-3',3'-dimethylglutarate~
Rapamycin- 14,31,42-tris (mono t-butyl-3'-methylglutarate)
Rapamycin-14,31,42-tris (monobenzylthiodiglycolate)
Rapamycin- 14,31,42-tris (monohexyldiglycolate)
Rapamycin-14,31,42-tris (monopropylphthalate)
Rapamycin-14,31,42-tris (monoethyl-2',6'-pyridinedicarboxylate)
Example 2.
Ra~amy~in-31.42-bis(monobenzvlsuccinatel
Fraction # 2, obtained from the procedure employed in Example 1, gave
1.25 g (17.7 %) of pure rapamycin-31,42-bis(monobenzylsuccinate) upon concen-
tration.
IH NMR (CDCl3, 400 MHz) ~ 7.351 (bs, 10 H, arom), 5.168 (d, J = 2.0 Hz,
1 H, CH-O2C), 5.125 (m, 4 H, CH2Ph), 4.680 (m, 1 H, CO2C~-CHOMe), 3.356
(s, 3 H, CH30-), 3.329 (s, 3 H, CH30-), 3.146 (s, 3 H, CH30-), 2.639 ( m, 8 H,
02CC~2CH2C02CH2Ph), 1.748 (s, 3 H, CH3C=C), 1.654 (s, 3 H, CH3C=C);
IR (KBr) 3450 (OH), 2940 (CH), 1740 (C=O), 1650, 1455, 1380, 1355, 1160, 1105,
995 cm-l; MS (neg. ion FAB) 1294 (M-), 1202, 1103, 1012, 590, 511, 475, 297,
207, 167, 148, 99 (100); High Res. MS (neg. ion FAB) Calcd for C73HggNOIg
1293.68108, found 1293.6811.
AnalysisCalcdforC73H99NOl9 H2o C 66.82; H 7.70; N 1.07
Found C 67.17; H 7.67; N 1.23
The following representative compounds can be prepared from rapamycin and
the appropriate half acid-ester by employing the method used to prepare the title
compound in Example 2.
Rapamycin-31,42-bis (monomethylsuccinate)
Rapamycin-31,42-bis (monophenyl-3',3'-dimethylglutarate)
Rapamycin-31,42-bis (mono t-butyl-3'-methylglutarate)
Rapamycin-31,42-bis (monobenzylthiodiglycolate)
Rapamycin-31,42-bis (monohexyldiglycolate)
Rapamycin-31,42-bis (monopropylphthalate)
Rapamycin-31,42-bis (monoethyl-2',6'-pyridinedicarboxylate)
AHP-9580
- 10-
Example 3.
Ra,~bi~Y~ccinate
s
Fraction # 3, obtained from the procedure employed in Example 1, gave
930 mg (15.4 %) of pure rapamycin-42-monobenzylsuccinate upon concentration.
IH NMR (CDC13, 400 MHz) o 7.355 (bs, 5 H, arom), 5.141 (m, 2 H,
CH2Ph), 4.680 (m, 1 H, CO2CH-CHOMe), 3.364 (s, 3 H, CH30-), 3.333 (s, 3 H,
CH30-), 3.141 (s, 3 H, CH30-), 2.698 ( m, 4 H, 02CCH2CH2CO2CH2Ph), 1.751
(s, 3 H, CH3C=C), 1.655 (s, 3 H, CH3C=C); IR (KBr) 3450 (OH), 2940 (CH), 1740
(C=O), 1645, 1455, 1380, 1165, 1105, 990cm-~; MS (neg. ionFAB) 1103 (M-),
1045, 1012, 624, 590, 167, 99 (100); High Res. MS (neg. ion FAB) Calcd for
C62HggNO16 1103.6181, found 1103.6048.
AnalysisCalcdforC62HggNOl6-H20 C 66.36; H 8.02; N 1.24
Found C 66.02; H 7.69; N 1.26
The following representative compounds can be prepared from rapamycin and
the appropriate half acid-ester by employing the method used to prepare the title
compound in Example 3.
Rapamycin-42-(monomethylsuccinate)
Rapamycin-42-monophenyl-3',3'-dimethylglutarate)
Rapamycin-42-(mono t-butyl-3'-methylglutarate)
Rapamycin-42-(monobenzylthiodiglycolate)
Rapamycin-42-(monohexyldiglycolate)
Rapamycin-42-(monopropylphthalate)
Rapamycin-42-(monoethyl-2',6'-pyridinedicarboxylate)
.
:
,
AHP-9580
035~a7
~
Example 4.
Rapamvcin-31 ~2-bishemigl~rate
To a solution of 2.0 g (2.2 mmol) of rapamycin in 10 mL of dry
dichloromethane was added 1.24 g (10.9 mmol) of glutaric anhydride followed by
881 uL (861 mg, 10.9 mmol) of pyridine. To this was added 200 mg of
4-dimethylaminopyridine and the reaction mixture was allowed to reflux for 8 h. The
solution was cooled to room temperature, poured into 2 N HCI, and extracted three
times with dichloromethane. The combined organic extracts were washed with brine,
dried over anhydrous sodium sulfate, decanted, and concentrated in vacuo to give a
yellow foam. The crude product was purified via reverse phase ~IPLC on a Clg
column eluting starting with 60 % acetonitrile/water. Collected, after, concentration,
586 mg (24 %) of rapamycin-31,42-bishemiglutarate.
IH NMR (CDC13, 400 MHz) ~ 5.398 (m, 1 H, -CO2CHCHOMe), 4.683
(m, 1 H, -CO2CHCHOMe), 3.364 (s, 3 H, CH30-), 3.362 ~s, 3 H, CH30-), 3.106
(s, 3 H, CH30-), 2.407 (m, 8 H, -O2CCH2CH2CH2CO2H), 1.960 (m, 4 H,
-O2CCH2CH2CH2CO2H). 1.770 (s, 3 H, CH3C=C), 1.653 (s, 3 H, CH3C=C);
13C NMR (CDC13, MHz) 211.45 (C=O), 206.84 (C=O), 200.44 (C=O), 177.83
(C=O), 177.04 (C=O), 172.43 (C=O), 171.20 (C=O), 165.27 (C=O), 159.08 (C=O);
IR (KBr) 3430 (OH), 2940 (CH), 2880 (CH), 1745 (C=O), 1685, 1625, 1580, 1450,
1385, 1330, 1200, 1140, 1100, 990 cm~l; MS (neg. ion FAB) 1140 (M-H), 1122,
1026, 990, 946, 913, 590, 475, 435, 321, 167, 148, 131 (100), 113; High Res.
MS (neg. ion FAB) Calcd for C61HgoOIgN (M-H) 1140.6107, Found 1140.6106.
AnalysisCalcdforC6lH9lOlsN H2o C 63.15; H 8.02; N 1.20
Found C 63.35; H 7.88; N 1.40
The following representative compounds can be prepared from rapamycin and
the appropriate anhydride by employing the method used to prepare the title compound
in Example 4.
Rapamycin-31,42-bishemi-3'-methylglutarate
Rapamycin-31,42-bishemi-3',3'-dimethylglutarate
Rapamycin-31,42-bishemi-3'-oxoglutarate
Rapamycin-31,42-bishemi-3'-thioglutarate
Rapamycin-31,42-bishemi-phthalate
Rapamycin-31,42-bishemi-2',3'-pyridine dicarboxylate
AHP-9580
- 12- ~ ~S~L7
Example 5.
Rapamycin-31,42-hemiglutarate bissodium salt
Purified bis-31,42-hemiglutarate of rapamycin (740 mg, 649 umol), prepared
as described in Example 4, was dissolved in 5 mL of 95 ~o ethanol and 107 mg
(1.27 mmol) of sodium bicarbonate was added. Water (I mL) was added to
completely dissolve the salt. Once dissolved, the light yellow solution was
10 concentrated in vacuo to give a foamy yellow solid. The foam was dried in a drying
pistol for 24 h, refluxing over acetone at reduced pressure to give 520 mg of the
bissodium salt.
lH NMR (d6-DMSO, 400 MH2) ~ 5.235 (m, 1 H, -CHO2C), 4.498 (m, 1 H,
MeOCHCHO2C-), 3.287 (s, 6 H, 2 CH30-), 3.236 (s, 3 H, CH30-), 2.245 (m, 8 H,
02CCH2CH2CH2C02-), 1.712 (s, 3 H, CH3C=C), 1.593 (s, 3 H, CH3C=C);
IR (KBr) 3420 (OH), 2920 (CH), 1725 (C=O), 1675, 1620, 1560, 1450, 1400, 1375,
1230, 1195, 1130, 1090, 980 cm~l; MS (neg. ion FAB) 1112 (M-l, free acid), 994,
589, 475, 297, 167, 148, 117, 99 (100); High Res. MS (neg. ion FAB) Calcd for
C61HggOlgNNa (M-Na) 1162.5926, Found 1162.5899.
AnalysisCalcdforC61HggOlgNNa2-H2O C 60.85; H 7.56; N 1.16
Found C 60.67; H 7.36; N 1.58
Example 6.
Rap~mycin-31.42-bishemigllltara~ç bistromethamine salt
Purified bis-31,42 hemiglutarate of rapamycin (950 mg,833 umol), prepared as
described in Example 4, was dissolved in 5 mL of 95 % ethanol and 197 mg
30 (1.63 mmol) of tris(hydroxymethyl)methylamine was added. Water (1 mL) was added
to completely dissolve the amine. Once dissolved, the yellow solution was
concentrated in vacuQ to give a foamy yellow solid. The very hygroscopic foam was
dried in a drying pistol for 24 h, refluxing over acetone at reduced pressure to give
900 mg (78 %) of the bistromethamine salt.
' ' ~ ' ',, . , '
AHP-9580
- 13- 2~.5~'7~
IH NMR (d6-DMSO, 400 MHz) o 5.253 (m, 1 H, -CHO2C), 4.523 (m, 1 H,
MeOCHCHO2C-), 3.347 (s, 6 H, 2 CH30-), 3.276 (s, 3 H, CH30-), 2.289 (m, 8 H,
02CCH2CH2CH2C02-), 1.681 (s, 3 H, CH3C=C), 1.595 (s, 3 H, CH3C=C);
IR (KBr) 3400 (OH), 2920 (CH), 1730 (C=O), 1620, 1555, 1450, 1400, 1370, 1185,
1060, 980 cm~1; MS (neg. ion FAB) 1140 (M-H, free acid), 1028, 167, 148, 131
(100), 113; High Res. MS (neg. ion FAB) Calcd for C~lHgoOlgN (M-H, free acid)
1140.6107, Found 1140.6069.
Analysis Calcd for C6gH103o2sN3 - 2 H2O C 58.77; H 7.58; N 2.98
Found C 58.47; H 7.94; N 3.58
Example 7.
Ra~amvcin-42-hemi-3'-oxoglutarate
To a solution of 3.0 g (3.3 mmol) of rapamycin in 20 mL of dry
dichloromethane was added 1.90 g (16.4 mmol) of diglycolic anhydride followed by1.32 mL (1.29 g, 16.4 mmol) of pyridine. To this was added 200 mg of
4-dimethylaminopyridine and the reaction mixture was allowed to stir at room
temperature for 2 days. The solution was cooled to room temperature, poured into 2 N
HCI, and extracted three times with dichloromethane. The combined organic extracts
were washed wi~h brine, dried over anhydrous sodium sulfate, decanted, and
concentrated vacu~ to give a yellow foam. The crude product was purlfied via
reverse phase HPLC on a Clg column eluting starting with 60 % acetonitrile/water.
After concentration, 870 mg ( 26 %) of rapamycin-42-hemi-3'-oxoglutarate and
500 mg (13 %) of rapamycin-31,42-bishemi-3'oxoglutarate were isolated.
lH NMR (CDC13, 400 MHz) o 4.768 (m, 1 H, CO2CH-CHOMe), 4.250
(m, 4 H, O2CCH2OCH2CO2), 3.356 (s, 3 H, CH30-), 3.331 (s, 3 H, CH30-),
3.139 (s, 3 H, CH30-), 1.759 (s, 3 H, CH3C=C), 1.653 (s, 3 H, CH3C=C);
IR (KBr) 3420 (OH), 2920 (CH), 2875 (CH), 1740 (C=O), 1720 (C=O), 1640, 1625,
1445, 1370, 1320, 1200, 1135, 1095, 980 cm-l; MS (neg. ion FAB) 1028 (M - H),
327, 167 (100), 148, 133, 115; High Res. MS (neg. ion FAB) Calcd for
CssH82ol7N (M - H) 1028.5597, Found 1028.5599.
Analysis Calcd for CssHg3O17N 3 H2O C 60.97; H 8.22; N 1.29
Found C 61.33; H 7.74; N 1.69
AHP-9580
14 2~5~7~
The following representative compounds can be prepared from rapamycin and
the appropriate half acid-ester by employing the method used to prepare the title
compound in Example 7.
S Rapamycin-42-hemi-3'-methylglutarate
Rapamycin-42-hemi-3',3'-dimethylglutarate
Rapamycin-42-hemi-3'-thioglutarate
Rapamycin-42-hemi-phthalate
Rapamycin-42-hemi-2',3'-pyridine dicarboxylate
Example 8.
Ra~amycip-~f 1.42-bishemi-3'-oxoglutarate
To a solution of 5.0 g (5.47 mmol) of rapamycin in 20 mL of dry
dichloromethane was added 3.17 g (27.3 mmol) of diglycolic anhydride followed by2.17 mL (2.12 g, 27.3 mmol) of pyridine. To this was added 400 mg of
4-dimethylaminopyridine and the reaction mixture was allowed to stir at reflux for 24 h.
The solution was cooled to room temperature, poured into 2 N HCI, and extracted three
times with dichloromethane. The combined organic extracts were washed with brine,
dried over anhydrous sodium sulfate, decanted, and concentrated ~n Yacuo to give a
yellow foam. The crude product was purified via reverse phase HPLC on a
Clg column eluting starting with 60 % acetonitrile/water. After concentration, 1.75 g
( 28 %) of rapamycin-31,42-bishemi-3'-oxoglutarate was isolated.
1H NMR (CDC13, 400 MHz) o 4.785 (m, 1 H, CO2CHCHOMe), 4.260
(m, 8 H, O2CCH2OCH2CO2), 3.360 (s, 3 H, CH30-), 3.343 (s, 3 H, CH30-),
3.143 (s, 3 H, CH30-), 1.775 (s, 3 H, CH3C=C), 1.656 (s, 3 H, CH3C=C);
13C NMR (CDC13, MHz) 211.12 (C=O), 207.73 (C=O), 193.11 (C=O), 171.90
(C=O), 171.59 (C=O), 170.15 (C=O), 169.35 (C=O), 168.83 (C=O), 166.63 (C=O);
IR (KBr) 3420 (OH), 2920 (CH), 2850 (CH), 1740 (C=O), 1645~ 1625, 1440, 1370,
1190, 11300, 980 cm~l; MS (neg. ion FAB) 1140 (M-H), 1122, 1026, 990, 946,
913, 590, 475, 435, 321, 167, 148, 131 ~100), 113; High Res. MS (neg. ion FAB)
Calcd for CsgH86O21N (M - H) 1144.5701, Found 1144.5702.
AnalysisCalcdforCsgHg7O21N C 61.82; H 7.65; N 1.22
Found C 61.59; H 7.36; N 1.84
AHP-9580
- 15 - 21~53
Example 9.
Rapamycin-31~42-bishemi-3'-oxo~lutarate disodium salt
S Purified bis-31,42 hemi-3'-oxoglutarate of rapamycin t720 mg, 629 umol),prepared by the procedure employed in Example 8, was dissolved in 10 mL of 95 %
ethanol and 106 mg (1.26 mmol) of sodium bicarbonate was added. Water (1 mL) wasadded to completely dissolve the salt. Once dissolved, the light yellow solution was
concentrated in vacuo to give a foamy yellow solid. The foam was dried in a drying
pistol for 48 h, refluxing over dichloromethane at reduced pressure to give 435 mg
(58 %) of the disodium salt.
lH NMR ~d6-DMSO, 400 MHz) o 4.975 (m, 1 H, -CHO2C), 4.593 (m, 1 H,
MeOCHC~O2C-), 4.135 (s, 2 H, -O2CCH2OCH2CO2R), 3.617 (s,2 H,
-O2CCH2OCH2CO2R), 3.299 (s, 6 H, 2 CH30-), 3.232 (s, 3 H, CH3C)-), 1.614
(s, 3 H, CH3C=C), 1.553 (s, 3 H, CH3C=C); IR (KBr~ 3420 (OH), 2920 (CH),
1735 (C=O), 1615, 1445, 1395, 1380, 1320, 1220, 1130, 1090, 980 cm~l;
MS (neg. ion FAB) 1188 (M-l), 1166 (M-Na), 1144, 1051, 1028, 590, 459, 167,
155 (100~, 148, 133, 115.
AnalysisCalcdforCsgHgso2lNNa2-2H2o C 57.79; H 7.26; N 1.14
Found C 57.94; H 7.11; N 1.26
Example 10.
25 Rapa,mvcin~ 42-~Q $hemi-~'-QxQ~lutarate bistromethamine salt
Purified bis-31,42 hemi-3'-oxoglutarate of rapamycin (1.01 g, 882 umol),
prepared by the procedure employed in Example 8, was dissolved in 10 mL of 95 %
ethanol and 213 mg (1.76 mmol) of tris(hydroxymethyl)- methylamine was added.
30 Water (1 mL) was added to completely dissolve the amine. Once dissolved, the yellow
solution was concentrated in vacuo to give a foamy yellow solid. The
hygroscopic foam was dried in a drying pistol for 48 h, refluxing over dichloromethane
at reduced pressure to give 805 mg (66 %) of the bistromethamine salt.
AHP-9580
- 16- 2~S~L7~.
lH NMR (d~s-DMSO, 400 MHz) ~ 4.955 (m, 1 H, -CHO2C), 4.600 (m, 1 H,
MeOCHCHO2C-), 4.149 (s, 2 H, -O2CCH2OCH2CO2R), 3.770 (s, 2 H,
-02CCH20CH2C02R), 3.407 (s, 6 H, 2 CH30-), 3.257 (s, 3 H, CH30-), 1.806
(s, 3 H, CH3C=C), 1.614 (s, 3 H, CH3C=C); IR (KBr) 3400 (OH), 2920 (CH),
1730 (C=O), 1620, 1550, 1450, 1395, 1370, 1200, 1060, 985 cm~l;
MS (neg. ion FAB) 1144 (M-H, free acid), 1028, 167, 148, 133 (100), 115.
AnalysisCalcdforC67HlogO27N3- H20 C 57.22; H 7.90; N 2.98
Found C 57.26; H 7.90; N 3.15
Example 11.
~?amycin-31.42-bishemisuccinate.
To a solution of 2.0 g (2.2 mmol) of rapamycin in 10 n~ of dry dichloro-
methane was added 1.19 g (10.9 mmol) of succinic anhydride followed by 881 uL
(861 mg, 10.9 mmol) of pyridine. To this was added 200 mg of 4-dimethylamino-
pyridine and the reaction mixture refluxed for 24 h. The solution was cooled to room
temperature, poured into 2 N HCl, and extracted three times with dichloromethane.
The combined organic extracts were washed with brine, dried over anhydrous sodium
sulfate, decanted, and concentrated in vacuo to give a yellow foam. The crude product
was purif1ed via reverse phase HPLC on a Clg column gradient eluting starting with
20 % acetonitrile/water to 60 % acetonitrile/water. Collected, after, concentration,
770 mg (31 %) of rapamycin 31,42-bishemisuccinate.
The purified bis-31,42 hemisuccinate of rapamycin (770 mg, 686 umol) was
dissolved in 10 mL of 95 % ethanol and 166 mg (1.37 mmol) of tris(hydroxymethyl)-
methylamine was added. Water (1 mL) was added to completely dissolve the amine.
Once dissolved, the yellow solution was concentrated in vacuo to give a foamy yellow
solid. The verv hygroscopic foam was dried in a drying pistol for 24 h, refluxing over
acetone at reduced pressure to give 890 mg (95 %) of the bistromethamine salt. The
bistromethane salt was evaluated in the standard pharmacological test procedures.
:
AHP-9580
2~ 7~L
- 17-
IH NMR (d6-DMSO, 400 MHz) 5.231 (m, 1 H, -CHO2C), 4.554 (m, 1 H,
MeOCHCHO2C-), 3.426 (s, 6 H, 2 CH30-), 3.249 (s, 3 H, CH30-), 2.431 (m, 8 H,
02CCH2CH2C02-), 1.700 (s, 3 H, CH3C=C), 1.554 (s, 3 H, CH3C=C); 13C NMR
(d6-DMSO, ) 211.28 (C=O), 205.23 (C=O), 199.59 (C=O), 174.86 (C=O), 173.62
(C=O), 171.72 (C=O), 171.50 (C=O), 166.56 (C=O), 166.53 (C=O); IR (KBr) 3420
(OH), 2940 (CH), 1735 (C=O), 1630, 1580, 1460, 1400, 1380, 1170, 1070,
990 cm-l; MS (neg. ion FAB~ 1112 (M-l, free acid), 994, 589, 475, 297, 167, 148,117, 99 (100).
AnalysisCalcdforC67HIogO2sN3-2H2O C 57.80; H 8.12; N 3.01
Found C 57.91; H 8.21; N 2.37,
: , .. ; . " . ~ ,
-
-- ,