Note: Descriptions are shown in the official language in which they were submitted.
2053571
" .
GAS REACTING APPARATUS AND ~RT~nn
This invention relates to gas/liquid, gas/liquid/
solid and gas/gas/liquid mass transfer, and more
particularly to a mechanically-aided gas reacting
apparatus and method for mass transferring solute gases
from an industrial or utility gas stream into a liquid
or slurry reacting medium, if necessary in conjunction
with simultaneous particulate matter removal, wherein
the mass transfer may be a purely physical phenomenon or
may involve solution of the solute gas in a liquid or a
slurry suspension, followed by chemical reaction with
one or more constituents in the liquid or slurry
reacting medium. This invention also relates to a gas
reacting apparatus for effecting gas-gas reactions in
fluid flow communication with an integrated wet
separation of the resultant reaction products which are
in the form of a finely divided particulate matter.
While not limited thereto, the invention is particularly
well suited for the removal of S02 and other gaseous
pollutants from waste gas streams such as those
emanating from electrical utilities, smelters and
industrial boilers.
For maximum efficiency, it is desirable to make a
gas rebcting apparatus wherein a high interfacial
surface area coupled with turbulent mixing and long
residence time are effected simultaneously and which, as
such, is capable of removing both solute gases and
particulate matter, either separately or simultaneously
with high efficiency, with the former being separated
via a gas-liquid, gas-liquid-solid or gas-gas-liquid
reaction, depending on the application. To-date, as far
as we are aware, there is currently no one type of gas
reacting apparatus available that is capable of
achieving high performance for all of the above
criteria, due to a compromise generally being made
between generation of very fine droplets for affecting
very high surface area on one hand and long residence
- 2053571
time on the other.
One of the methods for separating particulate
matter in solid or slurry form from a gas stream wherein
a dirty gas stream enters a conduit at one end and is
moved through it by a fan at the other end and where a
fine spray of liquid, preferably water, is cocurrently
discharged into such a gas stream upstream from the fan
is described in U.S. patent No. 4,067,703, issued
January 10, 1978. The patent disclosure, while showing
a highly-effective method for removal of particulate
matter from a gas stream, does not teach how the
apparatus can be used as a gas reacting apparatus for
removing solute gases. Also, in many aspects, the
technique disclosed therein does not provide the
absorbing and reacting environment required for
effecting high removal efficiency of solute gases. For
example, in the foregoing prior art patent, the mixture
of gas and particulate matter enters only a single
contact spray zone provided by one nozzle in which an
atomized liquid spray is injected cocurrent to the dusty
air stream flow. While this mode of operation as
disclosed has proven to be highly effective for
removing particulate matter and effecting lower pressure
drop in the apparatus wherein particles were collected
primarily by impaction upon the finely-divided water
droplets introduced, followed by further agglomeration
and impaction on the fan blades as the gas moves through
the device, the residence time available for mass
transfer is too short and the effective interfacial
surface area and turbulence generated by a single
contact spray cocurrently oriented to the gas stream are
not sufficient to effect high removal efficiencies of
solute gases of relatively low solubility in aqueous
solution. It is, therefore, desirable to provide for an
improved gas reacting apparatus and method which
overcome some of the shortcomings of the foregoing prior
20~3571
art apparatus in which increased available residence
time, interfacial surface area and turbulence are
generated to result in accelerated absorption and
reaction kinetics and intimate gas/liquid contact and
thus, in high removal efficiency of both solute gas and
particulate matter.
While high interfacial surface area, turbulent
mixing and long residence time for effecting accelerated
mass transfer of solute gases and effectively separating
particulate matter are the major criteria in gas
reacting apparatus selection, often a compromise must be
made between removal efficiency on one hand and
operating reliability on the other. Several other
factors then also enter into consideration, such as
slurry handling without plugging, turndown, and gas and
liquid distribution.
The basic processes for removal of solute gases
from gas streams, particularly flue gas desulfurization
processes, are based on readily-available, low-cost
absorbents in the form of an aqueous slurry, such as a
lime or limestone slurry, or a clear aqueous solution,
such as caustic or ammoniacal solutions. Various prior
art methods are in use to bring the above absorbing and
reacting media into intimate contact with the
pollutant-laden gas. Packed bed and perforated trays,
which are known to be efficient gas absorption and
reaction devices, are usually the first choice for
designers of flue gas desulfurization (FGD) systems, but
experience has shown that they are not completely
satisfactory. Both perforated trays which bubble the gas
through a thin layer of liquid, and packed beds, which
pass the gas over solid packing elements that are wetted
with the liquid have many narrow passages which are
subject to plugging especially if particulate loads are
heavy, or if precipitates are formed during the
chemisorption process. Such conditions can be minimized
205~71
~. ~.....
by careful process design, but the possibility of
scaling under upset conditions still exists and
compromises reliability. Another principal disadvantage
of both of the above types of scrubbers is their
extremely limited turndown capability.
Consequently, heretofore, the gas reacting devices
of preference and the ones that would seem to be the
answer have been the venturi or open spray tower wherein
the internal complexity is low and yet where a
relatively large surface area of the liquid is generated
per unit volume of gas treated. While the above devices
have evolved considerably over the last decade in a way
to improve their performance and to remove some of their
shortcomings, the current trend in the design
particularly of FGD systems, is away from venturis to
spray towers or combination towers. The venturi design,
although capable of producing a relatively large liquid
surface area for contact with the gas stream, was
abandoned largely because the very short liquid/gas
contact time (attributable largely to the absorbing
medium being introduced cocurrently to the gas stream in
the throat of the venturi) results in low sulfur dioxide
removal. Also, being a relatively high energy device, it
is incapable of producing an evenly distributed regime
of droplets at high density unless an 'overkill'
situation exists wherein excess energy in the form of
velocity pressure is added to the gas stream to provide
for the required uniform distribution. Spray towers, on
the other hand, have few internal components in the
gas/liquid contact zone and the use of sprays appears to
offer an easy way of increasing the surface area exposed
to the gas. However, the sprays are usually introduced
at the top of the spray tower and drop by gravity in
counter-current flow to the gas stream. To avoid being
entrained in the gas stream, the normal size of the
droplets sprayed is in the order of 1000 to 2500 microns
~ 20~3a71
s
in diameter. Thus, to increase the surface area exposed
to the gas phase and residence time, very high liquid to
gas (L/G) ratios and large towers must be employed, all
of which substantially increase the capital and
operating cost requirement. To effect good gas
distribution, a large number of spray nozzles must be
used, so that the tower cross-section is uniformly
covered with the spray pattern. However, failure of one
or two nozzles usually creates a path of least
resistance through which the gas can flow, thereby
reducing the efficiency of the apparatus.
In addition, the large size of droplets used in
spray towers reduces substantially the capability of the
apparatus to efficiently remove dust particles in the
low particle size range, typically less than 3 microns.
With the larger droplets, the decreased gas-liquid
surface area can be compensated for by increasing the
tower size, the number of spray headers, and
circulation rates of the scrubbing liquor, all of which
increase the tower space requirement, thereby initial
cost and energy consumption. Droplet entrainment and
mist elimination, while rather effectively being
addressed by the production of larger droplets, can
still be the "Achilles heel" of spray tower operation,
because it is the only part of the operation where gas
flow must be somewhat restricted. These limitations and
the fact that the spray and venturi apparatus each
offers advantages not shared by both, have given rise to
the development of combination gas reacting devices.
These combination arrangements generally combine the
features of venturi and spray apparatus into one
module. These recent designs offer greater performance,
allowing high removal efficiency of both gaseous
pollutants such as S02 and particulate matter such as
fly ash, but at a very high cost. It is, therefore,
desirable to provide an improved gas reacting apparatus
'' 20 ~ 5~ ~
which combines all of the advantages offered by
venturis and spray towers into one apparatus.
The problems and disadvantages associated with
prior art systems are overcome by the present invention
by providing a gas-reacting apparatus and a method
which is simple, economical and capable not only of
providing good turndown and gas-liquid distribution,
but also capable, on the one hand, of generating high
turbulence and many fine droplets of an aggregate
surface area many times larger than produced in a spray
tower of considerably larger size and, on the other
hand, of providing for a much longer available
residence time and higher surface area than in a
venturi, thereby effecting high removal efficiency of
both solute gases and fine particulate matter and yet
operating the apparatus with substantially decreased
amounts of liquid, low energy and space requirements.
It has been shown that the amount of liquid used by the
improved gas reacting apparatus is only about 2% of
that required by a suitable spray tower with comparable
efficiency.
In one aspect of the present invention, there is
provided a method for wet transferring at least one
solute gas from a process gas stream into a liquid
reacting medium capable of reacting with the at least
one solute gas, comprising: (a) providing an elongate
conduit having an inlet thereto and an outlet therefrom
and which is divided into a plurality of individual
gas-atomized liquid contact zones; (b) passing a gas
stream containing at least one solute gas into the
inlet end of the elongate conduiti (c) injecting the
liquid reacting medium capable of reacting with the at
least one solute gas directly into the gas stream under
a sufficient atomizing pressure from a plurality of
dual-fluid spray nozzles coaxially disposed in the
conduit one in each of the individual gas-atomized
L~
7 2~ 5357~ ~
liquid contact zones to form a spray pattern from each
of the nozzles filling homogeneously the cross-section
of the conduit in each of the individual gas-atomized
liquid contact zones and containing liquid droplets
ranging in size from about 5 to about 100 microns,
thereby to form a plurality of individual contact spray
zones whereby mass transfer of the at least one solute
gas into the reacting medium is carried out in a very
efficient way due to the large interfacial surface area
for mass transfer, turbulent mixing and relatively long
residence time generated therein; (d) contacting the
gas stream exiting each individual gas-atomized liquid
contact zone with demisting means to agglomerate and
remove entrained droplets from the gas stream before
the gas stream passes to the next such zone and out of
the outlet end; and (e) discharging a clean gas stream
from the outlet end of the conduit.
According to the invention, therefore, a gas
stream containing solute gases or both solute gases and
particulate matter is passed through a conduit and
contacted while flowing through the conduit by at least
two sprays of liquid or slurry, preferably injected
countercurrent to the gas stream, in separate gas-
liquid contact zones.
In the conduit, the liquid or slurry absorbing-
reacting medium is finely atomized by nonplugging,
dual-fluid nozzles, which are preferably centrally
disposed and spaced in series in the conduit to form
two or more contact spray zones, and adapted to spray
droplets in the range about 5 to about 100 microns,
more usually about 5 to 30 microns. By spraying such
liquids or slurries into a suitable reaction chamber, a
tremendous number of droplets are generated along with
very high surface area. For example, if only 5 micron
droplets are generated, each kilogram of water yields
' 2 0 5 3 5 7
7a
about 1.5 x 1013 droplets which have a surface area of
about 1200 square meters. On the other hand, in a
r~
2053~71
~ !~
traditional system, if only 1000 micron droplets are
generated, each kilogram of water yields about 1.9 x 106
droplets which have a surface area of about 6 square
meters. These surface area figures as shown above are
by orders of magnitude greater than generated by the
commercially-available devices presently used for this
service. Since the mass transfer that a given
dispersion can produce is often proportional to (l/D),
fine droplets are greatly favoured.
The droplets ejected from each nozzle agglomerate
and either contact the duct walls and drain or fall to
the bottom of the duct before the next nozzle stage.
Liquid droplets that remain entrained do not
significantly affect the reaction kinetics of the next
stage. Intermediate entrainment separators enhance this
effect. Where gas solubility is an important rate
governing factor, non-equilibrium conditions can be
employed, since the surface area of the droplets
decreases as they coalesce and are removed from the
system trapping the absorbed gases, resulting in very
high removal efficiencies under highly favourable
kinetics.
Upon intimate contact of the solute gas and
particulate matter with the sprayed absorbing-reacting
medium, transfer of the solute gas and particulate
matter from the gas stream to the absorbing-reacting
medium takes place. The removal of the solute gases so
effected may be a purely physical phenomenon of
absorption of the solute gas in the medium or may
involve solution of the solute gas in a liquid or a
slurry suspension, followed by chemical reaction with
one or more constituents in the liquid or the slurry
medium, to form a soluble by-product or a solid reaction
by-product. The term "reacting medium" used herein to
describe the medium in which the solute gas absorbed,
therefore, includes both media in which the solute gas
2053571
is absorbed and from which the solute gas subsequently
may be desorbed and media in which the solute gas is
both absorbed and chemically converted to another form.
The resultant liquid or slurry-laden gas stream may
be subsequently drawn into a slowly-turning fan that
provides turbulent mixing and additional residence time
plus an environment for continued absorption and
reaction, and for efficient coalescence or agglomeration
of the entrained, sprayed liquid or slurry and its
subsequent removal from the system by a simple gravity
drain in the fan casing. An entrainment separator is
located downstream from the fan to complete the removal
of agglomerated liquid phase (including slurries) from
the system. Where little or no particulate material is
present in the gas stream, the fan may be replaced by a
much more efficient I.D. fan downstream of the conduit
to draw the gas stream through a suitable static
demister.
The linear flow velocity of the gas stream
containing the at least one solute gas through the
conduit may be varied widely, generally at least about
1.5 meters per second, for example about 3 to 15 or more
meters per second. The flow normally is non-laminar in
the turbulent zone.
The conduit in which the solute gas is removed may
be provided in any desired cross-sectional configuration
which permits the fine liquid droplets to be distributed
across the cross-section sufficiently to effect the
desired absorption of solute gas. Often the conduit is
round but a square cross-section also may be employed,
along with other profiles which have a regular geometry.
The present invention broadly relates to the
removal of a solute gas from a gas stream using some
form of liquid scrubbing medium in which at least one
solute gas is absorbed. The invention is particularly
illustrated with respect to gas streams containing
2053571
.,
sulfur dioxide, with or without attendant particulate
material, but the principles thereof are broadly
applicable to a variety of situations where it is
desirable to remove a component from a gas stream prior
to its discharge.
Another important application of the present
invention is to the removal of bleach plant emissions
from a bleached pulp mill. Such emissions, primarily
chlorine and chlorine dioxide, may be treated in
multiple stages with a suitable solvent medium which
removes the chlorine and which reduces the chlorine
dioxide and neutralizes the resulting acids.
Another important application of the present
invention is to the removal of odiferous components,
mainly in the form of various sulfur compounds, from gas
streams arising in pulp mill recovery boilers, in sewage
treatment plants, meat rendering plants and the like, by
use of a suitable scrubbing medium.
A further application of the present invention is
to the removal of acid gas, such as hydrogen sulfide and
carbon dioxide, from various gas streams, using aqueous
alkanolamine solutions. Gas streams containing one or
both gaseous components arise from various sources,
including natural gas plants, refineries, ammonia
synthesis units and hydrogen plants. Suitable
alkanolamines which may be used include
monoethanolamine, diethanolamine, diglycolamine,
diisopropylamine and methyldiethanolamine.
Other acidic gas streams containing, for example,
HCl or SO3, may be treated with a suitable reacting
medium for the solute gas. In addition, gas streams
containing NOx gases may be treated by the process of
the invention to remove the same therefrom.
Removal of SO2 from gas streams may be effected by
contacting the gas stream with a suitable scrubbing
medium. For example, the scrubbing medium may be an
~ 2053~71
11
aqueous alkaline medium, such as an aqueous alkaline
slurry medium, for example, a limestone slurry in which
the absorbed SO2 reacts with the limestone.
Alternatively, the reacting medium may be a metal oxide-
based slurry medium, for example, an iron oxide slurryin which the absorbed SO2 reacts with the iron oxide.
One class of absorbing medium which may be used
with SO2 is one in which gas is reversibly soluble, such
as an amine solution. When such reversible absorbing
media are employed for absorption of SO2 or other solute
gases, the degree of loading of the medium by absorbed
gas is very high and generally beyond normally expected
levels, and sometimes beyond equilibrium levels. This
effect permits the process of the invention to operate
at lower liquid-to-gas ratios (L/G) that other solute
gas removal procedures and often less than 1.
The procedure of the present invention also may be
employed to remove slightly soluble unreactive gases,
i.e. VOC's, from gas phase to the liquid phase.
Examples of such gases include ethers, alcohols,
ketones, acetates, toluene, benzene, xylene etc. The
degree of transfer which can be obtained is up to many
times greater than would be predicted by Henry's law,
i.e. the vapor-liquid equilibrium relationship between
the concentration of at least one component to be
removed in the gas and the concentration of that
component in the liquid sorbent is linear.
The apparatus provided in another aspect of the
invention may also include means for quenching and
cooling a hot gas stream, such as that emanating from
electric utilities or smelters, with an aqueous medium
(water or other liquids), and to saturate the gas stream
with water vapor, prior to the removal of the solute
gases.
The apparatus of the invention may also include an
effluent hold tank for closed loop recycling of the
12 20 ~3 57 ~
reacting medium and its regeneration for recycle as
fresh make up feed, plus a pumping means to introduce
the absorbing-reacting medium into the spray nozzles at
the appropriate pressure.
In addition, demisters are provided between the
spray devices in the conduit, with the collected liquid
being reintroduced to the immediate upstream stage so
as to pass countercurrent to the flow of gases through
the conduit, if desired.
Overall, what has been developed is an improved
gas-reacting method and apparatus in which accelerated
absorption and, optionally, reaction of solute gases in
a reacting medium can be effected due to the large
surface area, intimate contact, relatively long
residence time, and turbulent mixing prevailing
therein, thereby overcoming the problems of the prior
art, as discussed above.
While the invention will be described further,
particularly with reference to the removal of solute
gases, either by absorption or absorption accompanied
by chemical reactions, it is to be understood that the
invention is also useful in the conduct of gas-gas
reactions and subsequent wet separation of the re-
sulting reaction product, in the removal of particulate
matter, in the humidification of gases and in reaching
a thermal equilibrium between a gas and a liquid.
In a preferred embodiment, the absorption, with or
without accompanied reactions, is conducted in the
improved gas reacting apparatus wherein the unexhausted
reacting medium and the reaction products are
agglomerated and thereby removed from the gas reacting
apparatus as a coherent liquid or slurry mass,
depending on the reacting system selected. In most
instances the resulting slurry can be recirculated
until some optimal concentration is reached, at which
point a
20S3~71
..,
13
bleed stream can be removed for further treatment to
recover product or for regeneration and recycling
purposes, while fresh makeup feed is introduced into the
system prior to recirculation.
One important feature of the improved gas-reacting
apparatus resides in its ability to remove both solute
gases and particulate matter simultaneously with high
efficiency, due to the large effective interfacial
surface area and the excessively large number of
droplets introduced to the system, coupled with
turbulent mixing and sufficient residence time that can
be effected therein. Still another significant advantage
of the improved gas reacting apparatus, particularly in
comparison with the venturis of the prior art, is its
ability to accommodate a very high turndown ratio
through a simple adjustment of the gas-side pressure
drop across the spray nozzles or the amount of liquid
sprayed or both simultaneously.
Yet another advantage is an ability to provide
spray zones of uniform density and, therefore, to yield
even gas distribution due to the nozzles being coaxially
spaced apart in series within the conduit. The spray
zones completely cover the cross sectional area of the
conduit and yet without overlapping one another, thereby
providing good gas and liquid distribution even under
upset conditions associated with a nozzle failure.
This distribution of spray zones is preferably
achieved with a unique dual-fluid, atomizing spray
nozzle design of the type depicted in the drawings
described below that has more precise gas-liquid mixture
control and allows for the flexibility required to
control size and number of droplets necessary for
efficient removal of solute gases. Such dual-fluid
spray nozzle design is covered by U.S. Patent No.
4,893,752, assigned to the applicant hereof. The dual-
fluid spray nozzles generally operate at about 20 to
2053571
14
about 100 psi, usually at about 20 to about 70 psi,
preferably about 25 to about 55 psi.
The cumulative results of the above-described
advantages is a gas reacting method and apparatus which
is more economical, more efficient, more compact and
easier to handle than any other more conventional
device. Also, being a relatively small piece of
equipment, it can be custom fitted/retrofitted or
configured to meet various specific site requirements.
In one preferred embodiment, a contact chamber is
provided located ahead of the scrubber for the removal
mainly of particulates from the incoming gas stream and
is useful, not only in the treatment of gas streams
containing solute gases which contain particulates but
also particulate-contaminated gas streams which do not
contain such solute gases. One example of the latter
gas stream is one containing submicron size particles,
for example, sodium chloride in a pulp mill boiler
product gas stream.
Where the gas stream contains both particulates and
solute gas, provided that at least about 90% of
particulates are removed ahead of the dual-fluid sprays
in a contact chamber, then a coalescing fan generally
is not required at the downstream end of the conduit but
rather a simple demister may be employed to remove the
liquid droplets from the gas stream.
In one such contact chamber, the entrance and exit
are located on opposite sides of a vertically-located
baffle extending normal to the gas flow. Such an
arrangement causes the incoming particulate-laden gas
stream to impinge on the baffle and then to pass under
the baffle to reach the exit. The performance of the
contact chamber is significantly enhanced by the
introduction of spray nozzles of the type described
above for removal of solute gases from the gas stream,
one of such nozzles being located to spray cocurrently
~ ~- 20~3~71
with the gas stream flow and the other located to spray
countercurrently to the gas stream.
The nozzles usually are located in the entrance and
exit respectively of the chamber and impinge on the
baffle and preferably are arranged so that the sprays
also substantially fill the inlet and outlet ducts and
the entrance of the gas stream to and the exit of the
gas stream from the contact chamber. The dynamic action
of these fine sprays on the particulate-laden gas stream
combined with the structure of the contact chamber
results in removal of significant quantities of
particulates from the gas stream, often up to 90% or
more, regardless of the particle size.
The gas stream passing from the contact chamber is
significantly depleted with respect to particulate
content, enabling very high overall efficiencies,
generally in excess of 98%, of particulate removal to be
effected when the contact chamber is used as a
prescrubber before the conduit in which the solute gas
and the remainder of the particulate material are
removed.
The contact chamber also provides the additional
residence time often required to achieve more than about
99% removal of certain acidic gases, notably SOx and
NOx, from the gas stream via the presence of suitable
reactants contained in the liquid sprayed into the
contact chamber, whether particulate materials are
present in the gas stream or not.
The contact chamber also may be used in a pre-scrub
mode to remove certain nuisance gases prior to treatment
for the removal of the target gas. For example, when
scrubbing for S02 removal and where the S02 is to be
recovered by regeneration of the scrubbing media, it is
necessary to remove S03 gas and H2S04 fume before
removal of S02. The removal of these gases can be
accomplished in a contact chamber using a recirculating
~ 16 2 ~ 5 3 S 7 ~ 1
mode which may contain H2SO4 + SO2 (saturated) and after
saturation with SO2, does not remove any further SO2
from the gas stream.
In addition, the contact chamber may serve to
provide effective quenching of hot gas streams to the
adiabatic dew point of the gas stream. In this way,
the gas stream becomes saturated with water vapor
before the gas stream enters the solute gas-removal
regime, so that the aqueous scrubbing medium does not
experience evaporative losses, which may give rise to a
variable level of solute gas absorption.
These and other characteristic features and
advantages of the invention disclosed herein will
become apparent and more clearly understood from the
further description given in detail hereinafter with
reference to the attached drawings which form a part
thereof.
The invention is described further, by way of
illustration, with reference to the accompanying
drawings, in which:
Figure 1 is a schematic sectional view of a gas
reacting apparatus and its accessories having two
separate single spray contact zones;
Figure 2 is an enlarged schematic sectional
illustration of a preferred single-orifice dual-fluid
nozzle (Turbotak* type) used in the apparatus of Figure
l;
Figure 3 is a schematic view of a portion of the
conduit shown in Figure 1 illustrating a cluster
(multiple orifice) nozzle incorporated into one contact
spray zone and includes a sectional view taken on line
C-C;
Figure 4 is a perspective schematic view of the
gas reacting apparatus of Figure l;
* - Trade-Mark
~,
17 2 0 5 3 5 ~
Figure 5 is a perspective schematic view of an
alternative fan arrangement used with the apparatus of
Figures 1 and 4 but having a common exhaust outlet for
both gas and coalesced liquid;
Figure 6 is a schematic representation of a two-
step application of the present invention to a typical
coal or oil-fired boiler exhaust gas stream for the
removal of gaseous pollutants and fly ash;
Figure 7 is a perspective, schematic represen-
tation of an embodiment of a gas reacting apparatus
illustrating a double-loop approach to absorption of
solute gases with low reacting medium usage; and
Figure 8 is a schematic representation of a
preferred form of a contact chamber for gas quenching
and particulate removal, as well as to provide
excellent contact for acidic or other gas removal;
Figure 9 is a schematic representation of an
alternative form of gas contact chamber for use in
environments where space constraints do not permit
normal horizontal flowi and
Figure 10 is a schematic illustration of an
alternative form of gas contacting apparatus,
constituting the present invention, consisting of three
contact stages for removal of a solute gas from a gas
stream, employing countercurrent flow of scrubbing
liquor and gas stream.
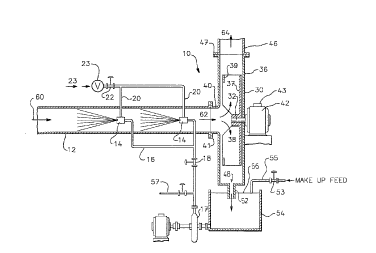
Referring to the drawings, the gas reacting
apparatus 10 shown in Figure 1 comprises in combination
an elongated housing 12 defining a primary reaction
zone and a fan 30 defining a post-reaction and
coalescence zone.
The elongated housing 12 comprises a relatively
straight conduit, preferably of circular cross-section,
having an inlet 60 for introduction of gas stream
containing solute gases alone or in combination with
r.
~5
17a 2 ~, S ~ 5 7 ~ ~
~ particulate matter and an outlet 62 at the other end
for the entry of the resultant liquid or slurry-laden
gas
B
2053~71
.
18
stream into the slowly turning fan 30. The conduit 12
may be positioned in any orientation with respect to the
ground level. However, the generally-horizontal
position or an orientation in which the conduit is
positioned at a slight angle with respect to the ground
level to permit gravitational flow through the conduit,
are preferred.
Within the conduit 12 are positioned a number of
atomizing spray nozzle means 14 for discharging liquid
or slurry sprays countercurrent as illustrated (or
cocurrent, if desired) to the gas stream flowing through
the duct 12. The atomizing spray nozzle means 14
provide a very fine spray and are capable of delivering
droplets in virtually any size distribution or quantity
required. Typically, the range of about 5 to about 30
microns liquid droplets is preferred. By spraying
liquid or slurry in the above droplet size range into
the conduit 12 a tremendous number of droplets having a
very large interfacial mass transfer area is produced.
For example, if only S micron droplets are generated,
each kilogram of liquid will yield about 1.5 x 1013
droplets which have a surface area of about 1200 square
meters. These figures are orders of magnitude greater
than generated by any other known contacting device.
In the preferred form as illustrated, liquid or
slurry atomizing spray nozzle means 14 comprises dual
fluid nozzles each capable of producing the above
droplet size distribution. The Turbotak dual-fluid
nozzle (shown in Figure 2) utilizing gas, i.e. air,
steam, etc., to impart the energy required to atomize a
liquid is very suitable for this purpose, particularly
when using a slurry as a reacting medium. One of the
features of the Turbotak nozzle of the type shown in
Figure 2 is that erosion of the orifices is virtually
non-existent. Multiple-orifice nozzles which may be
used in the present invention are described in the
2053571
19
aforementioned U.S. Patent No. 4,893,752.
For maximum turbulent mixing and gas-liquid contact
time, the scrubbing liquid or slurry preferably should
be introduced at a sufficient nozzle pressure and
velocity countercurrently to the gas stream to be
scrubbed to form the desired spray pattern needed to
cover substantially alI of the cross-sectional area of
the interior of the conduit 12 within a reasonable
distance, e.g. 5 feet from the nozzle. The geometry of
the spray issuing from the nozzles 14 and the exact
orientation of the nozzles 14 with respect to the
conduit 12, apart from being coaxially spaced, are not
critical. However, for a circular conduit 12, nozzles
14 producing a conically-shaped uniform spray pattern,
preferably with a spray angle of 15~ to 90~, is the
most advantageous to give adequate coverage of the
conduit cross-section. To effect maximum hold-up of
liquid and gas/liquid contact time, countercurrent flow
is used and the atomizing gas pressure preferably should
be high enough to impart to the liquid droplets
sufficient force to overcome the velocity of the
incoming main gas stream, so that no reversal of the
sprayed liquid by the high velocity incoming gas stream
can occur until a fully developed, conically-shaped
spray pattern, with its extremity touching the conduit
wall surface, is established, at which point the sprayed
liquid is turned back by the incoming gas stream and
becomes suspended therein. In this way, if all the
energy expended in the sprayed liquid is transferred to
the main gas with minimum loss to the conduit wall, a
very high degree of turbulence results as the liquid and
gas moving in opposite direction come together and the
liquid is forced to reverse direction. This high degree
of turbulence and increased liquid hold-up and
liquid/gas contact time provides extremely efficient
contact between liquid and gases to yield a very
- 20~3571
._
effective and accelerated mass transfer of solute gases
to the absorbing/reacting medium.
The rate of the flow and pressure of air through
the nozzle and thus the degree of atomization is
controlled by a pressure reducing valve 22 connected by
conduits 20 to the nozzles 14. Gas pressures in the
range of about 20 to about 60 psi, preferably about 25
to about 50, is supplied to the nozzle by a conduit
connected to a gas pressure source 23 through a gas
regulator (pressure reducing valve) 22. Under such
atomizing pressure conditions and a liquid usage of from
about 0.25 to 1.0 US gallon per 1000 acf (actual cubic
feet) of gas treated, the Turbotak dual-fluid nozzle has
been shown to be capable of generating liquid droplet
sizes in the range of about 5 to about 100 microns with
the majority of droplets having a size of about 5 to 30
microns.
For improved mass transfer operation, there may be
a number of atomizing spray nozzles 14 employed within
the conduit 12. The nozzles 14 are centrally disposed,
countercurrently or cocurrently oriented to the gas
flow, axially-spaced apart in series in the conduit and
adapted to spray droplets primarily in the size range
from about 5 to 30 microns, thereby creating a number of
well back-mixed zones in the conduit 12. Such
orientation of the nozzles results in very high
turbulent mixing and high interfacial surface area for
mass transfer. Depending on the atomizing pressure
employed, the spray nozzles preferably should be
positioned and spaced apart in series in the conduit,
so that the conically emerging spray patterns do not
substantially overlap each other. Generally, in the
above atomizing pressure range proposed, spacing of
approximately four to eight feet was found to be
adequate.
It has been found that the use of separated, spaced
21 2 ~ 5 3 5 ~ ~ ~
~~ spray nozzles 14 to provide at least two separate
gas/liquid contact spray zones in which oppositely
moving sprayed liquid and gas come together and the
sprayed liquid is forced to reverse direction, provides
for removal of typically over 99% of the sulfur dioxide
and over 99.6% of the particulate matter from a
synthetic gas stream when scrubbing with aqueous
caustic solution of 0.5M. This high efficiency is
accomplished with the use of about 0.5 USG per 1000 acf
of gas treated which is only 10% of that required by
most scrubbers with comparable efficiency.
An important feature of the scrubber apparatus of
Figure 1 resides is its low energy requirement. The
approximately < 1 to 4 H.P. expended into the liquid
per 1000 acf of gas treated and a gas pressure drop of
0" + W.G. measured across the device are considered to
be very low. To accomplish similar removal effects by
mechanically increasing the gas flow rate by means of
blowers pulling through a venturi throat involves
greater energy coupled with inferior results.
Another significant advantage of the gas reacting
apparatus of the present invention, particularly in
comparison with systems of the prior art, is its
ability to accommodate a high turn-down ratio when the
flow of the gas stream is decreased because of
decreased boiler load without the need for adjustments
by moving parts. As can be seen from Fig. 1, the
turndown capability of the gas reacting apparatus is
not affected by some mechanical limitation. In the gas
reacting apparatus of the present invention, the
interfacial area is not dependent on the gas flow rate
or the pressure drop. Hence, the solute gas removal
efficiency increases with reduction in gas flow. One
method to regulate make-up feed rate is by controlling
the effluent pH. Here a pH electrode probe activates a
signal that regulates the position of control valve 53
B
22 2~5~
~ to control the rate of make-up feed through line 55.
Other make-up feed control systems may be used, such as
controlling the inlet gas flow and solute gas
concentration or controlling the outlet solute gas
concentration as the control variable.
Although the gas reacting apparatus has been
described with reference to single orifice spray
nozzles creating separate spraying zones, it is desir-
able and practical in large scale applications where
conduits of large size are used to substitute for the
single spray nozzles with a multiple orifice nozzle
(i.e. a cluster nozzle such as described in the afore-
mentioned U.S. Patent No. 4,893,752) with a combined
spray pattern which substantially covers the cross-
section of the conduit in order to obtain maximumeffectiveness and space utilization. A schematic view
of a portion of the conduit illustrating a cluster
nozzle incorporated into one contact zone is shown in
Figure 3. In most large-scale applications, the ducts
in which the nozzles are mounted are rectangular in
cross-section, rather than circular. (see e.g. Figure
10) .
The apparatus 10 also comprises a low speed, motor
driven fan 30 downstream from the last spray nozzle 14.
The fan 30 is connected to the outlet of the conduit
12. In particular, fan 30 is of the radial-blade
centrifugal type and comprises a shaft 32 having a
bladed wheel 34 fixed thereto, the shaft and the bladed
wheel being coaxially positioned or supported in a
volute casing 36. In particular, fan wheel 34
(impeller) comprises a disc shaped member 37 fixed to
the shaft 32, a plurality of blade members 38 extending
from the disc 37 and equally-spaced around the shaft
32, and an annular rim 39 fixed to the edges of blades
38 and disposed in a plane parallel to the plane of the
disc 37. This type of structure, as shown in
B
2053571
23
Figure 1, was found to be self-cleaning and particularly
suitable for severe duty. other impeller types, such as
the forward curved, backward curved or inclined
structures, may be used but are not considered to be as
suitable as the simple radial-bladed fan illustrated.
The casing of the fan 30 is formed to include an
inlet 40 having an inner diameter smaller than the
diameter of annular element 39. Inlet 40 is connected
to the open end of conduit 12, and an annular joint 41
is provided to seal the connection. The fan opening
preferably should be sized to match the size of the
conduit or vice versa. However, a tapered inlet 40 or a
conduit gradually growing smaller toward the inlet of
the fan 30 can also be employed, causing the
compressible part of the gas stream to speed up, either
at the hub of the fan or in the conduit while the
incompressible part of the gas stream, i.e. "fly ash"
and liquid droplets, slows down, relative to the
velocity of the gas. The increase in the relative
velocity between the two phases results in lower gas
phase resistance and thus better scavenging action
against solute gas by liquid droplets. Also, the larger
differences in velocities of the liquid droplets and the
gas occur in such tapered inlet caused impaction and
results in better scrubbing action against particulates.
Another method to improve the turbulent mixing at the
inlet to the fan is by the use of variable guide vanes
to impart pre-rotation to the incoming gas stream in an
opposite direction to that of the impeller rotation.
The fan drive shaft 32 is connected to the drive
means in the form of an electric motor 42 for rotating
shaft 32 and fan wheel 34 at a relatively low speed.
The motor also includes suitable means 43 for
maintaining the speed in a desired range. Fan 30 also
includes means in the form of an gas exhaust passage
from fan 30. Exhaust passage 44 is at the upper portion
20~3~71
24
of casing 36 as viewed in Figures 1 and 4 and is
connected to one end of an output duct 46 for exhausting
clean gas from the apparatus. The connection of passage
44 to duct 46 is sealed by a joint 47. Fan 30 further
includes means in the form of an opening 48 provided in
volute casing 36 at the lower end, as viewed in Figures
1 and 4, for removing or collecting sprayed liquid and
particulate matter separated from the gas stream.
Opening 48 is in fluid flow communication with a
relatively short conduit or passage 52, disposed
generally vertically below the liquid surface of open
top tank 54 to provide a seal to prevent air passing
back into the fan and preventing proper drainage. In
slurry-based operation, the tank preferably should
include an agitator means (not shown in Figure 1) to
keep the solids in suspension. The tank 54 preferably
should include absorbing/reacting liquid 56 up to a
level above the bottom of the tube 52 to effect a proper
seal. In some cases, it may be desirable to exhaust
both the clean gas and the absorbing/reaction-liquid
from the same exhaust passage 44 for subsequent
separation. Such an arrangement is shown in Figure 5.
The distance between the last spray nozzle 14 and
inlet to the fan 30 is not critical when the nozzle is
countercurrently-oriented to the main gas stream flow.
However, in applications where the nozzle is directed
cocurrent to the gas stream flow, a minimum distance
upstream from the intake of the fan 30 generally is
required to permit the conically-shaped spray to fully
develop and fill completely the cross-section of the
conduit 12. In general, a distance of three to four
feet has been found to be satisfactory, depending on the
gas velocity in the duct.
The fan 30 also provides means to withdraw and move
the gas stream and to overcome the pressure losses
across the apparatus. In the fan, there is provided
2053~71
....
turbulent mixing and additional residence time plus
environment for continued absorption and effective
coalescence of the liquid droplets and their removal
from the system. Much turbulence can also be effected
in countercurrent operation as oppositely moving liquid
droplets suspended in an atomizing gas and the main gas
stream come together and the liquid droplets are forced
to reverse direction and to pass through at least two
spray contact zones formed by two or more spray nozzles.
The liquid-laden gas is drawn into the vortex of
the slowly-turning fan 30 in the direction indicated by
arrow 62 from which the solute gas-laden droplets and
the collected particulate matter exit into the liquid or
sludge trap provided by opening 48 at the bottom of fan
30, as viewed in Figure 1. Clean gas and some entrained
droplets not removed by the fan, exit through the fan
exhaust opening 46. The majority of the liquid droplets
first coalesced in the vortex created by the fan 30 grow
in size and then impinge and constantly coat the fan
blades 38, to form a layer of coalesced liquid and
solids that adheres to the rotary fan blades and is
separated from them mostly on the edges of the blades by
the effects of centrifugal force, moving outward in so
doing so as to form an attendant annular coarse spray
zone to further remove solute gas and particulate
matter. The liquid droplets adhering to the blades run
over the blades, washing them of collected particles.
The particulate matter and solute gas-laden liquid
is collected as it reaches the fan housing 36 and fan
casing and drains by gravity into a sump 54 through a
sealed drain 52. Because the impeller 38 and the fan
casing 36 are not coaxially aligned, the annular space
between the impeller and the fan casing increases toward
the exhaust opening, thereby preventing any blockage and
interference with the clean gas throughput capacity by
the sprayed liquid. Within the sprayed annular zones
2053~71
.."
26
formed as a result of centrifugal force imparted on the
liquid coating the blades the main gas stream that has
been agitated by the impeller 34 comes into intimate
contact with the reacting liquid discharged from the
blades 34, so that for all practical purposes,
additional removal of solute gases remaining in the gas
stream continues to take place.
The flow of clean gas with some entrained liquid
droplets not removed by the fan, continues through the
exhaust ducting 46 in the direction of arrow 64, from
which it can be discharged directly to the atmosphere or
into an entrainment separator for final removal of the
entrained liquid droplets. It was found that the fan,
upon rotating forward, can separate 85 to 90% of the
liquid droplets suspended in a gas stream while backward
rotation can separate some 95% of the suspended liquid.
Therefore, for gases containing heavy dust loadings or
where a higher overall removal efficiency is required, a
backward turning fan normally is recommended, although
the lower flow rate and static pressure may necessitate
either a larger fan or a booster fan in the system.
The provision of the fan, which constitutes an
integral part of the gas reacting apparatus, makes it
possible to use the same elements that serve to move the
gas and coalesce the liquid droplets also to provide the
turbulent mixing, additional residence time plus mass
transfer areas for continued absorption of solute gases
and removal of particulate matter from the gas that is
to be treated.
When the above-described scrubbing system is
employed solely as an absorber, i.e. where no
particulate matter is present, it is possible to
eliminate the coalescing fan as an integral part of the
scrubber and conduct the fine droplet laden gas stream
directly to an appropriately-designed mist eliminator
where the entrained liquid droplets are separated from
27 ~ ~ ~ 3 ~
the gas stream. The selection of the appropriate mist
eliminator is obvious to those skilled in this art. A
preferred mist eliminator consists of one or more banks
of chevron blades designed for this purpose. Other
devices can be employed, such as Kimre* fllament-type
preformed pads, etc.
The purpose in eliminating the fan is to reduce
the size and cost of this equipment which also provides
greater flexibility in equipment installation. The
pressure drop in the system is accommodated by an I.D.
fan which is normally placed between the absorption
equipment and the stack. Such I.D. fans have no pre-
imposed limits on fan speed and so are much less costly
and much more efficient than the style of scrubber fan
used in the scrubbing system when particulate levels
demand such.
In most coal fired power plants and similarly
large power producing plants, extensive particulate
control devices are conventional and are installed to
remove >99% of the particulates in the gas stream. For
such instances where SO2 control equipment is to be
retrofitted, it would be possible to eliminate the
scrubber fan as indicated above. One option to assist
in further removal of remaining particulate is to
install a contact chamber after the installed particu-
late removal equipment where the warm gas stream is
cooled to its adiabatic dew point and through the use
of the nozzles, up to 90% of the particulate still in
the gas stream can be scrubbed out while not effecting
removal of SO2 (see Figs. 8 & 9). SO2 removal then is
effected in the absorption section which then is
followed directly by a mist eliminator and again, a
scrubber fan is not required in this instance.
* - Trade-Mark
r~
j '.
27a 2 Q 5 3 5 ~
In bleach plant scrubbers where the off-gases con-
tain both chlorine (C12) and chlorine dioxide (Cl02),
multiple stage absorption of the above gases takes
place
r~
_ 205357 1
.
28
with agents that remove the chlorine and reduce the
chlorine dioxide and neutralize the resulting acids.
Again, since no particulate is present, it is possible
to eliminate the scrubber fan while adding an I.D. fan
to pick up the pressure drop and gas flow through the
system.
The entrainment separator 70 shown in Figure 4 to
which the clean gas is discharged from the fan 30 is
used to separate entrained liquid not separated by the
fan 30. Typically, a Chevron-type separator which may
be followed by a special mist eliminator packing (Kimre
preferred) both contained in the same housing were found
sufficient to clean the gas of any suspended liquid.
The clean gas then is discharged to the atmosphere via
duct 72 at 100% relative humidity, but virtually free of
liquid water content.
Referring again to Figures 1 and 4, a fresh make up
of reacting medium feed is added to a recirculation tank
54 through line 55. From the tank 54, the scrubbing
material is drawn through a pump 17 and is introduced
into the atomizing spray nozzles 14. Reacting liquid
recovered from the clean gas by the fan 30 and the
entrainment separator 70 is returned to tank 54 for
reuse, while spent scrubbing liquid is discharged
through line 57.
Since gas flow in the gas reacting apparatus 10 is
unrestricted, pressure drops are low, typically not
exceeding 2 to 3 inches W.G. including the entrainment
separator. This pressure drop is normally picked up by
the fan so that typically, the pressure drop across the
system, flange to flange, is zero inches W.G. If
desirable, the fan can also pick up total system
pressure drops up to about 6 to 8 inches W.G.
Figure 5 illustrates an alternate fan arrangement
in which a cyclonic separator 74 is used to effect
separation of gas and liquid drawn into the fan 30.
- - 20~3571
29
Referring to Figure 6, there is illustrated therein
the application of the present invention to a typical
coal- or oil-fired boiler exhaust gas for the removal of
S~2 therefrom.
As seen, the method lends itself to the use of
existing ductwork and I.D. fan, depending on the layout
of these in an available plant. Constraints of
residence time and temperature of a particular
application determine whether the existing layout is
practical.
As shown in Figure 6, the gas originating in a flue
gas duct 102 from a boiler 100 and exiting gas coolers
104 at temperatures normally ranging up to about 250~C,
but not limited to this range, enters a scrubbing area
15 106 for simultaneous S02 and fly ash removal using the
procedures described above. Adjacent the inlet of the
fan 108 a scrubbing medium is injected countercurrently
into the incoming flue gas stream through injection 110
to form at least two separate scrubbing zones covering
the cross-sectional area of the duct 112 adjacent to the
fan 108 whereby the flue gas is scrubbed. After
separation of the suspended liquid from the gas by the
fan 108 and a downstream entrainment separator 114 and
further reheating by gas heater 116, a clean gas is
discharged to the atmosphere through stack 118.
Quenching sprays (not shown in Figure 6) also may be
incorporated where the flue gases are hot to serve to
cool and saturate the gas stream with water vapour prior
to scrubbing.
Referring now to Figure 7, there is shown therein a
perspective schematic view of a double-loop slurry
approach to effect better utilization of slow reacting
solids in suspension such as limestone or iron oxide.
The use of a double-loop slurry procedure offers greater
flexibility because extreme operating conditions can be
segregated into discrete areas of the double loop
205~571
.1.~
system, allowing separate chemical and physical
conditions to be maintained. In the double loop slurry
procedure illustrated in Figure 7, a low pH slurry
solution contacts the entering gas stream in an initial
reacting loop 200 comprising an elongated conduit 202
and a plurality of atomizing spray nozzles 204 centrally
disposed and spaced apart from each other in the
conduit 202, and adapted to spray slurry into an
incoming gas stream whereby some solute gas removal
takes place. The slurry-laden gas stream exits from the
conduit 202, and enters a hydrocyclone 206 via a
tangential inlet 208 and swirls down about the vortex
finder. The swirling separated slurry concentrate
flows down the cone section to the apex opening 210
which is sealed by a joint to the top of a vertically
disposed conduit, the other end of which terminates in a
sludge effluent hold tank 212. The slurry-free,
partially-clean gas passes upwards through the vortex
finder to the outlet 214, then to another conduit 216
which is part of a second reacting loop designed for
almost complete removal of the remaining solute gas.
In the second loop, a high pH slurry or solution is
contacted with the partially clean gas in the conduit
216, where the bulk of the solute gas removal takes
place. The second loop comprises an elongated conduit
216, a plurality of spray nozzles 218 coaxially disposed
in series to form a number of reacting zones, a fan 220,
an effluent hold tank 222 and an entrainment separator
224. Spent slurry from this loop is discharged to
first loop via pump 226 and line 228 where the unused
reagent is consumed, thereby proving efficient reagent
utilization. Fresh make-up reagent need be added only
in the second reacting loop.
This type of design, incorporating two reacting
loops in conjunction with the gas reacting apparatus of
the present invention, takes advantage of the concept of
~ 2053~71
contacting a gas stream containing the highest solute
gas concentration with the lowest liquor alkalinity in a
first loop to effect good reagent utilization and
relatively low solute gas removal and the highest
liquor alkalinity with the lowest solute gas
concentration in a second loop to effect poor reagent
utilization, but good solute gas removal. The reduced
solute gas removal in the low pH loop (lower alkalinity)
is more than offset by improved performance of the
high-pH loop (higher alkalinity).
Referring now to Figure 8, there is illustrated
therein a preferred contact chamber 300 which effects an
initial treatment of the gas stream and provides a feed
to a scrubbing apparatus comprising a single spray
nozzle 310 spraying liquid into the gas flowing in the
duct 312 to a fan 314, operating in the manner
described previously. The nozzle 310 may be
supplemented by further nozzles, as required. The
contact chamber 300 is intended to increase turbulence
and residence time of the gas stream in a manner
superior to the three spray nozzle arrangement of Figure
4. This arrangement is of particular significance when
a mixture of acid gas and particulates is to be
processed with a high level of particulates.
The contact chamber 300 is enlarged in volume in
comparison with the duct 312 and comprises a baffle 316
located transversely to the gas flow and a pair of
nozzles 318, 320, each arranged to spray liquid at the
baffle 316. The contact chamber 300 is able to remove
over 90% of the particulates contained in the entering
gas stream in line and the resulting slurry is
conveniently drained, usually continuously, from the
lower portion of the chamber 300 by line 322.
It may be necessary to agitate the liquor contained
in the lower portion of the chamber to maintain
particulates in suspension to facilitate removal of the
2053571
slurry, especially if large quantities of particulates
are removed from the contact chamber relative to the
amount of liquid used therein.
For removal of fly ash and sulfur dioxide from a
coal-fired boiler, water sprays from nozzles 318 and 320
may be used in the contacting chamber 300, which would
remove substantial amounts of fly ash but only minor
quantities of sulfur dioxide. The solids may be
separated from the slurry removed by line 322 by
thickening and/or filtration and thereafter sent to
landfill. The aqueous phase from such separation, which
is acidic from the dissolved SO2, may be recycled with
make-up to the contacting chamber nozzles or may be made
basic and used as make-up liquor for the SO2 removal
stage at the nozzle 310. The nozzle 310 is fed with a
basic aqueous solution to remove the gaseous SO2 in the
duct 312 downstream of the contacting chamber 300.
In the removal of sulfur dioxide from a
particulate-laden gas stream, it generally is desirable
first to remove the particulates from the gas stream.
Such an operation may be achieved by using an aqueous
scrubbing medium which is recycled within contact
chamber 300 to the nozzles 318 and 320 and is saturated
with respect to sulfur dioxide, so that removal of
sulfur dioxide in the contact chamber 300 cannot be
effected to any degree. Following such contact, phase
separation is effected to remove all particulates
contained in the scrubbing liquor, prior to recycle
within the chamber 300.
Alternatively, a basic solution may be fed to the
nozzles 318, 320, which has the effect of removal of
larger quantities of SO2 in the chamber 300, so that
lesser quantities are required to be removed in the duct
312. If longer residence times are required, a second
contact chamber may be used and thereby enhance SO2
removal.
21~53571
33
It may be desirable under some circumstances to
employ an entrainment separator between the contact
chamber 300 and the nozzle 310 to remove droplets from
the gas stream and to assist in maintaining the specific
conditions conducive to each stage.
Any particulate material remaining in the gas
stream following the contact chamber 300 and entering
the scrubber section at nozzle 310 is removed from the
gas stream along with the SO2. Thickening or filtration
of the resulting scrubbing liquor separates out the
solids. If sufficient particulate removal is effected
from the gas stream in the contact chamber 300, then the
coalescing fan 314 may be replaced by a demister to
remove liquid droplets from the treated gas stream.
For the case where a water-soluble scrubbing agent
is used, for example, sodium hydroxide or sodium
sulfite, the filtered solution may be contacted with a
hydrated lime slurry in a conventional dual alkali
process with the basic sodium sulfite being returned to
the nozzle 310 as regenerated absorbing medium.
An alternative arrangement is shown in Figure 9, in
which inlet pipe 321 is in a vertically-downward
orientation, with the nozzle 318 again located in the
entrance to the chamber 300'. This arrangement is
useful where space constraints do not permit the normal
horizontal flow. An optional additional nozzle 326 may
be provided on the downstream side of the baffle 316 for
additional counter-current scrubbing, as required, both
in chamber 300 and 300'. Additional counter-current
nozzles 324, 328 can be added when it is desirable to
effect higher removal efficiencies of particulates in
the contact chamber 300 or 300'.
In cases where it is desirable to avoid a wet-dry
interface where particulates in a warm gas stream would
tend to deposit, such as the entrance to the chamber
310' where nozzles 318 is located, a downwardly-tapered
34 2û ~
~ entrance duct 321 oriented vertically downwardly is
employed, with a continuous film of water on the inner
wall of the duct 317 flowing downwardly, which can be
introduced by a wash ring 317.
In a further alternative arrangement, the dual-
fluid spray nozzle 318, in the embodiment of Figure 8
or 9, may be located to spray the aqueous contact
medium countercurrent to the direction of flow of the
gas stream entering the chamber 300. For example, the
nozzle 318 may be located vertically axially over the
lower closure to the contact chamber 300 or 300' to
spray contact medium vertically upwardly towards the
gas inlet port, parallel to the baffle plate 316. In
this arrangement, the spray nozzle 320 may be located
axially in the upper closure to the chamber 310 on the
gas discharge side of the baffle 316 to spray contact
medium vertically downwardly, countercurrent to the gas
flow direction, somewhat as shown for nozzle 324.
Referring to Figure 10, there is illustrated
therein an embodiment of apparatus 400 for removal of
solute gas from a gas stream which is relatively
particulate-free and which does not employ a droplet
coalescing fan, such as is employed in the embodiment
of Figure 1, and which is provided in accordance with
the present invention. Not shown in Figure 10 is a
contact chamber which would normally be present to
quench the gas stream to its adiabatic dew point
temperature, thereby saturating the gas stream before
entering the solute gas removal regime, otherwise water
would be lost to evaporation from the aqueous SO2
scrubbing medium, thereby giving a variable level of
dissolved concentration of SO2 in the scrubbing medium.
In particular, the sulfur dioxide-containing
gaseous emissions from a coal-fired power plant tend to
be relatively free from particulates, since the gas
34a 2 n 5 ~ ~ 7 1 ~
stream conventionally is passed through a high
efficiency electrostatic precipitator, and is
B
~ 2053a71
particularly suited to treatment by the apparatus 400.
Apparatus 400, which takes the form of an in-duct
scrubber, comprises a duct 410 which is divided into
three individual chambers 412, 414 and 416 by mist
eliminators 418, 420 and 421, which serve to coalesce
and remove liquid droplets from the gas stream passing
from chamber 412 to chamber 414, from chamber 414 to
chamber 416, and from chamber 416 out of the duct 410,
respectively. In each of the chambers 412, 414 and 416
is situated a dual-fluid spray nozzle 424, which is
arranged to form a uniform conical spray of absorbing
medium which is countercurrent to the direction of flow
of the gas stream 426, in each of the chambers 412, 414
and 416.
The downstream end of the chamber 416 is connected
through the mist eliminator 421 via a duct 428 to a high
efficiency mist eliminator 430, before the solute-gas
free and liquid droplet-free gas stream is discharged by
line 432. Any convenient fan mechanism may be used to
provide the necessary pressure difference to carry the
gas stream through the in-duct scrubber 400 to the exit
from the mist eliminator 430, such as an induced-draft
(I.D.) fan 434 located at the downstream end of the
entrainment chamber 430.
Scrubbing liquor for the solute gas passes
countercurrent to gas flow through the duct 410. Fresh,
or regenerated, scrubbing liquor is fed by line 436 to
the spray nozzles 424 in the chamber 416 to contact the
gas stream passing from the upstream chamber 414 and
remove any residual sulfur dioxide or other solute gas
remaining in the gas stream. The solute gas-containing
liquid droplets are coalesced and the resulting
coalesced liquid, containing dissolved solute gas is fed
to a scrubbing liquor storage vessel 444.
Coalesced liquid from the last section in the
demister 430 is passed by line 450 to the first liquid
2053571
36
storage vessel 442, while coalesced liquid from the
chamber 416 and from the other sections in the demister
430 pass by lines 452, 454 and 456 respectively to the
second liquid storage vessel 444. Liquor collected in
the first vessel 442 is recycled by line 458 to the
demister 430.
Liquor collected in the second vessel 442 is
forwarded by line 460 to the spray nozzle 424 in the
chamber 414 to contact the gas stream passing from the
upstream chamber 412 and to remove further quantities
of sulfur dioxide, or other solute gas from the gas
stream. The solute gas-containing liquid droplets in
the gas stream are coalesced in the demister 420 and
coalesced liquid is forwarded by line 462 to a third
liquid storage vessel 446.
The liquid in the third storage vessel 446 is
forwarded by line 462 to the spray nozzle 424 in the
chamber 412 to contact the gas stream 426 entering the
duct 410 and to remove sulfur dioxide or other solute
gas from the gas stream. The solute gas-containing
liquid droplets are coalesced in demister 418 and the
resulting coalesced liquid containing dissolved solute
gas is fed by line 464 to a fourth liquid storage
vessel 448. Pregnant scrubbing liquor is removed from
the fourth vessel 448 by line 466 for regeneration or
discard, as appropriate, depending on the nature of the
scrubbing liquor.
The nature of the scrubbing liquor which is
employed in the apparatus 400 depends on the solute gas
to be absorbed from the gas stream. The scrubbing
liquor generally is aqueous and one from which the
absorbed solute gas may be regenerated. For sulfur
dioxide, for example, as the solute gas, the scrubbing
liquor may be an aqueous amine solution in which the
sulfur dioxide is sorbed, and from which the SO2 is
desorbed to regenerate the amine solution for contact
20~3571
with further gas stream. The amine usually is a
diamine, which may be alicyclic, cyclic or heterocyclic,
and which generally contains secondary or tertiary amine
groups.
When using an amine to remove S02 from the gas
stream, complete removal (> 99~) of S~2 is achievable
in two or three scrubbing stages using an L/G <1 with
the solvent leaving the third stage fully loaded with
SO2 .
In the duct 410, therefore, the solute gas-
containing gas stream is contacted countercurrently by
the scrubbing liquor, so that the gas stream, itself
containing decreasing concentrations of solute gas, is
contacted by a scrubbing liquor having a decreasing
concentration of dissolved solute gas in the direction
of flow, and each contact stage is followed by a
demisting operation to remove solute-containing liquid
droplets from the gas stream before the next contact
step.
The in-duct contact apparatus 400 may be employed,
as noted above, for removal of sulfur dioxide from the
tail gas from coal-fired boilers. Similarly, the
apparatus may be used for the removal of chlorine
dioxide from bleach plant emissions. The ability to
employ simple mist eliminators for liquid droplet
removal and simple induced draft or forced draft fans
leads to a substantial cost savings in comparison to the
embodiment of Figure 1, where the fan is required to
perform turbulent mixing and agglomeration functions
associated with the presence of significant quantities
of particulates.
When the apparatus 400 is employed to effect
removal of SO2 or other solute gas from the gas stream,
it will be seen a staged counter-current flow of
absorbing medium and SO2-containing gas stream is
effected. The S02-depleted gas stream is exposed to the
38 2 ~ ~ ~ 5 7 ~ ~
"- regenerated absorption medium to ensure high overall
SO2 removal efficiency in the exiting cleaned gas
stream. At the same time, the partially-loaded
absorbing medium is exposed to the increasingly rich
gas stream containing the maximum amount of SO2, which
then ensures complete loading of the reagent with
absorbed SO2 prior to regeneration.
The apparatus 400 also may be adapted to employ
different reagents in the separate stages of scrubbing,
with each stage effectively being isolated from the
next stage by the demisters 418 and 420. Similarly,
rather than the countercurrent flow of the scrubbing
liquor with respect to gas flow, the same scrubbing
liquor may be fed in parallel to each of the several
stages of scrubbing, again with each stage of scrubbing
being isolated from the next stage by the demisters 418
and 420. The scrubbing liquor may be regenerated, as
required, prior to being recycled to the absorption
equipment.
The following specific Examples illustrate the use
of gas reacting apparatus for the purpose of removing
SO2 from synthetic gases by NaOH and NH3 aqueous
solutions and a lime slurry containing MgO.
EXAMPLE I
This Example illustrates the use of the gas
reacting apparatus of Figure 1 for the purpose of
removing SO2 from a synthetic gas stream containing
about 1100 ppm SO2, 21%V ~2 and the balance nitrogen,
by absorption into aqueous NaOH solution of sufficient
concentration of active sodium alkalinity.
In this type of removal, absorption accompanied by
chemical reaction takes place between the SO2 and NaOH
to form soluble sodium-based sulfite, bisulfite and
sulfate compounds, which effectively traps SO2 in the
solution. With the caustic system having an initial
20S3571
,~
39
active sodium concentration of 0.3 M (pH 12.4), a
liquid- to-gas ratio of 1.0 USG per 1000 acf of gas
treated and a ratio of active molar concentration of
sodium to moles of SO2 inlet of 2:1, 99% SO2 removal
s was effected. To effect the same degree of SO2 removal
but at a lower pH of 6.2, a L/G ratio of 4.75:1 was
required.
When the concentration of the aqueous NaOH solution
was increased to 0.5M active Na (pH 12.5), a liquid-to-
gas ratio as low as 0.5 USG per 1000 acf of gas treated
was required to operate the reactor to effect 99% SO2
removal. These results were obtained by using three
spray nozzles in series to form three separate reacting
zones within a single conduit. The nozzles were
oriented countercurrently to the gas stream flow. Thepressure drop across the conduit was less than 2 inches
W.G. during these tests.
EXAMPLE II
This Example further illustrates the use of the gas
reacting apparatus of Figure 1 for the removal of SO2
from synthetic gas stream containing about 1100 ppm SO2,
21%V ~2 and the balance nitrogen, by scrubbing with an
ammoniacal solution.
The SO2 removal efficiency averaged well above 95%
which was maintained at this level as long as the
NH3-t~-sO2 feed stoichiometry was higher than 1.9:1.
With an NH3-to-SO2 feed stoichiometry of from 1.8 to
2.0:1, the effect of liquid flow rate on SO2 removal
over the range of liquid rates of 0.005 to 0.5 USGM
(corresponding to a L/G ratio range from 0.17 to 1.15)
was observed to be minor and in general high removal
efficiencies were obtained ranging from 95 to 99%.
In these tests, the ammonia gas feed to the system
was introduced with the atomizing gas (air) into three
pneumatic, dual-fluid nozzles coaxially disposed in
series in a conduit and using recycled scrubbing liquor
2053571
., ~
as the liquid phase. It was observed that the high
turbulence, swirling and pressure conditions prevailing
at the nozzles enhanced substantially the chemisorption
of the sulfur dioxide in the sprayed liquid phase.
In this method of ammonia injection, there was also
evidence of substantial suppression of a plume (commonly
associated with ammonia scrubbing operations) exiting
the apparatus in all of the tests so conducted and it
may have been due to the manner in which the gaseous
ammonia was admitted to the system.
The Table below shows the S02 removal efficiency
obtained as a function of the reactor outlet pH and
NH3/So2 stoichiometry employed at an L/G of about 1.0
USG per 1000 acf of gas treated.
2053571
.. ~
Table
NH3/S02 Reactor pHS02 Removal Efficiency
Stoichiometry
1.09 3.9 55
1.24 5.4 81
1.52 6.6 92
1.89 7.4 95
2.00 8.5 99
EXAMPLE III
This Example further illustrates the use of the gas
reacting apparatus of Figure 1 for removal of SO2 from a
synthetic gas stream using a lime slurry containing MgO.
In this case a dolime assaying 35.9 wt% Ca and 20 wt% Mg
in the form of a finely divided powder, was slaked to
give a slurry of some 1.9 wt% solids loading.
A synthetic gas stream containing about 1200 to
1400 ppm SO2 and 21%V oxygen was produced at the rate
of 550 to 650 acfm by adding SO2 gas from cylinders to
the inlet air stream. The temperature of the gas was
ambient.
The system was operated in a recirculating mode
during which continuous addition of make up dolime
slurry was added at the rate of 0.32 lb/min for 145
minutes to provide for the required stoichiometric
amount of alkalinity and to maintain the recycled tank
pH at a prescribed level of between 6.0 and 7Ø Under
such pH conditions, it was found that a high
concentration of dissolved alkalinity (present as
magnesium sulfite) in the reacting liquor occurred,
resulting not only in a well-buffered reacting solution
but also in a scale-free operation of high reliability.
S~2 removal of 95 to 97% was achieved with the gas
reacting apparatus at gas-to-liquid ratio of 4.5 gal/103
acf. This scrubbing efficiency remained close to the
above values for the duration of the test.
Operating experience with the gas reacting
20~3~71
._
42
apparatus of Figure 1 using different commercially
available reacting agents has shown that, for most of
the systems studied, under optimal pH conditions and
reagent concentration, an L/G of only 1 to 5 USG per
1000 acf of gas treated appears to be adequate to
maintain uniformly and consistently high SO2 removal.
For reactive systems, such as the sodium and ammonia-
based systems, the apparatus provides excellent S02 gas
removal in excess of 99% and, if necessary, also
efficient simultaneous removal of particulate matter in
excess of 99.6%, and yet permitting a liquid-to-gas
ratio in the range from 0.17 to 0.5 USG per 1000 acf of
gas treated.
This low L/G ratio requirement employed by the gas
reacting apparatus of the invention should not only
reduce both capital and operating costs to a fraction of
the costs related to traditional removal devices, but
should also enable easy integration into flue gas
ductwork of existing oil or coal-fired boilers due to
its compact size. As shown particularly in Figure 6,
the apparatus of the invention can be configured to
easily meet site requirements.
In summary of this disclosure, the present
invention relates to the gas/liquid, gas/liquid/solid
and gas/gas/liquid mass transfer art and more
particularly to an improved method and gas reacting
apparatus for wet mass transferring of solute gases from
process gas streams into a liquid or slurry reacting
medium, wherein the mass transfer operation may be a
purely physical phenomenon or may involve solution of
the material in the absorbing liquid or slurry, followed
by reaction with one or more constituents in the
absorbing liquid or slurry medium.
The improvement provides an apparatus in which
accelerated absorption and reaction of solute gases can
be effected as a result of the large interfacial surface
20~3~71
43
area for mass transfer, plurality of reaction zones,
intimate contact, increased residence time and turbulent
mixing prevailing therein.
While an improved apparatus and method have been
described in detail, various modifications, alterations
and changes may be made without departing from the
spirit and scope of the present invention as defined by
the appended claims.