Note: Descriptions are shown in the official language in which they were submitted.
WO 91/06132 PCT/US90/05638
253887
CELL FOR MAKING SECONDARY BATTERIES
BACKGROUND OF THE INVENTION
The invention described herein arose in the course of
or under, Contract No. DE-AC03-76SF00098 between the
United States Department of Energy and the University
of California, and the United States Government has
rights in the invention.
The present invention relates to metal-sulfur type cells
for making secondary batteries, and particularly to
dells operating with all components thereof in the solid
state.
Secondary batteries are in widespread use in modern
society, particularly in applications where large
amounts of energy are not required. However, it is
desirable to use batteries in applications requiring
considerable power, and much effort has been expended
in developing batteries suitable for such high power
applications as electric vehicles. Of course, such
batteries are also suitable for use in lower power
applications such as cameras or portable recording
devices:
At this time, the most common secondary batteries are
probably the lead-acid batteries used in automobiles.
The batteries have the advantage of being capable of
operating for many charge cycles without any significant
WO 91/06132 5 ~ ~ 8 7 PCI"/US90/05638
-2-
loss of performance. However, these batteries have a
low power to weight ratio. In order to improve on
weight ratios, lithium batteries~have been thoroughly
investigated, and certain of these systems are promising
in certain applications. As improvements are made, it
will be appreciated that more widespread use will
follow.
Developments in lithium polyethylene oxide cells
typically have a figure of merit (FOM), which is
l0 computed by multiplying the number of cycles by the mean
cycle capacity and dividing by the excess installed
lithium capacity, of about 50. A typical example of
such a cell is to be found in U. S. Patent No. 4, 589, 197
describing a lithium/polyethylene battery system in
which the electroactive material is an intercalation
compound. This type of battery has also been shown to
be capable of scaling up to large sizes without any
significant loss of performance.
Another lithium type cell is to be found in United
States Patent No.4,833,048 which utilizes an
organosulfur positive electrode which has sulfur-sulfur
bonds in the charged state that are broken in the
discharged state to form organometal salts. This patent
discloses a cell which has an excellent weight ratio,
however, the disclosed electrode was utilized in the
liquid state and solvents were needed to provide the
desired current transport. The present invention
provides improvements over these patented systems.
Specifically, the present invention provides a cell
having a FOM of the order of 120 along with capability
of operation at room or ambient temperatures.
SUMMARY OF THE INVENTION
Therefore, it is a primary object of the invention to
provide a metal-sulfur type cell having a high figure
WO 91/06132 5 3 8 8 7 PCf/US90/05638
_.. -3 -
of merit, and which is capable of operating at ambient
temperatures.
Another object of the invention is to provide a cell in
which all of the components are in the solid state, and
which may be reliably fabricated into units having
reproducible performance values.
A further object of the invention is to provide a
battery having an energy to weight ratio far in excess
of the demands for load leveling and/or electric vehicle
l0 applications.
These and other objects will become apparent as the
specification continues.
In accordance with the invention, a composite positive
electrode and a battery system constructed with the
composite positive electrode system are provided. In
the fully charged states the positive electrode
comprises a 1-dimensional, 2-dimensional, or 3-
dimensional polymeric electroactive component. In the
one dimensional linear form this component can be
2o formulated as (SRS),.in which R is an organic moiety as
hereinafter defined and n is greater than 2 and
preferably greater than 20 in the charged state. The
half-cell reaction can be described as follows:
( SRS ) , + 2 n e' s n -SRS-
and the overall cell reaction can be described as
follows:
(SRS), + 2nLi = nLi~SRS
In the most general sense, the electroactive component
of the, solid-state organosulfur electrode can be
represented in the charged state by (RSy), wherein y is
2 to 6, n is greater than 2 and preferably greater than
20, and R is one or more different aliphatic or aromatic
WO 91/06132 ~ 3 ~ a 'f PCT/US90/05638
-4-
moieties having 1 to 20 carbon atoms which may include
one or more oxygen, phosphorus, silicon, sulfur or
nitrogen heteroatoms when R comprises one or more
aromatic rings, or one or more oxygen, phosphorus,
silicon, sulfur, nitrogen or fluorine atoms associated
with the chaff-n when R comprises an aliphatic chain,
wherein the aliphatic group may be linear or branched,
saturated or unsaturated, and wherein either the
aliphatic chain or the aromatic ring may have
substituted groups thereon, and wherein said
organosulfur positive electrode is further characterized
by a large number of sulfur-sulfur bonds when in the
charged state, which upon discharge of the cell are
broken to form an organo-metal salt with metal ions in
the cell.
The charge/discharge process in the positive electrode
can be viewed as a reversible redox polymerization (or
redox dimerization/scission in the case of monomeric
RSSR compounds). An example of a 2-dimensional (ladder
polymer) electrode can be illustrated by
polyethyleneimine disulfide as follows:
--CHzCHZN--CHzCHzN--CHiCHiN--CHzCH~N--
S S S S
S S S S
--CHiCHiN--CH=CHIN--CHzCHZN--CHiCHiN--
Although these polymeric electrode materials transport
alkali metal ions, in most cases it will be necessary
or desirable to include a suitable polymeric electrolyte
such as polyethylene oxide for rapid ion transport
within the electrode as is done with intercalation based
WO 91/06132 ~ 3 8 8 7 PCT/US90/05638
,_ -5-
electrodes. Furthermore, since the organosulfur
electrodes are not electrically conductive, it is
important to disperse a small amount of carbon black
(typically 7% by weight), or equivalent conductor
particles, in the composite electrode. The ranges of
the materials in the polymeric positive electrode is
from about 30% to 80% by weight of active organosulfur,
from about 20% to about 70% by weight of polymeric
electrolyte, and from about 1% to about 20% by weight
of conductor particles.
The desired mixture is achieved by dissolving or
dispersing the (SRS)o polymer, polyethylene oxide, and
carbon black powder in acetonitrile, and subsequently
evaporating the solvent to cast a thin film (say 10 to
200 microns) of solid composite electrode. In the
preferred case, the positive electrode is a composite
electrode composed of organosulfur redox polymer,
polyethylene oxide, and carbon black.
In the fully charged state the organosulfur positive
electrode is of the general formula (SRS)D with the
important feature being the formation of the sulfur-
sulfur bond upon oxidation of the alkali metal thio
salt. The preferred electrode is a polymeric disulfide,
but it is believed that monomeric disulfides (RSSR) as
described in U. S. Patent No. 4,833,048 will also be
operative in solid' state batteries. In the fully
discharged state, the organosulfur electrode comprises
polythio and/or dithio anions (-SRS-) dispersed in the
polymer electrolyte matrix. The final discharge product
depends, of course, on the type of R groups in the
polymer chain and the dimensionality of the fully
oxidized positive polymer electrode.
Another advantage of the invention resides in the
capability of the solid state electrodes to be
203887
-6-
reversible to various metals. While lithium has the lowest
equivalent weight and corresponding weight advantages, it
is more costly than sodium. In addition, the conductivity
of the preferred polyether electrolytes such as
polyethylene oxide is higher for sodium transport than for
lithium transport. Accordingly, while the intercalation
type cells require lithium as a practical matter, the
negative electrode of the battery system of the present
invention may be composed of many different metals.
Accordingly, any of the alkali or alkaline earth metals or
transition metals (the polyether electrolytes have been
shown to transport dications such as Zn'" ) are within the
ambit of the invention, and particularly mixtures
containing lithium and/or sodium.
The electrolyte used in the cells of this invention
functions as a separator for the electrodes and as a
transport medium for the metal ions. Therefore, any solid
material capable of transporting metal ions may be used.
For example, it has been shown that sodium beta alumina is
operative. Preferably, however, the solid electrolyte
separator is any suitable polymeric electrolyte such as
polyethers, polyimines, polythioethers, polyphosphazenes,
polymer blends, and the like in which an appropriate
electrolyte salt has been added.
In a broad aspect, therefore, the present invention relates
to a solid state metal-sulfur cell which comprises: (a) a
solid metallic anode; (b) a solid organo-sulfur cathode
comprising, in the charged state, a polymer having the
formula (R(S)Y)~ wherein y = 2 to 6, n is greater than 20,
and R is one or more of the same or different aliphatic or
aromatic moieties having 1 to 20 carbon atoms, which may
include one or more oxygen, sulfur, or nitrogen heteroatoms
when R comprises one or more aromatic rings, or one or more
oxygen, sulfur, nitrogen, or fluorine atoms associated with
the chain when R comprises an aliphatic chain, wherein the
-6(a)- 5 ~ ~ 8
aliphatic group may be linear or branched, saturated or
unsaturated, and wherein either the aliphatic chain or the
aromatic ring may have substituted groups thereon and
wherein said organo-sulfur positive electrode material is
further characterized by a sulfur-sulfur bond, when in the
charged state, which, upon discharge of the cell, is broken
to form an organo-sulfur metal salt with metal ions in said
cell; and (c) an electrolyte separator between said anode
and said cathode capable of ionic transport between said
anode and cathode comprising an organic polymer and an
electrolytic salt.
In another broad aspect, the present invention relates to
a solid state metal-sulfur cell which comprises: (a) a
solid lithium anode; (b) a solid organo-sulfur cathode
comprising, in the charged state, a polymer having the
formula (R(S)y)" wherein y - 2 to 6, n is greater than 20,
and R is one or more of the same or different aliphatic or
aromatic moieties having 1 to 20 carbon atoms, which may
include one or more oxygen, sulfur, or nitrogen heteroatoms
when R comprises one or more aromatic rings, or one or more
oxygen, sulfur, nitrogen, or fluorine atoms associated with
the chain when R comprises an aliphatic chain, wherein the
aliphatic group may be linear or branched, saturated or
unsaturated, and wherein either the aliphatic chain or the
aromatic ring may have substituted groups thereon and
wherein said organo-sulfur positive electrode material is
further characterized by a sulfur-sulfur bond, when in the
charged state, which, upon discharge of the cell, is broken
to form an organo-sulfur metal salt with metal ions in said
cell; and (c) an electrolyte separator between said anode
and said cathode capable of ionic transport between said
anode and cathode comprising an organic polymer and an
electrolytic salt.
253887
-6(b)-
In yet another broad aspect, the present invention relates
to a solid state metal-sulfur cell which comprises: (a) a
solid metallic anode; (b) a solid organo-sulfur cathode
comprising, in the charged state, a polymer having the
formula (R(S)Y)" wherein y = 2 to 6, n is greater than 20,
and R is one or more of the same or different aliphatic or
aromatic moieties having 1 to 20 carbon atoms, which may
include one or more oxygen, sulfur, or nitrogen heteroatoms
when R comprises one or more aromatic rings, or one or more
oxygen, sulfur, nitrogen, or fluorine atoms associated with
the chain when R comprises an aliphatic chain, wherein the
aliphatic group may be linear or branched, saturated or
unsaturated, and wherein either the aliphatic chain or the
aromatic ring may have substituted groups thereon and
wherein said organo-sulfur positive electrode material is
further characterized by a sulfur-sulfur bond, when in the
charged state, which, upon discharge of the cell, is broken
to form an organo-sulfur metal salt with metal ions in said
cell; and (c) an electrolyte separator between said anode
and said cathode capable of ionic transport between said
anode and cathode comprising an electrolytic salt, and an
organic polymer selected from the group consisting of
polyethers, polyimines, polythioethers, poly-phosphazenes,
and mixtures of same.
In another broad aspect, the present invention relates to
a solid state metal-sulfur cell which comprises: (a) a
solid lithium anode; (b) a solid organo-sulfur cathode
comprising, in the charged state, a polymer having the
formula (R(S)Y)" wherein y = 2 to 6, n is greater than 20,
and R is one or more of the same or different aliphatic or
aromatic moieties having 1 to 20 carbon atoms, which may
include one or more oxygen, sulfur, or nitrogen heteroatoms
when R comprises one or more aromatic rings, or one or more
oxygen, sulfur, nitrogen, or fluorine atoms associated with
the chain when R comprises an aliphatic chain, wherein the
aliphatic group may be linear or branched, saturated or
-6(c)- 2n 5 3 8 8 ~
unsaturated, and wherein either the aliphatic chain or the
aromatic ring may have substituted groups thereon and
wherein said organo-sulfur positive electrode material is
further characterized by a sulfur-sulfur bond, when in the
charged state, which, upon discharge of the cell, is broken
to form an organo-sulfur metal salt with metal ions in said
cell; and (c) an electrolyte separator between said anode
and said cathode, capable of ionic transport between said
20 anode and cathode, comprising one or more polyether
polymers and an electrolytic salt.
In another broad aspect, the present invention relates to
a rechargeable solid-state electrochemical generator
including an anode made of metal M which is generally pure
or alloyed and which can constitute a cation source of the
said metal, in contact with a polymer electrolyte made of
at least one salt of the said metal M of the anode
dissolved in a solvent polymer containing oxygen and/or
nitrogen heteroatoms capable of dissolving the cations of
the said metal M, M representing a metal capable of
entering into the composition of an anode, which can be put
in contact with the said polymer electrolyte, and also of
a cathode in contact with the said electrolyte and with an
electronic conductor made of at least one metal, carbon
black or an electronic polymer conductor; the said cathode
in at least a partially charged state containing at least
one polymer characterized by chains of the X-S-R-S- ( S-R-S
) a-
S-R-S-X' type where a is a number greater or equal to O, X
and X' are either a metal, including metal M, or hydrogen
or a terminal organic grouping, S is sulfur, and R
designates an organic grouping, cyclical and difunctional
consisting of carbons carrying both sulfur (S) atoms of
dithiol, the said carbons being chemically combined with at
least one nitrogen atom so as to permit the delocalization
by conjugation of the type S--C=N- -S=C-N'- of negative
charge and reversible electrochemical reduction of the
sulfur atoms when the S-S links are cut so as to result in
-6(d)_ 2 n 5 3 8 8 7
shorter links terminated at both extremities by -S-R-SM
groups, when the cathode is more or less in a discharged
state.
In a preferred form of such an electrochemical generator,
the R grouping is a heterocycle allowing each sulfur atom
related to that cycle to ensure a conjugation of the links
with at least one nitrogen atom of the heterocycle,
moreover, the R grouping may be chosen from amongst uracil,
thiadiazo.~e, triazine and pyrazine. The metal M may be
alkaline metal, an alkaline earth or a transition-bivalent
metal. The electrolyte is preferably made of a polymer
including polyether links. The electrolyte may be made of
ethylene polyoxide. The metal M may be lithium.
In another aspect, the present invention relates to said
generator, assembled in a partially or totally discharged
state, wherein the cathode is mainly in the form of short
links terminated at both extremities by SRSM groups where
S, R, and M are as defined above, and the said cathode
contains some metal M of the anode in the ionic form.
Further, some metal M of the anode may be present in the
cathode.
In another aspect of the said electrochemical generator the
metal M of the anode is present in the cathode in
symmetrical form MS-R-S- ( S-R-S ) m S-R-SM, where m is a number
smaller than a.
In a further aspect, the metal M of the anode may be
present in the cathode in an entirely reduced MS-R-SM form,
where M represents lithium, and R and S are as defined
above.
In another broad aspect, the present invention relates to
a cathode for a rechargeable solid-state chemical
6(e) 2 0 5 3 ~ 8 7
generator, the said generator including an anode of metal
M in a generally pure or alloyed form which can constitute
a cation source of the said metal, the said anode being in
contact with a polymer electrolyte made of at least one
salt of the said metal M of the anode dissolved in a
solvent polymer containing oxygen and/or nitrogen
heteroatoms and capable of dissolving the cations of the
said metal M, M representing a metal capable of entering
into the composition of an anode, which can be put in
contact with the said polymer electrolyte, the said
generator also including a cathode which is also in contact
with the said electrolyte and with an electronic conductor
made of at least one metal, carbon black or an electronic
polymer conductor characterized by the fact that the said
cathode in a charged or partially charged state, includes
at least one polymer with chains of the X-S-R-S-(S-R-S)Q-S-
R-S-X' type, where a is a number greater or equal to O, X
and X' representing a metal, including metal M, the
hydrogen or a terminal organic grouping, S is sulfur, and
R designates an organic grouping, cyclical and difunctional
consisting of carbons carrying at least two sulfur atoms of
thiol, the said carbons being chemically combined with at
least one nitrogen atom, so as to permit the delocalization
by conjugation of the type S--C=N-S=C-N-- of the negative
charge and the reversible electrochemical reduction of the
sulfur atoms when the S-S links are cut so as to result in
shorter links terminated at both extremities by -S-R-SM
groups, when the cathode is more or less in a discharged
state.
In a further aspect of said cathode, the R grouping is a
heterocycle allowing each sulfur atom related to that cycle
to ensure a conjugation of the links with at least one
nitrogen atom of the heterocycle. The R grouping may be
chosen amongst uracil, thiadiazole, triazine and pyrazine.
-6~f~- ~~53~~7
In another aspect, the present invention relates to said
cathode in a totally discharged state characterized by a
composition formula MS-R-SM, where M, S, and R are as
defined above.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a cross-sectional view of the main components
of a cell constructed according to the invention.
Figure 2 shows data in graphical form illustrating the
l0 operation of one embodiment of the invention and comparing
it with data of a prior art embodiment.
WO 91/06132 C~ ~ ~ ~ ~ PCT/US90/05638
-7_
DETAILED DESCRIPTION OF THE INVENTION
The metal-sulfur type cell as shown in Figure 1
comprises a current collector 11 in juxtaposition to a
negative electrode 12, a current collector 13 in
juxtaposition to a positive electrode 14, and an
electrolyte 15 sandwiched between the negative electrode
12 and the positive electrode 14. In a typical cell all
of these components will be enclosed in a suitable case
of plastic or the like (not shown) with only the current
collectors extending beyond the enclosure. In this way,
reactive metals such as sodium or lithium in the
negative electrode are protected. Similarly, protection
is provided for the other parts of the cell.
Suitable battery constructions may be made according to
the known art for assembling cell components and cells
as desired, and any of the known configurations may be
fabricated utilizing the invention. The exact
structures will depend primarily upon the intended use
for the battery unit. However, it will be appreciated
that the cell units are all in a substantially solid
state at ambient temperatures and in operation.
Referring again to Figure 1, current collectors 11 and
13 are sheets of conductive material such as stainless
steel which remain substantially unchanged during
discharge and charge of the cell, and which provide
current connections to the cathode and anode of the
cell. Negative electrode 12 is preferably an alkali
metal such as lithium or sodium with sodium being
preferred over lithium. The organo-sulfur cathode or
positive electrode 14 is foiled onto the current
collector 13 as described above, and the entire unit
pressed together with the electrolyte 15 sandwiched
between electrodes as shown.
PCT/US90/05638
WO 91/06132
-8-
In the drawing, the thicknesses of all of this ~~
components are exaggerated for the sake of illustration,
and all of these components are typically rather thin
sheets. For example, a typical lithium or sodium solid
anode 12 will be about 10 to 50 microns thick, a typical
solid composite polymeric cathode 14 will be about 50
to 100 microns thick, and a typical PEO electrolyte 15
will be about 10 to 100 microns thick.
The preferred electrolyte is a polyalkylene oxide such
l0 as polyethylene oxide into which a plasticizing
electrolyte salt such as LiN(CF~SOi)i has been added.
The effect of the plasticizing electrolyte salt is to
maintain the polyether in the amorphous (conductive)
state at low temperatures, thereby allowing low
temperature operation of the cell.
In accordance with the invention, the organo-sulfur
compound which comprises the novel positive electrode
of the invention is characterized by an organosulfur
material having at least one sulfur atom which forms a
first bond with an organic moiety and a second bond,
when the material is in its charged state, with another
sulfur atom which is also bonded to an organic moiety.
When the compound is in its discharged state, the
sulfur-sulfur bond is broken and a metal ion, such as
sodium, forms a salt with each of the resulting
organosulfur anions.
Thus, the positive electrode material comprises an
organosulfur material which includes the basic or
- backbone formula R-S-. In its charges state, the sulfur
3o atom (or atoms, as will be explained below) forms a
-S-S- bond with a sulfur atom of another R-S- group
forming R-S-S-R. Upon discharge, the S-S-bond is broken
and each R-S- group forms a salt with a metal ion such
as, for example, sodium, i.e., R-S-Na.
WO 91/06132 ~ ~ ~ ~ ~ ~ PCT/US90/05638
-g-
The R group, representing an organic moiety, as will be
explained below, may also have sulfur atoms bonded
thereto by double bonds, i.e., R=S, as well as the
sulfur atoms just described. The R group may also have
more than one sulfur atom bonded thereto by single bonds
thus making polymerization possible, for example in the
case of -S-R-S-. Branching may also occur when the R
group has three or more of such sulfur atoms single
bonded thereto.
Therefore, the general formula for the organosulfur
material comprising the novel positive electrode of the
invention, may be written, in its charged state, as:
(R(S)r), wherein y is 2 to 6; andhis greater than 20; and
R is one or more of the same or different aliphatic or
aromatic organic moieties having 1 to 20 carbon atoms,
which may include one or more oxygen, sulfur,
phosphorus, silicon, or nitrogen heteroatoms when R
comprises one or more aromatic rings, or one or more
oxygen, phosphorus, silicon, sulfur, nitrogen, or
fluorine atoms associated with the chain when R
comprises an aliphatic chain, wherein the aliphatic
group may be linear or branched, saturated or
unsaturated, and wherein either the aliphatic chain or
the aromatic ring may have substituted groups thereon.
When n in the general formula (R(S)y), is greater than
2, at least some of the organo-sulfur positive electrode
material comprises organic moieties containing more than
one sulfur atom, attached to the same organic moiety,
and capable of forming a sulfur-sulfur bond with a
sulfur attached to another organic moiety. Thus, in its
charged state, a polymer-like material may be formed
with the length of the polymer depending upon the
presence of impurities or chain stoppers such as mono
sulfide organic moieties, e.g., CHj-Cliz-S-Na, to
terminate polymerization. Such a polymer, for example,
WO 91/0613? ~ 3 ~ 8 7 PCT/US90/05638
y -10-
could comprise a linear aliphatic chain having such a
sulfur atom at each end of that chain, e.g.~
-S-CH2CH2-S-, permitting the formation of diners,
oligomers, etc. such as, -S-CH1-CHI-S-S-CHI-CHz-S-S-CH2-
CHz-S-, corresponding to the general formula (R(S)2)3.
Similarly, the organo-sulfur compounds may comprise
branched polysulfide materials containing more than two
sulfurs capable of forming sulfur-sulfur bond with
adjacent sulfur atoms on other organo-sulfur materials.
For example, when each R group contains three sulfur
atoms capable of forming sulfur-sulfur bonds, the
general formula could be written as (R(S)3)n.
Thus, y has been given a value of 1 to 6 in the general
formula in recognition of both the possibility of the
existence of double bonded sulfur atoms on the R group
as well as the presence of more than one sulfur atom
thereon capable of forming sulfur-sulfur bonds with
similar sulfur atoms on other molecules. The value of
n, in the general formula, while preferably greater than
20, has been given a range including 2 to 20 in
recognition of the possibility of the lower stages of
polymerization, such-~as by ring formation, and because
solid-state batteries have advantages with organosulfur
compounds that do not polymerize. 1"1o upper limit was
placed upon n because the degree of polymerization is
limited under charging conditions by the nature of the
organosulfur compound used.
The oxidation-reduction chemistry of the organo-sulfur
electrode is explained fully in United States Patent
No. 4,833,048. The present invention, while using
similar organo-sulfur electrodes differs by operating
at lower temperatures at solid state. Accordingly,
the present invention prefers organo-sulfur
WO 91/06132 20 5 3 8 8 7 P~/US90/05638
-11-
polymer which are in excess of 20 monomer units and
preferably higher than 50 units. In addition, the
positive electrode of this invention differs from that
of the cited patent by utilizing special current
transport additives.
The operating temperature of the solid-state cells is
in the range of -40 to 145°C, limited in the high range
by the melting point of either electrode or the
electrolyte. The preferred temperature range is from
ambient to 100°C. Sodium negative electrodes are
limited to temperatures below 98°C, but, sodium alloy
electrodes such as Na,Pb can be used at solid form at
well over 100°C.
The use of a solid polymeric electrolyte and a solid
redox polymerization cathode makes it possible to
manufacture all-solid-state without the difficulties
associated with the use of rigid or liquid electrolytes.
The adhesiveness and elastomerity of the solid polymeric
electrolyte and solid redox polymerization cathode
prevent loss of or serious reduction of electrical
contact between the solid electrolyte and the electrodes
during cell cycling;. In addition, the invention
provides improvements over the state of the art by
replacement of certain liquid and corrosive materials
with solid and safer compositions. This replacement
makes batteries utilizing the invention far easier to
manufacture and package by highly automated processes,
and provides cells that are non-corrosive to containment
materials.
The following examples of laboratory testing will serve
to further illustrate the invention.
Laboratory batteries were assembled with a sodium
negative electrode, sodium beta" alumina electrolyte,
WO 91/06132 5 ~ ~ ~ PCT/US90/05638
-12-
and a positive electrode made with (SRS)o, polyethylene
oxide and carbon particles. The (SRS)° polymer used was
a polymer of 2 , 5 dimercapto 1, 3 , 4 thiodiazole, and three
units of the polymer are shown in the following
structure:
N N N N N N
C C C C C C
/
- S S S S S S S S S -
The composite positive electrodes were cast to a
thickness of approximately 100 microns; which translates
to about 0.0115 g/cm2 of electrode surface area. The
available capacity of the 100 micron polymer films was
about 6.4 coulombs/cmi or 1.8 mAh/cm~. The assembled
cells were cycled to an end point of 6 coulombs (defined
as 100% of capacity). These cells were charged and
discharged at a variety of temperatures and current
densities for a total of 80 cycles with absolutely no
discernible evidence of deterioration of performance.
At an operating temperature of 130°C, the cells could
ba discharged to 100%.of available capacity at a current
density of 4mA/cm~, and could be completely recharged
at a current density of 3mA/cm~, with no adverse effects
on subsequent cycles. Furthermore, the cells could be
discharged at rates as high as 10 mA/cm= to 50% of
available capacity, and charged at rates as high as 6
mA/cmZ for 65t of available capacity. Moreover, these
exceptionally high charge/discharge current densities
did not harm the integrity of the solid polymer
electrode. The results of these studies demonstrated
the reversibility and reliability of the solid redox
polarization electrodes, even under harsh
electrochemical conditions.
WO 91/06132 a ~ 3 8 ~ 7 PCT/t1S90/05638
-13-
Cells made with lithium negative electrodes,
polyethylene oxide electrolyte, and positive electrodes
made with (SRS)n polymer, polyethylene oxide and carbon
particles were constructed to test the actual
performance of thin film batteries constructed according
to the invention. The solid electrolyte used in these
cells was polyethylene oxide doped with lithium trif late
(LiCF3S03) , lithium perchlorate (LiClO,) , or other
appropriate electrolyte salt. The concentration of
l0 electrolyte salt was 8 PEO monomer units (CHzCH2o) per
molecule of salt, abbreviated herein as PEOBLiX where
X is the salt anion. The organosulfur polymer used was
identical to that described above for the sodium cell.
Composite positive electrodes were constructed as
described above for the sodium-based cell, except that
two thicknesses of electrode were cast; a high capacity
6 coulomb/cm2 film (100 microns), and a lower capacity
3 coulombs/cm2 film (50 microns) for high power density
batteries. These Li/PEO/[(SRS),/PEO/C] cells had
theoretical energy densities of 1000 Wh/kg, and
assembled cells had practical energy densities of
338 Wh/kg (zero current drain) for the high capacity
films, and 304 Wh/kg-.for the low capacity films, based
on the weight of the actual electrodes, PEO films, and
a 4:1 excess of lithium (actual cells had a larger
excess of lithium). These cells were charged and
discharged at two different discharge levels for a total
of 3 50 cycles . The f irst 100 cycles were discharged to
depth 80% of capacity, and the remaining 250 cycles were
discharged to a depth of 50% capacity. The demonstrated
power densities and energy densities were exceptionally
high and exceed all known solid state intercalation
compound-based batteries, as can be seen from the table
below. These cells also outperform cells which operate
nt much higher temperatures such as the Na/beta"-
.~.-~'
WO 91 /06132 ~ 3 8 ~ 7 PCT/US90/05638
- -14- _
alumina/S cell (350°C), Li/LiCl/KC1/FeSZ cell (450°C),
and the like.
TABLE
TheoreticalPracticalVolumetricPower Power
Energy Energy Ensrpy DensityDensity
Density DensityDensity
Battery Whlkp Wh/kp Wh/1 WJkp W/1
1000 300 280 1B0 144
(OCV at :ero 350
~ 3.0)
LiIPE011SRS)" current cycles
284 2400 2200
at 0.5 1 cyclefor
mAlcmi 100!6 5
350 util. min.
cycles
Li/PEOITiS~480 120 150 100 1500
(OCV 5 sec
~ 2.1
)
Cd/Ni00H 245 ~ 35
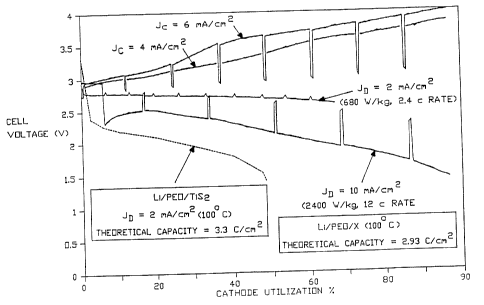
Figure 2, comparison data between Li/PEO/X and
Li/PEO/TiSi is shown graphically. In the graph, J~ shows
the cell under charge and 3D shows the cell under
discharge. The test was computer controlled, and the
peaks were printed during short off-times. Accordingly,
the true data lines are obtained by smoothing off these
peaks. As shown in the graph, the cells of the
invention maintained their voltage well through the
discharge period, whereas the comparison cell fell off
rapidly. In addition, the cells of the invention were
rechargeable from close to utilization of 100 of the
cathode.
From the foregoing description, it is seen that the
invention provides high specific energy and power cells
that exceeds that of highly developed systems now known
and in use. At the same time, the high energy and power
are available at room temperature or ambient operation.