Note: Descriptions are shown in the official language in which they were submitted.
C-4285
G-5077
METAL MAT~IX COMPOSITE COMPOSITION AND METHOD
Field of the Inv~n~ion
This invention relates to a metal matrix
composite which has a ceramic phase and an aluminum
alloy phase and a method of making it.
Back~round of the Invention
There is a continuing need to lighten and
strenythen motor vehicles and aircraft. As a result,
structural members and other parts are being
constructed from lighter materials such as aluminum and
magnesium. Despite the advantage of light weight
possessed by these materials, they have relatively
inferior mechanical characteristics, such as lower
yield strength, wear resistance and tensile strength.
Further, aluminum, magnesium and alloys thereo~ are
known to be relatively notch-sensitive and subject to
crack propagation. These deficiencies limit the
application of aluminum, magnesium and their alloys.
Therefore, it is desirable ~or such parts to be formed
from composite materials having a metal phase and a
reinforcement phase.
A composite structure is one which comprises
heterogeneous material, that is, two or more diferent
materials which are intimately combined in order to
attain desired properties of the composite. For
example, two different materials may be intimately
combined by embedding one in a matrix of the other or
impregnating the one with the other.
Metal matrix composites comprise a metal phase
and a strengthening or reinforcing phase, such as
ceramic particulates, whiskers and/or fibers. In a
2 ~ 8
metal matrix material, the reinforcing phase is
typically dispersed, distributed and/or embedded in the
metal phase. Generally, a metal matrix composite will
show an improvement in such properties as strength,
sti~fness, contact wear resistance, and elevated
temperature strength retention as compared to the metal
material alone. Metal matrix composites show great
promise for a variety of applications because they
combine the strength and hardness of the strengthening
phase with the ductility of the metal phase.
Aluminum matrix composites reinforced with a
ceramic such as alumina (Al2O33 or silicon carbide
~SiC) are of particular interest. Such metal matrix
composites potentially may provide the advantages of
the matrix alloy workability (i.e. aluminum is known
for its weight and workability advantages) while
avoiding disadvantages with regard to low strength and
crack propagation. The strength of a properly
~ormulated composite material is relatively high
compared to the same aluminum alloy without the
reinforcing phase. Moreover, the reinforcing ceramic
material should prevent the propagation of cracks
through the composite. However, enhanced properties
and an efficient, economical method to form the
composite are needed.
It has been suggested that metal matrix
composites be formed by a number of methods which
intermingle the ceramic and metal alloy together so
that the ceramic phase is distributed throughout the
composite. In one proposed method, the alloy is first
melted and then stirred while fibers are added. The
: . ,
'~
:,
stirred mixture is then permitted to cool. Another
proposed method includes powder metallurgy techniques,
where the metal, in the form of a powder, and the
ceramic reinforcing material, in the form of particles,
whiskers or fibers, are mixed and then either hot
pressed or extruded. Still other proposed methods for
intermingling the materials include high pressure
casting or infiltration processes. In an infiltration
method, the ceramic is formed into a structure and
molten metal is injected under the force of pressure
into voids or interstices in the structure.
It has been suggested that a bonding interface
exists between the metal matrix and the ceramic
reinforcement. It also has been suggested that the
strength of the interface is related to the composition
of the metal and good wetting of the ceramic by the
metal during formation of the composite.
There is a need for an improved composition
for the metal phase which provides a metal matrix
composite product having desired enhanced mechanical
propertiès and good bonding at the interface between
the ceramic and metal phases. There is also a need for
a new method ~or forming metal matrix composites which
produces such an improved composition and the desired
enhanced properties.
Sum~ary of the Invention
A new metal matrix co~posite of an aluminum
based alloy and a ceramic, and a method of making it
are provided.
In the preferred method, silicon carbide
ceramic is heated to an elevated temperature, generally
.:.
3 ~ ~ g
in the range of between about 750F and 2000F. An
alloy, comprising by weight about 3 to 6 percent
copper, about 0.5 to 5 percent magnesium and the
balance essentially aluminum, is heated to melt the
alloy. The heated ceramic and molten alloy are mixed
or intermingled. The intermingled ceramic and molten
alloy are then cooled at a rate sufficient to sustain
supersaturation of the copper and magnesium in the
aluminum down to a predetermined temperature. The
predetermined temperature is selected so as to permit
precipitation of a secondary metallic phase containing
the three elements copper, magnesium and aluminum. The
secondary ~etallic phase essentially consists of
between about 40 to 80 percent by weight copper,
ma~nesium in an amount between about S and 30 percent
by weight, and the balance essentially aluminum. This
forms a metal matrix composite having an aluminum-rich
primary metallic phase and a magnesium containing
copper-rich secondary metallic phase which has the
desired composition. The primary metallic phase can
contain up to lO percent of eutectic phase which is
generally present as a coarse network or as isolated
islands.
Preferably, cooling occurs immediately after
the step of intermingling and proceeds sufficiently
rapidly to a temperature below about 550F before
precipitation occurs. Hence, essentially all of the
precipitation occurs at a temperature below about 550F
and the precise composition of the precipitate will
depend on the temperature(s) selected. During
fabrication of components it is often not practically
possible to conduct a controlled, rapid cooling or
quench immediately after intermingling. Therefore, the
constraints of the manufacturing process may require
that the step of controlled cooling be deferred. If
such a deferral causes the composite to cool at an
uncontrolled rate, the composite must then be reheated
to a sufficiently high temperature as to dissolve or
solubilize the copper and magnesium in the aluminum
including that present in the eutectic phase. This can
readily be accomplished by heating the composite to
between about 900F and 1000F preferably for between
about 8 and 36 hours. Longer times or mechanical
working could be used to insure that the eutectic phase
is completely dissolved. After ~eheating, the cooling
step immediately follows, where t:he composite cools at
a rate sufficient to sustain supersaturation of the
copper and the magnesium in the aluminum preferably to
a temperature below about 550F to produce the desired
precipitate below about 550F.
Preferably, the aluminum alloy melt consists
of at least about 85 percent aluminum, about ~ to 5
percent copper, about 1.5 to ~.5 percent magnesium by
weight, and the method includes the step of forming a
self-supporting heated preform of a ceramic.
Preferably, the preform is of a silicon carbide ceramic
which is intermingled with melted alloy in an
infiltration-type intermingling or mixing process, by
applying about 11,500 PSI of pressure to a surface of
the molten aluminum alloy distal from the silicon
carbide preform so as to force the alloy into the
interstices of the preform (i.e. impregnate the
2 Q ~
preform).
In a preferred embodiment, a metal matrix
composite product is formed in accordance with the
invention, having a silicon carbide ceramic phase
distributed substantially uniformly throughout the
metal phase. Although silicon carbide ceramic is
preferred, other ceramics, such as crystalline alumina,
crystalline alumina-silica and glass, may also be
selected. The metal phase comprises the aluminum-based
primary metallic phase and the secondary metallic phase
distributed essentially uniformly throughout the
primary metallic phase wherein the secondary metallic
phase contains the three elements copper, magnesium and
aluminum and consists essentially of about 40 to 80
percent by weight copper, magnes.ium in an amount
between about 5 and 30 percent by weight, and the
balance essentially aluminum.
Desirably, the secondar~ metallic phase has
cubical shaped structures which are between about 300
to 500 angstroms or 30 to 50 nm on a side, and
preferably 400 angstroms (40 nm) on a side and
comprises at least about 50 percent by weight copper.
Preferably, the secondary metallic phase has a
volume fraction which is up to about 5 percent of the
volume frac~ion of the metal phase; and the primary
- metallic phase includes alpha-aluminum with a volume
fraction of at least about 95 percent of the volume
fraction o~ the metal phase. The primary metallic
phase can contain up to 10 percent of eutectic phase
which is generally present as a coarse network or as
isolated islands.
Desirably, the ceramic phase comprises silicon
carbide particles and an oxide. The oxide may be
present in the form of an oxygen containing binder,
(i.e. SiO2) admixed with silicon carbide particles.
Preferably, the ceramic phase comprises silicon carbide
particles and oxides thereof, (i.e. SiO2) formed by
surface oxidation of the SiC.
Objects, features and advantages of this
invention are to provide a unique metal matrix
composite with enhanced mechanica. properties and a
process of making it, which is efficient and
economical, and which facilitates the manufacture of
composite components.
It is also an object to provide a unique metal
matrix composite having a metal phase comprising an
alloy: which enhances the mechanical properties of the
composite; which provides the advantages of relativel~
high strength and low weight as compared to other
materials commonly used to form articles, such as
automotive, boat, airplane and other parts; which
provides high strength and low weight advantages needed
to increase fuel economy and recluce fuel consumption;
and which is readily adaptable to the process of
casting parts.
2~ Brief Description of the Drawings
These and other objects, features and
advantages of this invention will be apparent from the
following detailed description, appended claims and
accompanying drawings in which:
Fig. 1 is a transmission electron micrograph
of a metal matrix composite embodying the invention.
; 7
,
.
:,
Fig. 2 is an apparatus used in a method of the
invention.
Fig. 3 is a diagram o~ oxide layer formation
as a function of temperature.
5Fig. 4 is a diagram of atom percent of
magnesium as a function of distance from an interface.
Fig. 5 is a diagram of uniaxial tensile
strength as a function of weight percent magnesium.
Fig. 6 is a diagram of composite strength
compared to matrix strength.
Fig. 7 is a phase diagram of a Mg-Cu-Al
system.
Detailed Description of the Preferred Embodiments
In a preferred embodiment a metal matrix
composite of the invention 10, as shown in Fig. 1,
comprises a ceramic phase 11 dist:ributed substantially
uniformly throughout the composil:e 10, and a metal
phase 13 which comprises an aluminum-based primary
metallic phase 14 and a secondary metallic phase 15
distributed throughout the primary metallic phase 14.
The secondary metallic phase 15 contains copper,
magnesium and aluminum and consists essentially of
about 40 to about 80 percent by weight copper,
magnesium in an amount between about 5 and 30 percent
by weight and the balance essentially aluminum.
Preferably, the secondary metallic phase 15 ccmprises
cubical-shaped structures which are about 400 angstroms
on a side. The ceramic phase 11 comprises particles
preferably in the form of fibers or single crystal
whiskers having an aspect ratio (i.e. length to
diameter ratio), greater than 3 to 1 and preferably
2 ~ ~3 L~
greater than 10 to 1. Preferably, the ceramic phase 11
is of a silicon carbide material, however, other
ceramics, such as alumina, alumina-silicate glasses,
crystalline alumina-silica and the like may be used.
The preferred method of making the metal
matrix composite of the invention 10 includes the steps
o~:
a) heating the ceramic to a temperature
between about 750F and 2000F to preclude chilling of
the melt upon contact with the ceramic and promote
better wetting of the ceramic by the melt;
b) melting an alloy comprisinq, by weight,
about 3 to about 6 percent copper, about 0.5 to about 5
percent magnesium and the balance essentially aluminum;
c) intermingling the heated ceramic with the
melted alloy; and
d) cooling the intermingled ceramic and alloy
at a rate sufficient to sustain supersaturation of the
copper and magnesium until a predetermined temperature
is reached, the predetermined temperature being
selected so as to permit precipitation of a secondary
metallic phase containing copper, magnesium and
aluminum and consisting essentially of about 40 to 80
percent by weight o~ copper, magnesium in an amount
between about S to 30 percent by weight, and the
- balance essentially aluminum, thereby forming a metal
matrix composite having an aluminum-based primary
metallic phase and the secondary metallic phase
distributed throughout the primary metallic phase. The
primary metallic phase can contain up to 10 percent of
eutectic phase which is generally present as a coarse
.
.
g L~
network or as isolated islands.
The intermingling step is accomplished by a
number of methods, such as mixing and heating powdered
metal and ceramic; melting a metal and adding ceramic
S while stirring; or infiltrating a ceramic preform with
a melted metal. In essence, any method which achieves
intermingling or dispersion of ceramic in the metal
alloy may be used.
The intermingled alloy and ceramic (i.e. the
composite) must be hot enough to achieve a relatively
homogeneous metal alloy solution. Then the cooling
step is conducted to cool the composite from an
elevated temperature to a predetermined temperature at
or below which the precipitation of the secondary
metallic phase occurs. The cooling step is
sufficiently rapid as to preclude any substantial
precipitation from occurring before the predetermined
temperature is reached.
Preferably, the aforesaid rapid cooling occurs
immediately after the step of intermingling to achieve
a temperature below about 550F thereby causing
precipitation to occur below about 550F. Due to the
configuration of many components it is often not
practically possible to conduct a controlled, rapid
cooling or quench immediately after intermingling as
different portions of the component cool at different
rates. Therefore, the constraints of the manufacturing
prooess may require that the step of controlled cooling
be deferred. If such a deferral causes the composite
to cool at an uncontrolled rate, the composite must
then be reheated to a temperature sufficient to
. .
:,
redissolve or resolubilize the copper and magnesium in
the aluminum including that present in the eutectic
phase. Heating to a temperature between about 900F
and about 1000F preferably for between about 8 and
about 36 hours is adequate for this purpose thouqh any
temperature above about 900F would be effective.
Longer times or mechanical working could be used to
insure that the eutectic phase is completely dissolved.
After reheating, the cooling step immediately follows,
where the composite cools at a rate sufficient to
sustain supersaturation (i.e. of the copper and
magnesium in the aluminum) preferably to a temperature
below about 550F to produce the desired precipitate
below about 550F.
The rapid cooling is desirably conducted by
quenching in a liquid, preferably water, at a
temperature between about 80F and 200F. Then, if
desired, an aging step may follo~ the quench. The
aging step desirably occurs at about 150F to about
550F for about 4 to about ~8 hours, and preferably at
300F to 400F for 4 to 8 hours. A natural aging step
may be conducted at about room temperature for up to a
few days. It is well known in the art that aluminum
alloys generally exhibit an increase in strength over
time, sometimes for years after guenching. Thus, the
aging step will simply depend on the temper condition
desired at the time the part is placed in use.
In order to efficiently and economically
produce cast components, preferably the infiltration
~o method is used to achieve mixing or intermingling. In
this preferred method, a ceramic preform is contoured
11
, ~ .
2 ~
12
to the shape of the final part desired; heated;
impregnated with molten alloy metal under pressure; and
then cooled at a relatively rapid, controlled rate.
The resultant composite part will be in the shape of
the ~inal product desired, with little or no subsequent
machinin~ being required. Importantly, no subsequent
treatment such as mechanical work or heat treatment is
required to impart enhanced mechanical properties, as
further discussed below.
The metal matrix composite process and the
physical properties of the composite will be further
described by reference to the following examples.
Example 1
The preferred infiltration method was used to
cast a component part from a preEorm of the preferred
silicon carbide material, which was contoured to the
~inal shape desired for the part. In this method:
a) a porous ~i.e. 80% porosity) preform was
ormed of a ceramic material which included silicon
carbide whiskers bound together by a layer of oxides of
silicon (i.e. SiO2) on the surface of the whiskers;
b) the preform was heated to a temperature
between about 1450F and about 1500F;
c) an alloy comprising by weight, about 4.5
percent copper, about 0.45 percent magnesium, the
balance essentially aluminum, was melted at a
temperature between about 1450F and about 1500DF;
d) the heated ceramic preform was impregnated
with the melted alloy by applying about 11,500 PSI of
3~ pressure to a free surface of the molten aluminum alloy
distal from the preform;
12
.
13
e) the pressure was maintained for about 4
minutes to enable the metal to solidify, after which
the infiltrated preform and excess solidified metal was
ejected;
f) the impregnated preform was naturally
cooled from an ejection temperature of about 850F to
room temperature at an uncontrolled rate;
g) the impregnated preform was solution
treated by reheating to 977F by placing in a furnace
heated to 750F and ramping up to 977F at a rate not
exceeding more than 40F per hour, and holdiny ~or 16
hours followed by water quenching into 155F water at a
rate of about 400F per second; and
h) the solution treated impregnated preform
was aged for 5 hours at 370F and air cooled. At this
temperature a secondary metallic phase formed which
contained the three elements, copper, magnesium and
aluminum. The metallic phase essentially consisted of
about 40 to about ao percent by weight copper,
magnesium in an amount between about 5 and 30 percent
by weight and the balance essentially aluminum. By
this infiltration method, a metal matrix composite was
formed having an aluminum-based primary metallic phase
comprising about 95 percent by volume alpha aluminum
and the secondary metallic phase distributed throughout
the primary metallic phase. The primary metallic phase
can contain up to 10 percent of eutectic phase which is
generally present as a coarse network or as isolated
islands. The resultant metal matrix composite product
had a silicon carbide content of about 20 percent by
volume, a yield strength of 317 megapascals lMpa)~ a
13
. ~ :
2 ~ 8
14
tensile strength of 317 MPa and exhibited elongation of
zero percent.
~xa~ples 2, 3, 4 and 5
In Examples 2, 3, 4 and 5 the method of
Example 1 was followed except that the weight percent
of magnesium was increased to 0.9 percent, 1.1 percent,
1.58 percent, and 1.9 percent, respectively. The yield
strength was, respectively, 459 MPa, 476 MPa, 478 MPa
and 512 MPa; the tensile strength was, respectively,
562 MPa, 546 MPa, 543 MPa and 596 MPa; and the total
elongation was, respectively, 2%, 0.9%, 0.5% and 1.3%.
Table 1 is a summary of the properties obtained from
the several examples.
TABLE 1
Yield Tensile Total
Example Mg Strength Strength Elonga-
No. % (MPa) (MPa) (KSI) tion (~)
~
1 0.45 317 317 46 0.0
2 0.90 459 562 81 2.0
3 1.10 476 546 79 0.9
4 1.58 478 543 79 0.5
S 1.90 512 596 86 1.3
MPa - Megapascals
KSI = Thousands of Pounds per Square Inch
In Examples 1-5, infiltration was accomplished
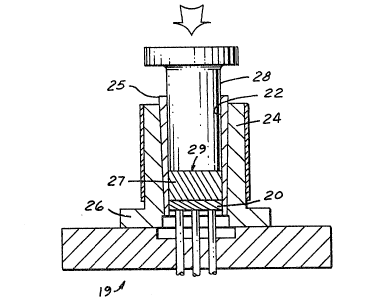
using a casting mold 19, (Fig. 2). The preform 20 was
made of the preferred silicon carbide particles, with
an oxide layer grown on the silicon carbide particles
14
.
by heating in air at an elevated temperature. The
oxide layer had a thickness of approximately 0.2
m-crons. The preform 20 was placed in a cylindrical
cavity 22 of an open top die 24 having a sleeve 25
resting on a base element 26 and heated to the
temperature specified. A charge of molten aluminum
alloy 27 was then ladled into cavity 22 and onto the
preform 20. A hydraulically driven punch 28 was
advanced into cavity 22 to apply a pressure of about
11,500 PSI to a free surface 29 of the molten alloy
charge 27 distal from the preform 20, to inject the
alloy 27 by force of pressure into the voids of the
preform 20 in about 15 to 30 seconds.
Althou~h silicon carbide particles were used
in Examples 1-5, composites were also formed with
either crystalline alumina-silica, or alumina-silicate
glass in place of silicon carbide. The silicon carbide
particles used in the examples were of the preferred
whisker type, having diameters less than two microns
and elongate with an aspect ratio generally greater
than about 3 to 1 and pre~erably significantly greater
than 10 to 1.
An oxide layer seemed to facilitate the
wettiny of the ceramic by the metal alloy. An oxide
layer may be present in the form of a binder added to
the ceramic, or grown "in situ" by surface oxidation of
the ceramic. One such binder is colloidal silica
(SiO2~ or colloidal alumina (Al~03).
Preferably, the oxide layer is grown in situ
by surface oxidation of the ceramic. It has been found
that the thickness of the oxide layer on the silicon
,:
- 2 0 ~ $
carbide may be controlled. Various thicknesses of
silicon oxides (i.e. SiO2) were formed by heating in
air to tempe~atures in the range of 800C to 1400C.
At the lower end of the range, an oxide thickness of
about 0.2 microns was achieved, at the higher end of
the range, an oxide thickness of about 0.5 microns was
achieved in about 10 to 16 hours. The thickness of
the oxide film in microns, is shown as a function of
temperature in Fig. 3.
In Examples 1-5, the alloy used was 206
aluminum available from any casting alloy supplier.
The 206 alu~inum is essentially a binary Al-4.5% Cu
alloy with trace amounts of other elements. Various
amounts of magnesium were added to the 206 Al-4.5~ Cu
alloy to form the Al-Cu-Mg alloy melts with Mg in the
range of 0.45% to 1.9% by weight.
It has been found that i3 range of about 0.5 to
about 5 percent Mg and a range of about 3 to 6 percent
Cu are each satisfactory, the balance being essentially
aluminum. Preferably, magnesium is present in the
alloy in a range of about 1.5 to 2.5 weight percent and
copper is present in the alloy in a range of about 4 to
5 weight percent. Other elements typically found in
alloys may also be present in low concentrations. Such
typical elements include manganese (Mn), chromium (Cr),
zinc (Zn), titanium (Ti), iron (Fe), vanadium (V),
zirconium (Zr), nickel (Ni), bismuth (Bi), palladium
(Pd), tin (Sn), beryllium (Be), silver (Ag), antimony
(Sb), cobalt (Co) and silicon (Si). These typical
elements may be present in concentrations up to about 3
percent but preferably are present in smaller
16
.
quantities. Silicon, up to about 20 weight percent,
may be tolerated.
It has been found that the preform should be
heated, prior to impregnation, to a temperature of
between about 750F and 2000F and preferably to a
temperature between about 1200F and 1750F.
Preheating of the preform will facilitate impregnation
and prevent the occurrence, for example, of premature
solidification of the molten alloy.
The pressure at which impregnation occurs in
an infiltration technique is not critical. Preferably,
the pressure is on the order of 5,000 pounds per square
inch or greater. Generally, the selection of the
pressure is determined by the desired length of the
15 infiltration step, so long as premature cooling does
not occur. ~igher pressures cause infiltration to
oc~ur more rapidly and lower pressures cause
infiltration to occur more slowly.
The coolin~ rate of about 400F per second
after completion of solidification was found to be
satisfactory. Other rates of cooling may be used so
long as cooling proceeds sufficiently rapidly to a
temperature below about 550F, so that essentially all
of the precipitation will occur at a temperature below
about 550~
The metal matrix composites of the invention
comprising the preferred silicon carbide ceramic and
the new second metal phase composition, as shown in
Fig. 1, have the strengths as shown in Table 1. The
new secondary metallic phase is in the form of cubical
shaped structures, about 300 to about 500 angstroms on
17
2 ~
a side and preferably about 400 angstroms (40 nm) on a
side. The structures are clearly visible on the
transmission electron micrograph of Fig. 1. Although
the secondary metallic phase consists of about 40 to 80
percent by weight copper, magnesium in an amount
between about 5 and 30 percent by weight, and the
balance essentially a~uminum, it has been found that
the atom percent of magnesium, at the interface between
the metal and the ceramic, in the as cast material, is
relatively high and drops off significantly with
distance from the interface (Fig. 4).
The metal matrix composite of the invention
clearly exhibits improved strength compared to the
unreinforced metal. As shown in Fig. 5, the reinforced
matrix (i.e. the composite) has a uniaxial tensile
strength (UTS) in the range of 70 to 90 thousands of
pounds per square inch (KSI), as magnesium is increased
from about 0.5 percent to about 2 percent. In
contrast, the unreinforced matrix metal alone has a UTS
which decreases from a high of about 65 KSI down to
about 15 KSI as the percentage of magnesium increases
from about 0.5 percent to about 2 percent.
The metal matrix composites 10 were compared
to prior art wrought composites obtained by repeated
working and/or heat treating. Fig. 6 is a graph having
matrix tensile strength on the X axis and composite
strength on the Y axis. Sigma C represents the
composite strength and Sigma M represents the matrix
strength. The diagonal line is formed by points where
Sigma C and Sigma M are equal. Thus, if the strength
of a composite is greater than the strength of the
` 18
:, .
'~ :
~, .
. .: ~ , . : .
matrix metal alone, such a composite would be
represented on the graph by a point above the diagonal
line. The composites of the invention 10, indicated on
Table 1~ generally exhibit strength well in excess of
500 MPa and approachinq 600 MPa; and the composites 10
are represented by the large cross located above the
diagonal line in Fig. 6.
Wrought composites, indicated by squares in
Fig. 6, exhibit a wide range of strengths and in one
case the strength of the wrought composite is worse
than that of the matrix metal. Such wrought composites
required significant additional treatment to obtain
their properties, as shown in Fig. 6. In contrast, the
metal matrix composites of the invention 10 in their
"as cast and heat treated condition", without
subsequent mechanical working treatment, have
properties comparable to or better than the wrought
composites.
Two comparative cast composites are indicated
on Fig. 6, by small crosses. These two comparative
composites were formed with either a 339 or 1275
aluminum alloy, each of which is different from the
alloy of the invention. The comparative composites
exhibit considerably less strength than the composite
of the invention 10. The composites of the invention
10 may be subjected to subsequent working and/or heat
treating to further enhance their properties over and
above the improved properties shown on Fig. 6.
Although not wishing to be confined to any
particular theory, it appears that the enhanced
properties o the composites of the invention 10 are
19
: '
: .:
, . : :
2 ~
achieved, at least in part, because: (1) magnesium
significantly improves bonding, as is well known; (2)
there is a limited amount of low melting eutectic phase
in the composite; (3) magnesium, and particularly
copper, provide a relatively large amount of the
secondary metallic phase which is a strengthening
precipitate; (4) the ceramic enhances stability o~ the
secondary metallic phase; (5) the oxide layer improves
strength by improving bonding at the metal-ceramic
interface; and (6) the ceramic and oxide may each
improve strenyth by contributing to the formation of
the copper-rich second metal phase, which has not
heretofore been observed in castings.
In order to produce these results the
invention takes advantage of the phenomena that an
alloy exists as a homogeneous solution at one
temperature and decomposes into its constituents at
some lower temperature. A more ~undamental description
of this phenomena, which occurs als cooling takes place
may be helpful. When metals dissolve in one another at
an elevated temperature, desired compositions and
properties may be obtained by controlling the cooling
of the metal solution from the elevated temperature.
For example, the solubilities of several alloying
elements in solid aluminum are much greater at elevated
temperatures than at room temperature. If an aluminum
alloy containing, for example, 5 percent by weight
copper is heated to well over 900F, all of the copper
will be in solution. If the alloy is then rapidly
cooled or quenched, it becomes supersaturated,
containing almost 5 percent more copper solute in
solution than it can retain under equilibrium
conditions, and particles of an aluminum-copper
metallic phase will precipitate. The final properties
will depend on the size and distribution of the
precipitated particles, which in turn depend on the
control of the cooling conditions. The rejection of
solute to form a precipitate generally occurs in a
similar manner whether the metal solution is a solid or
liquid. Thus, the changes that take place when a
liquid solution cools may also occur during the cooling
of a solid solution. The invention takes advantage of
the phenomena that an alloy exists as a homogeneous
solution at one temperature and decomposes into its
constituents at some lower temperature. Such
lS decomposition leads to the formation of a metal phase,
the structure of which is like that of the eutectic if
cooling occurs from a liquid, or a eutectoid if the
structure is the result of the decomposition of a solid
solution. Whether the cooling occurs from a liquid
homogeneous solution or a solid homogeneous solution is
not critical. What is critical is preventing the
rejection o~ solute until a desired (lower) temperature
is reached, to form the desired precipitate.
Correspondingly, if the composition of the precipitate
desired is known, the cooling conditions may be
controlled so as`to selectively generate the desired
precipitate.
As was described earlier, if a casting is made
and controlled cooling does not immediately take place,
the cast part will be permitted to cool at some random
uncontrolled rate. The desired composition of the
21
2 ~
metal phase will, therefore, not be achieved. In this
event, to take advantage of the precipitation hardening
reaction, it is possible to produce a supersaturated
solid solution by reheating the part, and then
conducting the rapid cooling step.
Transmission electron micrographs show the
existence of the cubic-shaped precipitate obtained by
the method of the invention. The precipitate has no~
been observed before in castings and was tentatively
determined to include one or more of the following
specific compositions: Cu6Mg2A15, CuA12, CuMg~12
and/or CuMgAl.
Relative to other systems, very little is
known about the Mg-Cu-Al system. Although some phases
having Mg-Cu-Al have been reported, many of those which
have been reported are said: (1) to be unstable and are
not in e~uilibrium with aluminum; and/or (2) to require
the presence of zinc, thus forming an Al-Cu-Mg-Zn
phase. It has been determined that the precipitate of
the invention, that is the secondary metallic phase of
the invention, has a composition in the range bounded
by the trapezoid shaped area sho~n on the intermetallic
phase diagram of Fig. 7. This phase consists of the
three elements copper, magnesium and aluminum and has
over 40 percent copper and magnesium in an amount
between about 5 and 30 percent by weight, with the
balance essentially aluminum. Such a phase has never
been reported in a cast metal matrix composite.
With regard to the phenomena of enhanced
bonding, Fig. 4 shows that the atom percent of
magnesium at the interace between the metal and the
2~
. . .
- ~ ' ' ''. . ' ,
:~ .
2 ~ 3
23
ceramic is relatively high and drops off significantly
with distance from the interface. It is believed that
enhanced bonding is also achieved by the magnesium
addition.
The enhanced mechanical properties of the
composites are believed to be produced by: (1) a
strong, thin bond (interface) for efficient load
transfer; (2) an interface which remains stable during
service; and (3) a strong, tough matrix which resists
crack propagation with particle and whisker
reinforcements. More specifically, the controlling of
the interfacial properties and the formation of the
second metàl phase which leads to the enhanced
mechanical characteristics is believed to be due to a
combination of the composition o~E the matrix metal
phase, the oxide layer on the reinforcement and the
controlled cooling and precipitation hardening of the
metal matrix.
The invention provides enhanced metal matrix
composite properties achieved by the specific
constituents of the matrix, their volume or weight
fraction, the method by which the metal matrix
composite is formed ~nd the temperature conditions
prevailing during formation of the metal matrix
composite.
The invention also provides optimized matrix
toughness, control of the matrix/reinforcement
interaction and the ability to maintain the desired
matrix composition during manufacture of a composite
and the service life of the composite. Metal matrix
composites of the invention achieve the advantages of
23
~.
2 ~ 8
24
relatively high strength and low weight as compared to
other materials commonly used to form articles.
Finally, the invention provides metal matrix
composites fabrica ed by a method which: (1) is
cost-effective because it utilizes an intermingling
impregnation process which provides a casting in the
shape of the part desired; and (2) permits,
alternatively, an immediate cooling step after
infiltration, or reheating and cooling steps to achieve
the enhanced metal matrix composite properties.
While the invention has been described
primarily in terms of specific example thereof it is
not intended to be limited thereto but rather only to
the extent set forth hereafter in the claims which
follow.
24
.: . . .