Note: Descriptions are shown in the official language in which they were submitted.
~ 205~39~
METHOl~ OF MAKTNG E~Tt:lJ STT.TCON,
LOW CARBON R~ TlT~A~ (:l~ATN OT~TT~'NTEll
SILICON ST~T~'T.
Jerry W. Schoen
~,,N,I~'AT. E~TT~'T.n
The invention relates to a process for producing
high silicon regular grain oriented electrical steel
with low melt carbon and in thicknesses ranging from
about 14 mils (0.35 mm) to about 6 mils (0.15 mm) or
less, and more particularly to such a process
including an intermediate anneal following the first
cold rolling stage having a very short soak time and
a two-part temperature-controlled cooling cycle, and
preferably an ultra-rapid anneal prior to
decarburization.
BACKGROUND ART
The teachings of the present invention are
applied to silicon steel having a cube-on-edge
orientation, designated (110) [001] by Miller'.s
Indices. Such silicon steels are generally referred
to as grain oriented electrical steels. Grain
oriented electrical steels are divided into two basic
categories: regular grain oriented and high
permeability grain oriented. Regular grain oriented
electrical steel utilizes manganese and sulfur
(and/or selenium) as the principle grain growth
inhibitor and generally has a permeability at 796 A/m
of less than 1870. High permeability electrical
steel relies on aluminum nitrides, boron nitrides or
other species known in the art made in addition to or
20~4395
in place of manganese sulphides and/or selenides as
grain growth inhibitors and has a permeability
greater than 1870. The teachings of the present
invention are applicable to regular grain oriented
silicon steel.
Conventional processing of regular grain oriented
electrical steel comprises the steps of preparing a
melt of electrical steel in conventional facilities,
refining and casting the electrical steel in the form
of ingots or strand cast slabs. The cast electrical
steel preferably contains in weight percent less than
about 0.1% carbon, about 0.025% to about 0.25%
manganese, about 0.01% to 0.035% sulfur and/or
selenium, about 2.5% to about 4.0% silicon with an
aim silicon content of about 3.15%, less than about
50 ppm nitrogen and less than about 100 ppm total
aluminum, the balance being essentially iron.
Additions of boron and/or copper can be made, if
desired.
If cast into ingots, the steel is hot rolled into
slabs or directly rolled from ingots to strip. If
continuous cast, the slabs may be pre-rolled in
accordance with U.S. Patent 4,718,951. If developed
commercially, strip casting would also benefit from
the process of the present invention. The slabs are
hot rolled at about 2550~ F (1400~ C) to hot band
thickness and are subjected to a hot band anneal of
about 1850~ F (1010~ C) with a soak of about 30
seconds. The hot band is air cooled to ambient
temperature. Thereafter, the material is cold rolled
to intermediate gauge and subjected to an
intermediate anneal at a temperature of about 1740~ F
(950~ C) with a 30 second soak and is cooled as by
205~395
-- 3 --
air cooling to ambient temperature. Following the
intermediate anneal, electrical steel is cold rolled
to final gauge. The electrical steel at final gauge
is sub~ected to a conventional decarburizing anneal
which serves to recrystallize the steel, to reduce
the carbon content to a non-aging level and to form a
fayalite surface oxide. The decarburizing anneal is
generally conducted at a temperature of from about
1525~ F to about 1550~ F (about 830~ C to about
845~ C) in a wet hydrogen bearing atmosphere for a
time sufficient to bring the carbon content down to
about 0.003% or lower. Thereafter, the electrical
steel is coated with an annealing separator such as
magnesia and is final annealed at a temperature of
about 2200~ F (1200~ C) for twenty-four hours. This
final anneal brings about secondary
recrystallization. A forsterite or "mill" glass
coating is formed by reaction of the fayalite layer
with the separator coating.
Representative processes for producing regular
grain oriented (cube-on-edge) silicon steel are
taught in U.S. Patent Nos. 4,202,711; 3,764,406; and
3,843,422.
In recent years, to lower the core loss of
regular grain oriented products, attention has been
turned to increasing the volume resistivity by
raising the silicon content to suppress macro-eddy
current losses. However, the expected improv t
from higher silicon content has generally not been
realized. A typical prior art approach has been to
increase both silicon and carbon in particular ratios
in an attempt to achieve improved magnetic quality.
It has been found that raising carbon and silicon
- 2054395
- 4 -
together will make the steel more prone to incipient
grain boundary melting during the high temperature
ingot/slab heating process and more brittle in
subsequent processing after hot rolling.
Particularly the handling and cold rolling
characteristics of the higher silicon and carbon
material are degraded. In the process of making
regular grain oriented silicon steel, decarburization
to 0.003~ carbon or less is required to provide
nonaging magnetic properties in the finished grain
oriented electrical steel. However, higher silicon
retards decarburization, making high silicon, high
melt carbon materials more difficult to produce.
The present invention is based upon the discovery
that in the production of regular grain oriented
electrical steel the nature of the intermediate
anneal following first stage of cold rolling, and its
cooling cycle, have a marked effect on the magnetic
quality of the final product. The volume fraction of
austenite formed during the anneal, the austenite
decomposition product and the carbide precipitate
formed during cooling are all of significant
importance. A cooling rate after the intermediate
anneal which does not allow for austenite
decomposition subsequent to the precipitation of fine
iron carbide produces lower pe ohility~ less stable
secondary grain growth, and~or an enlarged secondary
grain size. Added to this, higher silicon will raise
the activity of carbon, increasing the carbide
precipitation temperature and producing a coarser
carbide. As a result, the problems created by
improper cooling after the intermediate anneal are
aggravated at higher silicon. The tearh;ngs of the
present invention overcome these problems.
, .. . ... ... . ..
~ 2 0 5 4 3 9 ~
--5--
The present lnventlon ls dlrected to the productlon
of regular graln orlented slllcon steel starting wlth a melt
chemlatry havlng ln welght percent a slllcon content of from
about 3% to about 4.5~ and a low carbon content of less than
0.07%. The routlng of the present lnventlon follows the
conventlonal routlng glven above wlth three exceptlons.
Flrst of all, the hot band anneal can be elimlnated. This ls
partlcularly true at the lower end of the above glven slllcon
content range. Preferably, however, the routlng of the ==
present lnventlon lncludes such a hot band anneal.
Second, the present lnventlon contemplates a
modlfied intermedlate anneal procedure following the flrst
stage of cold rolllng. The modlfled lntermedlate anneal
procedure preferably has a short soak at a lower temperature
than the typlcal prior art lntermedlate anneal and lncludes a
temperature controlled, two-stage coollng cycle, as wlll be
fully descrlbed hereinafter.
The lntermedlate anneal coollng practice of the
present lnventlon provldes for austenlte decomposltlon ln the
Z0 flrst 810w stage of coollng prlor to preclpltation of fine
lron carblde in the second rapid stage of cooling. The short
soak feature and austenlte decomposltlon are facllltated by
the low melt carbon.
Finally, the routlng of the present lnventlon
preferably lncludes an ultra-rapld anneallng treatment prlor
to decarburizatlon. The ultra-rapld anneallng treatment
lmproves the overall magnetlc quallty by improvlng the
recrystalllzatlon texture. The ultra-rapld anneallng
r ~r~ 62804-1047
-6- 2 0 5 4 3 9 ~
treatment ls of the type set forth ln U.S. Patent 4,898,62Ç.~ ~
Brlefly, U.S. Patent 4,8g8,626 teaches that the
ultra-rapld anneallng treatment 18 performed by heatlng the
electrlcal steel at a rate in excess of 180~F (100~C) per
second to a temperature above the recrystalllzatlon
temperature, nomlnally 1250~F (675~C). The ultra-rapld
anneallng treatment can be performed at any polnt ln the
routlng after at least a flrst stage of cold rolllng and
before the decarburlzatlon anneal precedlng the flnal anneal.
A preferred polnt ln the routlng ls after the completlon of
cold rolllng and before the decarburlzatlon anneal. The
ultra-rapld anneallng treatment may be accomplished elther
prior to the decarburlzatlon anneal, or may be lncorporated
lnto the decarburlzatlon anneal as a heat-up portlon thereof.
DISCLOSURE OF TUE lNV~ V~
Accordlng to the lnventlon there ls provlded a
method for processlng regular graln orlented slllcon steel~ =
havlng a thlckness ln the range of from about 14 mlls
(0.35mm) to about 6 mlls (0.15mm) or less and havlng a
permeablllty at 796 A/m of less than 1870 comprlslng the
steps of provldlng a hot band of slllcon steel contalnlng ln
welght percent less than about 0.07% carbon, about 0.025% to
0.25% manganese, about 0.01~ to 0.035% sulfur or selenlum,
about 3.0% to 4.5% slllcon, less than about 100 ppm total
alumlnum, less than about 50 ppm nitrogen, the balance belng
essentlally lron, removlng the hot band scale, cold rolllng ~ ~
to lntermedlate gauge, sub~ectlng sald lntermedlate gauge
materlal to an lntermedlate anneal at a soak temperature of
~A
62804-1047
~ ~2~5~3Q 5
-7-
from about 1650~F ~900~C) to about 2100~F (1150~C~ for a soak
time of from about 1 second to about 30 seconds, conductlng a
slow coollng stage from sald soak temperature to a
temperature of from about 1000~F ~540~C) to about 1200~F
(6500C) at a cooling rate less than 1500~F (835~C) per
mlnute, thereafter conducting a fast coollng stage to a
temperature of from about 600~F (315~C) to about 1000~F
(540~C) at a rate greater than 1500~F (835~C) per mlnute
followed by water quench, cold rolllng sald slllcon steel to
flnal gauge, sub~ectlng said flnal gauge slllcon steel to a
decarburlzlng anneal, coatlng sald decarburlzed slllcon steel
wlth an anneallng separator, and sub~ectlng sald slllcon
steel to a final anneal to effect secondary
recrystalllzatlon.
Addltlons of boron and/or copper can be made, lf
deslred.
To thls end, the startlng materlal referred to as
"hot band" can be produced by a number of methods known in
the art such as lngot castlng/contlnuous castlng and hot
rolllng, or by strlp castlng.
The hot band ls preferably sub~ected to an anneal
at about 1850~F (1010~C) for a soak tlme of about 30 seconds,
followed by alr coollng to amblent temperature. It has been
found that thls hot band anneal can be omltted, partlcularly
when maklng a regular graln orlented electrlcal steel havlng
a slllcon content at the lower portlon of the range.
Thereafter, the electrlcal steel ls cold rolled to
lntermedlate gauge. The cold rolled lntermedlate thlckness
~A
~ 62804-1047
~ -~ 2~5439 ~
7a
electrlcal steel iB sub~ected to an intermediate anneal at
about 1650~F to about 2100~F (about 900~C to about 1150~C~
and preferably from about 1650~F to about 1700~F (from about
900~C to about 930~C) for a soak tlme of from about 1 to
about 30 seconds and preferably from about 3 to about 8
seconds. Following this soak, the electrical steel is cooled
in two stages. The flrst is a slow cooling stage from soak
temperature to a temperature of from about 1000~F to about
1200~F (about 540'C to about 650~C), a~d preferably to a
temperature of 1100~F + 50~F (595~C + 30~C) at a rate less
than about 1500~F (835~C) per minute, and preferably at a
rate of from about 500~F (280~C) to 1050~F (585~C) per
minute. ~he second stage is a fast cooling stage at a rate
of greater than 1500~F (835~C~ per minute and preferably at a
rate of
A
~ 62804-10~7
CA 020~439~ 1997-11-04
2,500~ F to about 3,500~ F (1,390~ C to 1,945~ C) per minute,
followed by a water quench at about 600~ F to about 1,000~ F
(about 315~ C to about 540~ C). Following the intermediate
anneal, the electrical steel is cold rolled to final gauge,
decarburized, coated with an annealing separator, and subjected
to a final anneal to effect secondary recrystallization.
In a preferred practice of the invention, the
electrical steel is subjected to an ultra-rapid annealing
treatment of the type described above. This can be performed
at any point in the routing after at least a first stage of
cold rolling, and before decarburization. It is generally
preferred to perform the ultra-rapid annealing treatment upon
completion of cold rolling and before the decarburization
anneal. As indicated above, the ultra-rapid anneal may be
incorporated into the decarburization annealing step as a
heat-up portion thereof.
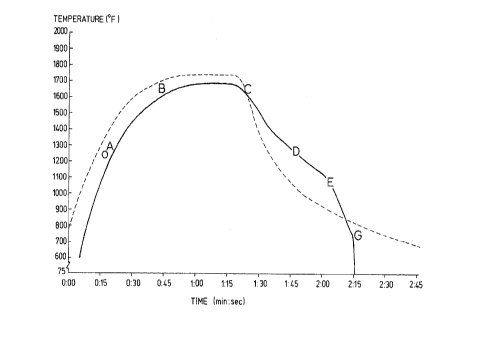
BRIEF DESCRIPTION OF THE DRAWING
The Figure is a graph illustrating the intermediate
anneal time/temperature cycle of the present invention and of
a typical prior art intermediate anneaL.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the practice of the present invention, the routing
for the high silicon, low melt carbon regular grain oriented
electrical steel is conventional and is essentially the same
as that given above with
62804-1047
205~3~
g
three exceptions. The first exception is that the
hot band anneal can be omitted, if desired. Where
equipment and conditions permit, the practice of a
hot band anneal is reC~mm~n~ since it makes the
high silicon regular grain oriented electrical steel
less brittle and more amenable to cold rolling.
Furthermore, it tends to contribute to more stable
secondary recrystallization. When practiced, a hot
band anneal is provided at a temperature of about
1850~F (1010~ C) at a soak time of about 30 seconds.
The hot band anneal is followed by air cooling to
ambient temperature. The second exception is the
development of the intermediate anneal and cooling
practice of the present invention following the first
stage of cold rolling. Finally, the third exception
is the optional, but preferred, use of an ultra-rapid
annealing treatment prior to decarburi~ation.
Following the first stage of cold rolling, the
silicon steel is subjected to an intermediate anneal
in accordance with the teachings of the present
invention. Reference is made to the Figure, which is
a schematic of the time/temperature cycle for the
intermediate anneal of the present invention. The
Figure also shows, with a broken line, the
time/temperature cycle for a typical, prior art
intermediate anneal.
A primary thrust of the present invention is the
discovery that the intermediate anneal and its
cooling cycle can be adjusted to provide a fine
carbide dispersion. The anneal and its cooling cycle
overcome the adverse effects of a higher silicon
content, described above.
20~43~5
- 10 --
During the heat-up portion of the intermediate
anneal, recrystallization occurs at about 1250~ F
(675~ C), roughly 20 seconds after entering the
furnace, after which normal grain growth occurs. The
start of recrystallization is indicated at "O" in the
Figure. Above about 1280~ F (690~ C) carbides will
begin dissolving, as indicated at "A" in the Figure.
This event continues and accelerates as the
temperature increases. Above about 1650~ F (900~ C),
a small amount of ferrite transforms to austenite.
The austenite provides for more rapid solution of
carbon and restricts normal grain growth, thereby
establishing the intermediate annealed grain size.
lS Prior art intermediate anneal practice provided a
soak at about 1740~ F (950~ C) for a period of at
least 25 to 30 seconds. The intermediate anneal
procedure of the present invention provides a soak
time of from about 1 to about 30 seconds, and
preferably from about 3 to 8 seconds. The soak
temperature has been detprmi n~d not to be critical.
The soak can be conducted at a temperature of from
about 1650~ F (900~ C) to about 2100~ F (1150~ C).
Preferably, the soak is conducted at a temperature of
from about 1650~ F (900~ C) to about 1700~ F
(930~ C), and more preferably at about 1680~ F
(915~ C). The shorter soak time and the lower soak
temperature are preferred because less austenite is
formed. Further, the austenite present in the form
of dispersed islands at the prior ferrite grain
boundaries is finer. Thus, the austenite is easier
to decompose into ferrite with carbon in solid
solution for subsequent precipitation of fine iron
carbide. To extend either the soak temperature or
time results in the enlargement of the austenite
islands which rapidly become carbon-rich compared to
CA 020~439~ 1997-11-04
the prior ferrite matrix. Both growth and carbon enrichment Gf
the austenite hinder its decomposition during cooling. The
desired structure exiting the furnace consists of a
recrystallized matrix cf ferrite having less than about 5%
austenite uniformly dispersed throuqhout the material as fine
islands. At the end of the anneal, the carbon will be in solid
solution and ready for reprecipitation on cooling. The primary
reason behind the redesign of the intermediate anneal time and
temperature at scak is the control of the growth of the
austenite islands. The lower temperature reduces the equil-
ibrium volume fraction of austenite which forms. The shorter
time reduces carbon diffusion, thereby inhibiting growth and
undue enrichment of the austenite. The lower strip temperature
the reduced volume fraction and the finer morphology of the
austenite make it easier to decompose during the cooling cycle.
Immediately after the soak, the cooling cycle is
initiated. The cooling cycle of the present invention contem-
plates two stages. The first stage extending from soak to the
point "E" on the Figure is a slow cool from soak temperature to
a temperature of from about 1,000~ F (540~ C) to about 1,200~ F
(650~ C) and preferably to about 1,100~ F + 50~ F (595~ C + 30~
C). This first slow cooling stage provides for the decomposi-
tion of austenite to carbon-saturated ferrite. Under
equilibrium conditions, austenite decomposes to carbon-saturated
ferrite between from about 1,650~ F (900~ C) and 1,420~ F (770~
C). However, the kinetics of the cooling process are such that
austenite decomposition does not begin in earnest until the mid
1,500~ F (815~ C) range and continues somewhat below 1,100~ F
(595~ C).
62804-1047
205~39~
- 12 -
Failure to decompose the austenite in the first
cooling stage will result in the formation of
martensite and/or pearlite. Martensite, if present,
will cause an enlargement of the secondary grain
size, and the deterioration of the quality of the
(110)[001] orientation. Its presence adversely
affects energy storage in the second stage of cold
rolling, and results in poorer and more variable
magnetic quality of the final electrical steel
product. Lastly, martensite degrades the mechanical
properties, particularly the cold rolling
characteristics. Pearlite is more benign, but still
ties up carbon in an undesired form.
As indicated above, austenite decomposition
begins at about point "C" in the Figure and continues
to about point "E". At point "D" fine iron carbide
begins to precipitate from the carbon-saturated
ferrite. Under equilibrium conditions, carbides
begin to precipitate from carbon-saturated ferrite at
temperatures below 1280~ F (690~ C). However, the
actual process requires some undercooling to start
precipitation, which begins in earnest at about
1200~ F (650~ C). It will be noted that the
austenite decomposition to carbon-rich ferrite and
carbide precipitation from the ferrite overlap
somewhat. The carbide is in two forms. It is
present as an intergranular film and as a fine
intragranular precipitate. The former precipitates
at temperatures above about 1060~ F (570~ C). The
latter precipitates below about 1060~ F (570~ C).
The slow cooling first stage, extending from point
"C" to point "F" of the Figure has a cooling rate of
less than 1500~ F (835~ C) per minute, and preferably
20543~
from about 500~ F to about 1050~ F (280~ C to 585~ C)
per minute.
5The second stage of the cooling cycle, a fast
cooling stage, begins at point "E" in the Figure and
extends to point "G" between 600~ F and 1000~ F
(315~ C and 540~ C) at which point the strip can be
water quenched to complete the rapid cooling stage.
10The strip temperature after water quenching is 150~ F
(65~ C) or less, which is shown in the Figure as room
temperature (75~ F or 25~ C). During the second
cooling stage, the cooling rate is preferably from
about 2500~ F to about 3500~ F (1390~ C to 1945~ C)
15per minute and preferably greater than 3000~ F
(1665~ C) per minute. This assures the precipitation
of fine iron carbide.
It will be evident from the above that the entire
intermediate anneal and cooling cycle of the present
invention is required in the process of obtaining the
desired microstructure, and precise controls are
critical. The typical prior art cycle time shown in
the Figure required at least 3 minutes, terminating
in a water bath, not shown, at a strip speed of about
25220 feet per minute (57 meters per minute~. The
intermediate anneal cycle time of the present
invention requires about 2 minutes, 10 seconds which
enabled a strip speed of about 260 feet per minute
(80 meters per minute) to be used. It will therefore
be noted that the annealing cycle of the present
invention enables greater productivity of the line.
No aging treatment after the anneal is either needed
or desired, since it has been found to cause the
formation of an enlarged secondary grain size which
degrades the magnetic quality of the final electrical
steel product.
205~395
- 14 -
The intermediate anneal is followed by the second
stage of cold rolling reducing the electrical steel
to the desired final gauge. At this stage, the
electrical steel can be decarburized, coated with an
annealing separator and subjected to a final anneal
to effect secondary recrystallization.
In the preferred practice of the present
invention, the electrical steel is given an
ultra-rapid annealing treatment after cold reduction
and prior to decarburization. To this end, the
electrical steel at final gauge is heated at a rate
above 180~ F (100~ C) per second to a temperature
above 1250~ F (675~ C). Preferably, the electrical
steel is heated at a rate of 1000~ F (540~ C) per
second. It is additionally preferred that the
ultra-rapid annealing treatment be performed as a
heat-up portion of the decarburizing anneal.
The preferred chemistry of the present invention
in weight % is as follows: less than 0.05% carbon,
about 0.04% to about 0.08% manganese, about 0.015% to
about 0.025% sulfur and/or selenium, about 3.25% to
about 3.75% silicon, less than 100 ppm aluminum, less
than 50 ppm nitrogen, additions of boron and/or
copper, can be made if desired, the balance being
essentially iron.
The ultra-rapid annealing treatment improves the
recrystallization texture after decarburization by
creating more (110)[001] primary grains. It also
contributes to smaller secondary grain size. When an
ultra-rapid annealing treatment is incorporated into
the process, the process is less sensitive to
intermediate and final gauge variations and the
CA 020~439~ 1997-11-04
magnetic characteristics of the regular grain oriented silicon
steel are improved and more consistent.
EXAMPLE I
Four heats were melted having the compositions in
weight % shown in Table I. The heats were prepared by
continuous casting into 8" (200 mm) thick slabs, prerolling the
8" thick slabs to 6" (150 mm), reheating to 2,550~ F (1,400~ C)
and hot rolling to 0.084" (2.1 mm) hot bands for subsequent
processing. The plant processing followed a routing using a
1,850~ F (1,010~ C) hot band annealing treatment and cold
rolling to various intermediate thicknesses; however, Heats A
and B were processed using a typical prior art intermediate
anneal with a 1,740~ F (950~ C) soak for 25-30 seconds followed
by normal ambient cooling while Heats C and D were intermediate
annealed according to the practice of the present invention.
After intermediate annealing, the materials were cold rolled to
final thicknesses of 7-mils (0.18 mm) and 9-mils (0.28 mm).
After completing cold rolling, the materials were decarburized
at 1,525~ F (830~ C) in a wet hydrogen-bearing atmosphere, MgO
coated and given a final anneal at 2,200~ F (1,200~ C). The~
resulting magnetic quality obtained in these trials are
summarized in Table III.
TABLE I
Code C Mn S Si Al Cu P N
A 0.0288 0.0590.0198 3.410.0013 0.092 0.006 0.0042
B 0.0296 0.0590.0209 3.420.0014 0.118 0.006 0.0038
C 0.0265 0.0580.0218 3.440.0012 0.097 0.005 0.0040
D 0.0274 0.0580.0212 3.360.0012 0.085 0.006 0.0035
62804-1047
TABLE II
Hot Inter- Inter-
Band mediate mediate
Heat End Thickness PlS H-10 Thickness P15 H-10
Conventional Practice: A Front 0.020" 0.393 1842 0.022" 0.413 1849
Back " 0.396 1833 " 0.442 1831
BFront " 0.399 1842 " 0.432 1842
Back " 0.420 1824 " 0.430 1840
Present Invention CFront 0.019" 0.383 08440.021" 0.411 1845
with Conventional Back " 0.380 1838 " 0.412 1843
Decarburization: DFront " 0.376 1845 " 0.408 1844
Back " 0.381 1840 " 0.410 1840
CFront 0.021" 0.373 18410.023" 0.411 1846
Back " 0.380 1838 " 0.423 1836
DFront " 0.368 1849 " 0.402 1849
Back " 0.379 1840 " 0.405 1846
CFront 0.025" 0.376 18380.025" 0.405 1844
Back " 0.376 1840 " 0.407 1846
DFront " 0.377 1841 " 0.405 1846
Back " 0.376 1837 " 0.406 1845
Averages:Conventional Practice0.022" 0.402 1835 0.429 1841
Present Invention: 0.019" 0.380 1842 0.410 1843
" " 0.021" 0.375 1842 0.410 1844
" " 0.025~ 0.376 1839 0.406 1845
T ~' of Present Invention: 5.5% 4.4X O
6.7% 4.5% ~p~
6.4% 5.5% C~
CD
~ =
205~39~
- 17 -
The results clearly show that the practice of the
intermediate anneal cycle of the present invention
provided improved core loss and enhanced stability of
secondary grain growth for these regular grain
oriented materials.
EXAMPLE II
Additional samples from Heats A and B were
secured during plant processing trials for laboratory
processing. Plant processing followed the
conventional routing of example I; however, after
cold rolling to intermediate thickness was completed,
the samples were secured in the plant and processed
in the laboratory in accordance with the teachings of
the present invention wherein the intermediate
annealing soak temperatures and times and controlled
cooling practice were employed and the more preferred
practice utilizing an ultra-rapid annealing treatment
after completion of cold rolling and prior to
decarburization was employed. In the practice of the
latter, a 1000~ F (556~ C) per second heating rate
from room temperature to 1375~ F was incorporated
into the heat-up portion of the decarburization
anneal. After the intermediate anneal, the materials
were cold rolled to 7-mils (0.18 mm) final thickness
and decarburized at 1525~ F (830~ C) in a wet
hydrogen-bearing atmosphere using either conventional
technigues and ultra-rapid annealing treatment during
heating. After decarburization, the samples were MgO
coated and given a final anneal at 2200~ F
(1200~ C). The results of these runs are summarized
in Table III.
20~43~
- 18 -
oooo oooooooo ooo a~_~
V ~ ~: ': o V o ~ ~ o
t~ . O O O O O
~ . .
H ~1 .~: V ,Y V ,b ~ .~C V ,1~
~ ~ ~ 3 .8 ~o ~d g ~ o t 3 ~a o ~
~ t~
..
51 e tQ 'C t~ e t~ t'
~ ~ 0
_ _tl,
~, . . O
~ ~ ~ ~ ';
V V .
~O ~ t~ ~ ~ ~ al
tll t ~ ~ t~ t~.
20~4395
-- 19 --
The results clearly show that the practice of the
intermediate anneal cycle of the present invention
provided improved core loss and enhanced the
stability of secondary grain growth for these regular
grain oriented materials. The more preferred
practice whereby an ultra-rapid annealing treatment
in addition to the intermediate anneal cycle of the
present invention further provided for still more
improvement in the magnetic guality.
Modifications may be made in the invention
without departing from the spirit of it.