Note: Descriptions are shown in the official language in which they were submitted.
2054966
TECHNICAL FIELD
This invention relates to compositions andmethods for treatment or prevention of colonic polyps.
~ACKGROUND OF THE INVENTlON
Each year in the United States alone,
approximately 60,000 people die from colon cancer, and
over 150,000 new cases of colon cancer are diagnosed.
For the American population as a whole, individuals
have a six percent lifetime risk of developing colon
cancer, making it the second most prevalent form of
cancer in the country. Colon cancer is also prevalent
in Western Europe.
To date, little progress has been made in the
prevention and treatment of colorectal cancer, as
reflected by the lack of change in the five-year
survival rate over the last few decades. The only cure
for this cancer is surgery at an extremely early stage.
Unfortunately, most of these cancers are discovered too
late for surgical cure, because most victims d~ not
experience symptoms untll the disease is advanced.
The incidence of colon cancer increases with
age, particularly ater the age of 40. Since the mean
--1--
- . - : . : .
' ' ' , : . ' ' ' ' : :~` '
2054966
ages of populations in America and Western Europe are
increasing, the prevalence of colorectal cancer should
increase in the future.
In view of these grim statistics, efforts in
recent years have concentrated on colon cancer
prevention. Colon cancer usually arises from pre-
existing benign growths known as polyps. Prevention
efforts have emphasized the identification and removal
of colonic polyps. Polyps are identified by x-ray
and/or colonoscopy, and usually removed by devices
associated with the colonoscope. The increased use of
colon x-rays and colonoscopies in recent years has
detected clinically significant precancerous polyps in
four to six times the number of individuals per year
that acquire colon cancer. During the past five years
alone, an estimated 3.5 to 5.5 million people in the
United States have been diagnosed with adenomatous
colonic polyps, and it is estimated that many more
people have or are susceptible to developing this
condition, but are as yet undiagnosed. In fact, there
are estimates that 10-12 percent of people over the age
of 40 will form clinically significant adenomatous
polyps.
Removal of polyps has been accomplished
either with surgery or fiber-optic ~ndoscopic
polypectomy -- procedures that are uncomfortable,
costly (the cost of a single polypectomy ranges between
$1,000 and $1,500 for endoscopic treatment and more for
surgery), and involve a small but significant risk of
colonic perforation. Overall, about $2.5 billion is
. ,
~' ' . "` ' ~; ' .
20~4~66
spent annually in the United States in colon cancer
treatment and prevention.
As indicated above, each polyp carries with
it a chance that it will develop into a cancer. The
likelihood of cancer is diminished if a polyp is
removed. However, many of these patients demonstrate
a propensity for developing additional polyps in the
future. They must, therefore, be monitored
periodically for the rest of their lives for polyp
reoccurrence.
In most cases (i.e. the cases of so-called
common sporadic polyps), polyp removal will be
effective to reduce the risk of cancer. In a small
percentage of cases (i.e. the cases of the so-called
polyposis syndromes), removal of all or part of the
colon is indicated. The difference between common
sporadic polyps and polyposis syndromes is dramatic.
Common sporadic polyp cases are characterized by
relatively few polyps, each of which can usually be
removed leaving the colon intact. By contrast,
polyposis syndrome cases can be characterized by many
(e.g. hundreds or more) of polyps -- literally covering
the colon in some cases, making safe removal of the
polyps impossible short of surgical removal of the
colon. Because each polyp carries with lt the palpable
risk of cancerous development, polyposis yndrome
patients invariably develop cancer if left untreated.
Many of these patients have undergone a severe change
in lifestyle as a result of surgery. Patients have
str ct dietary restrictions, and many must wear ostomy
appliances to collect their intestinal wastes.
' ~ ,: ' . ;
:: :
205~966
--4--
Recently, several non-steroidal anti-
inflammatory drugs ("NSAIDs"), originally developed to
treat arthritis, have shown effectiveness in inhibiting
and eliminating polyps. Colonic polyps virtually
disappear when the patient take the drug. However, the
prophylactic use of currently available NSAIDs, even in
polyposis syndrome patients, is marked by severe side
reactions that include gastrointestinal irritations and
ulcerations. Once NSAID treatment is terminated due to
such complications, the polyps return, particularly in
polyposis syndrome patlents.
SUMMARY OF THE INVENTION
This invention is a novel class of compounds
of formula I below that are effective in eliminating
and inhibiting polyps, but are not characterized by the
severe side reactions of NSAIDs.
This invention also relates to a method of
treating patients with common sporadic polyps and
polyposis syndromes to reduce or eliminate their polyps
by administering to a patient in need of such treatment
a physiologically effective amount of a compound of
formula I.
DETAILED DESCRIPTION OF THE INVENTION
-
As discussed above, the present inven~on i8
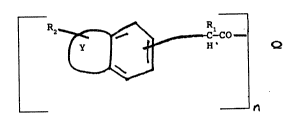
a class of compounds of formula I below:
.
--4--
:'
2054966
Q
wherein Y, together with the ring to which it
is fused forms a multi-ring system selected from the
group consisting of napthyl, [l]benzopyranol [2, 3-b],
pyridine, phenothiazine, carbazole, and benzoxazole;
R1 is lower alkyl or hydrogen;
R2 is one or more independently selected from
cyano, nitro, amino, alkylamino, hydrogen, halogen,
lower alkyl, lower alkoxy, hydroxy, phenyl or phenyl
substituted with halogen, alkyl or alkoxy;
wherein Q is the deprotonated residue of a
polymer or macromolecular structure having a molecular
weight of at Ieast 1000 containing at least two primary
and/or secondary amino groups and/or hydroxy groups;
and
n is an integer of at least 2.
As used herein, the term "halo" or "halogen"
refers to chloro, bromo, fluoro and iodo groups, and
the term "alkyl" or "alkoxy" refers to straight,
branched or cyclic groups. The term "lower alkyl" or
"lower alkoxy" refers to groups containing from 1-5
carbon atoms.
:, :
,:
:
,
~,' '..
20S4966
As used herein, the term macromolecule,
macromolecular structure, or polymer refers to
molecules having at least two primary and/or secondary
amino groups, and/or hydroxy groups. Examples of such
amino-containing polymers or macromolecules are
polyvinylamine, polyallylamine, polyethyleneimine,
chitosan, polyamino acids, polyamine exchange resins
(e.g. Amberlite), polyaminoalkanes, and the like.
Examples of hydroxy-containing polymers or
macromolecules are polyhydroxyalkanes,
polyvinylalcohols, carbohydrates (e.g. sucrose),
polyethylene glycols, and the like. The term
"deprotonated residuel' includes the situation where at
least some, but not all, of the amino and/or hydroxy
groups are deprotonated on the macromolecule or
polymer.
Compounds of this invention have unexpected
utility for suppression of colonic polyps in view of
the startling discovery that the effects of
conventional NSAID therapy on colonic polyps can be, in
fact, achieved via topical exposure to the agents.
This effect was discovered in a patient with familial
polyposis, a disease characterized by the presence of
numerous colonic polyps. In an attempt to avoid colon
cancer, the patient underwent surgical excision of the
colon with formation of a continent ileostomy, or
Kock's pouch. By this raraly performed surgical
procedure, a pouch is constructed from the terminal
portion of the small intestine. A colonic bacterial
environment developed within the pouch resulting in
extensive adenomatous polyp formation. Polyps also
' ' :~
:` . . :
. , , :' : , ' ':
' ' ''~ :
205496~
developed on the stoma, an external outlet from the
pouch constructed from a contiguous portion of small
intestine, and in the duodenum, the beginning of the
small intestine. One NSAID, when administered in oral
doses, led to the disappearance of the numerous polyps
located in the pouch but not the polyps on the stoma or
in the duodenum. Given the understanding of the
metabolic and excretory patterns of the drug as well as
of bacterial enzyme activation of the agent, these rare
findings suggested that high local concentrations of
the drug were responsible for the effects in the pouch.
It was apparent from the lack of response of the polyps
in the other locations, particularly the stomal polyps
which are close to the pouch, that the effect was
topical and that blood-borne or systemic delivery of
the drug was ineffectual.
The topical effect is particularly surprising
since the cells believed to be responsible for polyp
growth and subsequent malignancy are not only
epithelial cells deep within the crypts of the
intestine, but may also include cells which modulate
the local immunological defense mechanisms in deeper
mucosal and serosal layers of the intestinal wall.
Compounds of this invention deliver active
agents to the colon via the large macromolecular
structure to which the active agent is conjugated.
Colonic bacterial enzymes (or other colonic enzymes)
cleave the active agent from the macromolecule,
achieving locally high concentrations of the active
-7-
~" ' ~
~054966
agent and allowing the agent to contact the colon
itself leading to inhibition of polyp proliferation.
The advantage of this treatment ia that the
active agent can be concentrated where it is effective,
but whatever systemic levels are achieved are
minimized. The systemic levels are particularly low
because only passive absorption in the colon is
involved. The negligihle systemic levels are important
in that the maintenance of chronic systemic levels of
NSAIDs is complicated by a high incidence of gastric
ulcers rendering them useless in a long-term
prophylactic regimen.
Thus, contrary to prior approaches that
relied on the high systemic levels of active agent to
achieve the desired effect within the colon with the
consequent gastric complications, the compounds of the
present invention afford a different and safer
therapeutic approach in light of the topical effect of
these active agents on the colon itself.
The present invention also is a method for
treating patients having colonic polyps to reduce the
polyps which involves administering to the patient a
therapeutically effective amount of a compound of
formula I where R~, R2~ Y and n are as defined above,
but Q is the deprotonated resldue of a polyamine or
polyhydroxy compound.
Compounds of Formula I may be formulated into
compositions together with pharmaceutically acceptable
carriers for parenteral lnjection, for oral
administration in solid or liquid form, for rectal
,~
2054966
administration, and the like, although oral
administration is most preferred.
Compositions according to the present
invention for parenteral injection may comprise
pharmaceutically acceptable sterile aqueous or
nonaqueous solutions, suspensions or emulsions.
Examples of suitable nonaqueous carriers, diluents,
solvents or vehicles include propylene glycol,
vegetable oils, such as olive oil, and injectable
organic esters such as ethyl oleate. Such compositions
may also contain adjuvants such as preserving, wetting,
emulsifying and dispersing agents. They may be
sterilized, for example, by filtration through a
bacteria-retaining filter, by incorporating sterilizing
agents into the compositions, or irradiation. They can
also be manufactured in the form of sterile soled
compositions which can be dissolved in sterile water,
or some other sterile injectable medium immediately
before use.
Solid dosage forms for oral administration
include capsules, tablets, pills, powders, troches and
granules. In such solid dosage forms, the active
compound is admixed with at least one inert diluent
such as sucrose, lactose or starch. Such dosage forms
can also comprise, as is normal practice, additional
substances other than diluents, e.g., lubricating
agents such as magnesium stearate. In the case of
capsules, tablets, troches and pills, the dosage forms
may also comprise buffering agents. Tablets, pills and
granules can additionally be prepared with enteric
coatings.
_g_
~ . ~
20S4966 ``
--10--
Liquid dosage forms for oral administration
include pharmaceutically acceptable emulsions,
solutions, suspensions, syrups and elixirs containing
inert diluents commonly used in the art, such as water.
Besides such inert diluents, compositions can also
include adjuvants such as wetting agents, emulsifying
and suspending agents, and sweetening, flavoring and
perfuming agents.
Compositions for rectal administration are
preferably suppositories which may contain, in addition
to the active substance, excipients such as cocoa
butter or a suppository wax.
Actual dosage levels of active ingredient in
the compositions of the invention may be varied so as
to obtain an amount of active ingredient effective to
achieve polyp-eliminating activity in accordance with
the desired method of administration. The selected
dosage level therefore depends upon the nature of the
active compound administered, the route of
administration, the desired duration of treatment, and
other factors. If desired, the daily dose may be
divided into multiple doses for administration, e.g.
two to four times per day.
Compounds of this invention can be made by
one of the five general schemes below.
--10--
- :
.
:, - ~ .:
,
2054966
SCHEME I
~o~l - 1 ~ R-COo}'~ " r
l/~-- O~ C-I`I-R ~ \r I
~ . ~ 1~ 1
_ t~H,, _~ t~ ~ C- H- R~
~hi~os~n .~v ~c~) L O'C~
This scheme ls useful for cases, where Q is
water swellable polymer carrying aminogroups. The
water soluble carbodiimide allows acylation in the
alsoholic-aqueous phase, and the water soluble by-
product urea can be removed by water, from the acylated
polymer. The scheme allows acylation with carboxylic
acid sensitive to the conditions of acid chloride or
acid anhydride formation.
A chitosan gel (GlcN)m is prepared from
chitosan, according to the method of S. Hirano et al.
(Carbohyd. Res. 201 (1990) pp. 145-149) where m is the
number of repeating units within the chitosan molecule.
The gel is stirred in 70~ aqueous methanol solution, at
0-5 C, with the R-carboxylic acid (2 equivalents per
GlcN; where R is the group in the brackets in formula I
minus the attached carbonyl), and with a water-soluble
carbodiimide tR'-N-C=N-R"; 2 equivalents per GlcN) for
three days. (R' and R" are cycloalkyl or alkyl and the
like, containing also quaternary ammonium or sulfonate
salt for solubilization of the carbodiimide) The
resulting gel is homogenized, washed well with
distilled water, stirred with NaOH (1.2 equivalents per
GlcN) in water (50 ml per g of chitosan) for five days.
--11--
~:
4~66
The mixture is homogenized and washed to neutrality.
The gel is then dried to an amorphous powder.
SCHEME II
~ Y~ R-~oo~ - X-
1 J~ rC--~I ~ R ~IH
- R'~ Rt bC~R~
This scheme is useful for cases, where a salt
between R-COOH and a polyamine Q is swellable or
soluble in DMF. The carbodiimide is chosen, so that
the by-product urea is soluble in dichloromethane, and
therefore removable by extraction with it. Sodium
hydroxide is used to extract unreacted R-COOH, so this
scheme is useful for cases where acylation is difficult
and incomplete. The scheme especially allowæ acylation
with carboxylic acids that degrade under the conditions
of acid chloride or acid anhydride formation.
(GlcN)~ "chitosan," polylysine or similar
polyamine "X" (0.01 mol-NH2 groups; where m is the
number of repeating amino-containing units per molecule
of polyamine) are rapidly stirred in dimethylformanide
("DMF," 30 ml) at 50C until no further dissolution ls
apparent. The cooled (0) mixture is treated with
carbodiimide (R'-N=C=N-R"; 0.011 mol) with continued
stirring for two days. The resultant solution or
suspension is poured into ice water. The precipitate
is filtered off and washed with water. It is purified
-12-
20549~6
by being homogenized with and filtered from (a) CH2C12
(2x 50 ml); (b) 0.1 N-NaOH (2 x 50 ml); (c) 0.1N-HCl (2
x 50 ml); (d) H2O (2 x 50 ml); and (e) ether (2 x 50
ml). The resultant powder is dried.
~ ~' o_cl~~tC~J3)3
(cJI ) ~ ~ ( Y
cl~ X
3)3 ~C o~C~
~ l~nls scheme is suitable for cases wer~ y is a
hydroxyl group-containing polymer ~hat is soluble or
swellable in dimethyl formamide. The bulky t-butyl
group in pivalic acid prevents acylation by it, and
dimethylamino pyridine catalyzes the difficult O-
acylation. By-product pivalic acid is removable from
the acylated polymer by extraction with organic solvent
(e.g. toluene). The scheme is useful for carboxylic
acids that are sensitive to the conditions of
acylchloride synthesis.
Dry polyvinyl alcohol, methyl cellulose, or a
simllar swellable carbohydrate ([X-OH]~; 0.01 mol-OH;
where "m" is the number of repeating hydroxy-containing
units in the polymeric compound) is rapidly stirred in
absolute dimethyl formamide ("DMF," 50 ml) at 50C
-13-
. :..... . ; .;.: ~
2054966
until no further dissolution is apparent. Separately
the carboxylic acid (RCOOH; 0.01 mol) is dissolved in
absolute tetrahydrofuran (30 ml). At -10C, pivaloyl
chloride (0.01 mol) is added, followed by drop wise
addition of a tertiary amine (R'3N) (0.01 mol, e.g.,
triethylamine, ethyl diisopropylamine). The
precipitated amine hydrochloride is filtered off. The
solution is added, drop by drop, to the stirred and
cooled (-10C) polyol or carbohydrate mixture. The
combined mixture is treated at -10C with p-dimethyl
amino pyridine (0.0001 mol.), and is allowed to come to
room temperature and stay there for 15 hours. Toluene
(100 ml) is added, with stirring. The mixture is
evaporated to dryness in a rotary evaporator. The
residue is homogenized in and filtered from (a) toluene
(100 ml) and (b) water (2 x 100 ml). The filtercake is
dried in vacuo at 40C to constant weight.
..
-
-
' . .
~ ~ '
2os4966
SCHEME IV
C~ +~ ~ - C~ C
o~ - ~cl
~ ~C~ --Coz --~O
[~ [-~c--R1~
[ N~ NH~_R ~7
This scheme is useful for cases where Q is a
polymer swellable in dimethyl formamide (DMF), and
where it needs a highly reactive reagent for acylation.
The scheme is suitable for carboxylic acids that form
stable acid chlorides.
Carboxylic acid (R-COOH; 0.01 mol) i5
refluxed with thionylchloride or oxalylchloride (20 ml)
4 until solution is complete and gas evolution ceases.
Excess reagent is removed by evaporation. The residual
acid chloride is diluted with tetrahydrofuran (10 ml)
to give solution A.
-15-
,,
. ,
20S4966 ~
-16-
A polyamine ([-X-NH2]m) such as chitosan,
aminoethyl cellulose, polylysine (0.01 mol-NH2), or a
polyhydroxy compound ([-X-OH]~) such as polyvinyl
alcohol or a carbohydrate (e.g. methyl cellulose; 0.01
mol-OH) are heated in absolute dimethyl formamide at
50C, until no further dissolution is apparent.
Pyridine (0.01 mol) and p-dimethylaminopyridine (0.01
mol) are added. The mixture is cooled to -10C, and
solution A is added slowly with stirring. After 15
hours at room temperature, toluene (100 mol) is added,
and the solution is evaporated in vacuo. The residue
is homoqenized with and filtered from (a) water (2 x
100 ml); (b) ether (2 x 100 ml), and is dried.
R C~ C~o_c~O~
CI o"
~J d ~- R
- CO~ O
- R-o~ _ _ n
This scheme is useful, where Q is a polyamine
swellable or soluble in dimethylformamide. It is
especially suitable for cases where the removal of by-
products such as salts, acids, or bases) from the final
product is difficult, since in this case only carbon
dioxide and low molecular weight alcohol are produced
as by-products. The scheme is especially useful for
-16-
:
20S~966 -
-17-
carboxylic acids that decompose under th~ condition~ of
acid chloride synthesis.
Chitosan, amino ethyl cellulose, polylysine
or a similar polyamine ([-X-NH2]~; 0.01 mol-NH2 groups)
is rapidly stirred in dimethyl formamide (30 ml) at
50C until no further dissolution is apparent.
The carboxylic acid (RCOOH; 0.01 mol) is dissolved in
absolute tetrahydrofuran (30 ml). At first
trialkylamine (NR'3; 0.01 mol), and then
alkylchlorocarbonate (Cl-COOR"; 0.01 mol, where R" is
ethyl or isobutyl) is added. The precipitated
trialkylammonium chloride (R'3NHCl) is filtered off.
The filtrate is added, with ~tirring, to the cold (-
30C) polyamine solution. After being stored for 15
hours at -15C, the mixture is poured on ice (300 g),
with stirring. After the ice has melted, the
precipitate is filtered off, is thoroughly washed with
water, and is dried.
The foregoing may be better understood from
the following examples, which are presented for
purposes of illustration and are not intended to limit
the scope of the invention. As used in the following
examples, the references to compounds such as (1), (2),
(3) etc., and to substituents such as R, R1, R2 etc.,
refer to the correspondlng compounds and substituents
in the foregoing reaction schemes and in formula I.
EXAMPLE 1
Metiazinic Acid Amide Of Chitosan
Metiazinic Acid is conjugated to chitosan gel
(0.01 mol-NH2) according to Scheme I. The procedure
-17-
.: . , . .. ~ .,.,.~,
:: ~
2054966
-18-
yields the desired compound (R1 = hydrogen, R2 = CH3; Y
= phenothiazine; Q = chitosan; m > 50; n/m > 0.2; n >
10) .
EXAMPLE 2
Polvvinvl Alcohol Ester of Na roxen
A~ Naproxen acid chloride
Thionyl chloride 4.35 ml (0.059 mol) was
added to a suspended solution of 12.5 g (0.054 mol)
naproxen in 60 ml of toluene. The reaction mixture was
refluxed under nitrogen for 60 minutes. The solvent was
removed in vacuo to give naproxen acid chloride as a
white powder. IR (Nujor mull) 2954, 2923, 1790, 1604,
1460, 1377, 1229, 1026 cm~1.
8) Polyvinyl alcohol ester of naproxen
Polyvinyl alcohol (average molecular weight:
50,000) 2.39 g was added to 100 ml of pyridine. To
this suspended solution was added 6.7 g (0.27 mol) of
naproxen acid chloride. The react~on mixture was slowly
heated until all PVA dissolved, and then refluxed for
one hour. The clear solution was concentrated in vacuo
then washed with 200 ml of 1 N NaOH solution. The
aqueous part was decanted, and the residue oil was
washed with five 200 ml portions of water to remove
pyridine and unreacted PVA. The product was dried over
phosphorus pentoxide (P2O5) in a vacuum desiccator at
room temperature for 12 days. It gave 4.2 g of the
polyvinyl alcohol ester of naproxen as a yellow solld.
IR (film) 3453, 2927, 2852, 1729, 1606, 1264,
1218 cm~1.
-18-
.
~ ; ~
,
2()54966
Elemental analysis calculated for C1~H20O4; FW
300; ~ Theory: C, 72.00; H, 6.66; ~ Found : C, 71.77;
H, 7.10.
The procedure yields the desired compound R
= methyl; R2 = 6-methoxy; ~ = naphthyl; Q = polyvinyl
alcohol; n/m = 0.5.
EXAMPLE 3
PolY ~-~Pranoprofenyll Polylvsine
Pranoprofen (0.01 mol) is conjugated to
polylysine (0.01 mol) according to Scheme II omitting
the acid washing step. The procedure yields the
desired compound (R1 = methyl; R2 = hydrogen; Y =
[l]benzopyranol[2,3-b]pyridine; Q = polylysine; m > 50,
n/m > 0.2; n > 10).
EXAMPLE 4
PolY ~-Protizinvl PolYlYsine
Protizinic Acid (0.01 mol) is conjugated to
polylysine (0.01 mol-NH2) according to Scheme II
omitting the acid washing step. The procedure yields
the desired compound (R1 = CH3; R2 = 7-methoxy, 10
dimethyl; Y = phenothiazine; Q = polylysine; m > 50,
n/m ~ 0.2; n > 10).
EXAMPLE 5
Poly ~-Benoxaprofenyl PolylYsine
Benoxaprofen (0.01 mol) is con~ugated to
polylysine (0.01 mol-NH2) accordlng to Scheme II
omitting the acid washing step. The procedure yields
the desired compound (R1 = methyl; R2 = p-chlorophenyl;
Y = benzo~azole; m > 50; Q = polylysine; n/m > 0.2; n >
10) .
-19--
;
2()54966 -
-20-
EXAMPLE 6
Poly ~- E 2-(2-Phenyl-5-Benzoxazolyl)
Propionyll Polvlysine
A) 2-(2-Phenyl-5-Benzoxazolyl) Propionic Acid
(i) 2-(4-Hydroxyphenyl) propionitrile
Finely ground 2-(4-aminophenyl) propionitrile
(73 g., 0.5 mole) is suspended in concentrated
hydrochloric acid (125 ml.). The stirred suspension is
diazotised at 0 - 5C by the dropwise addition of a
solution of sodium nitrite (36.23 g., 0.525 mole) in
water (60 ml.) during 1-2 hours. The almost clear
solution is stirred for a further 20 minutes at 5 -
10C., then poured into a stirred, boiling solution of
concentrated sulphuric acid (250 ml.) in water (2.5
1.). After six minutes, it is cooled in an ice bath,
then extracted with ether (x4). The combined other
extracts are extracted with 2N-sodium hydroxide
solution (x6). The combined alkaline extracts are
cooled in an ice bath, acidified with concentrated
hydrochloric acid, and extracted with ether (x3). The
combined ether extracts are washed with saturated
sodium chloride solution (x3), dried (Na2SO4) and
evaporated to leave a dark brown oil (66.7 g.) which on
distillation gives 2-(4- hydroxyphenyl) propionitrile
(59.58 g.), h.p. 112C/0.125 mm., m p. 41 - 46C.
Analysis: Calculated C : 73.44, H : 6.16, N
: 9.51. Found C : 73.19, H : 5.91, N : 9.31.
(ii) 2-(3-Nitro-4-hydroxyphenyl)propionitrile
A solution of 2-(4-hydroxyphenyl)
propionitrile (7.79 g., 0.053 mole.) in glacial acetic
acid (10 ml.) is added at 7 - 10C., then for 30
-20-
:; ;
.
. ~ :
2054966
minutes at -10C to -15C. The suspension is diluted
with water ~approx. 90 ml.). Filtration yields 2-(3-
nitro-4-hydroxyphenyl)propionitrile as a yellow solid
(8.43 g.), m.p. 78 - 81C.
Analysis: Calculated C : 56.24, H : 4.19,
N : 14.57. Found C : 56.29, H : 4.24, N : 14.47
(iii)a. 2-(3-Amino-4-hydroxyphenyl) propionitrile
2-(3-Nitro-4-hydroxyphenyl) propionitrile
(123.8 g., 0.64 mole.) is suspended in absolute ethanol
(950 ml.) and added during 20 minutes, with cooling, to
a solution of stannous chloride dihydrate (437.8 g.,
1.94 mole.) in concentrated hydrochloric acid (591 ml.,
7 mole.). The addition is made at such a rate that the
temperature of the reaction mixture did not exceed
20C. Stirring of the mixture is continued for a
further 19 hours at room temperature. The resulting
solution, together with ice (1.75 kg.) is added during
one hour to a cooled solution of sodium hydroxide ~650
g.) in water (600 ml.). The temperature of the
reaction mixture is maintained at 15 - 20C during the
addition. The mixture is stirred for a further one
hour, and the pH then adjusted to 6 by the addition of
concentrated hydrochloric acid. The resulting
suspension is filtered, and the filtrate saturated with
sodium chloride, then extracted with ether (x6). The
combined ether extracts are dried (Na2SO4) and
evaporated to leave a solid (69.15 g.) whlch is
suspended in chloroform and extracted with 2N-
hydrochloride acid (x6). The combined acid extracts
are neutralized to pH 7-8 by the addition of sodium
bicarbonate. The resulting suspension is extracted
-21-
:. .
;
205496ti
-22-
with ether (x4). The combined ether extracts are
washed twice with water, dried (Na2SO4), and evaporated
to yield 2-(3-amino-4-hydroxyphenyl) propionitrile as a
light brown solid (62.85 g.), m.p. 110 - 112C.
Analysis: Calculated C : 66.64, H : 6.21,
N : 17.27. Found C : 66.45, H : 6.09, N : 16.99
(iii)b. 2-(3-Amino-4-hydroxyphenyl) propionitrile
was also prepared by the following method:
2-)3-Nitro-4-hydroxyphenyl) propionitrile
(38.4 g., 0.2 mole.) is suspended in absolute ethanol
(250 ml.) and hydrogenated at 4 atmospheres pressure
and room temperature over 10% palladium on charcoal.
Hydrogenation is complete in 3.8 hours. The catalyst
is removed by filtration. Evaporation of the filtrate
yields 2-(3-amino-4-hydroxyphenyl) propionitrile (17
g.), m.p. 110C.
(iv) 2-(3-Benzamido-4-hydroxyphenyl)
propionitrile
Benzoyl chloride (27.09 g., 0.19 mole.) is
added, with cooling, during 20 minutes to a stirred
solution of 2-(3-amino-4-hydroxyphenyl) propionitrile
(28.35 g., 0.175 mole.) in dry pyridine (200 ml.) at 0
- 3C. After addition is complete, the mixture is
heated at 100C. for 1 hour. It is then evaporated
under reduced pressure to yield crude 2-(3-benzamido-4-
hydroxyphenyl) propionitrile as an oil.
(v) 2-(2-Phenyl-5-benzoxazolyl) propionltrile
The oil from (iv) above is boiled for 30
minutes during or until the temperature of the vapor
above the oil rises to 130C. On cooling, the residue
solidifies. Recrystallization of the solid from
'~ ` . ' -
. . .
- ~
~05~5~66
methanol yields 2-(2-phenyl-5-benzoxazolyl)
propionitrile (27.65 g.), m.p. 118 - 120C.
Analysis: Calculated C : 77.39, H : 4.87,
N : 11.28. Found C : 77.23, H : 5.11, N : 11.34
(vi) 2-(2-Phenyl-5-benzoxazolyl) propionic acid
A solution of 2-(2-phenyl-5-benzoxazolyl)
propionitrile (24 g., 0.096 mole.) in concentrated
hydrochloric acid (220 ml) is refluxed for 2.5 hours.
The mixture is poured into ice water (1 liter). The
precipitated 2-(2-phenyl-5-benzoxazolyl) propionic acid
is filtered off and washed well with water. The dry
acid weighs 23 g. and has m.p. 177 - 179C.
Analysis: Calculated C : 71.89, H : 4.90,
N : 5.24. Found C : 72.13, H : 4.95, N : 5.39.
B) Poly ~-[2-(2-phenyl-5-benzoxazoly)propionyl]
polylysine
2-(2-phenyl-5-benzoxazolyl) propionic acid
(0.01 mol) is conjugated to polylysine (0.01 mol-NH2)
according to Scheme II, with the acid washing step
omitted. The procedure yields the desired compound (R,
= methyl; R2 = 2-phenyl; Y = 5-benzoxazolyl; m > 50,
n/m > 0.2, n > 10.
EXAMPLE 7
Polvvinyl Alcohol Ester of Flunoxaprofen
Flunoxaprofen (0.01 mol) is con~ugated to
polyvinyl alcohol (0.01 mol-OH) according to Scheme IV.
Specifically, thionylchloride is used to prepare the
acid chloride of flunoxaprofen. Triethylamine is the
base. The procedure yields the desired compound (R1 =
CH3; R2 = 2-(4-fluorophenyl); Y = benzoxazole; Q =
polyvinyl alcohol; m > 50; n/m > 0.2; n > 10).
-23-
;
~..
2a)~54966
-24-
EXAMPLE 8
Polynaproxenyl Polyethyleneimine
Naproxen acid chloride was prepared by the
procedure of Example 2A. Polyethyleneimine (purity 99
and average molecular weight 1800) 1.7 g was dissolved
in 30 ml of pyridine. To this stirred solution was
added 4.90 g (0.02 mol) of naproxen acid chloride. The
reaction mixture was refluxed for two hours.
Concentration in vacuo gave a viscous residue, which
was washed with 200 ml of 1 N sodium hydroxide
solution. The aqueous part was decanted and the
residue oil was washed with five 200 ml portions of
water to remove pyridine. The product was dried over
phosphorus pentoxide (P2Os) in a vacuum desiccator at
room temperature for 12 days. It gave 3.4 g of
polynaproxenyl polyethyleneimine as a yellow solid.
IR (film) 3407, 3296, 3064, 2971, 2936, 1721,
1714, 1667, 1651 cm~1.
Elemental analysis calculated for
Cl8H22N2O2-1.5 H2O FW 325;
% Theory: C, 66.46; H, 7.69; N, 8.61. ~ Found: C,
66.72; H, 7.41; N, 9.21.
The procedure yields the desired compound R
= methyl; R2 = 6-methoxy; Y = naphthyl; Q =
polyethyleneimine; n/m = 0.5.
It will be understood that various changes
and modifications can be made in the detalls of
procedure, formulation and use without departing from
the spirit of the invention, especially as defined in
the following claims.
-24-