Note: Descriptions are shown in the official language in which they were submitted.
CHEMICAL PROCESS ~5 ~9 ~
The present invention relates to novel 3H-purines
of thP general formula I, pharmaceutically acceptable
salts thereof, a process for the preparation of the
novel compounds as well as pharmaceutical compositions
containing compounds of the formula I and salts ther~of
as active ingredient.
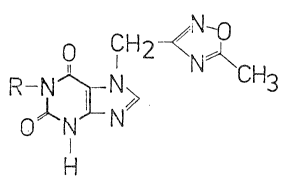
In the general formula I
R
o~,~ N
- H
R stands for C1_4 alkyl or hydrogen.
The compounds of the general formula I can be used
for the treatment of the diseases of the respiratory
organs. Various alkaloides and derivatives thereof, such
as codeine among the morphine based compounds have been
used for the treatment of the diseases of the
respiratory system as a cough reliever.
These compounds show numerous side effects of
central nervous system origin, therefore it was aimed to
prepare new cough relievers having less side effect.
A 4789-77 KY
- 2 - 2~
Recently various 3,5 disubstituted-1,2,4-oxadiazoles
have been prepared as novel cough relievers, and many of
these have been used in human therapy (oxolamine,
prenoxdiazine).
It has been also known that various xanthine
derivatives were advantageously used for the treatment
of the respiratory system due to their broncholytic
activity. The best known representative of these
compounds is theophylline. A new combination of 1,2,4~
oxadiazole ring and xanthine base is known, where cough
relieving and broncholytic activity occu- simultaneously
~HU-PS Nos. 190.377 and 197.574).
It should be emphasized however, that though on the
xanthtne derivatives used in therapy, the hydrogen of
the N-1, N-7 and N-3 nitrogen atoms of the purine ring
can be substituted by various groups, but common
characteristic thereof is that N-3 is always
substituted.
We have surprisingly found that the novel
oxadiazolylmethyl-3H-purines of the general formula I
exhibit a~vantageous pharmacological properties, mainly
for the treatment of the diseases of the respiratory
organs and these properties are par~icularly
advantageous in case of asthma bronchiale accompanied by
cough attacks.
When determining the cough relieving activity of
the compound of the formula Ia
-- 3
,~N-O
,~ Cl~
H3~NJ~-N ~h3
~ ~N~
I
H
as a reference compound the widely used cough reliever
codeine and dextromethorphane have been used and when
examining the broncholytic, antiasthmatic activity of
the compound of the formula Ia the well known
broncholytic theophylline has been used as reference.
The cough relieving activity of the compound of the
formula Ia was examined on cats by inducing cough due to
the electric stimulation of the nervous laryngeus
superior (R. Domenjoz, Arch. Exp. Path., 1952, l~).
The test compound when administered i.v. and by
gastric tube per os, the experiments show that compounds
of the formula Ia exhibit a significant cough reliever
activity both when using it ioV~ and p.o. The intensity
of the activity is the same like the activity of the
reference cough relievers, but the duration of activity
surpasses that of the references both at i.v. and p.o.
administration (Table 1)
-- 4 --
Table 1
Cough reliever p.o. ED50 value~ on cats upon cough induced by electric
nerve stimulation
Compound Ia Cod~ine HCl dextrometho~- ¦
phane HBr
p.o. EDso (+S.D-) 3.74 (~0-2) 4.33 (+0.1) 3.89 (~0-2)
mg/kg
p<O. 001
The cough reliever activity was examined on guirlea pig5
on reducing cough induced by 15 % citric acid spray (I.
Erdély, L. Tardos, Arzneim. Forsch. 1966, 617~. The
15 cough relievers were administered orally and the
activity was measured one hour later. The compound of
the formula Ia proved to be a significant cough reliever
in this test as well and the intensity of the activity
surpassed that of the reference cough relievers
20 (Table 2).
Table 2
ED50 values of cough relievers measured on guinea pigs 1 hour after
the oral administration and the cough was induced by
15 ~ citric acid ~pray
_
Compound Ia Codeine HC1 dextromethor
phane HBr
p.o. EDso (IS-D-) 3.04 (iO.10) 12,~4 ~I4.40) 5.26 (+1.72)
mg/kg
p<O . 001
_
_ 5 - 2~
The compound of the formula I does not show any
hreathing depressing activity as opposed to the
reference cough relievers, on the contrary the activity
of the bronthoconstrictors is eliminated on the basis of
both in vitro and in v.ivo experiments. The activity of
the compound of the formula Ia on trachea smooth muscle
of the guinea pig is similar to that of theophylline
when examining the activity against histamine by accumu-
lative dosage series (Table 3)
Table 3
Inhibition of contracting activity of histamine on
isolated trachea smooth muscle of guinea pigs
15 - Test compound p d 2' Emax%
_
Ia 5.48 (+1.42) 36
theophylline 5.38 (+1.24) 44
The inhibition of the increase of respiratory
resistance induced by histamine was examined on cats
(~.A. Wasserman~ D. W. Du Charme, Postaglandins, 1~77,
255).
The decrease of the increase of respiratory
resistance induced by histamine administered i.v~ at a
dosage of 3 ~glkg, was measured in 10-1~0 minute inter-
vals for 4 mg/kg intraduodenal doses of the compound of
the formula Ia and of theophylline. The compound of the
formula Ia proved to be almost twice as active as
theophylline and the activity lasted longer (Table 4).
- 6 -
Table 4
Decrease of the increase of tracheal resistance induced
~y histamine on cats upon 4 mg/kg intraduodenal doses
__ _ _ _
Test compound Max. activity % Relative efficacy
_ _ _ _
Ia 28.2 1.79
theophylline 15.7 1.00
The compound of the formula Ia shows a significant and
favourable effect-on guinea pigs in preventing
experimental asthma (anaphylaxis~. Guinea pigs were
sensitized by a single i.v. dosage of 1 ~g/kg of
ovalbumin (Andersson, Brattsand, Br. J. Pharmacol. 1982,
139), whereafter 40 days later the test compounds were
administered i.v. in a dosage of 10 mg/kg, and the
narcotized muscle relaxed and artificially breathed
animals were intratracheally administered 3 mg/kg of
ovalbumin. The prevention of l:he increase of the
tracheal resistanca of the an:imals was measured in
various intervals. The result-3 are shown in Table 5.
Records of guinea pig tests
Groups: normal, compound Ia, theophylline
Narcotization, preparation, artificial respiration after
the administration o~ a muscle relaxant
1. measurement: injection of 10 mg/kg of active ingre-
dient after 5 min and intratracheal provocation (0 min)
2. measurement: 3 min after intratracheal provocation
3. measurement: 6 min after intratracheal provocation
4. measurement: 12 min after intratracheal provocation
5. measurement: 24 min after intratracheal provocation
2 ~ ?~
Table 5
Resistance (R=Pa/ml/s)
Changes on ~3enzi~ized guinea pig5 after
intratracheal ovalbumin ()A) provocation
5(positive pressing respiration- 90/min
and 3 ml volume)
normal Ia ~heophylline
Case number ~n): 6 6 6
8Ody weight: 635t40 731+36 716+18
after 10 mg/kg i.v.: 10.72~1.50 8.07+0.93 11.04+0.26
-
3 min after OA:6.53+3.28 9.21+0.8412.87+1.06
~ .20+~.74 1.14*0.771.~3~1.05
6 min after OA:11.81+1.939.41+0.9114.65+0.93
~: 1.09'1.80 1.34+0.~63.51+1.04
2012 min after OA:16.32~2.1510.10+0.94 14.lB+0.54
~: 5.60tl.0~ 2.03+0.443.14~0.65
24 min after OA:18.16+2.459.92+1.1114.11+0.51
`~: 7.44~1.55 1.85+0.643.07+0.39
~: deviance from the value measured after 10 mg/kg
i,v. (own control) (The normal group obtained
physiological saline solution.)
Table 5 shows that compound of the Foxmula Ia
significantly reduces the experimental asthma on guinea
pigs at an extent surpassing the activity of
theophylli~e, i.e. the~trachPal resistance (R) incraase
is inhibited.
The broncholytic and antiasthmatic activities of
the compound of the Formula Ia are all the more
remarkable that the compound of the Formula Ia has no
undesired CNS and caxdial side effects which are
- 8
characteristic for theophylline.
The compound of the Formula Ia has further
fa~ourable toxicity data DLso>2000 mg/kg on mice when
administered orally. Considering the toxicity of
codeine, dextromethorphane and theophylline, which were
used as controll the thereap~utic index of the compound
of the Formula I is by one order of magnitude better
than that of the reference compounds (Codeine.HCl DL50
on mice: 359.2 mg/kg p.o., dextromethorphane.HBr DL50 on
mice: 165.9 mg/kg p.oO, theophylline DL50 on mice: 215
mg/kg p.o.). A special advantage of the compound of the
Formula Ia is that it absorbs in the organism
excellently and rapidly - in spite of the moderate water
- solubility - without salt formation in case of oral
administration.
The compounds of the Fonnula I according to the
invention can preferably be prepared by reacting a
compound of the general ~ormula II
N-o
X- CH 2~N9~
- wherein X stands for halogen or sulphonic acid ester
group -
with xanthine of the formula III -
~ 9
o H
R -i~ N~,
wherein R stands as given above -
in the presence of an organic solvent and/or diluent,
preferably dimethylformamide or alcolhols, preferably
butanol, and in the presence of an inorganic base,
preferably alkali hydroxide or alkali carbonate,
preférably potassium carbonate, or an alkali salt o the
xanthine of the formula III, preferably a sodium or
potassium salt thereof is reacted with a compound of t~e
formula II in the presence of an organic solvent and/or
diluent at a temperature of 20 to 150, preferably 40 to
10 100 C.
The compounds of the formula I are moderately
acidic and the salts with alkali metals can be prepared
by forming an aqueous solution of equimolar mixture of
the compounds of the general formula I and alkali
hydroxides, preferably sodium or potassium hydroxide,
and by evaporating this mixture. The so ~ormed sodium or
potassium salts are watersoluble.
The compounds of the general ormula I or salts
thereof can be used as such or in pharmac~utical
compositions. The pharmaceutical compositions can be
used in the ~orm of syrups, tablets, coated tablets,
2 ~
-- 10 --
capsules together with the usually used excipients. The
pharmaceutical compositions can contain 0.2 to 100 per
cent of active ingredients. The daily dosage depends on
the age of the patient, on the method of administration
and the aim thereof, as well of the weight of the
patient, and it can vary between 1 to 500 mg. The
further details of the invention are illustrated by the
following examples without limiting the scope of the
invention to the examples.
Example 1
3.32 g of 1-methyl-xanth:ine (K.A.Chkhidvadze, 0.
Magidson, Med. Prom. S.S.S.R.~ 1958, 16) and 1.45 g of
potassium carbonate are .suspended in 40 ml of dimethyl-
formamide, whereafter the mixture is stirred for 20
minutes at 85 C and at the same t~rmperature 3.43 g of
3-chloromethyl-5-methyl-1,2,4-oxadiazol2 are added drop-
wise within 15 minutss ~J.Wood, German Publication No.
1,915,495) and the mixture is stirred at this tempera-
ture for further 90 minutes. The mixture is filtered
while hot, the filtrate is evaporated at reduced
pressure and the residue is triturated with 15 ml of
water and the solid product is filtered by suction and
washed with water. 4.3 g of product are obtained and
crystalliæed first from 38 ml of 10 % aqueous hydro-
chloric acid and then 50 % aqueous ethanol clarifying
with active charcoal. 3 g (60 %) of 3,7-dihydro-1-
2 ~
methyl-7-[(5-methyl-1,2,4-oxadiazole-3-yl)-methyl]-3H
purine-2,6-dione are obtained ~ompound of the
formula I), me~ting point: 243-245 C.
lH NMR ([D6]DMS0) ~=2.56 ~s, 3H, CH33; 3.13 (s, 3H,
CH3); 5,65 (s, 2H, CH2); 8.1 ~s, lH, =(CH)-~; 12,16 ~b,
lH, 4 NH).
Example 2
A solution is prepared from 3.5 g of 1-methyl-
xanthine and 8.14 ml of 10 % sodium hydroxide solution
by shaking and a solid material precipitates ~rom the
mixture very soon. The water is distilled off at reduced
pressure and the ~races of water are removed ~rom the
residue by toluene azeotrope distillation. The obtained
l-methyl-xanthine sodium salt is suspended in dimethyl-
formamide, reacted with 3-chloromQthyl-5-methyl-1,2,~-
oxadiazole according to example 1 and worked up to a
compound of the formula Ia with the same yield.
Example 3
Tablets: Compound Ia 2.0 g
Starch 97.0 g
Calcium-phosphate 100.0 g
Magnesium-stearate 1.0 g
Total: 200.0 g
- 12 2~
The powdered mixture is converted to 1000 pieces of
200 mg tablet by method known per se, so that each
tablet contains 2 mg of active component.
Example 4
Syru~: Compound Ia ~.o g
Lemon syrup 200.0 ml
Benzoic acid solution 20.0 ml
Water 100.0 ml
Sugar syrup 1000.0 ml
The active component is dissolved in water while
hot, 500 ml of sugar syrup is added and the other
components are supplemented to lO00 ml by adding a sugar.
syrup. Each ml of the syrup solution co~tains 4 mg of
active component.