Note: Descriptions are shown in the official language in which they were submitted.
!,c ~
PHOSPHOLIPID-VITAMIN DERIVA11VES AND METHODS FOR PREPA~A~ON THEREOF
. .
BACKGROUND OF Tl iE INVENTION
This Inventlon relates to phosphollpld-vltamln derivatives and to a process for
their productlon by an enzymatlc transphosph~ldylatlon rooctlon. The process
described In this invention leads to the re~;lospecific synth0sis of phosphollpid-vHamln
10 derivatlves whlch are novel compounds possesslng valuable chemlcal and physlcal
properties. This Invention specifically pertalns to an enzymatlc process for produclng
a phospholipld-vHarnin derivative, wh~ch comprises reactin~ a phospholipid wHh awater-soluble v~ltamln or vitamin derlvative In the presence of a microblal
phospholipase-D, and to the resuHin~ phospholipid-vitamin derivative produced
15 thereby. By regiospecific~ is meant, In the context of this Invention, the site-speciflc
reaction of the phospholipid and vitamin derivative.
It has been previously shown that the phospholipase-D enzyme (EC 3.1.4.4)
from cabbage is capable of catalyzing the hydrolysis of the choline head ~roup from
20 either phosphatidylcholine or eg~ lecHhin, yieldin~ a hydrolyzate contalnin~
phosphatidic acid and the choline free base. It Is also known that the cabbage
phospholipase-D whlch catalyzes the hydroiysis of e~ lecHhin, also mediates a
transphosphatidylation reaction wherein the phosphatldyi moiety of
phosphatid~choline is transferred to one of the followin~ primary aicohols: ~Iycerol,
25 methanol, ethanol, ethanolarnine or serine.
The patent IHerature describes the use of cabba~e phospholipase-D as a
transphosphatidylation catalyst (BrHish Pat. No. 1,581,810). llle British patent relates
to the transfer reaction between a phospholipid and o primary alcohol having a
30 linear or branched alkyl ~roup of up to 5 carbon atoms, optionally substituted by a
hydroxyl, halogen or amino group. The patent states that when the
transphosphatidylation reaction is carried out with primary alcohols of more than 5
GZ- 1 .0
7 ~
the phosphatldyi molety from a phosphollpld donor to the primary 5'-hydroxyl ~roup
of varlous nucleosldes (S. Shuto et al,: Chern. Pharm. Bull., Va-i. 35, 447 (19873 ~nd S.
Shuto et al.: Chem, Pharm. i3u!1., Vol. 3~ 9 (1 98fl)). Som~ ot the resultln~ 5'~3-s~
phosphatldyi) nucleosldes prepared from anti-tumorlcldic nucleoskics (I,e. ~Fu, Ara
5 C) have been shown to have enhanced antlleukemlc activities, arK~i are o~ potentlal
therapeutlc Importance.
It is belleved that mlcroblal phosphollpase-D enzymes hove a number of
advanta~es compared to traditlonal cabba~e enzyme. For one, the relatlve
10 amount o~ phosphatldic acld produced by competitlve hydroiysis durin~ a
transphosphatlciylatlon reactlon Is greatly dlminlshed In the presence of some
microbial phosphollpase-D enzymes as opposed to the conventional cabba~e
enzyme, whlch may oflen aive phosphatidic aclci as the maJor product. Also, the
microbial enzymes, as described above, may have a wider substrate specificity for
15 the transphosphatidylation alcohol acceptor than does the cabi~e
phospholipase-D. This property may allow for the synthesis of struc~urally
complicated phospholipids, such as the aforementioned phosphaticiylsacchan'des
and phosphatidylnucleosides.
Accordin~ly, it is one aspect of this invention to provicie novel phospholipid-
vitamin derivatives which are lipophilic derivatives of a vHamin whl'ch has a primary
hydroxyi group.
It Is another aspect of the present invention to provide a method for the
synthesis of structurally novel phospholipid-vitamin derivatives.
It is still another ospect of this invention to provide vHamin derivatives w'lthenhanced lipophiiicity and therefore enhanced soiubiiity in lipids and enhanced
transport across cell membranes.
SUMMARY OF THE lNvENrloiN
The present invention relates to novel phospholij~id-vitamin derivatives and
GZ- 1 .0
`i~ ~ 2 ~
methods for thelr preparatlon. The metho~ of thls Inventlon Is an enzymatic
tronsphosphatidylallon reactlon wher~ln a new phosphollpl~vitamln derlvative Is
produced through lhe actlon of a mlcroblo! phosphollpas~-D which catcliyzes In are~lospeciflc manner the transfer o~ the phosphatl~lc acld m~ety from the startln~
s phosphollpld to the prirnary alcohol of the wc~ter-soluble v~tamln or vltamln derlvotive
used In the reactlon. The prociucts of th~s Inventlon may also ~e obtalne~ usln~techniqueS whlch Involve coupling a vHamin havlnç~ a pr~mary hydroxyl ~roup to aphosphc~tidlc acid.
Preferred embodiments of the present ~nventlon relote to phosphollpl~
vitamin derlvatives, represented by the following forrnula:
o
Il
A-O-P-O-B
~R6
wherein A is a moiety represented by the following formula:
~2Rl
~H2R2
2 5 ~H2 --
in which Rl and R2 both represent -O-COR3 or-O-COR4, or Rl represents-O-COR3
and R2 represents -OH, or Rt represents O-R5 and R2 represents {~COR4. in which
R3, R4 and R5 are identical or different and~each represents a saturated or
30 unsaturated aliphatic hydrocarbon group having from 1 to 21 carbon utoms, ~OHrepresents a vHamin having a primary hydroxy group, and R6 is a posrt~vely charged
counterion which forms a soluble salt of the phosphatidic acid. Representative
GZ2- 1 .0
'`s ~
~3~
counterions may be elther or~anlc or Inor~anlc specles or~ Incl~e H, Na, K, U, ivi~,
cyclohexylammonlum, ammonlum and ciimethyiamlnopyriciinlum,
The present Invention also relates to a proc~ss ~or prc~iucin~ phospholipi~
5 vitamln derlvatlves by reactln~ a phospholiplci represented by the formula:
Il .
A-O-P-O-D
OR6
15 wherein A and i?~ ,are as defined above, and ~ represents the ~roup
-(CH2)2N(CH3)3, -CH2CH(NH2)COOi i -CH2CH2NH2 -CH2CHOHa~20H~
-(CH2)nCH3, In which n represents a number from 1 to 5, with a vHamin or vitaminderivative having at least one primary hydroxyl group in the presence of a microbial
phospholipase-D.
More specifically, the vitamin component of the vitamin derivative may be
selected from, but is not limHeci to, the following: thiamine (Vrtamin Bl), riboflavin
(VItamin B2), pyridoxine (Vitamin 80, pyridoxal (Vitamin i~6 aldehyde), pyridoxamine
(vitamin i~ amine), ascorbic acid (Vitamin C), biotinol ~riamin H alcohol), ~
2s hydroxy-2,5,7,8- tetramethylchroman-2-carbinol (Vitamin E analo~ue), pantothenic
acid, pantothenol, or 3-pyridylmethanol (niacin alcohoi). The structures of these
particular vHamins or vitamin derivatives is well known in the art.
Additional aspects and understanding of the present invention may b
30 obtained by reference to the accompanying drawings.
GZ2- 1 .0
2 ~
BRIEF DESCi?lPrlON OF THi- Di?AWlNGS
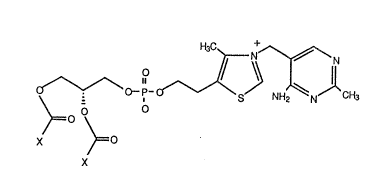
Fl~ure 1 is a structural representatlon of a phosphollpld-thiamine derivatlve
prepared accordin~ to the process of thls Inventlon.
Flgure 2 Is a structural representatlon of a phospholipld-pyricioxine derivatlveprepared accordln0 to the process of th!s Invention.
Flgure 3 is a structural representation of a phosphollpid-3-pyridYlCarCinol
derivative prepared accordin~ to the process of this Invention.
DETAILED DESCRli'TlON OF THE INVEiYTiON
It has now been discovered, as described in the present invention, that
another group of compounds, nameiy vitamins with primary hydroxyl ~roups and
their derivatives, are effective acceptors In the transphosphatidyiation reaction
catalyzed by microbial phospholipcse-D enzymes. Vrtamins such as ascorbic acld
(vitamin C), riboflavin ~Vitamin i32), thiamine ~itamln Bl), pyridox~ne (Vitamin B6),
biotinol ~riamin i-i alcohoi), and pantothenic acid are all distin~uished as containin~
primary hydroxyi groups. It is the presence of this primary hydroxyi ~roup whichallows for the regiospecrfic enzymc~ic prociuction of the phospholipid-vHamins in the
phospholipase-Dmediatedtransphosphatidylationreaction, i~nospholipid-vitamin
derivatives of vriamins containing primary hydroxyi groups may clternatively be
obtained by using chemical coupling of the vitamin to a phosphatidic acid.
The phospholipid-vitamin derivatives prociuced by this invention are novel
compounds. In the case of vitamlns possessing a primary hydroxyi group, the
intermediacy of a microbial phospholipase-D enzyme is a key factor in bypassin~ the
GZ2- 1 .0
2 ~
chemlcal synthesis of such derlvatlves whlch would, In neariy every case, be
expenslve and forrnldable duo to the numerous hydroxyi protect~on and
deprotectlon steps requlred (e.~. for Vit~mlns C, B2 and B6~. it is the newiy
discovered abllity of the mlcroblal phosphollpase-D enzYme to speclflcally cutalyze
5 ~he transfer of the phosphatidyl molety of the phospholipld to the primary alcohol of
She vHamin or vitamin derivatlve whlch characterizes the present invention.
The preferred process of the present inveotion comprises reactin~ a
phospholipld represented by the following formula:
o
Il
A-O-P-O-D
I
OR6
wherein A is a moiety represented by the following forrnula:
-CH2Rl
--CH2R2
--CH2--
2s in which R1 and R2 both represent -O-COR3 or -O-COR4, or R1 represents -O-COR3
~nd R2 represents -OH, or R1 represents O-R5 and R2 represents ~COR4, in whlch
R3, R4 and R5 are identical or different and each represents a saturated or
unsaturated aliphatic hydrocarbon aroup having from 1 to 21 cG~bon atoms, R~S Is a
positively charged counterion, preferably selected from the ~jroup consisting of H,
30 Na, K, Ll, Mg, cyclohexylammonium, ammonium, dimethyiaminopyridinium and D
represents the group -(CH2)2N(CH3)3, -CH2CH(NH2)COOH, -CH2CH2NH2,
~H2CHOHCH20H, or -(CH2)nCH3, in which n represents a number from 1 to 5, with a
vitamin or vitamin derivative having crt least one primary alcohoi ~roup in the
presence of a microbial phospholipase-D.
G22- 1 .0
f',. ~
2 ~ V
rhe phospholipld-vltamln derivatives descrii~d In thls Invention cannot be
easl1y prepared by uslng the conventlonal cabba~e phosphollpase-D. Thus,
incubatlon of phosphatidylchollne with a vHamin or vitamln derivativs in the
s presence of cabba~e enzyme showed oniy the productlon of phosphatidlc acid
and chollne. Chan~ln~ tha concentration of reactants or enzyme, or chan~in~ the
pH or temperature had no effect on the reaction product composition. The
phospholipase-D used for the production of the aforementloned phospholipid-
vitamin derivatives is ideaily derived from a mlcrobial source. The microbial source
10 can include a variety of selected mlcroor~anisms or other celis whlch have been
recombinantly en~ineered to contaln a gene encodin~ for the desired
phospholipase-D enzyme. The microbial phospholipase-D enzymes rnay be
distinguished from the cabbage enzyme in that they are caPable of catalyzin~ theformation of a phospholipid-vitamin derivative. Examples of suitable phospholipase-
15 D producin~ microorganisms are those beion~inç~ to the S~enus reptoverticillium sp.Tobie 1 shows the various strains of Streptoverticiiiiurn which produce useful
phospholipase-D enzymes:
Table 1
Streptoverticillium Species which prociuce Phosphoiipase-D Enzymes
CaPable of Phospholipid-Vitamin Forrnation
Stv. flavopersicum
Stv. hachqoensis
Stv. mediocidicus
Stv. roseoverticiilium
Stv. albireticull
Stv. Ieuteovert cl!ilum
Stv. hiroshimense
Stv. cinnamoneum
Stv. nseoca!num
GZ- 1 .0
The Streptoverticl! lum mlcroorganlsms are not the oniy mlcroblal sources of
phosphollpase-D capable of catalyzlng the productlon ot phospholipld-vitaminS.
Followin~ the method of thls Invention, numerous other mlcrobes have been examlned
5 for thelr ability to produce the requlsite enzymes. Bacterlà frorn the ~enus
Streptomyces and from ~he ç3enus CorYnebocterium have a~so been found to producea phospholipase-D capable of maklng the novel phospholipici-vitamlns.
The production of the aforementloned phosphoiipid-vitamins may be carried
o out uslng either purified mlcrobial phosphollpase-D enzymes or by usin~ the crude broth
following fermentatlon of the phosphollpase-D produclng or~anlsm. The
phospholipase-D enzymes may be used In solutlon or may be Immobilized onto solidsupports, For example, the following solici supports may be used to immobilize
phospholipase-D; octyl-sepharose CL-48, phenyl-sepharose CL-4B, butyl-toyopeari
15 ~550C, controlled glass beads, celite, Amberiite lon-exchcYnge resins, or glutaraldehyde
linked polvinylchloride.
The transphosphatidylation reactlon to produce a phospholipic~vHamin can
be advantageously conducted by contacting the phosphoiipid wHh the vitamln or
20 vitamin derivative in the presence of the microbial phospholipase D in a solvent rnixture.
The solvent mlxture may be either a monophasic aqueous buffer system or a biphasic
mlxture of an aqueous solvent and an organic solvent. The aqueous solvents may
contain additlves which act to promote the enzymatic reactivHy or to stabilize the
enzyme. For example, the aqueous solvents may contain buffers sucn as acetic ocid,
25 citric acid or phosphoric acid or neutral salts such as calclum acetate, calcium chloride
or sodium acetate. Examples of the organic solvents which may be used include
aliphatlc hydrocarbons such as hexane, pentane or octane, aromatic hydrocarbons
such as benzene or toluene, ethers such as tert-butyldimethyl ether, diethyl ether or
tetrahydrofuran, esters such as ethyl acetate and halogenated hydrocarbons such as
30 methylene chloride or chloroform. The preferred solvent system is a mixture of an
aqueous sodium acetate buffer and an ether. The mixing ratio of aqueous to or~anlc
solventscanbeintherangeof 100:1 to 1:100(v/v),witharat~of 1:1 (v/v)being
prefened.
GZ2- 1 .0
... . . . . .... ..
~ ~ rs~
rhe reactlon may be carrlcd out from about 0~C to abs~ut 80'C, preferabiy
between 20'C and 30~C for the StrePtov~rtlc Inum and Strepto~yces i~hosphollpase-D
catalyzed reactlons. The reactlon tlme may be frorn 10 mlnutes to 10 days, but is
5 preferably between 1 and 24 hours. If necessa~y, the course of the reactlon may i~
monitored by a technlque such as 11 C (thln iayer chromato~raphY) or HPLC thl0h
pressure llquld chromato~raphy). By monHoring the ~ormatlon of product and
disappearance of substrate, the proper reactlon tlme rnay be attained,
10The phospholipid-vitamin derivatlves formed may be isolated by a variety of
known technlques. These technlques Include column chromato~raphY on sllica or
alumina gel or on an lon-exchan~e resin, hi~hperformance llquici chromatography, or
by precipitation as various saits from or0anic solvents. These saH forms include sodlum,
potassium, barium, trialkylammonium or calclum saHs of the phospholipid-vHamin
lS derivative. The phosphoiipid-vHamin derivatives may be further purified by
recrystailization from organic soivents.
The phospholipid-vitamin derivatives of this inventlon are believed to be usefuias liposome-forming substances or as therapeutic açlents thernselves, particular~ In the
2 o treatment of diseases where there is evidence of a deflciency In the ability to transport
vitamins across the cellular membrane. By appending either the vitamin C (ascorbate)
or a vitamin E derivative to a phospholipid, novel materials with potent anti-oxidant
properties may be prepared. rhese anti-oxidant phospholipic~vitamins are usefui for
various applications in the scaven~in~ of oxy~en free-radicais which may cataiyze lipid
25 peroxidation. Thus, incorporation of the anti-oxidant phospholipid-vitamin derivatives
described in this invention into liposomal forrnulations contcinin~ other unsaturated
phospholipids wiil protect the liposomal components from the deleterious effect of
oxidative d~mage.
30One currentiy avaiiable commercial lipophilic vHamin derivative, aliithiamine, is
a lipid soluble form of Vitamin Bl which is administered to individuais suffering from
GZ2- 1 .0
~c~
3 ~ t7' ~
thiamlne deflclencles. AllHhiamlne is able to cross the cellu~r membrane bllayer with
moderate efficlency, and once In the cytoplasm allithlomlne is metabol!zed to
thiamlne, The phosphatldyi-thlamlne deriv~tive whlch Is readiiy ovallable accordln~ to
thls Inventlon may prove to be superlor to allHhlamlne In its ai~ility to cross the cellular
5 bilayer membrane, thus provldin~ an aHernate strate~y for the de ively of thlamine to
vitamln B1 deficlent cells.
Covalent lipophllic vitamln C derivatlv0s, wHh 6~palmitoyi ascorbate beln~
the most wldely describeci (Swern et ai., Soap, Vol 20, p.224, 1943), have a number of
o valuable commerclal applicatlons, for exam;~le as anti-oxldant aciciitlves In fooci and oll
preparations. Ronoxan A Is a commerclal formulatlon of ~O-paimHoyi ascorbate, a-tc~copherol (Vitamin i~, and e~j~ lecithin which Is useci as an antl-oxidant to protect fats
and oils containin~j unsaturated fatty acids a~ainst air oxidation (G. Pon0racz, Int. J.
Vitamin Nutr, Res. Vol. 43 (4) p. 517, (1973)). The ~palmitoyi ascorbate when
15 combined wrth tocopherol and lecithin has a syner~istic effect in protectin~ fats frorn
clir oxidation (see Table 2 below, from Pon~racz 1. J. V. N. R., 43(4) p. 517 (1973)).
Table 2
-
StabilHy of Butterfat to Oxidation
Anti- x'l~m~/a fat) Peroxide Values coPm)
3 4 5 6 (days)
Control 265 - - -
500 AP 74 280
200T 9.9 56.3 11û
500 AP+200 T 1.3 3.7 4.4 11
500AP+lOOT+500Lec 0.5 1.0 1.2 1.5
G22- 1 .0
.. . . .. .
! l ~ f~
Table 2
AP = ~PamltoylAscorbate
r = a-Tocopherol
Lec = E~ Lecithln
Althou~h Hs antloxldant propertles are Impressive, ~paimitoyl ascorbate
has poor solubility and a very slow dlssolution rate In fats and oiis ~i, Klaul p. 16 In
VHamin C, Edited by G. G. Birch and K. J. Parker John Wiley i~N, i~. 1974). These
10 drawbacks represent a serlous problern for many practlcal appllcotions of i~onoxan
A. One advanta~e of the phosphatldyl-ascorbate derivatlves described by the
present Inventlon Is that they display enhanced solubllity and Increased dlssolutlon
rates in fats and oiis.
The Interactlon of ~O-palm'rtoyl ascori~ate wHh the phospholipid bilayers of
DPPC, DPPE, and DMPE liposomes has been investi~cfed throu~h tt~e use of infrared
spectroscopic studies ~Kohler, Mantch, and Casal, J. Can. Chem. 6~ p. 983 (1988)). It
was found that incorporation of ~GpalmHoyl ascorbate into ~he pi~ospholipid bilayer
which makes up a liposome induced a lar~e increase (15-25C) n the phase
20 ~ransHion temperature for these phospholipids. The physical effect of this increase in
phase transition temperature is manifested by a correspondin~ increase in stability of
the liposome.
The ai~ove~escribed phospholipid-vrtamins producec accordin~ to this
25 invention may also be useful In research applications for the stuciy of vitamin
- transport across cellular membranes.
Further understanding of the invention will be had by a detailed study of the
followin~ examples. These examples are ~jiven by way of illustration and are not30 intended to limrt the inventlon.
Example 1 Preparation of 1 ~-Dimyristoyi-sn-Glycer~3-Phosphoryiffliamine (DMP-T) by
Transphosphatidylation of Dimyristoylphosphatidylcholine with Thicmine (VHamin B1),
in the Presence of Microbial Phospholipase-D.
GZ2- 1 .0
.. . . .
.
To 268 m~j of dlmyristoyiphosPhG~ldylCholine (DMPC) dissoved In 10 ml of
ether was added 20 ml of 0.11 M thlamlne-HCL In 50 mM socilum acetate (pH 5.0)
buffer. TQ the resultln~ blphaslc mlxture was added 10 ml ot pure StreptomVceS s~.
phosphollpase-D (60 U/ml, obtalned from Si~ma) In 50 mM socilum acetate buffer
(pH 6.2). Thc resuHln~;~ emulslon was vl~orously stirrod at room temperature and the
course of the transphosphatidylation reaction was monitored by thin-l~yer
chromato~raphy (rLC) by assayin~ for the disappearance of DMPC and the
simultaneous appearance of DMP-T. After stlrrin~ for 4 h, the rea~tion had
proceeded to completion. The reaction mixture was diluted with 60 ml of CHCI3, the
organic layer was separated and collected, dried over sodium suifate, and
concentrated in vacuo to ~ive 321 m~ of a whlte solid (67% molar yleld). This crude
solid was purified by silica ~el chromato~raphy usin~ 25:4 CHC13:MeOH:H20 as
eluent. Fractions containin0 UV active material which stained positive ~t phosphorus
(usin0 molybedenum stain) were pooied and concentrated in vacuo to ~jive 280 rn~(58~ molar yield) of pure DMP-T as a whHe solid. Characterization of the DMP-T ~ave
the following values: R~ = 0.26. silica ~el 60 F254 (Merck), CHC13-MeOH-H20 (65:25:4):
UV max (chloroform) 268 nm (E 3.9 mM): 400 MHZ ~H-NMi~ (CDC13)~10.0(s) 1 H, 8.10(s)
1 H, 5.62(s) 1 H, 5.18(s) 1 H,4.32(dd) 1 H, 4.05(m) 3H, 3.~m) 2H, 3.~s) 2H,3.10(m~ 2H,
2 0 2.5~(s) 3H, 2.45~s) 3H,2.25(dc~) 4H,1.40 1.18(m) 52i~i, 0.~8(t) 6H. The structure of this
product is shown In i~i~ure 1.
Example 2 Prepara~ion of 1 2-Dimyristoyi-sn-Glycero-3-Phophorylpyridoxine (DMP-P)
by Transphosphatidylation of Dimyristoyiphosphatidylcholine (DMPC) wHh Pyridoxine
(V'ltamin B~), in the Presence of Microbial Phospholipas~D.
To 500 m~ of dimyristoylphosphatidylcholine (DMPC) dissoived in 12 ml of
methylene chlofide was added 50 ml of 0.7 M sodium pyridoxine in 50 mM calcium
acetate (pH ~.0) buffer. To the resuHin~ biphasic mixture was ad~ed 1 ml of crude
Stre~toverticillium flavoPersicum phospholipase-D (10 U/ml, where U represents aDPPC hydrolysis activity unit) in a 50 mM sodium acetate (pH 6.2~ buffer.
13
GZ~- 1.0
!j',.,.; ~
J ~
The resultlng blphaslc solutlon was vlgorously stlrred at room temperatur
and the course of the transphosphatldyiatlon reactlon was monitored by thln iayer
chromatography (TLC) by assayln~ for the disappear<: nce of DMPC anci tha
S slrnultaneous appearance of DMP-P. After stlrrlng tor 18 hr., ti~? reactlon was Jud~ed
complete by rLC. The methylene chlorlde layer was separated and dried over
sodlum sulfate and concentrated In vacuo to ~Ive 500 mç~ of c~ white solid.
Characterization of the resultln~ DMP-P gave the followlr~: m.p. = 139-143C, Rf =
0.56, silica gel ~0 F254 (Merck), CHC13, MEOH, H20 (~5.25:4); HPLC 0a e
min. The structure of this product is shown In Fl~ure 2.
Example 3 Preparatlon of 1,2-Dimyristoy~sn-Glycer~3-Phosphory~i'yridylcarbinol
(DMP-i~ by Transphosphatidyiation of Dimyristoylphosphatldylcholine (DMi'C) wHh 3-
pyrldylcarbinol (niacinol) in the presence of Microblal Phospholipas~D.
To 500 m~ of dimyristoyl phosphatidylcholine (DMPC) d-~so~ed in 5 ml of
methylene chloride was added 10 ml of 0.9 M 3-pyridylcarbinol buffered to pH 6. To
the resuHln~ biphasic m~lxture was added 2 ml of crude StrePtoverticillium
flavoperslcum phospholipase D (10 Ulml, where U represents a DPPC hydrolysis
activity unit) in a 50 mM sodium acetate (pH ~.2) buffer.
The resultin~ biphasic solutlon was vl0orous~ stirred at room temperaturs
and the course of the transphosphatidylation reaction was monitored by thin layer
chromatography ~TLC) by assayin~ ~or the disappearance of DMPC and the
simultaneous appearance of D~P-N. A~ter stirrin0 for 18 hr. the reaction wasJudged
complete by ~C. The methylene chloride layer was sep~rated and the aqueous
layer washed wHh 10 rnl methylene chloride. The combined or0anic layers were
dried over sodium sulfate and concentrated in vacuo to ~Ive 258 mg of white solid
(510. Charactereation of the resulting DMP-N ~ave the followin~: mp 129-135C,
3 o RP: 0.4 ~UV active), silica ç~el 60 F254 (Merck), CHC13, MEOH, H20 (65:25:4). ll~e
structure of this product is shown in Figure 3.
G7~- 1 .0