Note: Descriptions are shown in the official language in which they were submitted.
'~~,~~J6~~~
-I-
NOVEL NOBLE METAL SUPPORTED
HYDROTREATIN6 CATALYSTS AND PROCESS
FIELD OF THE INVENTION
The present invention relates to catalysts for heteroatom
removal, particularly nitrogen, from petroleum and synthetic fuel
feedstocks. The catalyst is comprised of at least one Group VIII metal,
at least one Group VI metal, and a noble metal, on a refractory support.
The noble metal is incorporated onto the support by use of specific noble
metal precursors, such as xanthates and dithiocarbamates. The present
invention also relates to a hydrotreating process using said catalysts.
BACKGROUND OF THE INVENTION
Hydrotreating of petroleum feedstocks and various boiling
fractions thereof has become increasingly important because of more
stringent product quality requirements. Furthermore, the petroleum
industry foresees the time when it will have to turn to relatively high
boiling feeds derived from such materials as coal, tar sand , oil-shale,
and heavy crudes. Feeds derived from such materials generally contain
significantly more deleterious components, such as sulfur, nitrogen,
oxygen, halides, and metals. Consequently, such feeds require a
considerable amount of upgrading in order to reduce the content of such
components, thereby making them more suitable for further processing,
such as fluid catalytic cracking and/or cracking and/or catalytic
reforming.
Hydrotreating of hydrocarbonaceous feeds is well known in
the art and usually requires treating the feed with hydrogen in the
presence of a supported catalyst at hydrotreating conditions. The
catalyst is typically comprised of a Group VI metal with one or more
Group VIII metals as promoters on a refractory support. Hydrotreating
catalysts particularly suitable for hydrodesulfurization or
hydrodenitrogenation generally contain molybdenum on alumina promoted
with a metal such as cobalt, nickel, and iron. Cobalt promoted
molybdenum on alumina are most widely used for hydrodesulfurization,
while nickel promoted molybdenum on alumina catalysts are the most widely
used for hydrodenitrogenation.
N~~J~w.~tJ
-2-
Further, hydrotreating catalysts containing platinum are
known. For example, U.S. Patent No. 3,422,000 discloses hydrotreating
with a catalyst consisting essentially of 0.005 to 5 wt.% of a platinum
series metal and about 4 to 30 wt.% of molybdena on alumina, the catalyst
having been resulfided.
While catalysts containing molybdenum with nickel, cobalt,
or both, are in extensive commercial use today, they nevertheless have
limitations with respect to removing heteroatoms from heavy feeds, such
as heavy coker gas oil and coal derived gas oils. As the feeds become
heavier, the content of condensed aromatic hydrocarbons, with and without
heteroatoms, increases. These condensed aromatics can absorb strongly on
the catalyst sites, reducing both the rate and extent of heteroatom
removal. Consequently, there exist a need in the art for improved
hydrotreating catalysts having increased activity toward such heavy
feeds, particularly when the heteroatom to be removed is nitrogen.
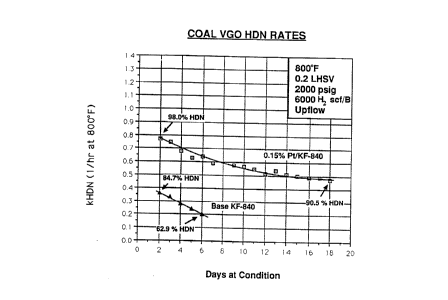
BRIEF DESCRIPTION OF THE FIGURE
The figure shows first order rate constants for
hydrodenitrogenation of a coal derived vacuum gas oil using a
commercially available Ni/Mo on alumina catalyst (KF-840) in a fixed bed
upflow reactor at 2000 psig hydrogen and 800°F. Rate constants for the
same commercial catalyst with 0.15% Pt added as PtEEX are also shown.
From the rate constants, it is apparent that the Pt promoted catalyst is
much superior.
SUMMARY OF THE INVENTION
In accordance with the present invention, there is
provided a catalyst composition comprised of: about 0.005 to 5.0 wt.f°
nobl a metal , about 0. 5 to 5 wt.~° of at 1 east one Group VI I I
metal , and
about 3 to 18 wt.% of a Group VI metal, and a refractory support, wherein
the noble metal is incorporated into the refractory support by use of a
precursor represented by ML2 when the nobl a metal i s Pt or Pd, and ML3,
when the noble metal is Rh or Ir, where M is the noble metal and L is a
ligand selected from the dithiocarbamates, dithiophosphates, xanthates,
thioxanthates, and further wherein L has organo groups having a
sufficient number of carbon atoms to render the noble metal complex
soluble in oil.
, , ~?
NI~~.~~:i~c~
Also, in accordance with the present invention, there is
provided an improved hydrotreating process for removing heteroatoms from
a hydrocarbonaceous feedstock which process comprises treating the
feedstock at hydrotreating conditions, in the presence of hydrogen, with
a catalyst composition comprised of: about 0.005 to 5.0 wt.% noble
metal, about 0.5 to 5 wt.% of at least one Group VIII metal, and about 3
to 18 wt.% of a Group VI metal, and a refractory support, wherein the
noble metal is incorporated into the refractory support by use of a
precursor represented by ML2 when the noble metal is Pt or Pd, and ML3,
when the noble metal is Rh or Ir, where M is the noble metal and L is a
ligand selected from the dithiocarbamates, dithiophosphates, xanthates,
thioxanthates, and further wherein L has organo groups having a
sufficient number of carbon atoms to render the noble metal complex
soluble in oil.
In one preferred embodiment of the present invention, the
noble metal is platinum and the platinum precursor is a xanthate or
dithiocarbamate.
In another preferred embodiment of the present invention,
the catalyst is comprised of about 5 to 15 wt.% Mo, 1 to 5 wt.% Ni(Co),
and 0.01 to 2.5 wt.% platinum, on an alumina support.
DETAILED DESCRIPTION OF THE INVENTION
A variety of feedstocks can be hydrotreated with the
catalysts of the present invention, including hydrocarbonaceous fractions
and whole feeds. Non-limiting examples of such feeds include organic
solvents, light, middle and heavy petroleum distillates, as well as
petroleum residual feeds, other feedstocks include coal derived liquids,
shale oil, and heavy oils derived from tar sands.
In the practice of the present invention, a heteroatom
containing feed, especially a high nitrogen containing feedstream, is
contacted with hydrogen at hydrodenitrogenation conditions in the
presence of a supported catalyst of the present invention. The catalyst
is comprised of a noble metal, at least one Group VIII metal, and at
least one Group VI metal, on an inorganic oxide support. The noble metal
is present in an amount ranging from about 0.005 to about 5 wt.%, based
on the total weight of the catalyst, preferably, about 0.01 to about 2.5
wt.%, most preferably 0.1 to 1%.
- 4 -
Noble metals suitable for use herein include platinum,
palladium, rhodium, and iridium. Preferred are platinum and rhodium, and
more preferred is platinum. The Group VIII metal is present in an amount
ranging from about 0.5.to 5 wt.%, preferably from about 1 to 4 wt.%.
Preferred Group VIII metals include Ni, Fe, and Co, with Ni being most
preferred. The preferred Group VI metal is Mo which is present in an
amount ranging from about 3 to 20 wt.%, preferably from about 5 to 15
wt.%, and more preferably form about 8 to 14 wt.%.
It is critical that certain noble metal precursors be used
in preparing the catalysts of the present invention. The noble metal
precursors suitable for use herein are represented by:
MLZ when M is Pt or Pd, and
ML3 when M is Rh or Ir
L is a ligand selected from the dithiocarbamates, dithio-
phosphates, xanthates, and the thioxanthates, wherein L contains organo
groups having a sufficient number of carbon atoms to render the noble
metal complex soluble or highly dispersed in a hydrocarbonaceous solvent
or feedstock. For example, the organo group can be selected from alkyl,
aryl, substituted aryl, and ether groups. Generally, the number of
carbon atoms of the organo group will be from about 4 to 30. Preferred
are the dithiocarbamates and xanthates. An example of the preferred
xanthates are the alkoxyalkylxanthates represented by the formula:
S
(Ry-0-R2-0-C-S)~ Mm
where R~ is an alkyl group (straight, branched, or cyclic); an alkoxy
substituted alkyl group; an aryl group; or a substituted aryl group,
R2 is a straight or branched alkylene group,
M is the noble metal,
n is an integer from 1 to 4, preferably from 2 to 3.
Preferably, R~ is a straight alkyl group, a branched alkyl
group, or an alkoxy substituted alkyl group. Most preferably, R~
comprises a straight chained alkyl group. Although the number of carbon
atoms in R~ can vary broadly, typically R~ will have from about 1 to 24,
preferably from 2 to 12, and more preferably from 2 to 8, carbon atoms.
Typically, R2 will have from about 2 to 8, preferably from 2 to 4, carbon
atoms. Most preferably, R1 and RZ will each have from 2 to 4 carbon
atoms. R~ and R2 together should contain a sufficient number of carbon
~:i~''1~J:,~J
-5-
atoms such that the metal alkoxyalkylxanthate is soluble in the oil.
Examples of suitable substituted groups in R1 include alkyl, aryl,
alkylthio, ester groups, and the like.
Examples of the various metal alkoxyalkylxanthates that
can be used in the practice of the present invention are platinum
bis(ethoxyethylxanthate), platinum butoxyethylxanthate, platinum
propyloxyethylxanthate, platinum isopropyloxyethylxanthate, platinum 2-
ethylhexyloxyxanthate, Rh trisethoxyethylxanthate, Rh
trisbutoxyethylxanthate, Rh tris(2-ethoxyethalxanthate) etc. Noble metal
dithiocarbamates can also be represented by general formula of ML2 and
ML3 wherein L is a dialkyl or monoalkyl dithiocarbamate group.
Any suitable support material may be used for the cata-
lysts of the present invention. Preferred are alumina and silica-
alumina. More preferred is alumina. Other refractory inorganic
compounds may also be present, non-limiting examples of which include
zirconia, titania, magnesia, and the like. The alumina can be any of the
aluminas conventionally used for hydrotreating catalysts. Such aluminas
are generally porous amorphous alumina having an average pore size from
about 50 to 200 A, preferably from about 70 to 150 A, and a surface area
from about 100 to about 350 m2/g, preferably from about 200 to 300 m2/g.
The Group VI and Group VIII (Ni, Fe, Co) metals can be
incorporated into the support using any suitable technique such as the
incipient wetness technique which is well known in the art. However, the
noble metals are incorporated into the catalyst by using the previously
mentioned precursor complexes disclosed in this invention. For example,
the catalysts can be prepared by first impregnating the support with an
aqueous solution of Group VIII and Group VI metal salts. The support can
then be dri ed, cal ci ned, and impregnated wi th the nobl a metal precursor
in a suitable solvent such as acetone etc.
The instant invention can also be practiced by adding the
noble metal precursor directly to the feed, to which a conventional
presulfided Mo and Ni and/or Co hydrotreating catalyst is added.
Another example of the preparation of catalysts of this
invention includes impregnating a presulfided hydrotreating catalyst as
given above with an solution of noble metal complex in a suitable organic
solvent.
-6-
Heteroatom removal conditions, especially hydrodenitro-
genation conditions, will vary considerably depending on such things as
the nature of the feed being treated, the nature of the nitrogen being
removed, the nature of the complexes being removed, the nature of the
complexes employed, and the extent of conversion, if any, desired. In
general, however, the following are typical conditions for
hydrodenitrogenation of a naphtha boiling within a range of about 25°C
to
about 210°C, a diesel fuel boiling within a range from about
170°C to
350°C, a heavy gas oil boiling within a range of from about
325°C to
about 475°C, a lube oil feed boiling within a range of from about 290
to
500°C, or residuum containing from about 10 percent to about 50 wt.% of
material boiling above about 575°C.
~t~~fi~~a
_,_
O O
CU O O O O O
N O O O
t!T
O O
Od N I ~ ~
C
i ~ ~ v
~
O 0
?~OC 0 0 0
Z .--m!f O .-vO
H ,-i N
Rf
C9
~1
i~
r
V
O O
r .--~ t0 ~ tn
i N
NZ i ~ i t i
M N .-~
N O O O O O
va
a
N
a
as
r
_
t0 H O O O O
H O O O O O
C) 00 -r N M t~
i ~ ~ i
O O O O O O
N 1~ tn 1~ O O
N r~ N N ~ O
d
i
a
U
AO O O O
O
P~ O M In
tn
M et at et
~
i ~ i
O
EO O t O
D ~
~ N N N
M
r
b ~r O
~ L r O 7
Q7 +- N
d ~= N 7 N ~r
LL a d b ~ N
f0 ~r d
2 D Z J 2
1
_g_
The following examples are presented to illustrate the invention
and should not be considered limiting in any way.
EXAMPLE 1
S~rnthesis of bis(2-ethoxvethvlxanthato~ Pt ~PtEEX~: To a mag-
netically stirred solution of 6.7g, of potassium 2-ethoxyethylxanthate,
(KEEX) in 200 ml. of deionized water, was added a filtered solution of
potassium tetra-chloroplatinate in 150 ml. of deionized water. The
initial reddish-brown solution turned turbid, and slowly a yellow
precipitate separated out. The mixture was allowed to stir for three
hours, the solid collected by filtration, and washed well with deionized
water. The solution was air dried and recrystallized from acetone-water
to give 4.5 g. (80% conversion) as yellow-orange crystals m.p. 83-84°C.
EXAMPLE 2
S_Ynthesis of bisy2-ethoxvethvlxanthato)Pd ~ PdEEX~: This com-
pound was prepared from 9.5 g. of (KEEX) and 6.52 g. of potassium tetra-
chloropalladate according to the procedure given above far PtEEX. The
product was obtained in 93% yield as a yellow shiny crystalline solid,
m.p. 70°C.
EXAMPLE 3
Synthesis of tris(2-ethoxyethYlxanthato)Rh, (RhEEXZ: This com-
pound was synthesized from 1.92 g. of sodium hexachlororhodium(III) and
4.2 g. of KEEX according to the procedure given above for PtEEX. The
product was obtained as a brown-orange crystalline solid, m.p. 75-76°C.
EXAMPLE 4
Samples of a commercial hydrotreating catalyst designated
KF-840, and available from Akzo Chemicals Inc., were presulfided and then
treated with various concentrations of the above noble metal precursors
in acetone to give the final catalyst as indicated in Table I below. KF-
840 is an alumina supported catalyst and is reported to contain about
12.7 wt.% Mo, and 2.5 wt.% Ni, and 6.4 wt.% P205, and has a surface area
of about i35 m2/g and a pore volume of about 0.38 cc/g.
~~ta~~ ~_~~
_g_
TabTable II
Catalyst
CatalystAdditive Abbreviation CatalystDescr~tion
CSZ
A 2.8 g. KF-840 Commercial A1203 talyst
Ni/Mo Ca
B KF/Pt KF-840(3.75g.)+ PtEEX(0.404g.)
C KF/Rh KF-840(3.75g.)+ RhEEX(0.87g.)
D KF/Pd KF-840(3.75g.)+ PdEEX(0.615g.)
E KF/Pt500 KF-840(3.75g.)+ PtEEX(0.101g.)
F KF/Pt500(imp) KF-840(3.75g.)+ PtEEX(0.101g.)
In all the runs, 32-42 mesh KF-840, which was presulfided with a
mixture of 10% HZS in HZ was used.
Activity measurements were performed on the above catalysts in a
gas flow-through autoclave fitted with a magnadrive. The reaction
temperature was maintained at 425°C, the pressure was 2000 psig H2,
residence time was 4 hours, the stirring rate was 1500 rpm and the H2
flow rate was 320 cc/minute. For each experiment, 75 g. of a coal-
derived gas oil as described below, and 3.75 g. of KF-840 (Mo+Ni = 6055
ppm) were used. In runs on Catalyst B, C, D, and E, EEX complexes of Pt,
Rh, and Pd, were added directly to the feed along with the presulfided
KF-840. For Catalyst F, the sulfided KF-840 was impregnated by a pore
filling method with a solution of PtEEX in acetone. The experiments were
conducted in the usual way, all the gaseous products were analyzed by
mass spectrophotomic analysis and the liquid products were characterized
by elemental analysis. The results are shown in Table II below.
The feed properties were as follows;
C = 88.97%; H = 7.99%; S = 0.321%; 0 (Calcd.) = 1.85%
H/C Ratio = 1.05
Fractions Boiling at:
IBP-400°F = 0%; 400-650°F = 10.8f°; 650°F+ =
89.2f°
- 10 -
Table II
Catalyst # HDS HDN H /C Ration
A KF-840 64.8 80.5 1.254
B KF/Pt 75.5 92.5 1.269
C KF/Rh 80.1 87.2 1.269
D KF/Pd 81.6 82.1 1.269
E KF/Pt500 90.3 94.2 1.267
F KF/Pt500(imp) 90.I 94.5 1.32
It is evident from Table II that Pt and Rh improve the HDN very
significantly. Very surprisingly, Pt at lower level has a significantly
larger benefit than at higher concentrations. Moreover, impregnation
seemed to increase the H/C ratio also. These results are highly
unexpected.
In Table III below comparative data on conversion to C1-C2
gases, total C1-C4, as well as 200°C- and 200-340°C liquids
produced are
given.
Table III
Catalyst # 1-C4 C1-C2 200C 00-340C
C
9.5 4.6 12.9 31.0
B 4.8 2.6 12.4 36.8
C 5.3 2.8 12.9 35.0
D 5~5 2.8 13.5 32.3
E 3.9 1.4 15.5 36.8
F 7.4 1.8 16.0 38.2
The advantage for HDN of the catalysts of the present invention
over KF-840, the conventional catalyst is unmistakable. In addition, the
catalysts of this invention lower the gas make and increase the naphtha
and distillate yields.
Examples given below describe preparation of Pt doped KF-840 by
a conventional incipient wetness technique using inorganic chloroplatinic
~~a~~
- 11 -
acid as well by the method of this invention using the precursor complex,
PtEEX.
EXAMPLE 5
Preparation of (0.4%1 Pt Doped Catalyst by Cloronlatinic Acid:
A sample of 100 g. of air exposed KF-840 was stored overnight over water
to equilibrate with moisture. The sample weight after the exposure was
119.2 g. It was transferred to a fritted funnel and was covered with
water so that the water layer was 1/8" above the solid. Carbon dioxide
was bubbled through the sample for 1 hr. To the sample, a solution of
1.0165g of hydrogen hexachloro-platinum(IV) in 16 ml of deionized water,
and 0.216 g. of concentrated hydro-chloric acid was added. Carbon
dioxide was bubbled through for an additional 4 hrs. The supernatant
liquid was decanted off and the sample was air-dried. It was then dried
in vacuum at 100°C overnight and calcined at 427°C for three
hrs. The
final product weighed 87.7 g. The catalyst was designated Catalyst G.
EXAMPLE 6
Preparation of 0.3% Pt Doped Catalyst by Using PtEEX: To 50.Og
of KF-840, which was sulfided with 10 % H2S/H2 at 345°C for 16 hrs, in
a
porcel ai n di sh, was added, dropwi se wi th manual sti rri ng, a sol uti on
of
0.404 g. of PtEEX in 40 ml of acetone. The small amount of the complex
on the side of the dish was washed down with 5 ml of acetone. The
catalyst was allowed to air dry for 1 hr and then dried in a vacuum
desiccator at 110°C overnight. The catalyst was designated Catalyst H.
EXAMPLE 7
Preparation of 0.15% Pt Doped Catalyst by Usin4 PtEEX: This
catalyst was prepared by the method described in Example 6 and was
designated Catalyst I.
EXAMPLE 8
Preparation of 0.05% Pt Doped Catalyst b Usina PtEEX: This
catalyst was prepared by the method described in Example 6 and was
designated Catalyst J.
~~)t~~i~ ii
- 12 -
XAMP E 9
In this experiment, the Catalysts G, H, J, and KF-840 were
compared for upgrading Baton Rouge light cat cycle oil (LCCO). The feed
characteristics were as given below:
S = 1.47%; N = 557 ppm; 10.6 °API.
Results are given in Table IV.
TABLE IV
Conditions: 315°C~ 2 0 LHSV; 3500 SCF/B~ 1200 psig
Catalyst G Catalyst H
KF- 40 tKF-840+0.4% Pt)* ~(KF-840+0.3% Pt/PtEEX
Product S9'° 0.36 0.39 0.38
Product N, ppm 192 138 101
°API 17.1 16.7 16.8
* KF-840 impregnated with 0.4% platinum using hydrogen hexachloro-
platinum as the precursor.
As is clear from this table, the catalyst of this invention
gives much better HON than the state-of-the-art catalyst KF-840, or KF-
840 impregnated with higher concentration of Pt, but by standard
impregnation technique using a precursor such as hydrogen
hexachloroplatinum.
EXAMPLE 10
In this experiment, catalysts H, I, J, and KF-840 were compared
for upgrading light catalytic cycle oil, as defined in Example 9 above.
The objective was to determine the effectiveness of Pt doping at even
lower concentration by the process of this invention. Results are given
in Table V.
~~~7~~ ~ j
- 13 -
TABLE Y
Conditions: 315°C: 0.85 LHSV~ 3500 SCF~/B; 1200 osia
Catalyst H Catalyst Catalyst
I J
(KF-840+ (KF-840+ (KF-840+
KF-840 .3%Pt ~ .15%Pt)Pt) .05%PtlPt1
Product S% 0.238 0.232 0.232 0.224
N ppm prod. 17.0 0.3 0.6 3,3
API 19.0 19.4 19.4 19.4
As can be seen from the table, all the catalysts of this
invention are much more active for HDN than the standard catalyst, KF-
840. Surprisingly, at lower Pt doping, the catalysts also maintain their
activity for HDS also.
EXAMPLE 11
A 1/2" diameter fixed bed upflow reactor was charged with 5 g.
of KF-840. Activity of the catalyst was determined at a variety of
conditions with a coal derived mid-distillate feed {0.270% S, 0.958% N
Distillate), and with a coal derived vacuum gas oil (0.318% S, 1.026% N
VGO). At the start of the run, the mid-distillate feed was spiked with
decanethiol (4% S on feed) to sulfide the catalyst. In all cases, the H2
pressure was 2000 psig, and treat gas rates were maintained at 6000
scf/bbl. Further conditions and results are given in Table VI. Upon
completion of the run, the catalyst was discharged from the reactor, and
replaced with 5 g, of pre-sulfided KF-840 which had been impregnated with
0.15° Pt using PtEEX. Pre-sulfiding was carried out with 10% H2S in H2
at 340°C. Impregnation was carried out by pore-filling from an acetone
solution, then drying in vacuum to remove residual acetone. Conditions
and results are given in Table VII.
i/ 1w :~
~,1 ~,' . 1
C, iyp k_ (,~
- 14 -
Tabte VI
Feed Tem . C HSY N in product kHDN
Distillate 365 0.2 96 O.gg
365 0.1 8 0.74
365 0.8 3526 0
94
365 0.4 1579 .
0.86
365 0.4 4653 0
68
400 0.4 70 .
0
96
425 0.4 50 .
0.53
VGO 425 0.4 850/2800 0.25/0.15
425 0.2 1600/3800 0.09/0.05
Table VII
Feed Tem . °C ~HSV N in product kHDN
Distillate 343 0.4 538 2.40
343 0.1 22 1
26
343 0.8 3475 .
1
75
365 0.4 227 .
1
58
365 0.2 41 .
1.27
343 0.4 2118 1.27
365 0.2 48 1.22
VGO 365 0.4 3164 0.54
365 0.2 1692 0.41
365 0.1 455 0.37
400 0.2 253 0.36
425 0.2 204/916 0.20/0.12
The data of the above Tables, evidences that the Pt promoted
catalyst is superior in HDN behavior to the commercial catalyst.
Furthermore, the plot of HDN rate constant, versus time on oil, given in
Figure 1 below, clearly shows that the Pt promotion dramatically
decreases the rate of deactivation of the catalyst.