Note: Descriptions are shown in the official language in which they were submitted.
WO 91/14944 1 2 0 5 6 4 4 7 PCI~/NL91/000~1
A ~e~
~pecif'i~ anti~dv sDe~i~s ~ ~_
out ~ai~ ~ethvd as w~ kit c~
~is invention concerns B ~ethod for t~e .coll~csion of
~ntibodies and the detection o~ ~ ~pec~fic ant~bod~ ~psc~es tn A fluid
~ample ~nd 8 device for carrying out ~aid lðod ~s well ~s a kit
co~prising said device.
European patent application 0200381 describes a solid ph~se
syste~ for use in B ligand-receptor Elssay for the detection Or a
~elected analyte in a fluid sample comprlsing a porous matrix, wherein
~icrospheres are entrapped, wherein said microspheres are bound wi~h a
receptor capAble o~ c~pturing a specific target ligand. Upon use Or
differen~ receptors either different target ligands cEn be bound or one
species Or target ligand c~n be bound to di~ferent plsces for examp}e
upon use of different antibodies that bind 'che antigen at different
non-interfering epitopes. The s~oli~ phase according to EP-A-0200381 can
consist of 8 membrane or filter made of glass fiber, nylon or cer~mic
~: ~aterial , wherein microspheres are ~ntrapped , whereby said microspheres
20 ~ ~re made of a polymeric material such as for example latex thht CE~Il
bind to the receptor substsnce. Preferably a ~y~tem is used here
consisting of membrsn2-microspheres-i~munological p~ir, whereby the
: : pa~ir consists of antibody~antigen or ~ntigen-antibody. The ~ethod thst
; is described in this`EP-A comprises the ap,plication of fluid sample
~; 75 Oh the solid phase system, whereby ~he receptor that is b~und to the
icrospheres specifioally binds ~he selected target ligand, as the
luid through pssses the solid phase system. Su~seguently a sslution of
a receptor eonjuga~e~is added, whereby s~id conjuEate is eapable of
specirically binding ~nd labelling~ ~he target ligand in order for the
bound target ligand to be ~detected ~n the ~olid~phase.: The term
"reeeptor conJuga~e" is directed at a complex co~prising a receptor and
a label~that can be !del~ec~ed.,I~ the ~arget ligand is ~n ~ntigen: the
~eceptor conjugate can be a labelled antibody preferably ~ ~onoclon~l
ntibody. 9n the other hand If the ~ar~et~ ligand is an antibody
~ ~ 35 labelled an~igen can ~e used~ as ~cepto~ conjuga~ç. Non-bound receptor
: ~ conjugate can subsequently be re~oved by rinsing. The pre~ence of bound
recep~or conjugste on the ~olid phase system implies the: presence of
2~64~7
W O 91/14944 2 PCT/NL91~000~1
~''" , .
.'the analyte in the ~emple. The EP-A also describes a device for
carrying out such a ~ethod, whereby ~id device compri5es the solid
phase sy~tem as described, whereby s~id device is provided with ~eans
permit~ing the flow of a fluid s~mple through the ~olid ph~se ~ystem. ::
It is known that protein A generally binds i~munoglobulin and
that it binds to Fc, it's constant region. (J.J. L~ngone, Advances in
~m~unology, Vol. 32, page 179-211).
Natural protein A is derived from aPl~yloco~cus aur~us, a
pathogen, and is thersfore not free o~ ~$~h~1Q~Q~suQ toxins.
American patent 4,189,466 desrribes the detection Or the
presence of rheumatoid f~ctor (RF) in an animal sample by mixing the
s8mple with a suspension of microbial cells contsining protein A that
have been sensitised with free Fc rrsgments of IgG for which RF i5
specific. If the mixing results in agglutination this indicates a
15^ positive result. ~:~
Further protein A is coupled to for example agarose beads via
chemically stable amide bonds and this complex is used in affinity
chromatography for sntibodies such as for example in American patent
4,879,211.
DuPont has ~o~e on ~o`the ~arket with a recombin~nt protein A
coupled to Seph~rose, whereby said protein is expressed in ~.coli, a
non~pathogen rather than in the pathogen StaDhvlococ~us aur~ from
~ which the natural protein A is o~tained, which implies that ~he ligand ~::
:~ is fre~ of toxins. DuPont uses recombinant protein A in
:~ 25 the~ per~lex 35RS ~ffinity column. The molecule contains 5 ho~ologous
binding regions which are highly speci~ic for the Fc portion of
munoglobulins. Immunoslo~ulin ~ (IgG) is~ the ~jor immunoglobulin
which binds to recombinant protein A. Other immunoglobulins will also
be sdsor~ed, however t~e class and sub-class ~pecificity varies fro~
species ~o ~pecies. The pro~ein A can therefore be us~d for purifying
IgG or ~or selectively removing I~G for analysis of the XG classes or
for absorbing i~munocomplexes ~or the purification of an`~igen`s. I
The ~-thod according to the inven ion is directed at ~ ~ethod
:~ ~or the collect~on o~ antibodies and the detectio~ of a specific ~:
~ntib~dy species in~a fluid shmple comprising the step~ of
: a) con~acting ~aid tluid sample with a solid phase ~a~rix. said
~ ~a~rix compr~sing porous ~embrane, latex particles distributed in said
~ ~ .
' .
W O 91/14944 2 0 5 6 4 4 7 PCT/NL~l/OVo~1 ~
,~.. ~, .
porous ~ewbrane, recombinant protein A or G being covalently bound to
~aid latex particles resulting in an aspec~fic blndin~ ~f antibodies
present in the fluid ~mple to ~orm a complex [ant~bodies-protei~ A/G-
latex-membrane],
b) optiorlally removing excess fluid ~ample rrom sald 50lid phase
~atrix,
~ ) adding an antigen t9 8aid complex tantibodie~-protein A/G-
; latex-membrane], ~aid antigen ~eing ~pecific for the antibody species
to be detected to allow for~tion of a complex containing said specific
antibody species: [antig~n-antibody-protein A/G-latex~m~mbrane],
d) optionally removing excess ~ntigen from ssid porous m~trix,
e) using a detection ~ethod specif$c for th~ antigen of step c).
The invention is directed a~ a method whereby ~ll antibodies
in a fluid sample are entrapped on a ~olid phsse matrix and
1~ ~ubsequently one or optiona11y more antibodies can specifically be
detected.
;The antigens and ~nti~odies that can be used with this ~ethod
,c:an be associated with bac~eria, fungi, parasites or virus. Gne can
consider a retrov:irus:such as for example a~HIV-1 virus. This invention
~: 20: :is directed 8~ a new method for the collec~ion of i~unoglobulins from
: for;example ~a body fluid for example ~or a diagnostic test. The
invention is directéd a~ the collection and dete~tion of ~ntibodies
f:rom:body fluids of a mammal (including ~ human being) such as ~west,
tears~,:blood~, urine or preferably sa1iva.~t is ther~fore possible to
use body~ fluids in which the a~ounts of antibodies ~re 1GW as fluid
sample. : : The ~ubstance that can bind i~munoglobulin is a recombina~nt
protein G ~r recombinRn~ pr~tein A derived ~rom E.coli or recombinant
pro~ein A and :G and thi~ pr~tein is cova1ently~ bound to 1 tex-: 30 parti~les. ~hese latex particles are ~urface modified for ~example by
carboxylate, amide and j~r hydroxy to ~na~le them to bind protein (page
; 30, Uniform ~atex Pa~ti~cles L~iR. Bangs). The 1atex~psrticle.~ boun~ with
protein G, A or G/A are entrapp~d ~n a porous ~embr~ne and form the
801id phase ~trix. The porous ~e~brane can ~e ~de ~r example ~rom
~ 35 c~llul~se, ceramic ~aterial, gla~s ~iber. nylon or plostic.
: Polystyrene l~tex~icrop~rticles possess an in~rinsic lsrge
surface whi~h allows a large contact area ~or ~he :immunogl~bulin
:: :
W O g1/14Q44 4 2 0 5 6 4 4 7 PcT/NL9l/ooQ~l ~
~j;J binding substance, r~combinant protein A/G, and also for the sample
fluid, resulting in a large amount of immunoglobulins that can be
entrapped on the natrix, whereby ~he sub~tra~e for a very sensitive
immunodiagnostic test is cr~ated. The high affinity of proteins G and A
for the Fc re~ion of i~munoglobulin permits a fast collection of
immunoglobulin G's [IgG) and in connection with the lar~e surface area
th~t is offered by latex microparticles this combination of protein
A/G-l~tex microparticles provides ~n ideal ~ystem for the collection
and detection of immunoglobulins in ~luids, p~rticularly in body fluids
such as saliva which contain a snaller smount of antibodies than seru~.
Si~ultaneously the antigen position of the ceptured an~ibodies remains
free as protein G and A bind to the Fc region of the antibodies so that
these captured antibodies can react wi~h their free specific regions
with the corresponding speci~ic antigens. The matrix consisting of
membrane-latex-protein A/G plays the p~rt of coll~ctor of
immunoglobulins and also serYes as solid phase carrier for the
identification of antibodies in an im~unodiagnostic test. It is
~herefore only necessary ~o use one specifically binding antigen for
~ ~he detectLon, in contrast to~ the test described in EP-A-0200381,
: 20 whereby at least two specifically blnding recepsors Or the analyte are
necessary.
,,
Furthermore 8 sample that is analysed according to the ~ethod :
.
of~ ~he invention can be examined for the presence o;f various ~pecific
antibodies by use of dirferent solutions w~th specif:ic antigens. This
: 25 was also~ possible w1th the method sccording ~to EP-A-0200381 however:
only by fillin~ various areas:of the matrix with microspheres bound to
: varlous specific receptors,~ wherebg the detection poss1bilities of
~: different antibodies with such a ~atrix are~ restricted ~o a few: antibodies determ1ned at the preparatio~ ~r the matr:ix. This contrast `~
w~th the ~atrix used in ~he method according ~o the invention in which,
in accordance with the antibody that is to be detected, a specific ~:corresponding anti~e~ s adde~after bindinglthe anti~odies ~nd whereby~,
~he matrix is suited ~or the binding of ~very antibody. In ~his ~ethod
: every poss~le antibody species that is present in~:the ~a~ple is bound
and therefore every antibody can be detected by use o~ a specific
: ~ antigen. ,~ :
The specificity Or ~his detection methcd depends on the
:;.
20~64~7
W O 91/14944 5 PC~/NL91/~00~1
t J antigen ~hat is selected as means of detection. In the ~ethod according
to ~he invention one can 5elect the detection nethod of step e) from
l~belling with an enzyme, with a radionuclide with ~luorescent agents,
phosphorous s~ents, polymers contai~ing eolouring agents or
chemilu~inescent portions, whereby preferably a detectio~ procedure is
used that will ~enerate a colour change which can be detected by
~nstruments or prefer~bly by ~ight. When lsbelling with on enzy~e lt is
possible So subsequently apply a substrate that brings about a colour
change. For example 4-rhloro-l-n~phthol or TM~ (tetr~methy}benzidin)
esn be used as substr~tes.
This invention is also directed at the use of ~ non-toxic,
non-infectious complex of [recombinant protein A or G-latex-membrane]
for immunological tes~s as well BS a device for carrying out
immunological tests according to the method of the inven~ion. This
15- device comprises a carrier containing the complex [protein A/G~latex-
~embr~ne]. I~e carrier comprises an absorbent means incorporated in ~n
elongated cont~iner with an opening at one end, where~y ~aid end
contains said complex and said opening enables the entrsnce Or fluid
via ~he co~plex absorbent ~eans. The absor~ent Leans is 2ssociated with
the solid phase sys~em complex iD such a manner that the absorbent
~eans draws fluid ssmple, that comes into contact with the sol~d ph~se
system, through th~-complex. Said absorbent means can ror example
contain capillaries~in a direction perpendicular to the surface over
whieh ,the solid phase system is placed so thst the capillaries are in
direct communication with the pores Or the solid phase system.
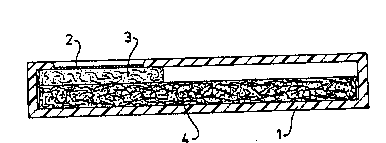
embodiment of the device accordin~ to the invention is
schematically illustrated in cros~s section 1n the drawing. In said
drawing l represents an elongated container of synthetic ~aterial
provided with an opening at one end in which a ~embrane permeablc for
f1uid 2 has been plac~d~ Porous carrier 3 containing ~he complex
~protein A or G-latex] i5 present under ~enbr~ne 2 in the container l.
Carrier 3 is in c~ntact with a fluid ~bsorbing ~a~eri~l 4 tha~ can
completely o~ partially fill the cont~iner l. Yarious ~aterials c~n be
u~ed ss absorbent ~e~ns ~uch as cellulose acetate ~bers, cotton.
psper, ~la tic or polyester. Th~ device can further ~or exsmp1e be msde
o~ plastic with the following dimensions, ~ length of approx~ately 4-
14 cm, a depth o~ 1-5 mm and a wid~h o~ 0,5-5 cm with ~n opening with a
W O 91/149~4 2 0 5 6 ~ 4 7 pT~g~uoo~l
.. . .
-' di~meter of ~pproximately l-4 cm ~t one end. The absorbent means can
have a length of 3-13 cm, a depth o~ 0,5-4,5 m~ and ~ width of 0,3-4,5
cm. ThB open end of th~ device cRn be cov~red with 8 membr~ne.
A preferred embodiment o~ ~he device is a device ~hereby the
open end of the device containing the substance that is capable of
binding immunoglobulin can be ~nserted ~nto the mouth Or a test person.
In particular for this ~pplication the i~port~nce of the use of
recombinant protein A without toxins that sre present upon use of
natural protein is an advantage. This is a new and extremely simple
~sst and handy method for collecting i~munoglobulins ~ and the further
use of said~im~unoglobulins in an immunodi~gnostic tes~ on ~ solid
phase matrix. It is only nece~sary to keep the device in the mouth for
the duration of l to 3 minutes. Saliva that comes into contact with ~he
~atrix passes through the latex particles bound with protein A, whereby
the immunoglobu}ins that are pre~ent wiIl bind quickly to protein A.
The size, and shape of the device Gske it simple to use and the
: discsmfort of collecting blood or urine for an immunodiagnostic test is
~ prevented. ::~
:~ Furthermore this same device in which ~he immunoglobulins ~re
collected serves as carrier to~be used in the identifiG~tion of desired
~: ~peci~ic antibodies associated with a cert~in dis~ase. Once the
..
sntibodies heve been bound to the protein A~latex particles membrhne;~
antigen of choice can be used to detec~ ~he specific antibodies from
; the collected saliva in an enzyme i-munotest. An e~ample of such a
specific experiment in which saliva of a patient~ was coI1ected is
escribed below. : ~
A patien:t known to be HIV-l-antibody~positive k~pt the device
according to: ehe invention under the tongue ~or: 1 minute. Af~er
c411eeting th~ saliva:O.5 ml of a buffer containing PBS/0,5X Tween~
20/2Z BSA/0.01% Thi~eros~l with pH 7.8 was pipet~ed on to the membrane
~o rinse the exces~ proteins.;Af~er the buffer~hsd ~een c~pletely
; ~bsorbed 0.2 ~l of a HIV-l-solution containing 1 micr~gram of`
:~iot~nilated protein recombinant envelope and ~ore HIV-~ in PBS/0,5%
. Tween-?0/0.l% gelatine was :add~d,and alss:~ab~orbed. The ~embrane was
&ubsequently washed wi~h 0.5 ~l PBS/0.5% Tween-20/2% BSA/0.01%
~: Thimeros~l pH 7,8 and l.5 microgra~ o~ HRP labelled avidin was pipetted
~ on t~ the ~embrane. 1~ is also possible to bind biotin to avidin in
: :
2056447
W O 91/14944 7 PCT/NL91/OOO~l
;.
`~;J ndvsnce 80 ~hat this step can be onitt~d during the tes~. The Dembrane
was rinsed ~gain with 1 ml of PBS/0.5~ Tween-20/2% BSA/D.01% Thimerosal
~th pH 7.8. The buffer was absorbed again. Then 100 ~icroliter of a
substrate, 4~chloro-1-naphthol, was pipetted on to ~he ~e~brane and
5 within 1 ~inute a positive blue colour developed, ~hara~terizing an
sn~ibody positive reaction. Ihe total time required for the test was
less than 5 minu~es. A ~imilar experiment was carried out on B patient
known to be HIV~ ntibody ne~ative ~nd no colouring reaction of the
device was observed, which is characteristic Or an antibody negative
reaction. These examples show the u~ility o~ the device for oollecting
antibodies from body fluid with a lower concentration ~f ~ntibodies
than ia serum. The antibodies ~hat are ~ollected in this ma~ner are
bound to a matrix in a concentration that is suited for a specific
immunodiagnostic test. Furthermore the ~pe~lficity of the test has been
: 15 demonstrEted upon use of a speci~ic entigen for detection of a certain
antibody.
The protein A/polystyrene nicrop~rticles were ooupled
covalently to each other in the rollowin~ manner:
: la~ex particles were used that had been c~rboxylate modified
,
(GML): with a diameter of 0.3-95 ~m and are co~ercially svailable from
Serydin, Inc., Indian~poIis. The COOH-groups on the surface enable a
~: fast and simple formation o~ æ~ide b~nds via activa~ion of water
soluble:carbodiamide. 1 ml of 10% solid latex particles with a diameter
of~ 0.3-95 mm was rinsed with 10 ~; o~ delonised water via
:25 centrifugation during 10 minu~es at 10-12,000 rpm. The water was
; c~refully~ removed by suction without sbsorbin~` the latex beads. The
b~ads were resuspended in lO::ml deionised H20 and soni~ied for a short
; period. The bçads were mixed by using a vsrtex:~nd centrifuged during
10 ninutes at 1~-12,000 rpm. T=hi~ ~tep was repeated twice. ~ ring this
rin~ing procedure the 0.07 uol~r carbQdiamide ~olution was prep~red: 67
~g of DC (ethyl-3-t3-dimethylamino-pr~pylcarbodiamide-HCl~ (Pearce
Rockford Ii~ with 5 ml of deionised H20 with i N NaOH~with pH 10 and 40
~g of sulf`o-~ (N-hydroxy~ulfosuccina~ide ~ were ~dded ~d this was
quickly dissolved. Af'ter the last rinsing step of the beads the w~ter
3~ w~s poured of~ and 3 ~l of the above-~entioned EDC/~ulfo-NHS-solution
~ere added. m e besds were uixed vigorously, onitied ~or a shor~
period ~f time and incubated at room te~perature under gentle agitation
W O 91/l4944 ~ 2 0 5 6 417 PcT/NLgl/ooosl
,,~t !,
t room temperature during 2 hours. After 2 hour~ o~ incubation the
~eads were rins~d another ~hree times with the dei~nl~ed waker as
described ~efore. During this rinsing ~tep the -amino-N-caproic acid
801ution was m~de by dissolving 1 g ~-amino-N-caproic acid in 0.5 ~olar
potassium phosphate with pH 7.4. The beads were resuspended in 3 ~1 of
~his ~olution. This wa5 power~ully sh~ken on a vortex and ~ncubated at
room tempersture in a ~ixer for 8 hours. After said 8 hours of
lncub~tion 0.3 ml of 1 molar ethanolamine was added to stop ~he
reaction ~nd the solution was incubated at room temperature ~or 1 hour:
the beads were wzshed snoter three ti~es in 10 ~1 of deionised water
and centrifuged during ten ~inutes st 12,000 rpm. During the rinsing
steps ~ new carbodi~ide solution was prepared a~s described earlier.
The carbodiami~e activstion was repeated and ~t the end of the 2 hours
of incubation the beads were rinsed ag~in in the deionised water as is
15 - described previously . The beads were resuspended in 1 ~l of 0. 5 KP04
with pH 7 . 4 and 1 mg of recombinsnt protein A was ~dded that had
dissolved in 2 ml of O . 5 KP04 of pH 7 . 4 and was incuba~ced at room
temperature over night. To stop the coupling reac~ion 0.3 ml of 1 nolar
ethanolamine was added and wss ~ncubated for 1 hour Bt room
~emperature. The latex beads coupled to protein were ri.nsed again ss
described ~ earIier wi~h deionised water. The latex beads were
.
~ resuspended in 2 ml of PBS and ~ixed vigorously. The ~ixture was
:~ sonified for 10 se~onds and vortexed again. This procedure was repeatedone more time. It is known that latex ~icr4particles:bind ~trongly to
25~ ~icroporous glass fiber. The selection of glass fiber ~embrane should
~: be: ~sde so that the latex particles cen be incorporated in the p~res ~f
: the ne~br~ne. In this example a Schleier and Schuell gla~s fiber
~embrane No. 34 was used. The freshly prepared microparticles bound
with protein A were dissolv~d 1:20 in PBS. 0.2 ml o~ dilu~ed letex
~: 30 microparticles/protein A was pipetted in the shape of a cros~ on~o thegl~ss ~iber ~embrane and allowed to dry at r~om te~perature. To prevent
the non-speci~ic binding ~ o'ther proteins that cQuld distu~b the
l~mun~çnzyme test the glass fi~er ~e~br~ne that contained la~ex-protein
~-c~plex was blocked with a blocklng buffer containing PBS/2~ BSA/0.1%
~elatine/~.5X Tw2en-20. In order to complcte the blockage khe ~embrane
was plac~d ~n an ~bsorbent cushion/filter paper snd 2 nI of the
b1ocking buffer were, added to the ~e=brane in order for the bufrer to
W 0 91~14944 9 2 D 5 6 ~ 4 7 PCT~NLgl/ooo~l
be absorbed by the membran~ fiO that ~n in depth block~ge of the glass
fiber m~trix was acco~plished. The ~atrix was allowed to dry at room
te~perature. The block~d ~atrix was stor~d before use in a plastic bag
with a desiccator. These glsss f'iber-protein bound lstex particles
serve as solid matrix for collecti~g immuno~lobulin ~ an increased
concentrstion, which is obtained by 8) the large surface ~rea that is
~ffered by the latex microparticles and b) the known high affinity of
protein A for immunoglobulin G.
'
;
: '
,
~.
:.
,
,: ~ : : : :
.
-
: