Note: Descriptions are shown in the official language in which they were submitted.
2~5~
PATENT APPLICATION OF
Adam Chi-Tung Hsu
FOR
HALOPPIOPARGYL MERCAPTOTRIAZOLE
COMPOUNDS, COMPOSITIONS, USES AND PROCESSES OF
PREPARATION
DN90-063 MBF:meb
Ba~kground of th~ Invention
Field of the Invention
This invention relates to control of microorganisms.
Description of the Prior Art
Certain classes of heterocyclic iodopropargyl compounds have
been proposed as fungicides or microbicides but no compound within
those classes has achieved commercial success.
Chemical Abstracts ~2:31696X(1970) shows synthesis of 2-(3-
iodo-2-propynyl-thio)benzimidazole, -benzothiazole, and benzoxazole of
the formula
~,~;, I
X = NH, O, S
U.S. Patent No. 4,170,704 to Brandmal1 et al. shows
iodopropargyl pyridyl and picolinyl ethers anà thioethers as antifungal
agents.
U.S. Patent No. 4,616,004 to Edwards discloses fungicidal activity
for compounds of the fornnula
Il .
R~,~N~ C~---I
/ =
RIS
U.S. Patent No. 4,639,460 to Rose shows compounds of the
formula
R~ ~--a-I
N
R>R3~0
as fungicides.
U.S. Patent No. 4,520,023 to Schmitt shows 3-(3-iodopropargyl)-
benzo-1,2,3-triazolin-4-ones and their use as microbicidal agents.
~6~2
There was no suggestion in the prior art that compounds within the
formula of the present invention would have utility in controlling
microorganisms.
Summary of the Invention
It is an object of the present invention to provide new compounds
for controlling microorganisms.
A further object is to provide methods of making such compounds,
methods of using them, compositions comprising such compounds, and
uses of such compositions.
These objects, and others which will become apparent from the
following clisclosure, are achieved by the present invention which
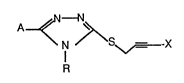
comprises in one aspect compounds of the formula
~\
N S ,~-X
R (I)
wherein A is selected from the group consisting of hydrogen,
alkyl, aryl, and heterocyclic;
R is selected from the group consisting of substituted or
unsubstituted alkyl, substituted or unsubstituted
alkenyl, substituted or unsubstituted alkynyl,
2 ~ 7 ~
substituted or unsubstituted phenyl, and substituted
or unsubstituted heterocyclic; and
X is selected from the group consisting of halogen.
In another aspect the invention comprises a method of preparing
such compound comprising reacting compound of the formula
A~ ~\
N S~-H
R (IV)
with a halogenating agent.
A further aspect comprises using a composition comprising the
compounds, or the compound itself, to protect a material se!ected from
the group consisting of wood, paint, adhesive, glue, paper, textile,
leather, plastics, cardboard, lubricants, cosmetics, food, caulking, feed
and industrial cooling water from microorganisms.
Detailed Description of tbe Invention and the Preferred Embodiment
The compounds of the invention are of formula ~1) as set forth
above. The more preferred embodiments are those wherein A is
selected from the group consisting of hydrogen; (C1-C18) straight or
branched alkyl; (C3-C8) cycloalkyl; (C3-C6) alkenyl; (C3-C5) alkynyl; (C7-
C12) aralkyl; (C6-C12) aryl; (C6-C12) aryl substituted with 1 to 3
2 ~ .~ fi ~ 7 ~
substituents selected from the group consisting of halogen, (C1-C4) alkyl,
(C1-C4) alkoxy, nitro, cyano, carboxyl (C1-C4) alkoxycarbonyl, (C1-C4)
alkyl thio, -S(O)nR2 where n is 1 or 2 and R2 is (C1-C4) alkyl; and a halo-
substituted, nitro-substituted, or un-substituteci moiety selected frorn the
group consisting of 2-thienyl, 3-thienyl, 2-furyl, 3-furyl, 2-pyridyl, 3-pyridyl,
and 4-pyridyl.
Preferred examples of A are hydrogen, 4-chlorophenyl,
3-chlorophenyl, 2-methylphenyl, 4-methylphenyl, 3-methylphenyl, 2-
thienyl, 2-nitrophenyl, 3-nitrophenyl, 4-trifluoromethylphenyl,
2-methoxyphenyl, 4-methoxyphenyl, 3-methoxyphenyl, 4-bromophenyl, 2-
fluorophenyl, 2-chloro-4-nitrophenyl, 2,4-dichlorophenyl,
3,4-dichlorophenyl, 2,4,5-trichiorophenyl methyl, iodopropargyl, t-butyl,
phenyi, 4-nitrophenyl, 3-fluorophenyl, 1-naphthyl, 2-naphthyl, 3-phyridyl,
3-bromophenyl, 3-ethoxyphenyl, n-propyl, 2-chlorophenyl, cyclohexyl,
and 2-furyl.
Suitable examples of R are methyl, ethyl, isopropyl, n-butyl,
phenyl, 4-chlorophenyl, allyl, 2-propynyl, 2-furyl, 2-thienyl, 3-iodo-2-
propynyl, 3-nitrophenyl, methoxyphenyl, and 4-methylphenyl. R can also
be hydrogen; (C1-G1a) straight or branched alkyl; (C3-Ca) cycloalkyl; (C3-
C6) alkenyl; (C3-C5) alkynyl; (C7-C~2) aralkyl; (C6-C12) aryl; (C6-C12) aryl
~'3
substituted with 1 to 3 substituents selected from the group consisting of
halogen, (Cl-C4) alkyl, (C1-C4) alkoxy, nitro, cyano, carboxyl (C1-C4)
alkoxycarbonyl, (C1-C4) alkyl thio, -S(O)nR2 where n is 1 or 2 and R2 is
(C1-C~4) alkyl; and a halo-substituted, nitro-substituted, or unsubstituted
moiety selected from the group consisting of 2-thienyl, 3-thienyl, 2-furyl, 3-
furyl, 2-pyridyl, 3-pyridyl.
The iodopropargyl compounds, i.e., those wherein X is 1, are
preferred.
As stated above, compositions comprising a compound according
to formula (I) and either an agronomically acceptable carrier, a cosmetic
agent, a cutting oil, a soap or synthetic detergent, a stabilizer, a film
forming material, or the like have a wide range of utility for protecting
against or controllins microorganisms from a wide variety of classes
includin~ fungus, bacteria, algae, viruses and yeasts. The preferred
utilities of the compositions are to protect wood, paint, adhesive, glue,
paper, textile, leather, plastics, cardboard, lubricants, cosmetics, food,
caulking, feed and industrial cooling water from microorganisms.
The following lists specific industries and applications of the
compounds or compositions:
2 ~ ~3
Industrv Appliça~
Adhesives, sealants adhesives
caulks
sealants
Agriculturelfood chain adjuvant preservation
agricultural active ingredient
agricultural chemical preservative
agricultural formulations preservation
animal feed preservation
dairy chemicals
fertilizer preservation
food preservation
food processing chemicals
grain preservation
post-harvest produce protection
sugar processing
tobacco
Construction products asphalt / concrete
cement modifiers
construction products
roof mastics
synthetic stucco
wall mastics
joint cement
Cosmetics and toiletries cosmetics
raw materials for cosmetics, toiletries
toiletries
Disinfectants, antiseptics antiseptic
disinfectant
~ ~ '3
Emulsions, dispersions aqueous dispersion
dispersed pigments
latex
photographic emulsions
pigment slurries
polymer latices
Formulated household fabric softeners
products polishes
waxes
hand dish detergents
raw materials
li~uid dete~ents
hand soaps
Industrial processing, misc electrodeposition paint, baths, rinses.
electrodeposition pre-treatment, post
rinses
industrial fluids preservation
pasteurization baths
process aid preservation
Industrial water treatment air washers
cooling towers
cooling water
water cooling
preservation/treatment of wooden
cooling tower slats and structural
members
can warmers
brewery pasteurization
closed loop water cooling systems
Laundry household laundry products
laundered goods
laundry wash water
sanitizers-laundry
2 ~
Leather, leather products leather and hide
leather and hide products
Lubricants, hydraulic aids automotive lubricants and fluids
conveyor lubricants
greases
hydraulic flwids
lubricants
Medical devices diagnostic enzymes
diagnostic kits
medical devices
Metalworking ~ related app's cutting fluids
metal cleaning
metalworking fluids
dor control (active ingredient) air conditioning
animal bedding
cat litter
chemical toilet prep'ns
deodorizers
humidifiers
industrial deodorants
sanitary formulations
toilet bowls
aints and coatingscoating emulsions
paints
Paper and wood pulp, absorbant materials of paper and wood
their products pulp
packaging materials of paper and wood
pulp
paper
paper products
paper treatment
soap wrap
2 ~ 7 ~
wood puip
wood pulp products
Paper mill paper mill slimicides
pulp and paper slurries
Petroleum refining, fuels aviation fuels (jet fuel, aviation gas)
crude oils
burner, diesel and turbine fuel oils
coal slurries
diesel fuel additives
diesel fuels
fuels
gasoline
heating oils
hydrocarbons
kerosene
liquefied petroleum gas
petrochemical feedstocks
petroleum products, storage,
transportation and production
recycled petroleum products
residual fuel oils
turbine oils
Photographic chemicals photographic processing - wash water,
and process rinses
photoprocessing
photoplate processing chemicals
(deveiopers, stabilizers etc)
Printing fountain solutions (printing)
ink components (pigments, resins,
solvents, etc)
inks
2 ~ r~ 2
Sanitizers (active) sanitizers
sanitizers-dairy
sanitizers-dental
sanitizers-fermentation
sanitizers-food preparation
sanitizers-food processing
sanitizers-medical
sanitizers-rendering
sanitizers-veterinary
Soaps, detergents, cleaners cleaners
detergents
household cleaners
industrial cleaners
liquid soaps
oil and grease remover
powdered soaps
raw materials for cleaning products
soaps
surfactants
Textiles, textile products bonded fabrics
burlap
canvas
canvas goods
carpet backing
carpets
. clothing
coated fabrics
curtains
draperies
engineering textiles
fibers
geotextiles
~oods made of textiles
knitted fabrics
nets
nonwoven fabrics
2 ~ 7 2
rope
rugs
textile accessories
textile products
textiles
upholstery
woven fabrics
yarn
Textile processing dye fixatives
dyes
fiber lubricants
hand modifiers
sizes
textile processing fluids
Therapeutic (active or animal health/veterinary
preservative) aquaculture
dental
human health
pharmaceutical /therapeutic
Water purification charcoal beds
deionization resins
filters
membranes
reverse osmosis membranes
ultrafilters
water purification
water purification pipes, tubing
Wood applications lazures (wood stains)
wood
wood products
12
2 ~ 7 ~
Miscellaneous alcohols
bedding incorporating water or gels
ceramic
contact lens cases-leachlng
electronic circuitry
electronics chemicals
enzymes-food production
en2ymes
enzymes-industrial
gel cushions
marine antifoulants
mildewcides
wood
plastics
laundry
mining
natural rubber latex
oil field injection waters including
enhanced recover injection fluids,
drilling, fracturing and completion
fluids
pipes
plastics
polymer systems
polymers and resins (synthetic and
natural)
reagent preservation
rubber
rubber products
skin remover
solid protective/decorative films
stains
swimming pools
waste treatment
water beds
2~6~7~
The amounts of the compound to be used depend on the
application. The useful amounts for a particular applicalion are similar to
amounts used for other microbiocide compounds.
The compound can be used in combination with other
microbicides. The term "microbicide" is considered equivalent to
"antimicrobial`' as used herein.
Compounds of formula I can be prepared by a variety of methods.
One suitable method comprises reacting a compound of the formula
A~ ~\~
N S~-H
F~ ~IV)
with an iodinating or brominating agent.
Suitable iodinating or brominating agents include, for example,
iodine, bromine, an iodine-amino compound such as morpholine-iodine
complex, morpholine-bromine complex, N-bromosuccinimide ("NBS")
and N-iodosuccinimide ("Nl~,n), the latter being the most preferred.
When an iodine, bromine, or iodo amino compound is used, base
should also be used, preferably sodiurn or potassium hydroxide, and
solvent such as methanol, ethanol, and aqueous ethanol should also be
used.
2 ~
When NIS or NBS is useci, a catalyst such as, for example, silver
nitrate, or the like, should be used in presence of solvent such as
acetone, methyl ethyl ketone, tetrahydrofuran, and the like.
Reaction times of about 20 minutes to about 24 hours have been
utilized successfully with reaction temperatures of about 0C to about
2~oc.
Another suitable method to obtain sompounds of formula (1) is from
compounds of Formula (Il) using a halopropargylating agent such as
Formula (111) in the presence of a base such as potassium carbonate and
a solvent such as acetone:
A~ + L~ N~S~X
(Il) (111) (1)
The meanings of A, R, and X have been defined before. L is a
leaving group such as bromine, chlorine, or 0-S02-R where R is methyl
or phenyl or p-tolyl. Examples of Formula (111) include 3-iodopropargyl
chloride, 3-iodopropargyl mesylate, and 3-iodopropargyi
p-toluenesulfonate. The reagants of Formula (111) may be prepared by
methods described in literature.
Suitable methods of application of compounds of formula (I) to
control fungi, bacteria, algae, viruses, yeasts, and the like are in amounts
and with carriers, etc., as well known in the art.
The following examples are presented to illustrate a few
embodiments of the invention. All parts and percentages are by weight
unless otherwise indicated.
.EXAMPL~S
Some representative compounds of the invention are the
following:
Compound #
1. 3-(3-lodopropargyl)mercapto-4-phenyl-1,2,4-triazole
2. 3-(3-lodopropargyl)mercapto-4~t4-chlorophenyl)-1,2,4-triazole
3. 3-(3-lodopropargyl)mercapto-4-methyl-5-phenyl-1,2,4-triazole
4. 3-(3-lodopropargyl)mercapto-4-(2-chlorophenyl)1,2,4-triazole
5. 3-(3-lodopropargyl)mercapto-4-(4-methoxyphenyl)-1,2,4-triazole
6. 3-(3-lodopropargyl)mercapto-4-(4-methylphenyl)-1,2,4-triazole
7. 3-(3-lodopropargyl)mercapto-4-(3-chlorophenyl)-1,2,4-triazole
8. 3-(3-lodopropargyl)mercapto-4-(n-hexyl)-1,2,4-triazole
9. 3-(3-lodopropargyl)mercapto-4-(n-butyl)-1,2,4-triazole
10. 3-(3-lodopropargyl)mercapto-4-(4-chlorophenyl)-5-methyl-1,2,4- triazole
11. 3-(3-lodopropargyl)mercapto-4-phenyl-5-methyl-1,2,4-triazole
12. 3-(3-lodopropargyl)mercapto-4,5-diphenyl-1,2,4-triazole
13. 3-(3-lodopropargyl)mercapto-4-(n-heptyl)-1,2,4-triazole
14. 3-(3-lodopropargyl)mercapto-4-(n-octyl)-1,2,4-triazole
15. 3-(3-lodopropargyl)mercapto-4-cyclohexyl-1,2,4-triazole
Table 1 shows the structures and the physical data of these
representative compounds.
16
2 ~ 7 ~
Table 1
Physiçal Data
Melting Point ~oC)
or NMR Chemical
Compound $~ A R Shift (ppm!
1. H ph 161 -163C
2. H 4-CI-ph 174-175C
3. ph Me 142-146C
4. H 2-CI-ph 65-70C
5. H 4-MeO-ph 125-136C
6. H 4-Me-ph 165-170C
7. H 3-CI-ph 170-173C
8. H n-C6H13 8.70 (1H, s)
4.07 (2H, s)
4.00 (2H, t)
1.70 (2H, m)
1.40-1.10 (6H, m)
0.86 (3H, t)
9. H n-C4Hg 8.24 (1 H, s)
4.08 (2H, s)
4.00 (2H, t)
1.80 (2H, tt)
1.50-1.10 (2H, m)
0.90 (3H, t)
10. Me 4-CI-ph 7.78-7.50 (4H, q)
4.04 (2H, s)
2.26 (3H, s)
11. Me ph 165-167C
12. ph ph 164C
13. H n-C7H15 8.24 (1H, s)
4.08 (2H, s)
4.00 (2H, t)
1.80 (2H, tt)
1.50-1.10 (8H, m~
o.go (3H, t)
14. H n-C8H17 8.22 (1H, s)
4.10 (2H, s)
4.00 (2H, t)
1.79 ~2H, tt)
1.50-1.10 (10H, m)
o.go (3H, t)
15. H cyclohexyl 120-1 25C
Example 1
Preparation of 3-(3-iodopropargyl)mercapto-4-methyi-
5-phenyl-1,2,4-triazole (Compound #3)
(a) Preparation of 1-Bçnzoyl-4-methyl-thiosemicarbazide:
To the suspension of benzoic acid hydrazide (6.8 g, 0.05 mole) in
tetrahydrofuran (75 mL) at room temperature with magnetic stirring was added
a solution of methyl isothiocyanate (3.7g, 0.05 mole) in tetrahydrofuran (5 mL)
from a dropping funnel. After addition, 3 drops of triethyl amine was added as
catalyst. The reaction rnixture turned to a very thick paste after 5 min. The
reaction mixture was stirred at room temperature for one hour. Hexane (100 ml)
was added and the resultant white precipitate was collected by suction-filtration
and washed with some hexane to give 7.0 g (yield: 66.6 %) of
18
2 ~ ,7 ~
1-benzoyl-4-methyl-thiosemicarbazide, mp= 197-200C. An NMR spectrum (in
DMSO-d6) showed the desired compound.
(b) Preparation of 3-phenvl-4-methyl-1 2~4-triazolin-5-thione:
Sodium hydroxide (1.15 g, 28.7 mmol) was dissolved in water (50 mL).
To the above basic solution was added 1-benzoyl-4-methyl- thiosemicarbazide
(6 g, 28.7 mmol). The resultant clear solution was gentiy refluxed on a heating
mantle for 2 hours with magnetic stirring. The reaction mixture was cooled down
to room temperature and was neutralized by conc. HCI to pH=~5. The white
precipitate was collected by suction-filtration and was washed with water to give
4.1 9 (yield: 74.8 %) of triazolin-5-thione as white powders. mp= i66-168C. An
NMR spectrum (in DMSO-d6) showed the desired compound.
(c) Preparation of 3-propar~vlme~capto-4-methyl-5-phenyl-1.2.4-triazole:
To the solution of 3-phenyl-4-methyl-1,2,4-triazolin-5-thione above (3.1 g,
16.23 mmol) in acetone (40 mL) at room temperature under nitrogen with
magnetic stirring was added anhydrous potassium carbonate (2.76 9, 2û
mmol), followed by propargyl bromide (2.7 9 of 80 % toluene solution, 18
mmol). The reaction mixture was refluxed on a heating mantle for 16 hours.
The mixture was cooled down to room temperature and the $olid was filtered off
by suction. The filtrate was concentrated on a rotary evaporator to a oily
residue. The residue was further purified by a short column silica gel eluted
2 ~ 7
with ethyl acetate-hexane (1:1 to 3:1, total 800 ml) to give 2.8 g ~yield: 75.3 %)
of 3-propargylmercapto-4-methyl-5-phenyi-1,2,4-triazole as yellow viscous oil.
An NMR spectrum (in DMSO-d6) showed the desired compound.
(d~ PreparatiQn of 3-(~-iodopropar~Ll)merca,~t~-4-melhvl-5-phen
1,2,4-triazole (Compound #3):
To the solution of 3-propargylmercapto-4-methyl-5-pheny!-1,2,4-triazole
(2 g, 8.73 mmol) in dry acetone (20 mL) at room temperature with magnetic
stirring was added silver nitrate (100 mg, 0.~8 mmol), followed by
N-iodosuccinimide (2 g, 8.8 mmol). After stirring for one hour, the resultant
cloudy mixture was diluted with some acetone and the solid was filtered off by
suction. The filtrate was ooncentrated to a residue. Ethyl acetate (200 mL) was
added and was washed with water (2 x 50 ml) and brine (3 x 50 ml~. The
organic layer was dried over sodium sulfate. After filtering and concentrating a
yellow residue was obtained. The residue was made a slurry with hexane and
suction-filtered to give 1.9 g (yield: 61.5 %) of
3-(3-iodopropargy!)rnercapto-4-methyl-5-phenyl-1,2,4-triazole (Compound #3)
as a yellow solid, mp = 142-146C. An NMR spectrum (in DMSO-d6) showed
the desired structure.
2~3~6~2
Example 2
Preparation of 3-(-3-iodopropargyl)merc:apto-4-(n-octyl)-1,2,4-triazole
(Compound #14):
(a) Preparation of 3-iodo,cropargvl mesvlate:
To the solution of 3-iodopropargyl alcohol (~ 9, 27.5 mmol) in methylene
chloride (150 mL) at 0C under nitrogen was added triethylamine (2.8 9, 27.5
mmol). To the above solution was dropwise added methanesulfonyl chloride
(3.2 g. 27.5 mmol) with magnetic stirring. The reaction mi~ture was stirred at Q
to 5C for 2 hrs. The reaction mixture was washed with 0.5 N HCI (50 mL),
diluted sodium bicarbonate (50 mL), water (3x50 mL), and brine. The organic
phase was dried over MgSO4 and filtered. The filtrate was concentrated on a
rotary evaporator to afford 5.5 g (yield: 76.9 %) as a tan oil. An 1 H NMR
spectrum (CDCI3) was consistent with the desired compound. This compound
was used for the following step without further purification.
(b) Preparation of 3-t3-iodopropargyl)mercapto-4-(n-octyl!-1.2.4-triazole
(Compound #141:
To the solution of 4-n-octyl-1,2,4-triazolin-5-thion (2 g,.12.55 mmol) in dry
acetone (mL) was added potassium carbonate (1.8 9,13.0 mmol), followed by
3-iodopropargyl mesylate (3.4 g, 13.18 mmol) at room temperature. The
21
2~tJ~7
reaction mixture was stirred at room for 20 hrs. The resultant solid was filtered
off by suction-filtration and the filtrate was washed with water and brine. The
organic phase was dried over MgSO4 and filtered. The filtrate was concentrated
on a rotary evaporator to give a brown oil. The pure compound was obtained by
column silica gel eluted with ethyl acetate affording 1.8 g (yield: 38 %) as a light
brown oil. An NMR spectrum showed the desired compound. Elemental
Analysis: Calcd for C13H201N3S: C = 41.4%, H=5.30%, S=8.30%, I=33.6%;
Found: C=42.03%, H=5.46%, S=8.56%, I=33.4%.
Example 3
Biocidal Evaiuations of Compounds
A minimum inhibitory concentration (MIC) value is obtained using a broth,
two-fold serial dilution test performed as follows: A stock solution or dispersion
of the test compound, typically at a concentration of 1%, is made in a 5:3:2
solvent solution of acetone, methanol, and water. A volume of the stock
solution is dispensed into culture media to give an initial starting test
concentration of 250 ppm compound.
When the test is ready to be done, each vessel in the dilution series,
except the first vessel, contains an equal volume of compound free broth. The
first vessel contains twice the volume of broth with the starting concentration of
test compound. One half of the broth from the first vessel is transferred to the
s~
second vessel. AHer being mixed, one half the resulting volume is removed
from the second vessel and transferred to the third vessel. The entire cycle is
repeated sufficiently to give a series of concentrations amounting to 250, 125,
63, 31,16, 8, and 4, 2,1, 0.5, n.25 and 0.12 ppm.
Each vessel is then inoculated with a cell suspension of the appropriate
test organism. Bacteria are grown in broth and fungi on agar slants for a lime
and at a temperature appropriate to the species being tested. At the end of the
growth period, the broth is vortexed to disperse the cells. In the case of fungi,
the spores are harvested by pipetting water onto the slant and dislodging the
spores with a sterile loop. The cell/spore suspension is standardized by
controlling incubation time, temperature, and the volume of the diluent. The
suspension is then used to inoculate the vessels containing the broth
compound. The vessels are then incubated at the appropriate temperature.
After the incubation, the vessels are examined for growth/no growth. The
minimum inhibitory concentration (MIC) is defined as the lowest concentration
of compound that results in complete inhibition of growth of the test organism.
The organisms tested to demonstrate biocidal activity include:
BACTERIA:
Pseudomonas aerugenosa (Psae), gram negative
Escherichia coli (Ecol), gram negative
Staphvlococcus aureus (Saur), gram positive
23
2~6~7~
FUNGI:
Asperaill_s niaer (Anig)
Aureobasidium pullulans (Apul)
ALGAE:
Chlorella Dvroidenosa (Chlor)
Table 2: Bioçidal Evaluation
The Result of Minimum Inhibitorv Concentration (MIC! Tests
MlC(ppm)
Compound # Psae Ecol Saur Aniq Apul Chlor
>250 >250 >250 8 4 <0.1 2
2 ,250 >250 2 2 1 ~250
3 250 >250 125 2 8
4 >250 >250 32 250 16
>250 >250 250 1 0.25 8
6 ,250 >250 250 125 63 >250
7 >250 >250 >250 63 8
8 >250 >250 32 <0.12 <0.12 32
9 >250 >250 63 1 1 4
1 0 >250 >250 125 1 6 1 25 1 25
11 >250 >250 32 1 0.25 8
1 2 >250 >250 32 125 2~0 250
1 3 >250 >250 <0.12 1 32
1 4 >250 >250 >250 <0.12 <0.12
1 5 1 25 >250 32 1 <0.12
24
2 ~
Example 4
Aqricultural Funaicide ~valuations of Compounds
The compounds of this invention were tested for fungicidal activity
in vivo against rice blast (RB), tomato late blight (TLB), wheat powdery
mildew (WPM), botrytis on tomato (BOT), cucumber anthracnose (CA),
rice helminthosporium (RH), wheat glume blotch (SNW) and wheat leaf
rust (WLR) and the results are shown in Table 3. In tests on cereals
(except for rice plants used for testing rice blast), the plants were trimmed
about 24 hours prior to the application of the fungicide compound to
provide a uniform plant height and to facilitate uniform application of the
compound and inoculation with the fungus. The compounds were
dissolved in a 2:1:1 mixture of water, acetone, and methanol, sprayed
onto the plants, allowed to dry (four to six hours), and then the plants
were inoculated with the fungus. Each test utilized control plants which
were sprayed with the water, acetone, and methanol mixture and
inoculated with the fungus. The remainder of the technique of each of
the tests is given below and the results are reported as percent disease
control (percentages of plants treated with the compounds of the present
invention lacking disease signs or symptoms compared to the untreated
control plants).
2 ~
Rice~last (F~BI:
Nato rice plants were inoculated with Piricularia oryzae (about
20,000 conidia per ml) by spraying the leaves and stems with an airbnush
until a uniform film of inoculum was observed on the leaves. The
inoculated plants were incubated in a humid environment (7~F to 85F)
for about 24 hours, then placed in a greenhouse environment (70F to
75F). Seven to eight days after inoculation, the percent disease control
was determined.
Io~L~):
Phvtophthora infestans was cultured on four week old Pixie
tomato plants in a controlled environment room (65F to 70F and 100%
relative humidity). After storage, the spores were washed from the leaves
with water and dispersed by DeVilbiss atomizer over three week old
Pixie tomato piants which had been sprayed previously with
experimental fungicides. The inoculated plants were placed in a
humidity ca~inet at 70F and constant mist for 24 hours for infection. The
plants were then moved to the controlled environment room as above
and scored after three more days incubation. Disease control levels
were recorded as percent control four days after inoculation and five
days after spraying the compounds.
2~6~72
Wheat PQwdery Mildew (WPM):
Ervsiphe araminis (f. sp. tritici) was cultured on Pennol wheat
seedlings in a controlled temperature room at 65F to 75F. Mildew
spores were shaken from the culture plants onto Pennol wheat seedlings
which had been sprayed previously with the fungicide compound. The
inoculated seedlings were kept in a controlled temperature room at 65F
to 75F and subirrigated. The percent disease control was rated 8 to 10
days after the inoculation.
heat Leaf Rust (WLR.~:
Puccinia recondita (f. sp. tritici Races PKB and PLD) was cultured
on seven day old wheat (cultivar Fielder) over a 14 day period in the
greenhouse. Spores were collected from the leaves with a cyclone
vacuum or by settling on aluminum foil. The spores were cleaned by
sieving through a 250 micron opening screen and stored or used fresh.
Storage employed sealed bags in an Ultralow freezer. When stored,
spores must be heat shocked for two minutes at 40F before use. A
spore suspension is prepared from dry uredia by adding 20 mg (9.~
million) per ml of Soltrol oil. The suspension is dispensed into gelatin
capsules (0.7 ml capacity) which attach to the oil atomizers. One capsule
is used per flat of twenty of the two inch square pots of seven day old
27
Fielder wheat. After waiting for at least 15 minutes for the oil to evaporate
from the wheat leaves, the plants are placed in a dark mist chamber
(1 8-20C and 100% relative humidity) for 24 hours. The plants are then
put in the greenhouse for the latent period and scored after 10 days for
disease levels. Protective and curative tests were inoculated one day
after and two days, respectively, before spraying the plants with the test
chemicals.
Botrytis on TomatQ (BOT):
Strains of ~3~trYtis ci~çrea to be used were inoculated singly onto
tomato plants to verify virulence. In tests, spore suspensions from at least
two strains were prepared and mixed in equal volume amounts. All
cultures were maintained on PDA (potato dextrose agar) and incubated
in closed plastic boxes at room temperature and room light for 2-3 weeks
before use in tests.
The inoculum was prepared as follows: dextrose solution (1.9
g/100 ml) in tap water was prepared and poured over each culture plate
of sporulating fungus. Plates were rubbed with a rubber policeman and
the solution filtered through 2 layers of cheesecloth. A spore suspension
of 5 - 6.5 x 105 spores/ml is desired. "Pixie" tomato plants, 3-4 weeks old
and about 8-10 cm tall were placed in indirect headhouse lighting tor at
28
~6~72
least two days before use (inoculation). The plants were inoculated
usin~ a DeVilbiss atomizer, by spraying the upper and lower leaf
surfaces and the stem of the plants until near run-off. The plants were
stored on a mist bench at 70F., 100% humidity in indirect room light and
scored for results in 5-7 days.
Cucumber AnthraGnose ((::A):
Colletotrichum las~enarium was cultured on potato dextrose agar
(PDA) in petri plates for 14 days under dark conditions. Plates were
inoculated by spreading inoculum over medium with an inoculating
needle. Spores were then harvested from plates by adding deionized
water to the PDA plates. The agar surface was scraped with a rubber
policeman and the spore suspension filtered through cheesecloth to
remove mycelial and agar fragments, followed by adjustment to a
concentration of 1-2 x 105 spores per ml.
Cucumber plants (var "Marketer" 14 day old seedlings) were
inoculated by sprayin~ the leaves (especially the underside) until a
uniform film of inoculum was observed on the plant. Inoculated plants
were then incubated in a humid environment at 70-75F for 24 hours and
then removed, allowed to dry and placed under greenhouse conditions.
Treatment comparisons were made 10-14 days after inoculation.
2~g~
F~Lçe Helminthocporium (RH):
Growth of the fungus was conducted on PDA under artificial light.
Spores grown for 3 weeks to 3 months were used. The plates were
flooded with Dl water and scraped with a spatula and the washings
filtered through a layer of cheesecloth.
Inoculation was done with a DeVilbis air inoculator by applying
100,000 to 500,000 spores per flat of 20 plants. The plants were
incubated in a humidity cabinet for 24 hours at 25C and then placed in
greenhouse conditions and scored after 6 days.
Wheat Glume Blotch (~NW):
Portions of inoculum from a 2 or 3 week old colony of Septoria
~Q~Qa~ (ascomycete stage, Leptosphaeria nodorum), were incubated
on Czapek-Dox V-8 agar plates for 48-72 hours in the dark at 20C with
alternating light and dark (12h/12h).
The spore solution for inoculation was prepared by adding
deionized water (one hundred mls per 10-15 plates) to a one-half gallon
jar and then transferring the fungal material from 10-20 culture plates to
the jar. The jar was shaken vigorously for 15-30 seconds and allowed to
stand for 5-10 minutes. The spore concentration was then filtered
through cheesecloth and diluted to the appropriate concentration
(approximately 3.0 x 106 spores per ml). Thi~ty to forty plates produced
about 1 liter of diluted inoculum. One week old Fielder (untreated seed)
wheat plants were used for disease evaluations. About 22 ml of
inoculum was applied to the wheat plants (i,n a flat of 20 pots) using a
DeVilbiss atomizen The flats were air-dried for 10-15 minutes and then
stored at 20C, 1 2hr/12 hr lighVdark at 100% humidity in a humidity
cabinet. The plants were incubated for 96 hours and then transferred to
an envirornmental cabinet for disease development for 8 days before
being scored for results.
Table ~
Green Hous~ Test Results of Plant dise~ses Control
Compound E~Q E~2I Ç~ ~ RH SNW IL@ ~J~ YE~
1 200 75 75 0 99 50 0 0 0
2 200 75 50 0 90 50 0 0 0
3 200 90 85 0 95 0 0 50 25
4 200 0 85 0 95 50 0 50 0
200 0 85 -0 95 50 0 0 0
6 200 75 50 0 90 0 0 o 0
7 200 50 75 0 99 0 0 0
8 200 75 75 50 99 50 0 90 0
9 200 - 75 0 95 50 0 80 0
200 90 80 0 95 50 0 50. 0
11 200 75 - 50 99 0 0 0 0
12 200 75 75 50 95 0 0 . 0 0
13 200 75 85 50 95 50 0 90 0
14 200 50 90 50 95 80 0 90 0
200 90 90 80 95 50 0 ~0 0
20~6672
While the invention has been described with reference to specific
examples and applications, other modifications and uses for the invention will
be apparent to those skilled in the art without departing from the spirit and
scope of the invention defined in the appended claims.