Note: Descriptions are shown in the official language in which they were submitted.
7129
CEPHALOSPORIN COMPOUNDS AND
PROCESSES FOR PRERARING T~E SAME
The present invention relates to novel
cephalosporin compounds having excellent antimicrobial
activities, processes for preparing the same and
intermediates therefor.
Prior Art
Hitherto, many cephalosporin antibiotics have been
known as an antimicrobial agent, for example, there has been
reported that (6R, 7R)-7-[(Z)-2-(2-aminothiazol-4-yl)-2-((2-
carboxyprop-2-yl)oxyimino)acetamido]-3~ pyridiniomethyl)-
ceph-3-em-4-carboxylate (general name: ceftazidime) has
potent antimicrobial activities [cf: Japanese Patent Second
Publication (Kokoku) No. 5916~1987].
Brief Description of the Invention
An object of the present invention is to provide
novel cephalosporin compounds having excellent antimicrobial
activities. Another object of the invention is to provide
processes for preparing the novel cephalosporin compounds.
Further object of the invention is to provide intermediates
for preparing the novel cephalosporin compounds. These and
other objects and advantages of the invention will be
apparent to those skilled in the art from the following
description.
Detailed Description of the Invention
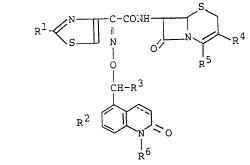
The cephalosporin compounds of the present
~ 2Q~7129
invention have the following formula [I]:
N ~ C-CON~ ~ ~
S N ~/ I R4
O R5
1H--R3 [I]
~ ~0
R6
wherein Rl is amino group or a protected amino group, R2 is
hydroxy group, a protected hydroxy group or a lower alkoxy
group, R3 is carboxyl group or a protected carboxyl group,
R4 is hydrogen atom, a lower alkyl group, a lower alkenyl
group or a group of the formula: -CH2R41 ~wherein R41 is a
nucleophilic residue), R5 is carboxyl group, a protected
carboxyl group or a group of the formula: -COO , R6 is
hydrogen atom or a lower alkyl group and the dotted line
means the presence or absence of a double bond.
The novel cephalosporin compounds [I] of the
present invention and pharmaceutically acceptable salts
thereof have excellent antimicrobial activities against the
wide range of microorganisms including Gram positive
bacteria and Gram negative bacteria, especially against Gram
positive bacteria. The cephalosporin compounds [I] of the
present invention also show high stability to various 3-
lactamase-producing bacteria, and show high absorbability in
the living tissues, and further the treatment effects
.
_ 3 _ 2 ~ 2 9
thereof last for a long time. In view of these advantages,
the compounds [I] of the present invention can be used as an
antimicrobial agents, for example, in prophylaxis and
treatment of infectious diseases caused by the above various
microorganisms as a chemotherapeutic drug for mammals
including human being and also an additive for animal
feeds.
The desired compounds of the present invention
include, for example, the compounds of the formula [I],
wherein R4 is hydroqen atom, a lower alkyl group such as
methyl, a lower alkenyl group such as vinyl, or a group of
the formula: -CH2R41 {wherein R41 is a conventional
nucleophilic residue used in this field, for example, a
lower alkanolyoxy group such as acetoxy; a nitrogen-
containing heterocyclic group such as pyridinio group,
quinolinio group, iso~uinolinio group, 2,3-propanopyridinio
group, 5,6,7,8-tetrahydroquinolinio group or 2,3-
pentanopyridinio group [these heterocyclic compounds may
optionally have one or two substituents selected from the
group consisting of a halogen atom ~e.g. chlorine, bromine,
fluorine, etc.), a lower alkyl group (e.g. methyl, etc.), a
lower alkoxy group (e.g. methoxy, etc.), a lower
alkoxycarbonyl group (e.g. methoxycarbonyl, etc.), amino
group, carbamoyl group, cyano group, formylamino group and a
hydroxy(lower)alkyl group (e~g. hydroxymethyl, etc.)3, a
thio group substituted by a nitrogen-containing heterocyclic
group such as thiadiazolylthio group, tetraæolylthio group
20~7129
and pyridiniothio group [these nitrogen-containing
heterocyclic groups may optionally have a substituent
selected from the group consisting of a lower alkyl te.g.
methyl, etc.), a lower alkenyl (e.g. vinyl, allyl, etc.)]).
Further, the compounds [I] of the present invention
include, for example, the compounds of the formula [I],
wherein Rl is amino group, R3 is car~oxyl group, R5 is
carboxyl group or a group of the formula: -COO , R6 is
hydrogen atom or methyl group.
Moreover, the compounds [I] of the present
invention include, for example, the compounds of the formula
[I], wherein R2 is hydroxy group or methoxy group which
substitutes at 8-position of the 2-oxo-lH-quinoline ring or
the 2-oxo-1,2,3,4-tetrahydroquinoline ring.
The more preferred compounds of the invention as a
medicament are compounds of the formula [I], wherein R4 is a
group of the formula: -CK2R41 (wherein R41 is acetoxy group,
pyridinio group, 3-chloropyridinio group, 3-amino-2-
methylpyridinio group or quinolinio group).
The most preferred compounds of the invention as a
medicament are compounds of the formula [I], wherein R4 is a
group of the formula: -CH2R41 (wherein R41 is pyridinio
group, 3-chloropyridinio group, 3-amino-2-methylpyridinio
group or quinolinio group).
When Rl of the compound [I~ of the present
invention is a protected amino group, the protecting group
for amino group includes various conventional protecting
20~7129
groups which are usually used in the synthesis of peptides,
for example, a lower alkanoyl group; mono-, di- or
trihalogeno(lower)alkanoyl group; a lower alkoxycarbonyl
group; a substituted or unsubstituted phenyl(lower)-
alkoxycarbonyl group such as benzyloxycarbonyl group, p-
~lower)alkoxybenzyloxycarbonyl group; a substituted or
unsubstituted phenyl(lower)alkyl group such as benzyl group,
p-(lower)alkoxybenzyl group, 3,4-di(lower)alkoxybenzyl
group; and di- or triphenyl(lower)alkyl group, and the
like.
When R2 of the compound [I] of the present
invention is a protected hydroxy group, the protecting group
for hydroxy group includes, for example, a substituted or
unsubstituted phenyl(lower)alkyl group such as benzyl,
diphenylmethyl, and the like; a lower alkanoyl group such as
acetyl group, benzoyl group, and the like.
Moreover, when R3 or/and R5 of the compound ~I] of
the present invention are a protected carboxyl group, the
protecting group for carboxyl group are preferably a
protecting group which can be easily removed from the
carboxyl group by a conventional method such as hydrolysis,
acid-treatment and reduction, and the like. Such protecting
groups are, for example, a lower alkyl group; a substituted
or unsubstituted phenyl(lower)alkyl group such as benzyl, p-
(lower)alkoxybenzyl, p-nitrobenzyl, diphenylmethyl, and the
like; and tri(lower)alkylsilyl group.
According to the present invention, the desired
- 6 20~71 29
compounds ~I] and pharmaceutically acceptable salts thereof
can be prepared by condensing an oxyiminoacetic acid
compound of the formula [II]:
S N
CH-R3 [II]
R2 ~,~;,1~
R6
wherein the symbols are the same as defined above, a salt or
a reactive derivative thereof, with a 7-aminocephalosporin
compound of the formula [III]:
H2N~;~
~ R4 [III]
R5
wherein the symbols are the same as defined above, or a salt
thereof, or by reacting an oxyiminocephaloporin compound of
the formula [IV]:
1 ~ N ~ C-CONH ~ ~ [IV]
OH R5
wherein the symbols are the same as defined above, or a salt
thereof, with a carbostyril compound of the formula [V~:
_ 7 _ ~ ~ ~7~3
C~-R3 [V]
O
R6
wherein Z is a reactive residue, R2, R3, R6 and the dotted
line are the same as defined above, or a salt thereof.
Among the desired compounds [I] of the present
invention, the compound wherein R4 is a group of the
formula: -CH2R41 can be prepared by reacting a cephalosporin
compound of the formula [VI]:
S ~ N ~ ~ CH2Xl
CH-R3 [VI]
O
R6
wherein Xl is a reactive residue, Rl, R2, R3, R5, R6 and the
dotted line are the same as defined above, or a salt
thereof, with a nucleophilic compound [VII] or a salt
thereof.
When the desired compounds [I] thus prepared are
the compounds wherein Rl is a protected amino group, R2 is a
protected hydroxy group, and/or R3 and/or RS are a protected
carboxyl group, if desired, the said protectin~ groups may
2057129
optionally be removed. Besides, the desired compounds [I3
may optionally be converted into a pharmaceutically
acceptable salt thereof.
The salts for he starting compounds [II], [III],
[IV], [V] and [VI] may preferably be any salt with a
conventional inorganic or organic amine.
The starting nucleophilic compound [VII] includes
any conventional compounds which are usually used in this
field, for example, a lower alkane acid (e.g. acetic acid,
etc.), a nitrogen-containing heterocyclic compound (e.g.
pyridine, quinoline, isoquinoline, 2,3-propanopyridine,
tetrahydroquinoline, 2,3-pentanopyridine, etc.) which may
optionally have substituents selected from the group
consisting of a halogen atom, a lower alkyl, a lower alkoxy,
a lower alkoxycarbonyl, amino, carbamoyl, cyano, formylamino
and a hydroxy(lower)alkyl, and a mercapto- or thio-
subsituted nitrogen-containing heterocyclic compound ~e.g.
mercaptothiadiazole, mercaptotetrazole, thiopyridone, etc.)
which may optionally have a substituent selected from the
group consisting of a lower alkyl and a lower alkenyl.
These nucleophilic compounds [VII] may be used in the form
of a salt thereof such as mineral acid salt or alkali metal
salt.
The condensing reaction between the free
oxyiminoacetic acid [II~ and the 7-aminocephalosporin
compound ~III] may be carried out in the presence of a
dehydrating agent. The dehydrating agent may be any
2ù~ ~129
conventional dehydrating agent, for example,
dicyclohexylcarbodiimide, phosphorus oxychloride, thionyl
chloride, oxalic chloride or a Vilsmeier reagent which is
prepared by reacting dimethylformamide with phosphorus
oxychloride, oxalic chloride, phosgene or thionyl
chloride. This reaction is preferably carried out under
cooling or at room temperature.
The condensation reaction between a reactive
derivative (e.g. acid anhydride, mixed acid anhydride,
active ester, etc.) of the oxyiminoacetic acid compound [II]
and the 7-aminocephalosporin compound [III] or a salt
thereof may be carried out in the presence or absence of a
base. The base may preferably include, for example, an
alkali metal hydroxide, an alkali carbonate, an alkali
hydrogen carbonate, bis(trimethylsilyl)acetamide,
trialkylamine, N,N-dialkylaniline, pyridine, and the like.
This reaction is preferably carried out under cooling or at
room temperature ~e.g. -60C to 25C).
The reaction between the oxyiminocephalosporin
compound [IV] and the carbostyril compound [V3 is carried
out in the presence of an dehydrating agent or a base. The
reactive reside for Z of the carbostyril compound [V] is,
for example, hydroxy group, a halogen atom, a lower
alkylsulfonyloxy group, and the like. The dehydrating agent
may be, for example, a mixture of a phosphorus compound
(e.g. triphenylphosphine, diphenylmethylphosphine,
diphenylethylphosphine, etc.) and an azodicarboxylic acid
2l~7129
-- 10 --
di(lower)alkyl ester. The base is preferably an alkali
metal hydroxide, a trialkylamine, N,N-dialkylaniline,
pyridine, and the like. This reaction is preferably carried
out under cooling or with warming (e.g. -40C to 30C).
The reaction between the cephalosporin compound
[VI] and the nucleophilic compound [VII] is preferably
carried out in a suitable solvent. In this reaction, the
cephalosporin compound [VI] may be a compound of the formula
[VI] wherein the reactive residue for Xl is, for example, a
carbamoyloxy group, a lower alkanoyloxy group or a halogen
atom. This reaction is preferably carried out at room
temperature, under cooling or with heating (e.g. -40C to
40C), at pH 2 - 8, more preferably at pH 5 - 8. If
desired, in order to promote the reaction, there may be
added to the reaction system an alkali metal halide, a
tri(lower)alkylsilyltrifluoromethanesulfonate, an alkali
metal thiocyanate, an alkali metal hydrogen carbonate, a
quaternary ammonium salt having surface activity [e.g.
tri(lower)alkylbenzylammonium halide] or phosphate buffer
solution, and the like.
The reactions mentioned above may, if desired, be
carried out in a suitable solvent such as dimethylformamide,
dimethylacetamide, dioxane, acetone, alcohol, acetonitril,
methylene chloride, chloroform, pyridine, dimethylsulfoxide,
toluene, tetrahydrofuran, water or a mixture thereof.
In the above reactions, optical active compounds
E I] of the present invention may be obtained from optical
20~71~9
active starting compounds [II], [V] or [VI].
The compounds ~I] wherein Rl is a protected amino
group, R2 is a protected hydroxy group, and/or R3 and/or R5
are a protected carboxyl group, may optionally be subjected
to removal of the protecting groups. The removal of these
protecting groups is carried out in a conventional manner,
for example, hydrolysis, solvolysis, acid-treatment,
reduction, and the like, or a combination thereof, which is
selected depending on the kind of the protecting groups to
be removed.
The compounds [I] of the present invention and
pharmaceutically acceptable salts thereof have excellent
antimicrobial activities against the wide range of Gram
positive bacteria, such as microorganisms of the genera
Enterococcus (e.g. Enterococcus faecalis), Staphylococcus
(e.g. Staphylococcus aureus, Staphylococcus epidermidis),
Morganella (e.g. Morganella morganii) and Pseudomonas(e.g.
Pseudomonas aeruginosa) and Gram negative bacteria.
Especially, unlike the above mentioned conventional
cephalosporin compounds, the compounds [I] of the present
invention and pharmaceutically acceptable salts thereof have
potent antimicrobial activities against Gram positive
bacteria (e.g. microorganisms of Staphylococcus, etc.).
For example, in compared with the above mentioned
ceftazidime, the compound of this invention, 7B-{(Z)-2-(2-
aminothiazol-4-yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido}-3-(1-quinoliniomethyl)-3-
2~712~
cephem-4-carboxylic acid has excellent antimicrobial
activities of more than 32 times stronger against
Staphylococcus aureus 20CiP JC-l, and 7~-{~Z)-2-(2-amino-
thiazol-4-yl)-2-~(8-hydroxy-2-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido}-3-(1-pyridiniomethyl)-3-
cephem-4-carboxylic acid and 73-{(Z)-2-(2-aminothiazol-4-
yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxy-
iminc]acetamido}-3-(3-amino-2-methyl-1-pyridiniomelhyl3-3-
cephem-4-carboxylic acid have excellent antimicrobial
activities of more than 16 times stronger against
Staphylococcus epidermidis Kawamura.
Moreover, the desired compounds [I] of the present
invention and pharmaceutically acceptable salts thereof
characteristically have higher stability to various ~-
lactamase-producing bacteria. For example, the compounds of
the present invention, 3-(1-quinoliniomethyl) derivative and
3-(1-pyridiniomethyl) derivative of 73-{(Z)-2-(2-amino-
thiazol-4-yl)-2-[(B-hydroxy-2-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido}-3-cephem-4-carboxylic
acid have excellent antimicrobial activities of more than 4
times stronger against Citrobacter freundii GN346 than
ceftazidime.
The desired compounds [Il of the present invention
and pharmaceutically acceptable salts thereof also have
e~cellent antimicrobial activities a~ainst microorganisms of
the genera Proteus (e.g. Proteus morganii), Citrobacter
(e.g. Citrobacter freundii), Escherichia (e.g. ~scherichia
- 13 - 2~7~2~
coli) r Klebsiella (e.g. Klebsiella pneumoniae), or
Enterobacter, Serratia, Shigella and Salmonella.
The most important character of those compounds is
to show potent effects on clinical isolates of methicillin
resistant Staphylococcus aureus (MRSA) which is resistant to
many B-lactam antibiotics. For example, antibacterial
activity (90 % inhibitory concentration) of 7R-{(Z)-2-(2-
aminothiazol-4-yl)-2-[(8-hydroxy-~-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido}-3-(3-amino-2-methyl-1-
pyridiniomethyl)-3-cephem-4-carboxylic acid against clinical
isolates of MRSA is about 16 times superior to that of
ceftazidime. Further, antibacterial activity (90 %
inhibitory concentration) of the above-mentioned compound of
the invention against clinical isolates of P. aeruginosa is
about 2 times superior to that of ceftazidime.
Moreover, the desired compounds [I] or
pharmaceutically acceptable salts thereof have high
absorbability in the living tissues, and the treatment
effects thereof last for a long time so that the compounds
[I] of the present invention show excellent defense effect
in infectious diseases caused by various microorganisms
including Staphylococcus aureus and Pseudomonas
aeruginosa. In addition, the desired compounds [I~ of the
present invention and pharmaceutically acceptable salts
thereof have low toxicity and show high safety as a
medicament. For example, when acute toxicity of 7B-{(Z)-2-
~2-aminothia~ol-4-yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)-
- 1~ - 20~71 29
(carboxy)methyloxyimino]acetamido~-3-(3-amino-2-methyl-1-
pyridiniomethyl)-3-cephem-4-carboxylic acid was tested with
intravenous administration using mice, no mouse of five mice
which were administered at dose of 1 g/kg was died in five
days after the administration thereof.
The cephalosporin compounds [I] of the present
invention may be used either in the free form or in the form
of a pharmaceutically acceptable salt thereof. The
pharmaceutically acceptable salt includes, for example, non-
toxic metal salts such as sodium salt, potassium salt,
calcium salt, magnesium salt, aluminum salt, and the like;
salts with non-toxic amines such as trialkylamine (e.g.
triethylamine), pyridine, ethanolamine, triethanolamine,
dicyclohexylamine, and the like; salts with an inorganic
acid such as hydrochloric acid, sulfuric acid, hydrobromic
acid, and the like; salts with an organic acid such as
oxalic acid, tartaric acid, and the like; addition salts
with an amino acid such as glycine, lysine, arginine,
aspartic acid, glutamic acid, and the like.
The salt of the compound [I] may also be a salt
with a resin such as a polystyrene resin having an amino
group, quaternary amino group or sulfonic acid group, or a
resine having a carboxyl group such as a resin comprising
polyacryl acid resin. The compounds [I] of the present
invention may also be in the form of a complex with a metal
such as iron, copper, and the like, or with an ammonium salt
such as ammonium chlorider and the like. Thus, the desired
- 15 - 2~7~29
compounds [I] of the present invention and salts thereof
include inner salts, addition salts, complexes, solvates and
hydrates thereof.
The cephalosporin compounds [I] or pharmaceutically
acceptable salts thereof can be administered either by oral
route or parenteral route (e.g. intravenous, intramuscular
or subcutaneous route). They can be used in the form of a
pharmaceutical preparation suitable for oral or parenteral
administration in admixture with a pharmaceutical acceptable
carrier or diluent, for example, tablets, granules,
capsules, powder, injections, and the like.
The daily dose of the compounds [I] of the present
invention may vary depending on administration rcute, age,
weight and conditions of the patient, and kinds of the
diseases to be cured, but it is usually in the range of
about 2 to 200 mg/kg of body weight, preferably about 5 to
40 mg/kg of body weight.
In this specification and claims, the partial
structure of the formula: -C-CONH-
N
l
means, if not determined otherwise, the geometrical isomers
of the following formulae:
-C-CONH- -C-CONH-
ll or 11
N-O- -O-N
IZ)-isomer (E)-isomer
- 16 - 2~ 7 1 ~ 3
or a mixture thereof. However, the compounds lI] or
pharmaceutically acceptable salts thereof wherein the
oxyimino group has (Z)-configuration are preferably used as
a medicament, and show more excellent biological
activities.
The desired compounds [I] of the present invention,
the starting compounds [II] and [VI] include either optical
isomers or racemic mixture thereof owing to the partial
structure of the following formula:
--C--
N
*CH-R3
R 2 ~
R6
wherein the mark * means an asymmetric carbon atom, R2, R3,
R6 and the dotted line are the same as defined above.
The starting compound [II] is a novel compound, and
can be prepared, for example, by reacting the carbostyril
compound [V] with a compound of the formula ~VIIT]:
N ~ C-C02Y
S N ~VIII]
OEI
wherein a group of -C02Y is carboxyl group or a protected
carboxyl group, Rl is the same as defined above, in the
presence of a base under cooling or with heating to give a
2~71 29
- 17 -
compound of the formula [II-a]:
l ~ N~ ~ C~COOY
S N
CH-R3 [II-a]
~ O
R6
wherein the symbols are the same as defined above,
optionally followed by removal of the protecting group from
the compound [II-a]. When a group of the formula: -C02Y is
a protected carboxyl, the protecting group therefor may be
any one as exemplified above for the protecting group for R3
and R5. Alternatively, the compound [II] may also be
prepared by reacting the carbostyril compound [V] with N-
hydroxyphthalimide or an alkali metal salt thereof, followed
by treating the reaction mixture with a lower alkylhydrazine
under cooling to give a compound of the formula [IX]:
IONH2
CH-R3 [IX]
~ O
R6
wherein the symbols are the same as defined above, further
by reacting the compound ~IX~ with a glyoxylic acid compound
of the formula ~X]:
- 18 ~ 7 1 2 ~
_~ ~ rCOC02Y [X]
s
wherein the symbols are ~he same as defined above, in the
presence or absence of a base under cooling or with heating,
optionaily followed by subjecting the product to removal of
the protecting group therefrom.
Through the specification and claims, the lower
alkyl, lower alkenyl, lower alkoxyl and lower alkanoyl
groups have 1 to 6 carbon atoms, 2 to 6 carbon atoms, 1 to 6
car~on atoms and 2 to 6 carbon atoms, respectively.
The present invention is illustrated in more detail
by the following Examples and Reference Examples, but should
not be construed to be limited thereto.
Example 1
~ (a) To a mixture of 2-(8-diphenylmethyloxy-2-
oxo-lH-quinolin-5-yl)-2-hydroxyacetic acid diphenylmethyl
ester (47.7 g), ~-hydroxyphthalimide (41.2 g),
triphenylphosphine (55.2 g) and tetrahydrofuran (2000 ml) is
added dropwise azodicarboxylic acid diethyl ester (36.6 9)
under argon atmosphere at 5 to lO~C. The mixture is stirred
at the same temperature for one hour, and further at room
temperature for 70 hours. The mixture is concentrated, and
the resulting residue is purified by silica gel column
chromatography (eluent; he-xane/ethyl acetate and
chloroform), recrystallized from chloroform/ether to give 2-
(8-diphenylmethyloxy-2-oxo-1~-quinolin-5-yl)-2-~phthal-
imidoxy)acetic acid diphenylmethyl ester (44.5 g).
- 19 - 2~7~29
M.p. 206 - 207C (decomposed)
IR (Nujol): 3170, 3130, 3065, 3030, 1790, 1760,
1730, 1660, 1610 cm~
MS (m/z): 713 (~+)
(l)-(b) A suspension of 2-(8-diphenylmethyloxy-2-
oxo-lH-quinolin-5-yl)-2-hydroxyacetic acid diphenylmethyl
ester (0.57 9) and calcium carbonate (0.2 g) in tetra-
hydrofuran (10 ml) is cooled to -5 to 0C and stirred under
argon atmosphere. To the mixture is added dropwise
phosphorus tribromide (0.38 g), and the mixture is further
stirred for one hour. The mixture is poured into a cold
mixture of ethyl acetate (10 ml) and 2N-aqueous sodium
hydroxide solution (3 ml) with stirring at a temperature
below 2C, and basified with saturated aqueous sodium
hydrogen carbonate solution, and separated. The aqueous
layer is extracted with ethyl acetate, and the extract is
combined with the above obtained ethyl acetate layer, washed
with saline solution, dried, and thereto is added calcium
carbonate (5 mg). ~he mixture is evaporated to remove the
solvent to give 2-bromo-2-(8-diphenylmethyloxy-2-oxo-1~-
quinolin-5-yl)acetic acid diphenylmethyl ester.
A suspension of N-hydroxyphthalimide potassium salt
(0.25 9) in dimethylformamide (2 ml) is cooled to -10C
under argon atmosphere, and thereto is added dropwise a
solution of the above compound in dimethylformamide (3 ml),
and the mixture is stirred at 0 to 2C for one hour. The
mixture is poured into a mixture of ice-water (20 ml)/ethyl
- 20- 20~7~29
acetate (30 ml), and separated. The organic layer is
collected, washed with aqueous sodium hydrogen carbonate
solution and water, dried and concentrated. The resulting
residue is purified by silica gel column chromatography to
give 2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)-2-
(phthalimidoxy)acetic acid diphenylmethyl ester (0.30 g).
The physicochemical properties of this product are the same
as those of the preparation obtained in above step (a3.
(2) A solution of the product (36.5 g) obtained
above in methylene chloride (500 ml) is cooled to -60C
under argon atmosphere, and thereto is added dropwise
methylhydrazine (3.0 ml). The mixture is stirred at -60 to
0C for 1.5 hour, and further at 0C for 30 minutes. The
precipitated crystals are removed by filtration, and washed
with methylene chloride. The washings and the filtrate are
combined, and evaporated. The residue is crystallized from
ether and the precipitated crystals are collected by
filtration, washed with ether and dried to give 2-aminooxy-
2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)acetic acid
diphenylmethyl ester (29.8 9) as colorless crystals.
M.p. 178 - 180C (decomposed)
IR (Nujol): 3230, 3160, 1750, 1730, 1650, 1600 cm 1
MS (m~z): 583 (MH+)
This product is recrystallized ~rom methanol to
give crystals having the different I~ spectrum.
M.p. 170 - 171C
IR (Nujol): 3650, 3550, 33C0, 3240, 1760, 1740,
20~7129
1660, 1610 cm-l
~ 3) The product (28 g) obtained above and 2-
tritylaminothiazol-4-yl-~lyoxylic acid (18.65 g) are
dissolved in a mixture of tetrahydrofuran (800 ml),
acetonitril (280 ml) and water (280 ml), and the mixture is
stirred at room temperature for five hours, and evaporated
to remove the solvent. The residue is crystallized from
ether and the precipitated crystals are collected by
filtration, washed with ether, and dried to give 2-(2-
tritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-2-oxo-lH-
quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino~-
acetic acid (41.6 g) as colorless crystals.
M.p. 154 - 156C (decomposed)
IR ~Nujol): 3400, 3200, 3060, 3030, 2800 - 2400,
1740, 1720, 1660, 1610 cm~
Mz (m/z): 979 (MH+)
(4) To dimethylformamide (2.23 9) are added
successively phosphorus oxychloride (2.85 g) and methylene
chloride (10 ml) with ice-cooling under argon atmosphere,
and the mixture is stirred at room temperature for one hour,
and then cooled to -55 to -50C. To the mixture is added
dropwise a solution of 2-(2-tritylaminothiazol-4-yl)-2-[(8-
diphenylmethyloxy-2-oxo-1~-quinolin-5-yl)(diphenylmethyloxy-
carbonyl)methyloxyimino]acetic acid (13 g) in methylene
chloride (300 ml~, and the mixture is stirred at -60 to
-50C for two hours (the resulting solution is referred to
as Reaction Solution A). Alternatively, bis(trimethyl-
20~7129
- 22 -
silyl)acetamide (14.8 ml) is added to a suspension of 7-
aminocephalosporanic acid (5.43 9) in methylene chloride (48
ml) with ice-cooling under argon atmosphere, and the mixture
is stirred at the same temperature for 1.5 hour ~the
resulting solution is referred to as Reaction Solution B).
Reaction Solution B is added dropwise to Reaction Solution A
at -60 to -55C, and the mixture is stirred at -60 to -30C
for one hour, then at -30DC for 1.5 hour. After completion
of the reaction, the mixture is poured into ice-water and
separated. The aqueous layer is extracted with methylene
chloride, and the extract is combined with the methylene
chloride layer, washed with saline solution, dried and
evaporated to remove the solvent to give 7~-{(Z)-2-(2-
tritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-2-oxo-lH-
quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino]-
acetamido}cephalosporanic acid (16.48 g) as light yellow
powder.
M.p. 160 - 162C (decomposed)
IR (Nujol): 3380, 3230, 2800 - 2400, 1790, 1740,
1670, 1610 cm-
~S (m/z): 1233 (MH+)
Example 2
7~-~(Z)-2-(2-Tritylaminothiazol-4-yl)-2-[(8-
diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)~diphenylmethyloxy-
carbonyl)methyloxyimino]acetamido}cephalosporanic acid
(12.48 g) is dissolved in ~ormic acid (160 ml), and thereto
is added water (40 ml) under ice-cooling, and the mixture is
_ ~3 _ 2 ~ ~ 7 1 2 9
stirred at room temperature for 8.5 hours. The precipitates
are separated by filtration, and washed with 80 %
aqueous formic acid solution. The filtrate and the washings
are combined, and evapor~ted to remove the solvent. To the
residue is added ethanol, and the precipitate powder is
collected by filtration, washed with ethanol and ether,
dried and dissolved in aqueous sodium hydrogen carbonate
solution. The pH value of the mixture is adjusted to about
pH 8.0 with saturated aqueous sodium hydrogen carbonate
solution, and purified with a column packed with a nonionic
adsorbing resin (tradename: Diaion HP-20 manufactured by
Mitsubishi Kasei Corporation, hereinafter referred to as HP-
20) (eluent; water and 5 ~ aqueous methanol). The fractions
containing the desired compound are combined, concentrated,
and lyophilized to give 7B-{(Z)-2-(2-aminothiazol-4-yl)-2-
[(8-hydroxy-2-oxo-lH-~uinolin-5-yl)(carboxy)methyloxyimino]-
acetamido}cephalosporanic acid disodium salt (4.2 g) as
light yellow powder.
M.p. 230 - 240C (decomposed3
IR (Nujol): 3320, 3200, 1770, 1730, 1650, 1600 cm 1
MS (m/z): 703 (MH+)
NMR (D2O) ~: 2.11 and 2.12 (3H, sx2), 2.88 and 2.92
(lH, dx2, J=18Hz and 18Hz), 3.36 and 3.39 (lH, dx2, J=18Hz
and 18Hz), 4.66 (lH, d, J=12Hz), 4.83 (lH, d, J=12Hz), 4.89
and 4.92 ~lH, dx2, J=4.9Hz and 4.4Hz), 5.56 and 5.66 (lH,
dx2, J=4.9Hz and 4.4Hz3, 5.97 (lH, s), 6.75 and 6.76 (lH,
dx2, J=9.8Hz and 9.8Hz), 6.99-7.05 (2H, m), 7.21 and 7.23
- 24 - 2~7129
(lH, dx2, J=8.3~z and 7.8Hz), 8.28 and 8.35 (lH, dx2,
J=9.8Hz and 9.8 Hz)
Example 3
(l) To a solution of quinoline (900 mg) in
methylene chloride (9 ml) is added trimethylsilyltrifluoro-
methanesulfonate (l.l ml) under argon atmosphere, and the
mixture is stirred at room temperature for lO minutes. To
the mixture is added 7B-{(z)-2-(2-tritylaminothiazol-4-yl)
2-[(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)(diphenyl-
methyloxycarbonyl)methyloxyimino]acetamido}cephalosporanic
acid (l 9), and the mixture is stirred at room temperature
for 45 minutes, and then refluxed with stirring for 1.5
hours. The solvent is distilled off, and the resulting
residue is dissolved in formic acid (12 ml), and thereto is
added water. The mixture is stirred at room temperature for
6.5 hours. The precipitates are separated by filtration,
washed with 80 % aqueous formic acid solution, and the
washings and the filtrate are combined. The solvent is
distilled off, and the residue is dissolved in saturated
aqueous sodium hydrogen carbonate solution and acetonitril,
and the pH value thereof is adjusted to about pH 8.
Acetonitril is distilled off from the mixture, and the
aqueous layer is washed with chloroform, and purified by HP-
20 column chromato~raphy (eluent; water and 5 - 30 % aqueous
metanol). The fractions containing the desired compound are
combined, concentrated, and lyophilized to qive 7B-~(Z)-2-
(2-aminothiazol-4-yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-
- 25 _ 2 0 ~ 7 ~ 2 9
yl)(caboxy)methyloxyimino]acetamido}-3-(1-quinoliniomethyl)-
3-cephem-4-carboxylic acid sodium salt (160 mg) as slightly
yellow powder.
M.p. 205 - 210C (decomposed)
IR (Nujol): 335C, 3200, 1770, 1660, 1610 cm 1
MS (m/z): 750 (MH+)
NMR (D2O) ~: 2.58 and 2.65 ~lH, dx2, J=19Hz and
l9Hz), 3.12 and 3.15 (lH, dx2, J=19Hz and l9Hz), 4.91 and
4.94 (lH, dx2, J=4.9Hz and 4.9Hz), 5.62 and 5.68 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.70 and 5.85 (2H, each br d), 5.93 (lH,
s), 6.60 and 6.64 (lH, dx2, J=9.8Hz and 9.8Hz), 6.71 and
6.86 (lH, dx2, J=8.3Hz and 8.3Hz), 6.92 and 6.94 (lH, sx2),
7.12 and 7.18 (lH, dx2, J=8.3Hz and 8.3Hz), 7.80-8.34 (6H,
m), 8.98-9.10 (2H, m)
(2) The product (100 mg) obtained above is
subjected to high performance liquid chromatography to
isolate each isomer, and further purified by HP-20 column
chromatography to give ~-isomer and ~-isomer, separately.
Retention time (Rt) of ~-isomer: 25 minutes
Retention time (Rt) of B-isomer: 40 minutes
[Conditions]
Solid phase; C18-silica gel (tradename: YMC-Pack S-343)
Eluent; Acetonitril : 30 mM phosphate buffer (pH 7.0) =
8.5 : 91.5
Flow rate; 5 ml~minute
-Isomer
Yield: 30 mg
- ~6 - 2~7129
M.p. >175C (decomposed)
IR (Nujol): 3380, 3200, 1770, 1660, 1610 cm 1
MS (m/z): 750 (MH+)
NMR (D20) ~: 2.62, 3.17 ~2H, dx2, J=18Hz and 18~z),
4.94 (lH, d, J=4.9Hz), 5.62 (lH, d, J=4.gHz), 5.75, 5.91
(2H, dx2, J=16Hz and 16Hz), 5.95 (lH, s), 6.66 (lH, d,
J=9.8Hz), 6.79 (lH, d, J=8.3Hz), 6.99 (lH, s), 7.13 (lH, d,
J=8.3Hz), 7.90-8.40 (6H, m), 9.10 (2H, m)
B-Isomer
Yield: 27 m~
M.p. >175C (decomposed)
IR (Nujol): 3380, 3200, 1770, 1660, 1610 cm 1
MR (m/z): 750 (MH+)
NMR ~D20) ~: 2.57, 3.13 (2H, dx2, J=18Hz and 18
Hz), 4.91 (lH, d, J=4.9Hz), 5.69 (lH, d, J=4.9Hz), 5.73,
5.88 (2H, dx2, J=16Hz and 16Hz), 5.96 (lH, s), 6.68 (lH, d,
J=lOHz), 6.94 (lH, d, J=8.3Hz), 6.98 (lH, s), 7.20 (lH, d,
J=8.3Hz), 7.80 - 8.38 (6H, m), 9.12 (2H, m)
Example 4
To a mixture of 7g-{(Z)-2-(2-aminothiazol-4-yl)-2-
[(8-hydroxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxyimino]-
acetamido}cephalosporanic acid disodium salt (0.59 g),
pyridine (0.34 ml) and sodium iodide (1.25 9) in water (6
ml) is added acetic acid in order to adjust the pH value of
the mixture to pH 6.6. The mixture is stirred at 70C under
arson atmosphere for 30 minutes, and then cooled. The pH
value of the mixture is adjusted to pH 7.9 with saturated
- 27 - 2~7.~2~
aqueous sodium hydrogen carbonate solution, and the mixture
is purified by HP-20 column chromatography (eluent; water
and 5 ~ aqueous methanol~. The initially eluating part, the
middle part and the las'~ part of the ~ractions containing
the desired compound are combined separately, and
concentrated. The each resulting residue is lyophilized to
give ~-isomer, a mixture of ~-isomer and 3-isomer, and B-
isomer of 7~-{(Z)-2-(2-aminothiazol-4-yl)-2-[(8-hydroxy-2-
oxo-lH-quinolin-5-yl)(carboxy)methyloxyimino]acetamido}-3-
(l-pyridiniomethyl)-3-cephem-4-carboxylic acid sodium salt.
~-Isomer
Yield: 24 mg
M.p. >200C (decomposed)
IR (Nujol): 3350, 3320, 1770, 1660 - 1620, 1600 cm 1
MS (m/z): 722 (MNa+), 700 (MH+)
NM~ (D2O) ~: 2.61 (lH, d, J=18Hz), 3.34 (lH, d,
J=18Hz), 4.96 (lH, d, J=4.9Hz), 5.28 (lH, d, J=14Hz), 5.49
(lH, d, J=14Hz), 5.70 (lH, d, J=4.9Hz), 5.96 (lH, s), 6.67
(lH, d, J=9.8Hz), 6.90 (lH, d, J=8.3Hz), 7.19 (lH, d,
J=8.3Hz), 8.13 (2H, m), 8.32 (lH, d, J=9.8Hz), 8.60 (lH, m),
8.90 (2H, m)
3-Isomer
Yield: 16 mg
M.p. >200C (decomposed)
IR (Nujol): 3350, 3320, 1770, 1660 - 1620, 1600 cm 1
MS (m/z): 722 (MNa+), 700 (MH+)
NMR (D2O) ~: 2.64 (lH, d, J=18Hz), 3.34 (lH, d,
- 28 - 2~712~
J=18Hz), 4.97 (lH, d, J=4.4Hz), 5.28 (1~, d, J=lSHz), 5.51
(lH, d, J=15Hz), 5.61 (lH, d, J=4.4Hz), 5.96 (lH, s), 6.64
(lH, d, J=8.3Hz), 6.69 (lH, d, J=9.8Hz), 7.06 (lH, d,
J=8.3Hz), 8.13 (2H, m), 8.22 (lH, d, J=9.8Hz), 8.60 (lH, m),
8.90 (2H, m)
A mixture of ~- and 3-isomers
Yield: 91 mg
M.p. >200 C
IR (Nujol): 3350, 3200, 1770, 1660 - 1620, 1600 cm 1
MS (m/z): 722 (MNa+), 700 (MH+)
NMR tD2O) ~: 2.16 and 2.64 (lH, dx2, J=18Hz and
18Hz), 3.34 (lH, d, J=18Hz), 4.96 and 4.97 (lH, dx2, J=4.9Hz
and 4.4Hz), 5.28 tlH, br d), 5.49 and 5.51 (lH, dx2, J=14Hz
and 15Hz), 5.61 and 5.70 (lH, dx2, J=4.4Hz and 4.9Hz), 5.96
(lH, br d), 6.66 and 6.90 (2H, m and d, J=8.3Hz), 7.00 (lH,
s), 7.06 and 7.19 (lH, dx2, J=8.3Hz and 8.3Hz), 8.13 (2H,
m), 8.22 and 8.32 (lH, dx2, J=9.8Hz and 9.8Hz), 8.60 (lH,
m), 8.90 (2H, m)
Examples 5 - 26
73-{(Z)-2-(2-Tritylaminothiazol-4-yl)-2-[(8-
diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)(diphenylmethyl-
oxycarbonyl)methyloxyimino]acetamido}cephalosporanic acid
and a nitrogen-containing heterocyclic compound are treated
in the same manner as in Example 3 to give the compounds of
the following Table 1.
20~7~.~9
2q
Table 1
N ~ C-CONH
O COO-
I
CH-COONa
~0
OH H
Ex. Compound
No.
_ R41 Physicochemical properties
M.p. 210-220C (decomposed)
5 -N ~ IR v nu~ol cm 1 3350, 3200, 1770, 1660,~
~ NMR (D2O) ~: 2.58 and 2.66 (lH, dx2,
/ \ J=18Hz and 18Hz), 3.19 and 3.22 (lH,
~\ // dx2, J=18Hz and 18Hz), 4.96 (lH, d,
~__\ J=4.9Hz), 5.63 and 5.68 (lH, dx2,
C1 J=4.9Hz and 4.9 Hz), 5.74 (2H, br s),
5.91 and 5.95 (lH, sx2), 6.61 and 6.65
~lH, dx2, J=9.3Hz and 9.3Hz), 6.67 and
6.83 (lH, dx2, J=7.8Hz and 7.8Hz), 6.87
and 6.92 (lH, sx2), 7.13 and 7.17 (lH,
dx2, J=7.8Hz and 7.8Hz), 7.90-8.37 (SH,
m), 8.88 (lH, m), 9.09 (lH, m)
_
M.p. 205-210C (decomposed)
6 Br IR v nujol cm 1 3340, 3200, 1770, 1660,
/ max 1610
+ ~ NMR (D2O) ~: 2.62 and 2.69 (lH, dx2,
-N \~ J=17Hz and 17Hz), 3.22 and 3.28 (lH,
~ dx2, J=17Hz and 17 Hz), 4.97 ~lH, d,
/ \ J=4.9Hz), 5.64 and 5.69 (lH, dx2,
J=4.9Hz and 4.9 Hz), 5.77 (2H, br s),
S.91 and 5.96 (lH, sx2), 6.64 and 6.65
(lH, dx2, 3=9.8Hz and 9.8Hz), 6.70 and
6.88 (lH, dx2, J=8.3Hz and 8.3Hz), 6.91
and 6~95 (lH, sx2), 7.21 (lH, d,
J=8.3Hz), 8.15 (lH, m), 8.03-8.38 (4H,
m), 9.15 ~lH, s), 9.20 and 9.34 (lH,
sx2)
_
2Q~7i29
- 30 -
M.p. 200-210C (decomposed)
7 -N ~ IR v nujol cm 1 3350, 3200, 1770, 1660
~ NMR (D2O) ~: 2.57 and 2.66 (lH, dx2,
/ \ J=18Hz and 18Hz), 3.22 (lH, d, J=18Hz), I
4.97 (lH, d, J=4.9Hz), 5.63 and 5.68
\ (lH, dx2, J=4.9Hz and 4.9Hz), 5.74 (2H,
Br br s), 5.91 and 5.96 (lH, sx2), 6.61 and
6.65 ~lH, dx2, J=lOHz and 10Hz), 6.65 anc
6.83 (lH, dx2, J=7.3Hz and 8.3 Hz), 6.88
and 6.93 (lH, sx2), 7.13 and 7.16 (lH,
dx2, J=7.3Hz and 8.3Hz), 7.96-8.31 (5H,
m), 8.85 (lH, m), 9.13 (lH, m)
i M.p. 190-200C (decomposed)
8 ¦ + IR v nujol cm~l 3340, 3200, 1770, 1660,
-N ~ max 1610
MS (m/z): 790 (MNa+), 768 (MH+)
/ \ NMR (D2O) ~: 2.58 and 2.67 (lH, dx2,
// J=18Hz and 18Hz), 3.18 and 3.23 (lH, dx2,
J=18Hz and 18Hz), 4.95 and 4.97 (lH, dx2,
F J=4.9Hz and 4.9Hz), 5.63 and 5.69 (lH,
dx2, J=4.9Hz and 4.9Hz), 5.79 (2H, br s),
I 5.93 (lH, s), 6.63 and 6.66 (lH, dx2,
! J=9.8Hz and 9.8Hz), 6.71 and 6.87 (lH,
dx2, J=8.8Hz and 8.8Hz), 6.90 and 6.93
(lH, sx2), 7.13 and 7.18 (lH, dx2,
J=8.8Hz and B.8Hz), 7.85-8.09 (3H, m),
8.20 and 8.29 (lH, dx2, J=9.8Hz and
9.8Hz), 8.43 (lH, m), 8.98 (lH, m), 9.10
(lH, d, J=5.4Hz)
~ M.p. 190-200C (decomposed)
9 + ~ IR v nujol cm~l 3330, 3200, 1770, 1660,
-N/ ~) max 1$10 +
~S (m/z): 802 (MNa ~, 780 (~H )
\ NMR (D2O) ~: 2.56 and 2.65 (lH, d, J=18Hz
// and 18Hz), 3.14 and 3.17 (lH, dx2, J=18Hz
___\ and 18Hz)~ 3.87 and 3.89 (3H, sx2), 4.95
OCH3 and 4.96 (lH, dx2, J=4.4Hz and 4.4Hz),
5.63 and 5.68 (lH, dx2, J=4.4Hz and
4.4Hz), 5.69 (lH, br s), 5.89 and 5.92
(lH, sx2~, 6.56 and 6.61 (lH, dx2,
J=8.8Hz and 9.3 Hz), 6.64 and 6.79 (lH,
dx2, J=8.3Hz and 8.3Hz), 6.84 and 6.90
(lH, sx2), 7.11 and 7.15 (lH, dx2,
J=8.3Hz and 8.3Hz), 7.40 (lH, m), 7.63
(lH, m)! 7.85 (lH, m), 8.13-8.29 (2H, m),
8.74 (lH, m), 8.87 (lH, m)
- 31 - 2~712~
M.p. 195-205C (decomposed)
+ IR v nujol cm 1 3350, 3200, 1770, i650
-N ~ CH3 max 1600
~ MS (m/z): 786 (MNa+), 764 (MH+)
/ \ NMR (D2O) ~: 2.58 and 2.67 (lH, dx2,
J=18Hz and 18Hz), 2.89 and 2.90 (3H,
sx2), 3.13 and 3.16 (lH, dx2, J=18Hz and
18Hz), 4.92 and 4.94 (lH, dx2, J=4.9Hz
and 4.9Hz3, 5.59 (lH, br d, J=14Hz), 5.61
and 5.66 (lH, dx2, J=4.9Hz and 4.9Hz),
5.76 (lH, br d, J=14Hz), 5.91 and 5.93
(lH, sx2), 6.57 and 6.62 (lH, dx2,
J=9.8Hz and 9.8Hz), 6.65 and 6.83 (lH,
dx2, J=8.3Hz and 8.3Hz), 6.88 and 6.92
(lH, sx2), 7.13 and 7.17 (lH, dx2,
J=8.3Hz and 8.8Hz), 7.83 (2H, m), 8.00-
8.29 (4H, m), 8.85 (lH, m)
l M.p. 195-205C (decomposed)
11 ¦ + IR v nujol cm~l 3350, 3200, 1770, 1650,
I -N ~ max 1610
¦ ~ MS (m/z): 786 (MNa+), 764 (MH+)
/ \ NMR (D2O) ~: 2.43 and 2.46 (3H, sx2),
2.57 and 2.66 (lH, dx2, J=18Hz and 18Hz),
\ 3.13 and 3.15 (lH, dx2, J=18Hz and 18Hz),
CH3 4.95 and 4.97 (lH, dx2, J=4.4Hz and
4.4Hz), 5.63 and 5.69 (lH, dx2, J=4.4Hz
and 4.4 Hz), 5.69 (2H, br s), 5.90 (lH,
s), 6.55 and 6.61 (lH, dx2, J=9.8Hz and
9.8Hz), 6.70 and 6.83 (lH, dx2, J=8.3Hz
and 8.3Hz), 6.85 and 6.90 (lH, sx2), 7.10
and 7.17 (lH, dx2, J=8.3Hz and 8.3Hz),
7.78-7.93 (3H, m), 8.13-B.29 (2H, m),
8.78 (lH, m), 9.00 (lH, m)
_ M.p. >180C (decomposed)
11 ~-Isomer 1
IR nujol cm~l: 3360, 3200, 1770, 1660,
+ ~ max 1610
-N ) MS (m/z): 786 (MNa+), 764 ~MH+)
~ NMR (D2O) ~: 2.53 (3H, s), 2.63 (lH, d,
/ ~ J=18Hz), 3.15 (lH, d, J=18Hz), 4.95 (lH,
d, J=4.4Hz), 5.64 (lH, d, J=4.4Hz), 5.67
(lH, d, J=16Hz), 5.82 (lH, d, J=16Hz),
CH3 5.91 (lH, s), 6.58 (lH, d, J=9.8Hz), 6.71
(lH, d, J=8.3Hz), 6.95 (lH, s), 7.10 (lH,
d, J=8.3Hz), 7.90-7.98 (3H, m), 8.14-8.2
(2H, m), 8.88 (lH, d, J=8.3Hz), 9.02 (lH,
d, J=5.9Hz)
- 32 - 2 0 ~
*1 M.p. ~180C (decomposed)
11 3-Isomer
IR nujol cm~l: 3350, 3200, 1770, 1660,
_ ~ MS (m/z): 786 (MNa$), 764 (MH )
NMR (D20) ~: 2.48 (3H, s), 2.54 (lH, d,
/ ~ J=18Hz), 3.13 (lH, d, J=18Hz), 4.94 (lH,
d, J=4.4Hz), 5.68 (lH, d, J=16Hz), 5.69
\ (lH, d, J=4.4Hz), 5.77 (lH, d, J=16Hz),
CH3 5 93 (lH, s), 6.64 (lH, d, J=9.8Hz), 6.86'
(lH, d, J=8.3Hz), 6.88 (lH, s), 7.18
(lH, d, J=8.3Hz), 7.85-7.93 (3H, m), 8.20
(lH, d, J=9.3Hz), 8.29 (lH, d, J=9.8Hz),
8.82 (lH, d, J=7.3Hz), 8.99 (lH, d,
J=6.8Hz)
M.p. 185-195C (decomposed)
12 + IR v nujol cm 1 3340, 3200, 1770~ 1650
-N--~ max 1610
MS (m/z): 750 (MH )
\==/ NMR (D2O) ~: 2.71 and 2.74 (lH, dx2,
\ J=18Hz and 18Hz), 3.41 (lH, d, J=18Hz),
5.07 and 5.08 (lH, dx2, J=4.4Hz and
4.4Hz), 5.26 and 5.31 ~lH, dx2, J=14Hz
and 14Hz), 5.61 (lH, d, J=14Hz), 5.65 and
5.72 (lH, dx2, J=4.4Hz and 4.4Hz), 5.86
and 5.89 (lH, sx2), 6.50 and 6.73 (lH,
dx2, J=7.8Hz and 7.8Hz), 6.54 and 6.57
(lH, dx2, J=9.8Hz and 9.8 Hz), 6.85 and
6.89 (lH, sx2), 6.99 and 7.10 (lH, dx2,
J=7.8Hz and 7.8Hz), 7.68-8.24 (6H, m),
8.49 (lH, m), 9.59 (lH, br s)
M.p. >175C (decomposed)
13 + IR v nujol cm 1 3350, 3200, 1770, 1660,
-N ~ max 1610
~ MS (m/z): 740 (MH+)
/ ~ NMR (D2O) ~: 2.2g (2H, m), 2.64 and 2.69
(lH, dx2, J=18Hz and 18Hz), 3.11-3.30
(5H, m), 4.96 and 4.98 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.21 (lH, d, J=15Hz), 5.36
and 5.38 (lH, dx2, J=15Hz and 15Hz), 5.64
and 5.71 (lH, dx2, J=4.9Hz and 4.9Hz),
5.97 (lH-, s), 6.69 and 6.72 (lH, dx2,
J=9.8Hz and 9.8Hz), 6.91 and 7.01 (lH,
dx2, J=7.8Hz and 8.3Hz), 6.97 and 6.98
(lH, sx2), 7.19 and 7.23 (lH, dx2,
J=7.8Hz and 8.3Hz), 7.68-7.79 (lH, m),
8.23-8.45 (3H, m)
_
- ` - 2 (~ 2 ~
M.p. >175C ~decomposed)
¦ 14-~ ~ max 1610
MS (m/z): 754 (MH+)
NMR ~D~O) ~: 1.79-1.91 (4H, m), 2.63 and
2.67 (-H, dx2, J=18Hz and 18Hz), 2.93
(4H, m), 3.15 and 3.17 (lH, dx2, J=i8Hz
and 18Hz), 4.97 and 4.98 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.23 (2H, br s), S.64
and 5.71 (lH, dx2, J=4.9Hz and 4.9Hz),
5.97 (lH, s), 6.69 and 6.73 (lH, dx2,
J=9.8Hz and 9.8Hz), 6.89 and 7.00 (lH,
dx2, J=7.3Hz and 7.8Hz), 6.96 and 6.97
(lH, sx2), 7.20 and 7.24 ~lH, dx2,
J=7.3Hz and 7.8Hz), 7.60-7.78 (lH, m),
8.14 (lH, m), 8.26 and 8.35 (lH, dx2,
J=9.8Hz and 9.8Hz), 8.47 (l~i, d, J=6.3Hz)
M.p. >160C (decomposed)
15-N ~ max cm : 3350, 3200, 1770 1660
MS (m/z): 790 (MNa+), 768 (MH+)
NMR (D2O) ~: 1.50-1.90 (6H, m), 2.58 and
V 2.63 (lH, dx2, J=18Hz and 18Hz), 2.90-
3.30 (5H, m), 4.93 (lH, m), 5.34, 5.73
(2H, ABq, J=17Hz), 5.61 and 5.67 (lH,
dx2, J=4.4Hz and 4.4Hz), 5.97 (lH, s),
6.71 and 6.74 (lH, d, J=9.8Hz and 9.8Hz),l
6.94 and 7.02 (lH, dx2, J=8.3Hz and
8.3Hz), 6.99 (lH, s), 7.20 and 7.24 (lH,
dx2, J=8.3Hz and 8.3Hz), 7.70 (lH, m),
8.21 (lH, d, J=9.3Hz), 8.26 ad 8.35 (lH,
dx2, J=9.8Hz and 9.8Hz), 8.50 (lH, d,
J=5.4Hz)
M.p. >160C (decomposed)
16-N ~ max 1610
~ MS (m/z): 736 (MNa+), 714 (MH+)
CH NMR (D2O) ~: 2.61 and 2.66 (lH, dx2,
3 J=18Hz and 18Hz), 2.79 and 2.80 (3H,
sx2), 3.21 and 3.24 (lH, dx2, J=18Hz and
18Hz), 4.96 and 4.99 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.27 (lH, d, J=lSHz), 5.45
(lH, d, J=15Hz), 5.63 and 5.71 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.98 (lH, s), 6.70
and 6.73 (lH, dx2, J=9.8Hz and 9.8Hz~,
6.90 and 7.01 (lH, dx2, J=8.3Hz anà
8.3Hz), 7.00 (lH, s), 7.18 and 7.23 ~lH,
dx2, J=8.3Hz and 8.3Hz), 7.91 (2H, m),
8.22-8.46 (2H, m), 8.67 (lH, d, J=5.9Hz)
_ _ _ _ _ . . . _ .
- 34 ~ 2~7129
M.p. >175C (decomposed)
17 + IR v nujol cm-l 3360, 3200, 1770, 1660
-N ~ ~ max 1640, 1610
MS (m/z): 752 (MNa+), 730 (MH+)
/ NMR (D2O) ~: 2.76 and 2.81 ~lH, dx2,
OCH3 J=18Hz and 18Hz), 3.17 and 3.20 (lH, dx2,
J=18Hz and 18Hz), 4.24 (3H, s), 4.87 (lH,
s, J=4.9Hz), 4.90 (lH, d, J=15Hz), 5.60
and 5.67 (lH, dx2, J=4.9Hz and 4.9Hz),
5.66 (lH, d, J=15Hz), 5.98 (lH, s), 6.71
and 6.73 (lH, dx2, J=9.8Hz and 9.8Hz),
6.99 (lH, s), 7.00 and 7.04 (lH, dx2,
J=8.3Hz and 8.3Hz), 7.21 ad 7.24 (lH,
dx2, J=8.3Hz and 8.3Hz), 7.46-7.57 (2H,
m), 8.26 and 8.34 (lH, dx2, J=9.8Hz and
9.8Hz), 8.34-8.46 (2H, m)
l M.p. >175C (decomposed)
18 -N ~ IR v nujol cm 1 3340, 3200, 1770, 1660,
MS (m/z): 736 (MNa+), 714 (MH+)
\ NMR (D2O) ~: 2.52 and 2.55 (3H, sx2),
CH3 2.59 and 2.65 (lH, dx2, J=18Hz and 18Hz),
3.35 and 3.36 (lH, dx2, J=18Hz and 18Hz),
4.99 and 5.00 (lH, dx2, J=4.9Hz and
4.9Hz), 5.21 and 5.24 (lH, dx2, J=15Hz
and 15Hz), 5.44 (lH, d, J=15Hz), 5.63 and
5.71 (lH, dx2, J=4.9Hz and 4.9Hz), 5.96
(lH, s), 6.69 (lH, d, J=9.8Hz), 6.83 and
7.13 (lH, dx2, J=8.3Hz and 8.3Hz), 6.99
(lH, s), 7.22 (lH, d, J=8.3Hz), 7.95 (lH,
m), 8.23 and 8.32 (lH, dx2, J=9.8Hz and
9.8Hz), 8.31-8.40 (lH, m), 8.70 (lH, d,
J=7.8Hz), 8.72 (lH, br s)
_
M.p. >180C (decomposed)
19 -N ~ IR v nu~ol cm 1 3340, 3200, 1770, 1660,
MS (m/z): 621 (MH+-3-cyanopyridine)
\ NMR (D2O) ~: 2.69 and 2.76 (lH, dx2,
CN J=18Hz and 18Hz), 3.45 and 3.47 (lH, d`x2,
J=18Hz and 18Hz), 5.02 and 5.03 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.33 and 5.34 (lH,
dx2, J-14Hz and 14Hz), 5.66 (lH, d,
J=14Hz), 5.66 and 5.74 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.96 (lH, s), 6.69 (lH, d,
J=9.3Hz), 6.88 and 7.00 (1~, dx2, J=8.3H
and 8.3Hz), 6.99 (lH, s), 7.16 and 7.22
(lH, dx2, J=8.3Hz and 8.3Hz), 8.21-8.38
(2H, m), 8.99 (lH, br d), 9.25 (lH, d,
J=4.9Hz), 9.56 (lH, br d)
- 35 - 20~129
IM.P. ~175C (decomposed)
+ ~/ IR v nujol cm-l 3330, 3200, 1770~ 1740
-N ~ max 1650, 1610
MS (m/z): 780 (MNa+), 758 (~H+)
NMR (D2O) ~: 2.66 and 2.75 (lH, dx2,
COOCH J=18Hz and 18Hz), 3.46 and 3.47 (lH, dx2,
3 J=18Hz and 18Hz), 4.01 and 4.04 (3H,
sx2), 5.03 and 5.04 (lH, dx2, J=4.9Hz and
4.9Hz), 5.28 and 5.33 (lH, dx2, J=15Hz
and 15Hz), 5.61 (lH, d, J=15Hz), 5.65 and
5.72 (lH, dx2, J=4.9Hz and 4.9Hz), 5.94
(lH, s), 6.66 and 6.68 (lH, dx2, J=9.8Hz
and 9.8Hz), 6.83 and 6.97 (lH, dx2,
J=8.3Hz and 8.3Hz); 6.93 and 6.95 (lH,
I sx2), 7.14 and 7.20 (lH, dx2, J=8.3Hz and
8.3Hz), 8.17-8.23 (lH, m), 8.24 and 8.31
(lH, dx2, J=9.8Hz and 9.8Hz), 8.98-9.05
(lH, m), 9.14 (lH, d, J=6.4Hz), 9.55 (lH,
i br s)
M.p. >160C (decomposed)
21 + IR v nujol cm 1 3340, 3200, 1770, 1660,
-N ~ max 1610
MS (m/z): 750 (MNa+), 728 (MH+)
NMR ~D2O) ~: 2.50 ~3H, s), 2.68 (4H, m),
CH3 CH3 3.13 and 3.15 (lH, dx2, J=18Hz and 18Hz),
4.95 and 4.98 (lH, dx2, J=4.9Hz and
4.9Hz), 5.27-5.56 (2H, m), 5.63 and 5.70
(lH, dx2, J=4.9Hz and 4.9Hz), 5.98 (lH,
s), 6.70 and 6.73 (lH, dx2, J=9.8Hz and
9.8Hz), 6.92 and 7.01 (lH, dx2, J=8.3Hz
and 8.3Hz), 6.99 (lH, s), 7.20 and 7.23
(lH, dx2, J=8.3Hz and 8.3Hz), 7.77 (lH,
m), 8.24 (lH, m), 8.26 and 8.31 (lH, dx2,
J=9.8Hz and 9.8Hz), 8.50 (lH, d, J=5.7Hz)
- 36 - 2 ~ 7
M.p. >160C ~decomposed)
22 + IR nujol cm~l: 3350, 3200/ 1770, 1660,
-N ~ CH3 max 1610
~==/ MS (m/z): 750 (MNa ), 728 (MH )
CH NMR (r~20) ~: 2.35 and 2.41 (3H, sx2),
3 2.48 and 2.53 (3H, sx2), 2.63 and 2.65
(lH, dx2, J=18Hz and 18Hz), 3.34 (lH, d,
J=18Hz), 5.00 (lH, d, J=4.9Hz), 5.10 and
5.14 ~lH, dx2, J=14Hz and 14Hz), 5.35
(lH, d, J=14Hz), 5.62 and 5.69 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.94 and 5.95 (lH,
sx2), 6.68 (lH, d, J=lOHz), 6.73 and 6.95
(lH, dx2, J=8.3Hz and 8.3Hz), 6.92 and
6.97 (lH, sx2), 7.11 and 7.21 (lH, dx2,
J=8.3Hz and 8.3Hz), 7.73 and 7.79 (lH,
dx2, J=6.4Hz and 6.4Hz), 8.24 and 8.32
(lH, dx2, J=lOHz and lOHz), 8.51 (lH, d,
J=6.4Hz), 8.53 (lH, s)
. _
M.p. 185-190C (decomposed)
23 + IR nujol cm~l: 3350, 3200, 1770, 1660,
_ ~ max 1610
MS (m/z): 740 (MNa+), 718 (MH+)
NMR (D20) ~: 2.65 and 2.71 (lH, dx2,
F J=18Hz and 18Hz), 3.40 and 3.42 (lH, dx2,
J=18Hz and 18Hz), 5.00 and 5.01 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.29 and 5.32 (lH,
dx2, J=15Hz and 15Hz), 5.59 (lH, d,
J=15Hz), 5.64 and 5.73 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.96 (lH, s), 6.69 (lH, d,
J=9.8Hz), 6.85 and 7.00 (lH, dx2, J=8.3Hz
and 8.3Hz), 6.99 (lH, s), 7.14 and 7.22
(lH, dx2, J=8.3Hz and 8.3Hz), 8.18 (lH,
m), 8.24 and 8.32 (lH, dx2, J=9.8Hz and
9.8Hz), 8.50 (lH~ m), 8.87 (lH, d,
J=5.4Hz), 9.11 (lH, br s)
_ 37 _ 2Q~7129
M.p. >180C (decomposed)
?4 IR ~ nujol cm 1 3350, 3200, 1770, 1660,
max 1610
Cl MS (m/z): 6a4 (MNa+-3-chloro-5-
+ ~ methoxypyridine)
-N \~ 621 (MH+-3-chloro-5-
~==J methoxypyridine)
\ NMR (D2O) ~: 2.67 and 2.73 (lH, dx2,
OCH3 J=18Hz and 18Hz), 3.43 (lH, d, J=18Hz),
3.95 and 4.01 (3H, sx2), 5.04 (lH,
d, J=4.9Hz), 5.14 and 5.19 ~lH, dx2,
J=15Hz and 15Hz), 5.50 (lH, d, J=lSHz),
I 5.65 and 5.71 (lH, dx2, J=4.9Hz and
4.9Hz), 5.95 (lH, s), 6.69 and 6.70 (lH,
dx2, J=9.8Hz and 9.8Hz), 6.85 and 6.98
(lH, dx2, J=8.3Hz and 8.3Hz), 6.94 and
6.96 (lH, sx2), 7.17 and 7.22 (lH, dx2,
J=8.3Hz and 8.3Hz), 8.15 (lH, m), 8.25
and 8.33 (lH, dx2, J=9.8Hz and 9.8Hz),
8.69 (2H, m)
_
M.p. >190C (decomposed)
25-N ~ max 1610
MS (m/z): 765 (MNa+), 743 (MH+)
\ NMR (D2O) ~: 2.63 and 2.71 (lH, dx2,
CONH2 J=18Hz and 18Hz), 3.42 (lH, br d,
J=18Hz), 5.01 (lH, m), 5.29 and 5.32 (lH,
dx2, J=14Hz a~d 14Hz), 5.61 (lH, d,
J=14Hz), 5.63 and 5.72 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.95 (lH, s), 6.66 and 6.68
(lH, dx2, J=3.8Hz and 9.8Hz), 6.77 and
6.96 (lH, dx2, J=8.3Hz and 8.3Hz), 6.97
(lH, s), 7.11 and 7.20 (lH, dx2, J=8.3Hz
and 8.3Hz), 8.19-8.32 (2H, m), 8.91 (lH,
m), 9.11 (lH, d, J=5.9Hz), 9.37 (lH, s)
_
_ 3~ _ 2~ 71 ~3
M.p. >175C (decomposed)
26 + ~ IR nujol cm~l: 3350, 3200, 1770, 1660,
-N \~ max 1610
MS (m~z): 758 (MNa++2), 756 (M+Na+),
736 (MH +2), 734 (NH )
Cl NMR (D2O) ~: 2.66 and 2.71 (lH, dx2,
J=18Hz and 18Hz), 3.41 and 3.42 (lH, dx2,
J=18Hz and 18Hz), 5.01 and 5.02 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.24 and 5.26 (lH,
dx2, J=lSHz and lSHz), 5.55 (lH, d,
J=lSHz), 5.65 and 5.73 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.96 (lH, s), 6.69 (lH, d,
J=9.8Hz), 6.85 and 7.00 (lH, dx2, J=8.3Hz
and 8.3Hz), 6.97 (lH, s), 7.15 and 7.22
(lH, dx2, J=8.3Hz and 8.3Hz), 8.03-8.13
(lH, m), 8.23 and 8.32 (lH, dx2, J=9.8Hz
and 9.8Hz), 8.59-8.66 (lH, m), 8.91 (lH,
d, J=6.4Hz), 9.14 (lH, br s)
.
M.p. >170C (decomposed)
26 -Isomer 2 IR nujol cm~l: 3350, 3200, 1770, 1660,
max 1610
+ ~ MS (m/z): 758 (MN+a++2), 756 (MNa+),
-N ~ 736 (MH +2), 734 (MH+)
NMR (D2O) ~: 2.69, 3.42 (2H, ABq,
C J=18Hz), S.01 (lH, d, J=4.9Hz), 5.27,
1 5.56 (2H, ABq, J=14Hz), 5.64 (lH, d,
J=4.9Hz), 5.96 (lH, s), 6.69 (lH, d,
J=9.8Hz), 6.85 (lH, d, J=7.8Hz), 7.01
(lH, s), 7.15 (lH,d, J=7.8Hz), 8.12 (lH,
dd, J=8.3Hz, 5.9Hz), 8.22 (lH, d,
J=9.8Hz), 8.67 (lH, d, J=8.3Hz), 8.92
(lH, d, J=5.9Hz), 9.14 (lH, s)
_ 39 _ 2 ~ ~ 7 ~
M.p. >170C (decomposed)
26~-Isomer 2 1610
+ ,,--~ MS (m/z): 736 ~MNat), 734 ~MH+)
-N' ~ NMR (~2O) ~: 2.63, 3.41 ~2H, ABq,
~=~/ J=18Hz), 5.00 ~lH, d, J=4.9Hz), 5.26,
\ 5.56 (2H, ABq, J=14Hz), 5.72 ~lH, d,
Cl J=4.9Hz), 5.97 ~lH, s), 6.71 (lH, d,
J=g.8Hz), 7.00 (lH, s), 7.02 (lH, d,
J=7.8Hz), 7.23 ~lH, d, J=7.8Hz), 8.10
(lH, dd, J=8.3Hz, 5.9Hz), 8.33 (lH, d,
J=9.8Hz), 8.63 (lH, d, J=8.3Hz), 8.91
_ (lH, d, J=5.9Hz), 9.15 (lH, s)
*1: Each isomer is isolated by high performance liquid
chromatography [solid phase; C18-silica gel (YMC-
Pack S-343), eluent; acetonitril/30 mM phosphate
buffer (pH 7.0) = 1 : 9)].
*2: Each isomer is isolated by high performance liquid
chromato~raphy [solid phase; C18-silica gel (YMC-
Pack D-ODS-10), eluent; acetonitril/30 mM phosphate
buffer (pH 7.0) = 6 : 94].
~7129
-- .. o
Example 27
(1) To dimethylformamide (0.38 g) are added
dropwise phosphorus oxychloride ~0.48 g) and methylene
chloride (2.2 ml) with ice-cooling under argon atmosphere,
and the mixture is stirred at room temperature ~or 1.5 hour,
and cooled to -55 to -50C. To the mixture is added
dropwise a solution of 2-(2-tritylaminothiazol-4-yl)-2-[(8-
diphenylmethyloxy-2-oxo-lH-~uinolin-5-yl)~diphenylmethyloxy-
carbonyl)methyloxyimino]acetic acid (2.2 g) in methylene
chloride (54 ml). The mixture is stirred at -65 to -55C
for 1.5 hour, and then at -55 to -50C for 30 minutes (the
resulting solution is referred to as "Reaction solution
A"). Separately, bis(trimethylsilyl)acetamide (3.08 g) is
added to a suspension of 7B-amino-3-(3-amino-2-meth
pyridiniomethyl)-3-cephem-4-carboxylate (1.57 g) in
methylene chloride (32 ml) with ice-cooling under argon
atmosphere, and the mixture is stirred at the same
temperature for two hours (the resulting solution is
referred to as "Reaction solution B"). The Reaction
solution B is added dropwise to the Reaction solution A, and
the mixture is stirred at -55 to -30C for 40 minutes, and
then at -30C for 1.5 hour. After completion of the
reaction, the mixture is poured into water, and evaporated
to remove methylene chloride. The resulting precipitates
are collected by filtration, washed with water, and dried.
The resulting residue is added to a mixture of anisole (10
2~7~29
ml) and trifluoroacetic acid (10 ml) at 15 to 20C, and the
mixture is stirred at room temperature for two hours. To
the mixture is added isopropyl ether (180 ml) under ice-
coolin~, and the mixture is stirred for 15 minutes. The
precipitates are collected by filtration, washed with
isopropyl ether, dried, and suspended in water. The pH
value of this suspension is adj~sted to pH 7.7 with
saturated aqueous sodium hydrogen carbonate solution, and
purified by HP-20 column chromatography (eluent; water and
20 % aqueous methanol). The fractions containing the
desired compound are combined, concentrated, and lyophilized
to give 7B-{(Z)-2-(2-aminothiazol-4-yl)-2-[(8-hydroxy-2-oxo-
lH-quinolin~5-yl)(carboxy)methyloxyimino]acetamido}-3-(3-
amino-2-methyl-1-pyridiniomethyl)-3-cephem-4-carboxylic acid
sodium salt (0.75 g) as li~ht yellow powder.
M.p. >160C (decomposed)
IR (Nujol): 3360, 3200, 1770, 1650, 1610 cm 1
MS (m/z): 751 ~MNa+), 729 (MH+)
NMR (D2O) ~: 2.50 and 2.52 (3H, sx2), 2.54 and 2.57
(lH, dx2, J=17Hz and 18Hz), 3.06 and 3.09 (lH, dx2, J=17Hz
and 18Hz), 4.92 and 4.g5 (lH, dx2, J=4.4Hz and 4.4Hz), 5.22
(lH, d, J=15Hz), 5.50 and 5.51 (lH, d, J=15Hz and 15Hz),
5.60 and 5.68 (lH, dx2, J=4.4Hz and 4.4Hz), 5.99 (lH, s),
6.71 ~nd 6.74 (lH, dx2, 3=9.8Hz and 9.8Hz), 6.91 and 7.01
(lH, dx2, J=8.3Hz and 8.3Hz), 7.01 (lH, s), 7.18 and 7.22
2V~7~2~
- 4~ -
(lH, d~2, J=8.3Hz and 8.3Hz), 7.52-7.62 (lH, m), 7.69 and
7.71 (lH, dx2, J=8.3Hz and 8.3Hz), 8.00 (lH, d, J=6.3Hz),
8.24 and 8.34 (lH, d, J=9.8Hz and 9.8Hz)
(2) The product (70 mg) obtained above is
subjected to high performance liquid chromatography to
isolate each isomer, and purified by HP-20 column
chromatography to give ~-isomer and B-isomer, separately.
Rt of ~-isomer: 45 minutes
Rt of 3-isomer: 75 minutes
[Conditions]
Solid phase; C18-silica gel (tradename: YMC-Pack D-ODS-10)
Eluent; Acetonitril : 30 mM phosphate buffer (pH 7.0) =
6 : 94
Flow rate; 5 ml/minute
~-Isomer
Yield: 20 mg
M.p. >160C (decomposed)
IR (Nujol): 3360, 3200, 1770, 1650, 1610 cm 1
MS (m/z): 751 (MNa+), 729 (MH+)
NMR (D2O) ~: 2-52 (3H, s), 2.57, 3.09 (2H, ABq,
J=18Hz), 4.95 (lH, d, J=4.4Hz), 5.22, 5.51 (2H, ABq,
J=15Hz), 5.60 (lH, d, J=4.4Hz), 5.98 (lH, s), 6.71 (lH, d,
J=9.8Hz), 6.91 (lH, d, J=8.3Hz), 7.01 (lH, s), 7.18 (lH, d,
J=8.3Hz), 7.59 (lH, dd, J=8.3Hz, 5.9Hz), 7.71 (lH, d,
J=8.3Hz), 8.00 (lH, d, J=5.9Hz), 8.24 (lH, d, J=9.8Hz)
~-Isomer
- ~3 - 2 ~ 7~ 2
Yield: 20 mg
M.p. >160C (decomposed)
IR ~Nujol): 3360, 3200, 1770, 1660, 1610 cm 1
MS (m/z): 751 (!~Na+), 729 (MH+)
NMR (D2O) ~: 2.50 (3H, s), 2.54, 3.06 (2H, ABq,
J=17Hz), 4.92 (lH, d, J=4.4Hz), 5.22, 5.50 (2H, ABq,
J=15Hz), 5.68 (lH, d, J=4.4Hz), 5.99 (lH, s), 6.74 (lH, d,
J=9.8Hz), 7.01 (lH, s), 7.01 (lH, d, J=8.3Hz), 7.22 (lH, d,
J=8.3Hz), 7.56 (lH, dd, J=7.8Hz, 6.3Hz), 7.69 (lH, d,
J=7.8Hz), 8.00 (lH, d, J=6.3Hz), 8.34 (lH, d, J=9.8Hz)
Examples 28 - 33
2-(2-Tritylaminothiazol-4-yl)-2-[(8-diphenylmethyl-
oxy-2-oxo-lH-quinolin-5-yl)(diphenylmethyloxycarbonyl)-
methyloxyimino]acetic acid and the corLesponding starting
compounds are treated in the same manner as in Example 27
except that the removal of the protecting groups are carried
out by using 80 % aqueous formic acid solution to give the
compounds oF the following Table 2.
_ ~4 - 2~7129
Table 2
In the following Table 2, R5 is COO in Examples 28
- 31, and COONa in Examples 32 - 33.
4 N ~ C-CONH ~ -CH2-R4
O R
I
CH-COONa
N ~ O
OH H
Ex.¦ Compound
No. ¦ Physicochemical properties
M.p. >170C (decomposed)
28 IR v nujol cm 1 3340, 3200, 1770~ 1650
OCH3 MS ~m/z): 767 (MNa$), 745 (MH )
+ ~ NMR (D2O) ~: 2.58 and 2.62 (lH, dx2,
-N \) J=18Hz and 18Hz), 3.32 (lH, d, J=18Hz),
3.83 and 3.88 (3H, sx2), 4.70-4.90 (lH),
5.00 (lH, d, J=4.9Hz), 5.26 (lH, d,
NH2 J=15Hz), 5.61 and 5.68 (lH, dx2, J=4.9Hz
and 4.9Hz~, 5.95 and 5.96 (lH, sx2), 6.68
and 6.69 (lH, dx2, J=9.8Hz and 9.8Hz),
6.80 and 6.96 (lH, dx2, J=7.8Hz and
7.8Hz), 6.96 and 6.97 (lH, sx2), 7.10 and
7.15 (lH, br sx2), 7.14 and 7.21 (lH,
dx2, J=7.8Hz and 7.8Hz), 7.78 (lH, br s),
7.82 (lH, br s), 8.23 and 8.31 (lH, dx2,
J=9.8Hz and 9.8Hz)
- 45 ~ 29
l M.p. >150C (decomposed)
29 ¦ + IR nujol cm~l: 3380, 3200, 1770, 1660,
I _ ~ r~lax 1610
¦ / ~ \ ~S (m/z): 779 (MNa+), 757 ~MH+)
j NHCHO NMR (D2O) ~: 2.54 and 2.66 (lH, dx2,
CH3 J=18Hz and 18Hz), 3.22 and 3.26 (lH, dx2,
J=18Hz and 18Hz), 2.73 (3H, s), 4.96 and
5.00 (lH, dx2, J=4.9Hz and 4.9Hz), 5.37
l and 5.39 (lH, dx2, J=16Hz and 16Hz), 5.54
i (lH, d, J=16Hz), 5.64 and 5.70 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.99 (lH, s), 6.72
and 6.74 (lH, dx2, J=9.8Hz and 9.8Hz),
6.93 and 7.02 (lH, dx2, J=8.3Hz and
8.3Hz), 7.02 (lH, s), 7.19 and 7.22 (lH,
dx2, J=8.3Hz and 8.3Hz), 7.90-8.02 (lH,
m), 8.25 and 8.34 (lH, dx2, J=9.8Hz and
9.8Hz), 8.50 (lH, s), 8.63 and 8.67 (lH,
dx2, J=5.9Hz and 6.4Hz), 8.73 llH, d,
J=8.3Hz)
M.p. >150C (decomposed)
29~-Isomer* IR v nujol cm 1 3370, 3200, 1770, 1660,
max 1$10 +
+ ~ MS (m/z): 779 (MNa ), 757 (~H )
-N' ~) NMR (D~O) ~: 2.66, 3.25 (2H, ABq,
/ ~ J=18Hz~, 2.73 (3H, s), 5.00 (lH, d,
NHCHO J=4.9Hz), 5.37, 5.54 (2H, ABq, J=16Hz),
CH3 5.64 (lH, d, J=4.9Hz), 5.98 (lH, s), 6.7
(lH, d, J=9.8Hz), 6.93 (lH, d, J=8.3Hz),
7.02 (lH, s), 7.19 (lH, d, J=8.3Hz), 7.9
(lH, dd, J=8.3Hz, 5.9Hz), 8.25 (lH, d,
J=9.8Hz), 8.49 (lH, s), 8.63 (lH, d,
J=5.9Hz), 8.73 (lH, d, J=8.3Hz)
M.p. >150C (decomposed)
29~-Isomer* IR nujol cm-l: 3380, 3200, 1770, 1660,
max 1$10 +
+ fi--~ MS (m/z): 779 (MNa ), 757 (MH )
N" NMR (D O) ~: 2.54, 3.22 (2H, ABq,
~ \ J=18Hz~, 2.73 (3H, s), 4.96 (lH, d,
/ NHCHO J=4.9Hz), 5.39, 5.53 (2H, ABq, J=16Hz~,
CH3 5.70 (lH, d, J=4.9Hz), 5.99 (lH, s), 6.7
(lH, d, J=9.8Hz), 7.02 (lH, s), 7.02 (lH,
d, J=8.3Hz), 7.22 (lH, d, J=8.3Hz), 7.95
(lH, dd, J=8.8Hz, 6.4Hz), 8.34 (lH, d,
J=9.8Hz), 8.50 (lH, s), 8.67 (lH, d,
J=6.4Hz), 8.72 (lH, d, J=8.8Hz)
_
- ~6 - 2 ~ a 7
l M.p. >165C (decomposed)
30 1 + IR v nujol cm~l: 3300, 3200, 1770, 1660,
-N ~ max 1610
MS (m/z): 752 (MNa+), 730 (MH+)
CH OH NMR (~2) ~: 2.61 and 2.67 (lH, dx2,
2 J=18Hz and 18Hz), 3.36 and 3.38 (lH, dx2,
J=18Hz and 18Hz), 4.88 and 4.90 (2H,
sx2), 4.99 and 5.00 (lH, dx2, J=4.9Hz anc
4.9Hz), 5.25 and 5.27 (lH, dx2, J=14Hz
and 14Hz), 5.52 (lH, d, J=14Hz), 5.63 anc
5.71 (lH, dx2, J=4.9Hz and 4.9Hz), 5.96
(lH, s), 6.69 (lH, d, J=9.8Hz), 6.82 and
6.99 (lH, dx2, J=8.3Hz and 8.3Hz), 6.99
(lH, s), 7.12 and 7.21 (lH, dx2, J=8.3Hz
and 8.3Hz), 8.07 (lH, m), 8.22 and 8.31
(lH, dx2, J=9.8Hz and 9.8Hz), 8.51 (lH,
m), 8.83 (lH, d, J=5.9Hz), 8.92 (lH, s)
_
M.p. ~170C (decomposed)
31 OCH IR v nujol cm-l 3350, 3200, 1770, 1660,
~ 3 max 1610
+ ~ MS (m/z): 795 (MNa+), 773 (MH+)
-N ~) NMR (D2O) ~: 2.61 and 2.71 (lH, dx2,
J=18Hz and 18Hz), 3.40 and 3.44 (lH, dx2,
\ J=18Hz and 18Hz), 3.92 and 3.95 (3H,
NHCHO sx2), 5.03-5.07 (2H, m), 5.49 (lH, d,
J=14Hz), 5.62 and 5.71 (lH, dx2~ J=4.9Hz
and 4.4Hz), 5.94 tlH, 5), 6.64 and 6.69
(lH, dx2, J=9.8Hz and 9.8Hz), 6.77 and
6.93 (lH, dx2, J=8.3Hz and 8.3Hz), 6.91
and 6.93 (lH, sx2), 7.13 and 7.21 (lH,
dx2, J=8.3Hz and 8.3Hz), 7.94 and 8.01
(lH, br sx2), 8.22-8.45 (3H, m), 8.97 anc
9.00 (lH, br sx2)
~7 20~7129
M.p. 175-185C (decomposed)
32 ~ INN max cm : 3340, 3200, 1760 1650
- S ~ MS (m/z): 783 (MNa+), 761 (~H+)
NMR (D2O) ~: 2.91 and 2.94 (lH, dx2,
J=18Hz and 18Hz), 3.33 and 3.37 (lH, dx2,
J=18Hz and 18Hz), 3.90, 4.28 and 3.98
(2H, ABq, J=14Hz and br s), 4.89 and 4.91
(lH, dx2, J=4.4Hz and 4.4Hz), 5.48 and
5.56 (lH, dx2, J=4.4Hz and 4.4Hz), 6.00
(lH, s), 6.67 and 6.75 (lH, dx2, J=9.8Hz
and 9.8Hz), 6.92 and 6.99 (lH, dx2,
J=7.8Hz and 8.3Hz), 7.02 and 7.04 (lH,
sx2), 7.16 and 7.25 (lH, dx2, J=8.3Hz anc
7.8Hz), 8.28 and 8.36 (lH, dx2, J=9.8Hz
and 9.8Hz), 8.59 and 8.67 (lH, sx2)
M.p. 195-205C (decomposed)
33 ~ I max 1600
-S/ N~ N MS (m/z): 781 (MNa+), 759 (MH+)
l NMR (D2O) ~: 2.95 and 2.97 (lH, dx2,
CH3 J=18Hz and 18Hz), 3.44 (lH, br d,
J=18Hz), 3.97 and 4.01 (lH, dx2, J=14Hz
and 13Hz), 4.02 (3H, s), 4.18 and 4.28
(lH, dx2, J=13Hz and 14Hz), 4.87 and 4.88
(lH, dx2, J=4.9Hz and 4.4Hz), 5.50 and
5.60 (lH, dx2, J=4.9Hz and 4.4Hz), 6.00
(lH, s), 6.77 (lH, d, J=9.8Hz), 7.02 and
7.03 (lH, sx2), 7.06 and 7.08 (lH, dx2,
J=7.8Hz and 7.8Hz), 7.22 and 7.26 (lH,
dx2, J=7.8Hz and 7.8Hz), 8.28 and 8.37
(lH, dx2, J=9.8Hz and 9.8Hz)
_
*: The isomers are isolated by high performance liquid
chromatography [solid phase: C18-silica gel (YMC-
Pack D-ODS-10), eluent: acetonitril/30 mM phosphate
buffer (pH 7.0) = 6 : 94].
_ 4~ ~ 2~7129
Exam~le 34
~ mixture of 4-(7-amino-3-methyl-3-cephem-4-
carboxymethyl)anisole (502 mg), 2-(2-tritylaminothiazol-4-
yl)-2-[(8-diphenylmethyloxy-2-oxo-1~-quinolin-5-yl)-
(diphenylmethyloxycarbonyl)methyloxyimino]acetic acid (979
mg), dicyclohexylcarbodiimide (227 mg), butyl alcohol (149
mg) and tetrahydrofuran (15 ml) is stirred at room
temperature for 18 hours. The precipitates are removed by
filtration, and the filtrate is concentrated, and the
residue is purified by silica gel column chromatography
(eluent; toluene/ethyl acetate = 1 : 1). The resulting
foamy product is dissolved in anisole (5 ml), and thereto is
added dropwise trifluoroacetic acid (5 ml) under ice-
cooling, and the mixture is stirred at room temperature for
two hours. To the reaction mixture is added a mixture of
isopropyl ether (100 ml) and hexane (30 ml), and the
precipitates ~re collected by filtration, washed with
isopropyl ether, dried, and dissolved in water. The pH
value of the mixture is adjusted with saturated aqueous
sodium hydrogen carbonate solution to about pH 8.0, and
purified by HP-20 column chromatography (eluent; water and
20 % aqueous methanol). The fractions containing the
desired compound are combined, concentrated, and lyophilized
to give 7~-~(Z)-2-(2-aminothiazol-4-yl)-2-[(8-hydroxy-2-oxo-
l~-quinolin-5-yl)(carboxy)methyoxyimino]acetamido}-3-methyl-
3 cephem-4-carboxylic acid disodium salt (400 mg) as light
- 49 ~ 7 1 2 ~
yellow powder.
M.p. 185 - 210C (decomposed)
IR (Nujol): 3320, 3200, 1755, 1650, 1600 cm 1
MS (m/z): 645 ~MH+)
NMR (D2O) ~: 1.87 and 1.88 (3H, sx2), 2.74 and 2.76
(1~, dx2, J=18Hz), 3.30 ~lH, dx2, J=18Hz), 4.87 and 4.88
(lH, dx2, J=4.5Hz), 5.49 and 5.58 (lH, dx2, J=4.5Hz), 5.98
and 5.99 (lH, sx2), 6.76 and 6.77 (lH, dx2, J=9.8Hz), 7.02
and 7.03 (lH, sx2), 7.04 and 7.05 (lH, dx2, J=8Hz), 7.22 and
7.24 (lH, dx2, J=8Hz), 8.29 and 8.37 (lH, dx2, J=9.8Hz)
Examples 35 - 36
The corresponding starting compounds and 2-(2-
tritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-2-oxo-lH-
quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino]-
acetic acid are treated in the same manner as in Example 34
to give the compounds of the following Table 3.
_ 50 - ~a~7~23
Table 3
S ~ ~ O ~ ~ R4
O COONa
I
CH-COONa
~ ~0
¦ H
OH
Ex. Compound
No. _
R4 ¦ Physicochemical properties
_
M.p. 165-175C (decomposed)
H IR nujol cm~l: 3320, 3200, 1760, 1650,
max 1605
MS (m/z): 630 (MH+)
NMR (D2O) ~: 2.95 and 3.01 (lH, ddx2,
J=18Hz and 7Hz), 3.37 and 3.40 (lH, ddx2,
J=18Hz and 2.5Hz), 4.89 and 4.90 (lH,
dx2, J=5Hz), 5.60 and 5.69 (lH, dx2,
J=5Hz), 5.99 (lH, s), 6.22 (lH, m), 6.75
and 6.77 (lH, dx2, J=lOHz), 7.02 and 7.03
(lH, sx2), 7.04 and 7.05 (lH, dx2,
J=8Hz), 7.22 and 7.25 (lH, dx2, J=8Hz),
8.28 and 8.36 (lH, dx2, J=lOHz)
_
M.p. 180-187C (decomposed)
36 -CH=CH2 IR ~ nujol cm 1 3340, 3200, 1760, 1650
MS (m/z): 657 (MH+)
NMR (D O) ~: 3.16 and 3.18 (1~, dx2,
J=18Hz~, 3.32 and 3.34 (lH, dx2, J=18Hz),
4.93 and 4.95 (lH, dx2, J=SHz), 5.27 (lH,
dx2, J=llHz), 5.40 (lH, dx2, J=17.5Hz),
5.54 and 5.63 (lH, dx2, J=5Hz), 5.99 and
6.00 (lH, sx2), 6.77 (lH, d, J=lOHz), 6.8
(lH, m), 7.02 and 7.04 (lH, sx2), 7.04
and 7.08 (lH, dx2, J=8Hz), 7.23 and 7.26
(lH, dx2, J=8Hz), 8.30 and 8.38 (lH, dx2,
J=lOHz)
_
- 51 - 2~J~7129
E~ample 37
(1) A mixture of S-oxalo-8-benzyloxycarbostyril
~3.23 g), wet 10 % palladium-carbon (4 g, moisture: 50 %),
and a mixture of tetrahydrofuran (100 ml), water (50 ml) and
acetic acid (50 ml) is shaken at 45C for 40 hours under 4
atoms of hydrogen, and then the catalyst is removed by
filtration. The catalyst is washed with aqueous
tetrahydrofuran, and the washings and the filtrate are
combined and evaporated to remove the solvent therefrom.
The residue is dissolved in 50 ~ aqueous tetrahydrofuran
(200 ml), and thereto is added diphenyldiazomethane (2.9 g),
and the mixture is stirred at room temperature for 20
hours. The solvent is distilled off, and to the resulting
residue is added ether to crash the solid, and the solid is
collected by filtration, and washed with ether, and dried
under reduced pressure. The resulting solid is dissolved in
dimethylformamide (20 ml), and thereto is added potassium
carbonate (2.6 g). To the mixture is added dropwise a
solution of benzhydril bromide (4.64 g) in dimethylformamide
(5 ml), and the mixture is stirred at room temperature for
five hours. The reaction solution is poured into ice-water
(100 ml), and extracted with chloroform. The extract is
washed successively with water and saturated saline
solution, and dried. The solvent is distilled off, and the
resulting residue is purified by silica gel column
chromatography (eluent; hexane/ethyl acetate) to give 2-(8-
- 52 -- 2~7~2~
diphenylmethyloxy-2-o~o-1,2,3,4-tetrahydroquinolin-5-yl)-2-
hydroxyacetic acid diphenylmethyl ester ~2.85 9, 50 %) as
colorless crystals.
M.p. 165 - 16~C
IR (Nujol): 3250, 1750, 1670, 1610, 1590 cm 1
MS (m/z): 570 (MH+)
(2) The product obtained above is treated in the
same manner as in Example 1, (l)-(a) to give 2-(8-diphenyl-
methyloxy-2-oxo-1,2,3,4-tetrahydroquinolin-5-yl)-2-
(phthalimidoxy)acetic acid diphenylmethyl ester.
M.p. 180 - 183C
IR (Nujol): 3240, 1790, 1760, 1740, 1670, 1610,
1590 cm~
MS (m/z): 715 (MH+)
~ 3) The product obtained a~ove is treated in the
same manner as in Example 1-(2) to give 2-aminooxy-2-(8-
diphenylmethyloxy-2-oxo-1,2,3,4-tetrahydroquinolin-5-yl)-
acetic acid diphenylmethyl ester.
M.p. 166 - 168C
IR (Nujol~: 3410, 3320, 3240, 1750, 1680, 1610,
1590 cm-
MS (m/z): 585 (MH+)
(4) The product obtained above is treated in the
same manner as in Example 1-(3) to give 2-(2-tritylamino-
thiazol-4-yl)-2-~(8-diphenylmethyloxy-2-oxo-1,2,3,4-tetra-
hydroquinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxy-
- 53 - 20~7~29
imino]acetic acid.
M.p. 142 - 144C (decomposed)
IR (Nujol): 3400, 3220, 1740, 1700 (sh), 1680 cm 1
MS (m/z): 981 (MH+)
(5) The product obtained above is treated in the
same manner as in Example 1-(4~ to give 7B-{(Z)-2-(2-trityl-
aminothiazol-4-yl)-2-[(8-diphenylmethyloxy-2-oxo-1,2,3,4-
tetrahydroquinolin-5-yl)~diphenylmethyloxycarbonyl)methyl-
oxyimino]acetamide}cephalosporanic acid.
M.p. 138 - 142C (decomposed)
IR (Nujol): 3400, 3250, 1790, 1740, 1690 cm 1
MS (m/z): 1235 (MH+)
Example 38
The corresponding starting compounds are treated in
the same manner as in Example 2 to give 7B-{(Z)-2-(2-amino-
thiazol-4-yl)-2-[(8-hydroxy-2-oxo-1,2,3,4-tetrahydro-
quinolin-5-yl)(carboxy)methyloxyimino]acetamido~-
cephalosporanic acid.
M.p. >170C (decomposed)
IR (Nujol): 3300 (br), 3200 (sh), 1780, 1730,
1670 (br) cm~
MS (m/z): 661 (MH+)
Disodium salt of this product
M.p. >170C (decomposed)
IR (Nu jol): 3340 (br), 3200 (sh), 1760, 1660, 1600 cm 1
MS (m/z): 727 (MNa+), 705 (MH+)
5~ 2~7~2~
NMR (D2O) 6: 2.13 and 2.15 (3H, sx2), 2.61-2.68
(2H, m), 3.01-3.13 (2H, m), 2.96, 3.46 (2H, ABq, J=18Hz),
4.68 and 4.71 (lH, dx2, ~=13Hz and 12Hz), 4.77 and 4.89 (lH,
dx2, J=13~z and 12~z), 5.01 and 5.03 (lH, dx2, J=4.4~z and
4.4Hz), 5.64 and 5.68 (lH, dx2, J=4.4Hz and 4.4Hz), 5.69 and
5.70 (lH, s), 6.82 and 6.83 (lH, dx2, J=8.3Hz and 8.3Hz),
6.96 and 6.99 (lH, dx2, J=8.3Hz and 8.3Hz), 7.02 (lH, s)
Example 39
(1) To a suspension of 2-(8-hydroxy-2-oxo-lH-
quinolin-5-yl)-2-hydroxyacetic acid (11.8 g) in 17 % aqueous
tetrahydrofuran (480 ml) is added diphenyldiazomethane (15.6
g), and the mixture is stirred at room temperature for three
days. The solvent is distilled off, and to the resulting
residue is added diethyl ether (250 ml) in order to crash
the solid, and the solid is collected by filtration. The
solid is washed with diethyl ether (300 ml) and dried under
reduced pressure to give 2-(8-hydroxy-2-oxo-lH-quinolin-5-
yl)-2-hydroxyacetic acid diphenylmethyl ester (17.16 g).
M.p. 218.5 - 219.5C (decomposed)
IR (Nujol): 3365, 3240, 2720-2580, 1730, 1645,
1630, 1590 cm-
MS (m/z): 402 (MH+)
(2) To a solution of the product (16.15 g)
obtained above in dimethylformamide (81 ml) are added
successively potassium carbonate (4.2 g) and methyl iodide
(7.8 ml~, and the mixture is stirred at room temperature for
- 55 - 2Q~ 7 ~ 2~
7 hours. To the mixture is added ice-water (480 ml) under
ice-cooling, and the mixture is stirred. The precipitates
are collected by filtration, washed with water, and dried
under reduced pressure to give 2-(8-methoxy-2-oxo-lH-
quinolin-5-yl)-2-hydroxyacetic acid diphenylmethyl ester
(16.05 9).
M.p. 177 - 182C
IR (Nujol): 3330, 3160, 1740, 1650, 1630, 1600 cm 1
MS (m/z): 416 (M~+)
(3) The product obtained above is treated in the
same manner as in Example i, (l)-(a) to give 2-(8-methoxy-2-
oxo-lH-quinolin-5-yl)-2-(phthalimidoxy)acetic acid diphenyl-
methyl ester.
M.p. 206 - 209C
IR (Nujol): 3170, 1790, 1740, 1660, 1610 cm 1
MS ~m/z): 561 (MH+)
(4) The corresponding starting compounds are
treated in the same manner as in Example 1-(2) to give 2-
aminooxy-2-(8-methoxy-2-oxo-1~-quinolin-5-yl)acetic acid
diphenylmethyl ester.
M.p. 166 - 168C
IR (Nujol): 3280, 3230, 3150, 1750, 1660, 1610 cm 1
MS (m/z): 431 (MH+)
(5) The corresponding starting compounds are
treated in the same manner as in Example 1-(3) to give 2-(2-
- 56 - 2~712~
tritylaminothiazol-4-yl)-2-[(8-methoxy-2-oxo-lH-quinolin-5-
yl)(diphenylmethyioxycarbonyl)methyloxyimino3acetic acid.
M.p. 157 - 158.5C (decomposed)
IR (Nujol): 3380, 3220 (br), 1740, 1670, 1610 cm 1
MS (m/z): 827 (MH+)
(6) The corresponding starting compounds are
treated in the same manner as in Example 1-(4) to give 7B-
{(Z)-2-(2-tritylaminothiazol-4-yl)-2-[(8-methoxy-2-oxo-lH-
quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino]-
acetamido}cephalosporanic acid.
M.p. 154 - 156C
IR (Nujol): 3220 (br), 1790, 1740, 1660, 1610 cm 1
MS (m/z): 1081 (MH+)
Example 40
The corresponding starting compounds are treated in
the same manner as in Example 2 to give 73-{(Z)-2-(2-amino-
thiazol-4-yl)-2-[(8-methoxy-2-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido~cephalosporanic acid.
M.p. >160C (decomposed)
IR (Nujol): 3300 (br), 3200 (br), 1780, 1730,
1660 (br), 1610 cm 1
MS (m/z): 673 (MH+)
Disodium salt of this product
M.p. >160C (decomposed)
IR (Nujol): 3350 (br), 3200 (sh), 1770, 1730 (sh),
1660, 1610 cm-l
_ 57 _ 2 ~ ~ 7
MS (m/z): 717 (MH+)
NMR (D2O) ~: 2.12 and 2.13 (3H, sx2), 2.87 and 2.93
(lH, dx2, J=18Hz and 18~z), 3.38 and 3.41 (lH, dx2, J=18Hz
and 18Hz), 4.01 and 4.02 (3H, sx2), 4.68 and 4.69 (lH, dx2,
J=13Hz and 13Hz), 4.85 and 4.87 (lH, dx2, J=13Hz and 13Hz),
4.93 and 4.95 (lH, dx2, J=4.9Hz and 4.9Hz), 5.60 and 5.68
(lH, dx2, J=4.9Hz and 4.9Hz), 5.99 (lH, s), 6.77 and 6.79
(lH, dx2, J=9.8Hz and 9.8Hz), 7.02 and 7.03 (lH, sx2), 7.18
and 7.20 (lH, dx2, J=8.3Hz and 8.3Hz), 7.34 and 7.36 (lH,
dx2, J=8.3Hz and 8.3Hz), 8.29 and 8.37 (lH, dx2, J=9.8Hz and
9.8Hz)
Example 41
(1) To a suspension of sodium hydride (750 mg) in
dimethylformamide (50 ml) is added dropwise a solution of 2-
(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)-2-(phthal-
imidoxy)acetic acid diphenylmethyl ester (10.6 g) in
dimethylformamide (60 ml) over a period of 25 minutes under
argon atmosphere, and the mixture is stirred at room
temperature for 1.5 hour. The reaction solution is cooled
to 0C, and thereto is added dropwise methyl iodide (8.44
g), and the mixture is stirred at the same temperature for
1.5 hour. The mixture is poured into 0.05N-hydrochloric
acid (330 ml), and the precipitates are extracted with ethyl
acetate. The extract is washed with water, dried, and
evaporated to remove the solvent therefrom. The resulting
residue is purified by silica gel column chromatography
- 58 - 20a7~2~
(eiuent; hexane/ethyl acetate) to give 2-~8-diphenyl-
methyloxy-l-methyl-2-oxo-lH-quinolin-5-~1)-2-(phthal-
imidoxy)acetic acid diphenylmethyl ester ~5.28 g).
IR (Nujol): 1790, 1760, 1740, 1660, 1610, 1590 cm 1
MS (m/z): 727 (MH+)
NMR (CDC13) ~: 3.89 (3H, s), 6.21 ~lH, s), 6.41
(lH, s), 6.70 (lH, d, J=9.8Hz), 6.82-7.40 (23H, m), 7.69-
7.80 (4H, m), 8.34 (lH, d, J=9.8Hz)
(2) The product obtained above is treated in the
same manner as in Example 1, ~l)-(a) to give 2-aminooxy-2-
(8-diphenylmethyloxy-1-methyl-2-oxo-lH-quinolin-5-yl)acetic
acid diphenylmethyl ester.
IR (Nujol): 3310, 3240, 1750, 1660, 1610, 1590,
1560 cm~
MS (m/z): 597 (MH+)
NMR (CDC13) ~: 3.88 (3H, s), 5.55 (lH, s), 5.91
(2H, brs), 6.20 (lH, s), 6.61 (lH, d, J=9.9Hz), 6.79-7.43
(23H, m), 8.00 (lH, d, J=9.9Hz)
(3) ~-he product obtained above is treated in the
same manner as in Example 1-(2) to give 2-(2-tritylamino-
thiazol-4-yl)-2-[(8-diphenylmethyloxy-1-methyl-2-oxo-lH-
quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino]-
acetic acid.
M.p. 140 - 145C (decomposed)
IR (Nujol)~ 3220 (br), 1740, 1660, 1610 (sh), 1590 cm 1
MS (m/z): 993 (MH+)
20~7129
N~R (CDC13) ~:3.83 (3H, s), 6.16 (lH, s), 6.19 (lH,
s), 6.52 (lH, d, J=9.8Hz), 6.70 (lH, s), 6.79-7.36 (38H, m),
7.50 (lH, br), 8.02 (lH, d, J=9.8Hz)
(4) The product obtained above is treated in the
same manner as in Example 1-(3) to give 73-{(Z)-2-(2-
tritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-1-methyl-2-
oxo-lH-quinolin-5-yl)(diphenylmethyloxycarbonyl)-
methyloxyimino]acetamido}cephalosporanic acid.
M.p. 139 - 141C (decomposed)
IR (Nujol): 3240 (br), 1790, 1740, 1680, 1660 cm 1
MS (m/z): 1247 (MH+)
NMR (DMSO-d6) ~: 2.00 and 2.03 (3H, sx2), 3.00-3.60
(2H, m), 3.86 (3H, s), 4.65 and 4.68 (lH, dx2, J=13Hz and
13Hz), 4.90 and 4.98 (lH, dx2, J=13Hz and 13Hz), 4.94 and
5.08 (lH, dx2, J=4.9Hz and 4.9Hz), 5.64 (lH, m), 6.18 (lH,
s), 6.50 and 6.51 (lH, dx2, J=9.8Hz and 9.8Hz), 6.67, 6.75,
6.78, 6.80 and 6.82 (3H, each s), 7.03-7.57 (37H, m), 8.08
and 8.10 (lH, dx2, J=9.8Hz and 9.8Hz), 8.87 and 8.90 (1~,
sx2), 9.59 and 9.63 (lH, dx2, J=7.8Hz and 7.8 Hz)
Example 42
The compound obtalned in Example 41 is treated in
the same manner as in Example 2 to give 7~-{(Z)-2-(2-amino-
thiazol-4-yl)-2-[(8-hydroxy-1-methyl-2-oxo-lH-quinolin-5-
yl)(carboxy)methyloxyimino]acetamido}cephalosporanic acid.
M.p. >170C (decomposed)
IR (Nujol): 3300 - 3200, 1780, 1740, 1650, 1590 cm 1
- 60 - 2 ~ 5 7 1 2
Disodium salt of this product
M.p. >145C (decomposed)
IR ~Nujol): 3340 (br), 3200, 1770, 1650, 1590 cm 1
NMR (D2O) ~: 2.13 and 2.14 (3H, sx2), 2.87 and 2.94
(lH, dx2, J=18Hz and 18Hz), 3.37 and 3.40 (lH, dx2, J=18Hz
and 18Hz), 3.96 and 3.99 (3H, sx2), 4.69 (lH, brd, J=llHz),
4.79-4.93 (lH, m), 4.87 and 4.91 (lH, dx2, J=4.9Hz and
4.9Hz), 5.56 and 5.66 (lH, dx2, J=4.9Hz and 4.9Hz), 5.99
(lH, s), 6.76 and 6.78 (lH, dx2, J=9.8Hz and 9.8Hz), 7.02
and 7.03 (lH, sx2), 7.14 and 7.15 (lH, dx2, J=8.3Hz and
8.3Hz), 7.25 and 7.28 (lH, dx2, J=8.3Hz and 8.3Hz), 8.16 and
8.25 (lH, dx2, J=9.8Hz and 9.8Hz)
Examples 43 - 48
The corresponding starting compounds are treated in
the same manner as in Example 3 to give the compounds of the
following mable 4.
- 61 - '3~7 t ,~
Table 4
N ~ C-CONH
O COO
I
C~-COO~ia
la
Ex. Compound
No.
Ra IR41 Physicochemical properties
M.p. >160C (decomposed)
43 + fi__~ IR (Nujol): 3300(br), 3200(sh), ~770,
-N \> 1660, 1610, 1590 cm
~ ~==/ ~MR (D2O) ~: 2.45-2.66 (3H, m), 2.75-
l~ l~ J~ ~ ~ 3.07 (2H, m), 3.23 and 3.24 (lH, dx2,
~ N ~O ~ J=18Hz and 18Hz), 4.99 and 5.04 (lH,
H~ H dx2, J=4.9Hz and 4.9Hz), 5.65 (lH,
s), 5.57 and 5.70 (lH, dx2, J=4.9Hz
and 4.9Hz), 5.78 and 5.79 (lH, dx2,
J=15Hz and 15Hz), 5.92 (lH, d,
J=15Hz), 6.43 and 6.62 (lH, dx2,
J=8.3Hz and 8.8Hz), 6.85 and 6.91
(lH, dx2, J=8.8Hz and 8.3Hz), 6.95
and 6.96 (lH, sx2), 7.97-8.47 (5H,
m), 9.15-9.27 (2H, m)
M.p. >165C (decomposed)
l IR (Nujol): 3380(br), 3200~sh), 1780,
44 1660, 1610 cm~
MS (m/z): 736 (MNa+), 714 (~H+)
+ ~NMR (D2O) ~: 2.63 and 2.72 (lH, dx2,
-N J=18Hz and 18~z), 3.39 and 3.48 (1~,
~ N~- O \==~ dx2, J=18Hz and 18Hz), 3.86 and 3.93
CH30 H (3H, sx2), 5.01 and 5.04 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.27 and 5.30
(lH, dx2, J=15Hz and 15Hz), 5.53 and
5.55 (lH, dx2, J=15Hz and 15Hz), 5.67
and 5.73 (lH, dx2, J=4.9Hz and
4.9Hz), 5.94 and 5.97 (lH; sx2), 6.69
and 6.70 (lH, dx2, J=9.8Hz and
9.8Hz), 6.95 and 7.10 (lH, dx2,
J=8.3Hz and 8.3~z), 6.98 (lH, s),
7.26 and 7.34 (lH, dx2, J=8.3Hz and
8.3Hz), 8.07-8.15 (2H, m), 8.25 and
8.33 llH, dx2, J=9.8Hz and 9.8Hz),
8.61 (lH, m), 8.89-8.95 (2H, m)
. _ ,
- 62 - 20~7129
M.p. >165C (decomposed)
IR (~ujol): 3380(br), 3200~sh), 1770,
166C, 1610 cm~
MS (m/z): 726 (MH+ free acid)
Cl NMR (D2O) 6: 2.69 and 2.78 (lH,
J + ~ dx2, J=18Hz and 18Hz), 3.43 and 3.52
I `N ` O -N \) (lH, dx2, J=18Hz and 18Hz), 3.88 and
CH30 H ~=) 3.93 (3H, sx~), 5.04 and 5.06 (lH,
dx2, J=4.9Hz and 4.9Hz), S.26 and
5.29 (lH, dx2, J=lSHz and 15Hz), 5.55
ard 5.57 (lH, dx2, J=15Hz and lSHz),
5.68 and 5.74 (lH, dx2, J=4.9Hz and
I 4.9Hz), 5.94 and 5.97 (lH, sx2), 6.69
and 6.71 (lH, dx2, J=9.8Hz and
9.8Hz), 6.96 and 6.97 (lH, sx2), 6.98
and 7.11 (lH, dx2, J=8.3Hz and
8.3Hz), 7.27 and 7.34 (lH, dx2,
J=8.3Hz and 8.3Hz), 8.10 (lH, m),
8.26 and 8.34 (lH, dx2, J=9.8Hz and
9.8Hz), 8.63 (lH, m), 8.91 and 8.95
(lH, dx2, J=6.3Hz and 6.3Hz), 9.16
(lH, brs)
~ M.p~ >165C (decomposed)
46l IR (Nujol): 3380(br), 3200~sh), 1770,
1660, 1610 cm~
~ MS (m/z): 786 (MNa+), 764 (~H+)
I I + ~-~ NMR (D20) ~: 2.62 and 2.66 (lH, dx2,
~ ~N' ~ N~ ~ J=18Hz and 18Hz), 3.14 and 3.17 (lH,
CH3~ H ~ dx2, J=18Hz and 18Hz), 3.68 an 3.78
(3H, sx2), 4.96 and 4.98 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.65 and 5.70
(lH, dx2, J=4.9Hz and 4.9Hz), 5.60-
5.95 ~2H, m), 5.91 and 5.93 (lH,
sx2), 6.62 and 6.66 (lH, dx2, J=9.8Hz
and 9.8Hz), 6.80 and 6.99 (lH, dx2,
J=8.3Hz and 8.3Hz), 6.88 and 6.91
(lH, sx2), 7.21 and 7.30 (lH, dx2,
J=8.3Hz and 8.3Hz), 7.87-8.40 (6H,
_ m~, 9.01-9.21 (2H, m)
- 63 - 2~7123
M.p. >170C (decomposed)
47 IR (Nujol): 3350~br), 3200~sh), 1770,
1650, 1590 cm~
+ h--~ MS (m/z): 786 (MNa+), 764 (MH+)
NMR (D2O) ~: 2.56 and 2.65 (lH, dx2,
J=18Hz and 18Hz), 3.13 and 3.15 (lH,
dx2, J=18Hz and 18Hz), 3.46 and 3.55
N'~ O~ (3H, sx2), 4.90 and 4.91 (lH, dx2,
OH ¦ J=4.4Hz and 4.4Hz), 5.62 and 5.69
CH3 (lH, dx2, J=4.4Hz and 4.4Hz), 5.68-
l 5.95 (2H, m), 5.91 and 5.92 (lH,
i sx2), 6.57 and 6.62 (lH, dx2, J=9.3H
and 9.3Hz), 6.75 and 6.91 (lH, dx2,
J=8.3Hz and 8.3Hz), 6.90 and 6.93
(lH, sx2), 7.12 and 7.21 (lH, dx2,
J=8.3Hz and 8.3Hz), 7.82-8.38 (6H,
m), 9.01 and 9.04 (lH, dx2, J=7.3Hz
and 7.3Hz), 9.17 (lH, brd, J=5.8Hz)
l M.p. >170C (decomposed)
48l ~-Isomer* IR (Nujol): 3340(br), 3200 1770,
l 1650, 1590 cm~i
I + fi__~ MS (m/z): 786 (MNa+), 764 (MH+)
N \~ NMR (D2O) ~: 2.60, 3.13 (2H, ABq,
J=18Hz), 3.58 (3H, s), 4.91 (lH, d,
J ~ ~ J=4.9Hz), 5.62 (lH, d, J=4.9Hz), 5.76
N ~O ~ 5.90 (2H, ABq, J=16Hz), 5.92 (lH,
OH ¦ s), 6.58 (lH, d, J=9.8Hz), 6.74 (lH,
CH3 d, J=7.8Hz), 6.97 (lH, s), 7.11 (lH,
d, J=7.8Hz), 7.90-8.39 (5H, m), 9.10
(lH, d, J=8.3Hz), 9.20 (lH, d,
J=5.9Hz)
M.p. >170C (decomposed)
48 B-Isomer* IR (Nujol~: 3350(br), 32001 1770,
1650, 1590 cm~
+ ~ MS (m/z): 786 (MNa+), 764 (MH+)
-N ~) NMR (D2O) ~: 2.54, 3.12 (2H, ABq,
J=18Hz), 3.48 (3H, s), 4.90 (lH, d,
J~ ~ J=4.9Hz), 5.69 (lH, d, J=4.9Hz),
N ?o ~ 5.76, 5.88 (2H, ABq, J=16Hz), 5.93
OH l ;lH, s), 6.64 (lH, d, J=9.8Hz), 6.93
CH3 (lH, s), 6.94 (lH, d, J=8.3Hz), 7.21
(lH, d, J=8.3Hz), 7.95-8.05 (2H, m),
8.16-8.28 (3H, m), 8.39 (lH, d,
J=8.8Hz), 9.07 (lH, d, J=8.3Hz), 9.1
(lH, d, J=5.4Hz)
*: The isomers are isolated by high performance liquid
chromatography [solid phase; C18-silica gel (YMC-Pac~
D-ODS-10), eluent; acetonitril/30 mM phosphate buffer
(pH 7.0) = 1 : 9].
- 64 - 20~712~
Example 49
The compoulld obtained in Example 39 (disodium salt)
and l-allyl-4-thiopyridone are treated in the same manner as
in Example 4-except that 50 ~ aqueous acetonitril is used as
a solvent to give 7B-{(z)-2-(2-aminothiazol-4-yl)-2-[(8-
methoxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxyimino]-
acetamido}-3-(1-allyl-4-pyridiniothio)-3-cephem-4-carboxylic
acid sodium salt.
M.p. >175~C (decomposed)
IR (Nujol): 3370 (br), 3200 (sh), 1770, 1660, 1630,
1610 cm~l
MS (m/z): 808 (MNa+), 786 (MH+)
NMR (D2O) 6: 2.94 and 2.96 (lH, dx2, J=18Hz and
18Hz), 3.28 and 3.34 (lH, dx2, J=18Hz and 18Hz), 4.15 (lH,
d, J=14Hz), 4.23 and 4.34 (lH, dx2, J=14Hz and 14Hz), 4.91-
4.98 (3H, m), 5.38 (lH, d, J=17Hz), 5.49 (lH, d, J=lOHz),
5.56 and 5.61 (lH, dx2, J=4.9Hz and 4.9Hz), 5.92-6.12 (lH,
m), 5.96 and 5.97 (lH, sx2), 6.71 and 6.74 (lH, dx2, J=9.8Hz
and 9.8Hz), 6.95 (lH, s), 7.08 and 7.13 (lH, dx2, J=8.3Hz
and 8.3Hz), 7.29 and 7.36 (lH, dx2, J=8.3Hz and 8.3Hz), 7.77
(2H, d, J=6.8Hz), 8.24-8.39 (3H, m)
Examples 50 - 52
7~-Amino-3-(3-amino-2-methyl-1-pyridiniomethyl)-3-
cephem-4-carboxylate and the corresponding starting
compounds are treated in the same manner as in Example 27 to
give the compounds of the following Table 5.
- 65 - 2 ~ ~ 7 ~ ~ ~
Table '
N C-CONH ~ S ~ CH3 NH2
S ~ N // N ~ CH2-
O
O COO-
H-COONa
la
Ex. Compound
No.
Ra Physicochemical properties
M.p. >170C (decomposed)
IR (Nujol): 33S0, 32~0, 1770, 1660,
1610 cm~
MS (m/z): 731 (MH+)
NMR (D~O) ~: 2.53 (3H, s), 2.46-2.73 (2H,
m), 2.80-3.21 (2H, m), 5.00 and 5.05 (lH,
N ` o dx2, J=4.9Hz and 4.9Hz), 5.24 and 5.28
ok H (lH, dx2, J=15Hz and 15Hz), 5.50 (lH,
brd), 5.65-5.70 (2H, m), 6.62 and 6.73
(lH, dx2, J=8.3Hz and 8.3Hz), 6.90 and
6.95 (lH, dx2, J=8.3Hz and 8.3Hz), 7.01
(lH, s), 7.6'-7.54 (lH, m), 7.72 (lH, d,
J=8.8Hz), 8.01 and 8.08 (lH, dx2, J=5.9Hz
and 6.4Hz)
M.p. >165C (decomposed)
IR (Nujol): 3340(br), 3200~br), 1770,
51 1660, 1600 cm-
MS (m/z): 765 (MNa+), 743 (MH+)
NMR (D2O) ~: 2.49 and 2.51 (3H, sx2),
J~ 2.54 and 2.60 (lH, dx2, J=18Hz and 18Hz),
`r' N ` O 3.06 and 3.10 (lH, dx2, J=18Hz and 18Hz),
H3Cd H 3.85 and 3.9~ (3H, sx2), 4.94 and 4.97
(lH, dx2, J=4.9Hz and 4.9Hz), 5.22 and
5.24 (lH, dx2, J=16Hz and 16Hz), 5.50
(lH, d, J=16Hz), 5-.63-5.70 (lH, dx2,
J=4.9Hz and 4.9Hz), 5.96 and 5.98 (lH,
sx2), 6.70 and 6.74 (lH, dx2, J=9.8Hz anc
9.8Hz), 6.98 and 6.99 (lH, sx2)l 6.99 anc
7.10 (lH, dx2, J=8.3Hz and 8.3Hz), 7.29
and 7.34 (lH, dx2, J=8.3Hz and 8.3Hz),
7.55 (lH, ~), 7.67-7.69 (lH, dx2, J=8.3Hz
and 8.3Hz), 7.99 (1~, m), 8.25 and 8.35
(lH, dx2, J=9.8Hz and 9.8Hz)
.
- 66 - 2 ~ ~ 71 2 5
i -
M.p. ~160C (decomposed)
IR (Nujol): 3350, 32~0, 1770, 1640,
52 1590 cm~
MS (m/z): 765 ~MNa+), 743 (MH+~
NMR (D2O) ~: 2.49 (3H, s), 2.56 and 2.65
~ ~ (lH, dx2, J=18Hz and 18Hz), 3.02 and 3.04
l~ ll J (lH, dx2, J=18Hz and 18Hz), 3.75 and 3.77
N ~O (3H, sx2), 4.90 and 4.91 (lH, dx2,
dH ¦ J=4.4Hz and 4.4Hz), 5.20 and 5.23 (lH,
CH3 dx2, J=15Hz and 15Hz), 5.51 (lH, d,
J=15Hz), 5.59 and 5.70 (lH, dx2, J=4.4Hz
and 4.4Hz), 5.95 and 5.97 (lH, sx2), 6.66
and 6.71 (lH, dx2, J=9.8Hz and 9.8Hz),
6.93 and 7.04 (lH, dx2, J=8.3Hz and
8.3Hz), 6.98 (lH, s), 7.18 and 7.25 (lH,
dx2, J=8.3Hz and 8.3Hz), 7.41-7.60 (lH,
m), 7.65 and 7.67 (lH, dx2, J=8.3Hz and
8.3Hz), 7.96 and 7.99 (lH, dx2, J=5.9Hz
and 5.9Hz), 8.08 and 8.22 (lH, dx2,
J=9.8Hz and 9.8Hz)
M.p. >160C tdecomposed)
IR (Nujol): 3350, 32~0, 1770, 1650,
52 ~-Isomer* 1590 cm
MS (m/z): 765 (MNa+), 743 (MH+)
NMR (D~O) ~: 2.51 (3H, s), 2.56, 3.05
(2H, A~q, J=18Hz), 3.76 (3H, s), 4.92
J ~ (lH, d, J=4.4Hz), 5.25, 5.51 (2H, ABq,
N ~ O J=16Hz), 5.60 (lH, d, J=4.4Hz), 5.97 (lH,
OH ¦ brs), 6.65 (lH, d, J=9.8Hz), 6.91 (lH, d,
CH3 J=8.3Hz), 6.99 (lH, s), 7.18 (lH, d,
J=8.3Hz), 7.58 (lH, dd, J=7.8Hz and
6.4Hz), 7.69 (lH, d, J=7.8Hz), 8.00 (lH,
d, J=6.4Hz), 8.06 (lH, d, J=9.8Hz)
.
2~'71~
- 6~ -
_
M.p. >160C ~decomposed)
52 B-Isomer* IR (Nujol): 3350, 31~0, 1770, 1650,
MS (m/z): 765 (MNa+), 743 (MH+)
i NMR (D2O) ~: 2.49 (3H, s), 2.55, 3.02
~ !2H, ABq, J=18Hz), 3.75 (3H, s), 4.90
l~ l~ J~ (lH, d, J=4.9Hz), 5.21, 5.51 (2H, ABq,
N'- O J=16Hz), 5.70 (lH, d, J=4.9Hz), 5.96 (lH,
OH ¦ s), 6.71 (lH, d, J=9.8Hz), 6.98 (lH, s),
CH3 7 04 (lH, d, J=8.3Hz), 7.25 ~lH, d,
J=8.3Hz), 7.52 (lH, dd, J=8.3Hz, 5.9Hz),
7.66 (lH, d, J=8.3Hz), 7.97 (lH, d,
J=5.9Hz), 8.23 (lH, d, J=9.8Hz)
*: The isomers are isolated by high performance liquid
chromatography [solid phase; C18-silica gel (YMC-
Pac~ D-ODS-10), eluent; acetonitril/30 mM phosphate
buffer (pH 7.0) = 8 : 92].
~ 68 - 2 ~ ~ 7 ~ 2~
E~ample 53
(1) 2-(8-Diphenylmethyloxy-2-oxo-lH-quinolin-5-
yl)-2-hydroxyacetic acid diphenylmethyl ester (5.03 9) is
dissolved in 17 % aqueous tetrahydrofuran (96 ml), and to
the mixture is added dropwise 2.ON-aqueous sodium hydroxide
solution (8.8 ml) with stirring under ice-cooling, and the
mixture is stirred at room temperature for two hours. To
the mixture is added dropwise 2.4N-hydrochloric acid (7.4
ml) under ice-cooling, and then added a~ueous tetrahydro-
furan (q.s.). The mixture is made homogeneous, and
tetrahydrofuran is distilled off. The precipitates are
collected by filtration, washed successively with water and
diethyl ether, and dried under reduced pressure at 60C to
give 2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)-2-
hydroxyacetic acid (3.42 g) as a colorless solid.
M.p. 186 - 188C (decomposed)
IR (Nujol): 3360, 3160, 1710, 1640, 1600 cm 1
MS (m/z): 401 (M+)
(2) The product (145.8 g) obtained above is
reacted with (R)-2-amino-3-methyl-1,1-diphenylbutan-1-ol
(92.8 g), and the resulting salt is recrystallized twice
from methanol to give optically active 2-(8-diphenylmethyl-
oxy-2-oxo-lH-quinolin-5-yl)-2-hydroxyacetic acid (R)-2-
amino-3-methyl-1,1-diphenylbutan-1-ol salt (47.7 g) as
colorless crystals.
M.p. 208.5 - 209.5C (decomposed)
69 ~ 20571 2~
IR (Nujol): 3440, 3400, 3360, 3260, 1660, 1630,
1600, 1590 cm~
MS (m/z): 657 (MH+)
[~] 20 -53.6 Ic=1.00, methanol)
(3) To a suspension of the product (111 g)
obtained above in tetrahydrofuran (3.3 liters) is added
diphenyldiazomethane (49.2 g), and the mixture is stirred at
room temperature for two days. The resulting dark red-
purple solution is evaporated to remove tetrahydrofuran
therefrom, and the residue is subjected to azeotropic
distillation with diethyl ether (200 ml) twice to give
crystals. To the crystals is added diisopropyl ether (1
liter) in order to crash the crystals, and the crystals are
collected by filtration, washed with diethyl ether (1
liter), and dried under reduced pressure at 45C to give
optically active 2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-
yl)-2-hydroxyacetic acid diphenylmethyl ester (94.6 9).
M.p. 194 - 196C
IR (Nujol): 3320, 1740, 1640, 1600 cm 1
MS (m/z): 568 (MH+)
[~] D0 -6.2 (c=1.00, chloroform)
Optical purity: j98 % ee [determined by high
performance liquid chromatography (solid phase; cellulose
derivative-coating silica column; tradename: Opti-Pak XC,
eluent; hexane/ethanol = 3 : 2]
7 205712~
(4) The product obtained above is treated in the
same manner as in Example 1, (l)-(a) to give optically
active 2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-yl)-2-
(phthalimidoxy)acetic acid diphenylmethyl ester.
M.p. 194 - 196C
IR (Nujol): 3180, 3060, 3020, 1790, 1760, 1740,
1650, 1610 c~-
MS (m/z): 713 (MH+)
(5) The product obtained above is treated in the
same manner as in Example 1-(2) to give optically active 2-
aminohydroxy-2-(8-diphenylmethyloxy-2-oxo-lH-quinolin-5-
yl)acetic acid diphenylmethyl ester.
M.p. 182 - 184C (decomposed)
IR (Nujol): 3320, 3300, 3220, 3160, 1750, 1730,
1650, 1600 cm-
MS (m/z): 583 (MH+)
(6) The product obtained above is treated in the
same manner as in Example 1-(3) to give optically active 2-
(2-tritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-2-oxo-
lH-quinolin-5-yl)(diphenylmethyloxycarbonyl)methyloxyimino]-
acetic acid.
M.p. 154 - 156C
IR (Nujol): 3400, 3200 (br), 1740, 1670, 1610 cm 1
MS (m/z): 979 ~MH+)
(7) The product obtained above is treated in the
same manner as in Example 1-(4) to give optically active 73-
- 71 - 2 ~ ~ 71 2 v
{(Z)-2-(2-~ritylaminothiazol-4-yl)-2-[(8-diphenylmethyloxy-
2-oxo-lH-quinolin-5-yl)(diphenylmethyloxycarbonyl)methyl-
oxyimino]acetamido}cephalosporanic acid.
M.p. 142 - 145C (decomposed)
IR (Nujol): 3400, 3250 (br), 1790, 17~0, 1670, 1600 cm 1
MS (m/z): 1233 (MH+)
NMR (DMSO-d6) ~: 1.99 (3H, s), 3.09, 3.35 (2H, ABq,
J=18Hz), 4.62, 4.91 (2H, ABq, J=13Hz), 4.94 (lH, d,
J=4.9Hz), 5.62 (lH, dd, J=7.8Hz, 4.9Hz), 6.13 (lH, s), 6.46
(lH, d, J=9.8Hz), 6.77-7.38, 7.72-7.76 (40H, m), 8.15 (lH,
d, J=9.8Hz), 8.90 (lH, brsj, 9.59 (lH, d, J=7.8Hz), 11.0
(lH, brs)
Examples 54 - 55
The optically active cephalosporanic acid compound
obtained in Example 53-(7) and the corresponding starting
compounds are treated in the same manner as in Example 3-(1)
to give the following compounds.
(54) Optically active 7B-{(Z)-2-(2-aminothiazol-4-
yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxy-
imino]acetamido}-3-(1-quinoliniomethyl)-3-cephem-4-
carboxylic acid sodium salt
The physicochemical properties of the above
compound are the same as those of the B-isomer obtained in
Example 3-(2).
(55) Optically active 7B-~(z)-2-(2-aminothiazol-4
yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxy-
- 72 - 2~5 7 s ~
imino~acetamido}-3-(3-chloro-1-pyridiniomethyl)-3-cephem-4-
carboxylic acid sodium salt
The physicochemical properties of the above
compound are the same as those of the B-isomer obtained in
Example 26.
Example 56
The optically active oxyiminoacetic acid compound
obtained in Example 53-(6) and the corresponding starting
compounds are treated in the same manner as in Example 27-
(1) to give optically active 7g-{(Z)-2-(2-aminothiazol-4-
yl)-2-[(8-hydroxy-2-oxo-lH-quinolin-5-yl)(carboxy)methyloxy-
imino]acetamido~-3-(3-amino-2-methyl-1-pyridiniomethyl)-3-
cephem-4-carboxylic acid sodium salt.
The physicochemical properties of the above
compound are the same as those of the B-isomer obtained in
Example 27-(2).
Example 57
(1) To a solution of the compound (2.45 9)
obtained in Example 53-(6) in tetrahydrofuran (20 ml) are
added with ice-cooling l-hydroxybenzotriazole (405 mg) and
dicyclohexylcarbodiimide ~567 mg) under argon atmosphere,
and the mixture is stirred at room temperature for three
hours (the resulting suspension is referred to as "Reaction
solution A"). Separately, a solution of 7B-phenylacetamido-
3-chloromethyl-3-cephem-4-carboxylic acid 4-methoxybenzyl
ester (1.58 g) in dichloroethane (40 ml) is cooled with ice
- 73 ~ 20~7~.~J
under arson atmosphere, and thereto are added pyridine (0.49
ml) and phosphorus pentachloride (1.01 g), and the mixture
is stirred at the same temperature for one hour, and then at
room temperature for 30 minutes. To the mixture is added
dropwise methanol (24 ml) under ice-cooling, and the mixture
is reacted at room temperature for 15 minutes. After
cooling with ice, to the mixture is added 0.5N-aqueous
potassium dihydrogen phosphate solution (48 ml), and the
mixture is stirred at room temperature for 1.5 hour. The
reaction solution is extracted with dichloromethane, and the
extract is washed with sal~ne solution, dried, and the
solvent is distilled off. The resulting residue (1.87 g) is
dissolved in tetrahydrofuran (15 ml) (the resulting solution
is referred to as "Reaction solution B"). To Reaction
solution A is added dropwise Reaction solution B, and the
mixture is stirred at room temperature for 17 hours. The
precipitates are removed by filtration, and the filtrate is
evaporated to remove the solvent therefrom. The oily
residue is purified by silica gel column chromatography
(eluent; hexane/ethyl acetate) to give optically active 7B-
{(Z)-2-(2-tritylaminothiazol-4-yl)-2-L(8-diphenylmethyloxy-
2-oxo-lH-quinolin-5-yl)(diphenyloxymethylcarbony)methyloxy-
imino]acetamide}-3-(chloromethyl)-3-cephem-4-carboxylic acid
4-methoxybenzyl ester (2.12 g).
IR (Nujol): 3380, 3280, 3200, 1790, 1740, 1660,
1610 cm~l
7~ 2~ J 29
(2) To a solution of the product (800 mg) obtained
above in dimethylacetamide (8 ml~ are added with ice-cooling
successively zinc iodide (77 mg) and 1-allyl-4-thiopyridone
(272 mg) under argon atmosphere, and the mixture is stirred
at the same temperature for 22 hours. To the reaction
mixture are added water (20 ml) and saturated saline
solution (20 ml) under ice-cooling, and the precipitates are
collected by filtration, washed successively with water and
ethyl acetate, and concentrated to dryness under reduced
pressure at room temperature overnight. The resulting solid
(840 mg) is added to a mixture of trifluoroacetic acid (2.5
ml)/anisole (2.5 ml) under ice-cooling, and the mixture is
stirred at room temperature for two hours. The mixture is
cooled with ice, and thereto is added diisopropyl ether (50
ml), and the mixture is stirred for 15 minutes. The
precipitates are collected by filtration, washed with
diisopropyl ether, and dried under reduced pressure at room
temperature. The resulting solid (555 mg) is suspended in
water (5 ml), and the pH value of the mixture is adjusted to
pH 8.0 with saturated aqueous sodium hydrogen carbonate
solution, and purified by HP-20 column chromatography
(eluent; water and 20 ~ aqueous methanol). The fractions
containing the desired compound are combined, concentrated
and lyophilized to give optically active 7~-{(Z)-2-(2-
aminothiazol-4-yl)-2-[~8-hydroxy-2-oxo-lH-quinolin-5-yl)-
(carboxy)methyloxyimino]acetamido}-3-[(1-allyl-4-pyridinio)-
2~7129
thiomethyl]-3-cephem-4-carboxylic acid sodium salt (104
mg).
M.p. >165C (decomposed)
IR (Nujol): 3350 (dr), 3180 (br), 1770, 1660, 1630,
1610 cm~l
NMR (DMSO-d6) ~: 2.96, 3.34 (2H, ABq, J=18Hz), 4.79
(?H, brs), 4.90-4.93 (3H, m), 5.33 (lH, d, J=17Hz), 5.47
(1~, d, J=9.8Hz), 5.90-6.10 (lH, m), 5.97 (lH, s), 6.74 (lH,
d, J=9.8Hz), 6.95 (lH, s), 7.01 (lH, d, J=8.3Hz), 7.23 (lH,
d, J=8.3Hz), 7.73 (2H, d, J=6.8Hz), 8.29 (2H, d, J=6.8Hz),
8.35 (lH, d, J=9.8Hz)
Example 58
To a solution of the compound (1.33 9) obtained in
Example 57-(1) in acetonitril (13 ml) are added with ice-
cooling sodium iodide (164 mg) and 3-chloropyridine (454 m~)
under argon atmosphere, and the mixture is stirred at 8C
for 14 hours. The mixture is evaporated under reduced
pressure to remove acetonitril therefrom, and to the
resulting residue is added a mixture of trifluoroacetic acid
(8 ml)/anisole (8 ml) under ice-cooling, and the mixture is
stirred at room temperature for two hours. The mixture is
cooled with ice, and thereto is added diisopropyl ether (150
ml), and the mixture is stirred for 15 minutes. The
precipitates are collected by filtration, washed with
diisopropyl ether and dried under reduced pressure at room
temperature. The resulting solid is suspended in water (20
- 76 - 2~ 71 29
ml), and the pH value thereof is adjusted tc pH 8.0 with
saturated aqueous sodium hydrogen carbonate solution, and
purified HP-20 column chromatography (eluent; water and 2Q %
aqueous methanol). The fractions containing the desired
compound are combined, concentrated and lyophilized to give
optically active 7B-{(Z)-2-(2-aminothiazol-4-yl)-2-[(8-
hydroxy-2-oxo-lH-quiolin-S-yl)(carboxy)methyloxyimino]-
acetamido}-3-(3-chloro-1-pyridiniomethyl)-3-cephem-4-
carboxylic acid sodium salt (220 mg).
The physicochemical properties of the above
compound are the same as those of the ~-isomer obtained in
Example 26.
[Preparation of the starting compounds]
Reference Example 1
(1) 5-(2,2-Dihydroxyacetyl)-8--(benzyloxy)-
carbostyril (58 g) is suspended in tert-butanol (1000 ml)
and 5 % aqueous potassium dihydrogen phosphate solution (650
ml), and to the suspension is added dropwise with stirring
to a mixture of potassium permanganate (56.3 9 ) and water
(930 ml) at 5 - 7C, and the mixture is stirred at 5 - 7C
for seven hours, and then at room temperature for 17
hours. To the mixture is added saturated aqueous sodium
sulfite solution (535 ml) under ice-cooling, and the pH
value thereof is adjusted to pH 1--2 with conc. hydrochloric
acid, and the mixture is stirred for 30 minutes. The
precipitates are collected by filtration, washed with water
205712~
and dried to give 5-oxalo-8-(benzyloxy)carbostyril (36.7
9) -
M.p. 217 - 219C (decomposed)
IR (Nujol): 3200 - 2400, 1700, 1670, 1640, 1600 cm 1
MS (m/z): 323 (M+)
(2) To a solution of the product (74 9) obtained
above in tetrahydrofuran (2000 ml) and water (1500 ml) is
added wet 10 % palladium-carbon (37 9, moisture: 50 %), and
the mixture is stirred for three hours under hydrogen
atmosphere, and the catalyst is separated by filtration.
The catalyst is washed with a mixture of tetrahydrofuran and
water, and the washings and the filtrate are combined, and
evaporated to remove the solvent therefrom, and dried to
give 2-hydroxy-2-(8-hydroxy-2-oxo-lH-quinolin-5-yl)acetic
acid (54 9).
M.p. 259 - 261C (decomposed)
IR (Nujol): 3160, 3080, 2780 - 2560, 1725, 1695,
1640, 1600 cm-
MS (m/z): 236 (MH+)
(3) The product (39 9) obtained above is dissolved
in tetrahydrofuran (3000 ml) and water (600 ml) with
heating, and then cooled to 40C. To the mixture is added
diphenyldiazomethane (96.6 9), and the mixture is stirred
for one hour, and then at 55C for 20 hours. To the mixture
is added additional diphenyldiazomethane (96.6 9), and the
mixture is stirred for 24 hours. The solvent is distilled
7~ 20~7i~
off, and to the residue is added ether to give crystals.
The precipitated crystals are collected by filtration,
washed with ether and dried to give 2-(8-diphenylmethyloxy-
2-oxo-lH-quinolin-5-yl)-2-hydroxyacetic acid diphenylmethyl
ester (57.2 9).
M.p. 183 - 185C (decomposed)
IR (Nujol): 3330, 1750, 1650, 1600 cm 1
MS (m/z): 568 (MH+)
[Effects of the Invention]
The desired compound [I] of the present invention
and pharmaceutically acceptable salts thereof have excellent
antimicrobial activities against the wide range of various
microorganisms including Gram positive bacteria and Gram
negative bacteria, more particularly, have high stability to
3-lactamase-producing bacteria, and hence, these compounds
can be used as chemotherapeutics in treatment of various
infectious diseases caused by above mentioned microorganisms
for mammals including human beings or as additive for animal
feeds.