Note: Descriptions are shown in the official language in which they were submitted.
A- 18483/A
Sllbstituted dibenzo~d.~lrl,3.21dioxaphosphocin~
The present invention relates to novel substituted dibenzo[d,g][1,3,2]dioxaphosphocins, to
the use thereof for stabilising organic material and to compositions which contain these
compounds.
Dibenzo[d,g][1,3~2~dioxaphosphites have been known for some time as processing
stabilisers for organic polymers (q.v. CA-A-l 15g 845). Later, dibenzo[d,g][l,3,2]-
dioxaphosphocins which are substituted at the phosphorus atom by aromatic groups and
which contain sulfur in 12-position or carry hydroxyl groups at the benzene rings, were
disclosed as stabilisers for synthetic resins and polyolefins (US-A-3 297 631;
SU-A-897 797; JP-A-71 17896). Bis-dibenzo[d,g][1,3,2]dioxaphosphocins which are
linked through aromatic groups have also been proposed for this utility ~US-A-4 481 317).
It has now been found that compounds of this class which carry alkyl or aralkyl radicals at
the phosphorus atom and which are also linked through alkylene groups are surprisingly
good stabilisers for organic material, especially processing stabilisers ~or synthetic
polymers.
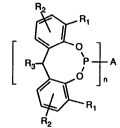
~Pl
Specific311y, the in~ention relates to compuond3 of fottnule I [ Ft3~p ~ A (I)
R2 Rl
wherein Rl and R2 are each independently of the other Cl 18alkyl, Cs l2cycloalkyl, phenyl
or Cl 4alkyl-substituted phenyl or phenyl-CI 4alkyl, R3 is hydrogen or methyl, n is 1 or 2
and A, when n = 1, is Cl 30alkyl, Cs l2cycloalkyl, phenyl-CI4alkyl or C2 l8alkyl which is
interrupted by -NR-, -S- or -O-, and, when n = 2, is a direct bond, Cl l2alkylene or
C2 l2alkylene whic11 is interrupted by -NR-, -S- or -O-, where R is Cl l2alkyl or phenyl.
2~81~
- 2 -
Rl and R2 defined as alkyl may be branched or unbranched radicals. The numerical range
prefixed by the symbol "C" denotes the number of possible carbon atoms. Thus Rl und R2
defined as Cl l8alkyl are typically methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl,
tert-butyl, pentyl, isopentyl, hexyl, heptyl, octyl, 2-ethylhexyl, nonyl, decyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, 2-ethylbutyl,
isoamyl, l-methylpentyl, 1,3-dimethylbutyl, 1,1,3,3-tetramethylbutyl, 1-methylhexyl,
isoheptyl, 1-methylheptyl, 1,1,3-trimethylhexyl or l-methylundecyl. Alkyl radicals
containing 1 to 8 carbon atoms are preferred, and those containing 1 to 4 carbon atoms are
particularly preferred.
A defined as Cl 30alkyl is, in addition to the meanings given for Cl 18alkyl, typically
icosyl, henicosyl, docosyl or triacontyl. Preferably A is Cl 22alkyl, and most prefeMbly
Cl 18alkyl.
A defined as Cl 12alkylene typically includes methylene, ethylene, l-methylethylene,
propylene, 1-methylpropylene, 2-methylpropylene, n-butylene, 2-methylbutylene,
pentylene, hexylene, heptylene, octylene, 2-ethylhexylene, 2-ethylbutylene,
l-methylpentylene, 1,3-dimethylbutylene, 1,1,3,3-tetramethylbutylene, 1-methylhexylene,
I-methylheptylene, decylene or dodecylene. If A is interrupted by -O-, -S- or -NR-, then
preferably the structural units -CH2-CH2-O-, -CH2-CH2-S- or -CH2-CH2-NR- are forrned.
The chain may be interrupted by one or by a plurality of the groups -NR-, -O- and -S-.
Rl and R2 defined as Cs l2cycloalkyl typically include cyclopentyl, cyclohexyl,
cyclohepty;, cyclooctyl, cyclodecyl and cyclododecyl. Cyclopentyl, cyclohexyl and
cycloheptyl are preferred, and cyclohexyl is especially preferred.
Rl and R2 and A defined as phenyl-Cl4alkyl are typically benzyl, phenylethyl,
3-phenylpropyl, a-methylbenzyl and a,a-dimethylbenzyl. Benzyl is preferred.
Rl and R2 defined as Cl4alkyl-substituted phenyl typically include: methylphenyl,
dimethylphenyl, trimethylphenyl, ethylphenyl, diethylphenyl, isopropylphenyl,
tert-butylphenyl, di-tert-butylphenyl or methyl di-tert-butylphenyl. The number of alkyl
groups is preferably 1 to 3, typically 1 or 2. The total number of carbon atoms of all alkyl
substituents is preferably 1 to 12, more particularly 1 to 6.
.
.
.
.
2 ~
- 3 -
In particularly preferred compounds of formula I, R2 is in meta-position to Rl. The
especially preferred meaning of Rl and R2 is alkyl, more particularly Cl4alkyl.
Useful compounds of formula I are those wherein Rl and R2 are each independently of the
other Cl l8alkyl, Cs l2cycloalkyl, phenyl or Cl4alkyl-substituted phenyl or
phenyl-Cl4alkyl, R3 is hydrogen or methyl, n is 1 or 2 and A, when n = 1, is Cl 30alkyl,
Cs 12cycloalkyl or C2 l8alkyl which is interrupted by -NR-, -S- or -O-, and, when n = 2, is
a direct bond, Cl l2alkylene or C2 l2alkylene which is interrupted by -NR-, -S- or -O-, and
R is Cl l2alkyl or phenyl.
Preferred compounds of formula I are those wherein Rl and R2 are each independently of
the other Cl 8alkyl, Cs 7cycloalkyl or phenyl-CI4alkyl, R3 is hydrogen or methyl, n is 1 or
2, and A is Cl l8alkyl or phenyl-CI4alkyl or Cl 12alkylene.
Particularly preferred compounds of formula I are those wherein Rl and R2 are each
independently of the other Cl 8alkyl, Cs 7cycloalkyl or phenyl-CI4alkyl, R3 is hydrogen or
methyl, n is 1 and A is Cl l8alkyl or phenyl-CI4alkyl.
Compounds of forrnula I meriting special interest are those wherein Rl and R2 are each
independently of the other Cl 8alkyl, n is 1 or 2, and A is Cl l8alkyl, phenyl-CI4alkyl or
Cl 8alkylene, and also those wherein Rl and R2 are each independently of the other
Cl 8alkyl, n is 1 or 2, and A is Cl 18alkyl or Cl 8alkylene, especially those wherein Rl and
R2 are each independently of the other Cl 8alkyl, n is 1 and A is Cl l8alkyl or
phenyl-CI4alkyl.
The compounds of formula I are admirably suitable for stabilising organic materials
against light-induced, thermal and/or oxidative degradation. Accordingly, the invention
also relates to compositions comprising an organic material which is susceptible to such
degradation reactions and at least one compound of formula I, and to the use of
compounds of formula I as stabilisers for protecting organic materials against the
aformentioned kinds of degradation. Exemplary of such materials are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut- 1-ene, polymethylpent-1-ene, polyisoprene or polybutadiene, as well as polymers
of cycloolefins, for example of cyclopentene or norbornene, polyethylene (which can be
uncrosslinked or crosslinked), for example high density polyethylene (HDPE), low density
2~8~
-4-
polyethylene (LDPE and linear low density polyethylene (LLDPE).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/H~PE,
PP/LDPE) and mixtures of different types of polyethylene (for example LDPEI~ PE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers, for exarnple ethylene/propylene copolymers linear low density polyethylene
(LLDPE) and mixtures thereof with low density polyethylene (LDPE),
propylene/but- l-ene copolymers, ethylene~exene copolymers, ethylene/methylpentene
copolymers, ethylene/heptene copolymers, ethylene/octene copolymers,
propylene/butadiene copolymers, isobutylene/isoprene copolymers, ethylene/alkyl acrylate
copolymers, ethylene/alkyl methacrylate copolymers, ethylene/vinyl acetate or
ethylene/acrylic acid copolymers and their salts (ionomers), as well as terpolymers of
ethylene with propylene and a diene such as hexadiene, dicyclopentadiene or
ethylidenenorbornene; and also mixtures of such copolymers with each other and with
polymers mentioned in 1) above, for example polypropylene/ethylene propylene
copolymers, LDPEIEVA,LDPE/EAA,LLDP~EVA and LLDPE/EAA.
3a. Random or alternating copolymers of a-olefins with carbon monoxide.
3b. Hydrocarbon resins (for example C5-C9), including hydrogenated modificationsthereof (for example tackifiers).
4. Polystyrene, poly-(p-methylstyrene), poly-(a-methylstyrene).
5. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for
example styrenel[ch]butadiene, styrene/acrylonitrile, styrene/alkylmethacrylate,styrenelbutadiene/alkylacrylate, styrene/ maleic anhydride, styrene/acrylonitrile/methyl
acrylate; mixtures of high impact strength from styrene copolymers and another polymer,
for example from a polyacrylate, a diene polymer or an ethylene/propylene/diene
terpolymer; and block copolymers of styrene, for example styrene/butadiene/styrene,
styrenerlsoprene/styrene, styrene/ethylene/butylene/styrene or
styrene/ethylene/propylene/styrene.
6. Graft copolymers of styrene or a-methylstyrene, for example styrene on polybutadiene,
.. . . .
. . ' ~ .. '.
.
-
. ~ .
2 ~ 6
styrene on polybutadiene/styrene or polybutadiene/acrylonitrile; styrene and acrylonitrile
(or methacrylonitrile) on polybutadiene; styrene and maleic anhydride or maleimide on
polybutadiene; styrene, acrylonitrile and maleic anhydride or maleimide on polybutadiene;
styrene, acrylonitrile and methyl methacrylate on polybutadiene, styrene and alkyl
acrylates or methacrylates on polybutadiene, styrene and acrylonitrile on
ethylene/propylene/diene terpolymers, styrene and acrylonitrile on polyalkylacrylates or
polyaLkylmethacrylates, styrene and acrylonitrile on acrylate/butadiene copolymers, as
well as mixtures thereof with the copolymers listed under 5), for example the copolymer
mixtures known as ABS, MBS, ASA or AES polymers.
7. Halogenated polymers such as polychloroprene, chlorinated rubbers, chlorinated or
sulfochlorinated polyethylene, copolymers of ethylene and chlorinated ethylene,
epichlorohydrin homo- and copolymers, pr~ferably polymers of halogenated vinyl
compounds, for example poly- vinylchloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluoride, as well as copolymers thereof, for example vinyl
chloride/vinylidene chloride, vinyl chloride/vinyl acetate or vinylidene chloride/vinyl
acetate copolymers.
8. Polymers derived from o~"~-unsaturated acids and derivatives thereof, such aspolyacrylates and polymethacrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers of the monomers mentioned under 8) with each other or with other
unsaturated monomers, for example acrylonitrile/butadiene copolymers, acrylo-
nitrile/alkylacrylate copolymers, acrylonitrile/alkoxyalkylacrylate or acrylonitrile/vinyl
halide copolymers or acrylonitrile/alkylmethacrylate/butadiene terpolymers.
10. Polymers derived from unsaturated alcohols and amines or the acyl derivatives or
acetals thereof, such as polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate, polyvinyl
benzoate, polyvinyl maleate, polyvinylbutyrate, polyallyl phthalate or polyallylmelamine;
as well as their copolymers with the olefins mentioned in 1) above.
11. Homopolymers and copolymers of cyclic ethers such as polyalkylene glycols,
polyethylene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
12. Polyacetals such as polyoxymethylene and those polyoxymethylenes which contain
ethylene oxide as a comonomer, polyacetals modified with thermoplastic polyurethanes,
. .
- 6 -
acrylates or ~I~S.
13. Polyphenylene oxides and sulfides and mixtures thereof with polystyrene or
polyamides.
14. Polyurethanes which are derived from polyethers, polyesters or polybutadienes
caIrying terminal hydroxyl groups on the one hand and aliphatic or aromatic
polyisocyanates on the other, as well as precursors thereof.
15. Polyamides and copolyamides which are derived from diamines and dicarboxylicacids andlEch]or from aminocarboxylic acids or the corre- sponding lactams, such as
polyamide 4, polyarnide 6, polyarnide 6/6, 6/10, 6/9, 6/12 and 4/6, polyamide 11,
polyamide 12, aromatic polyamides obtained by condensation of m-xylene, diamine and
adipic acid; polyamides prepared from hexamethylenediamine and isophthalic andVor
terephthalic acid, with or without an elastomer as modifier, for example poly-2,4,4,-
trimethylhexamethylene terephthalamide or poly-m-phenylene isophthalamide; blockcopolymers of the aforementioned polyamides with polyole~lns, ole~m copolymers,
ionomers or chemically bonded or grafted elastomers; or with polyethers, for example
with polyethylene glycol, polypropylene glycol or polytetramethylene glycol; and also
polyamides or copolyamides modified with EPDM or ABS, and polyamides condensed
during processing (RIM polyamide systems).
16. Polyureas, polyimides and polyamide-imides and polybenzimidazoles.
17. Polyesters derived from dicarboxylic acids and diols and/or from hydroxycarboxylic
acids or the corresponding lactones, such as poly-ethylene terephthalate, polybutylene
terephthalate, poly-1,4-dimethylolcyclohexane terephthalate, polyhydroxybenzoates as
well as block-copolyether esters derived from hydroxyl-terminated polyethers; and also
polyesters modified with polycarbonates or MBS.
18. Polycarbonates and polyester carbonates.
19. Polysulfones, polyether sulfones and polyether ketones.
20. Crosslinked polymers which are derived from aldehydes on the one hand and phenols,
ureas and melamines on the other hand, such as phenoVformaldehyde resins,
- 7 -
urea/formaldehyde resins and melamine/formaldehyde resins.
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins which are derived from copolyesters of saturated and
unsaturated dicarboxylic acids with polyhydric alcohols and vinyl compounds as
crosslinking agents, and also halogen-containing modifications thereof of low
flammability.
23. Crosslinkable acrylic resins derived from substituted acrylic esters such as epoxy
acrylates, urethane acrylates or polyester acrylates.
24. Alkyd resins, polyester resins or acrylate resins which are cross-linked with melamine
resins, urea resins, polyisocyanates or epoxy resins.
25. Crosslinked epoxy resins which are derived from polyepoxides, for example from
bisglycidyl ethers or from cycloaliphatic diepoxides.
26. Natural polymers such as cellulose, rubber, gelatine and chemically modifiedhomologous derivatives thereof such as cellulose acetates, cellulose propionates and
cellulose butyrates, or the cellulose ethers, such as methylcellulose; as well as rosins and
their derivatives.
27. Mixtures (polyblends) of the aforementioned polymers, for example PP/EPDM,
Polyamide 6/EPDM or ABS, PVC/EVA, PVS/ABS, PVC/MBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/therrmoplastic PUR, PC/thermoplasticPUR, POM/acrylate, POM/MBS, PPE/~IPS, PPE/PA 6.6 and copolymers, PA/HDPE,
PA/PP, PA/PPE.
28. Naturally occurring and synthetic organic materials which are pure monomericcompounds or mixtures of such compounds, for example mineral oils, animal and
vegetable fasts, oil and waxes, or oils, fats and waxes based on synthetic esters (e.g.
phthalates, adipates, phosphates or trimellitates) and also the mixtures of synthetic esters
with mineral oils in any weight ratios which are used as spinning compositions, as well as
aqueous emulsions of such materials.
2 ~
- 8 -
29. Aqueous emulsions of natural or synthetic rubber, for example natural latex or latices
of carboxylated styrene/butadiene copolymers.
The composidons of this invention conveniently contain the compounds of formula I in an
amount of 0.01 to 10, typically 0.05 to 5, preferably 0.05 to 3 and, 0.1 to 2 %, by weight.
The compositions may contain one or more of the compounds of formula I, and the
percentages by weight are based on the total amount of said compounds. The computation
is based on the total weight of the organic material without the compounds of formula I.
Incorporation in the organic materials can be effected by blending them with, or by
application thereto, of the compounds of formula I and further optional additives by
methods which are commonly employed in the art. If the organic materials are polymers,
especially synthetic polymers, the incorporation can be effected before or during the
fabrication of shaped articles or by applying the dissolved or dispersed compounds to the
polymer, with or without subsequent evaporation of the solvent. In the case of elastomers,
these may also be stabilised as lattices. A further means of incorporating the compounds
of formula I in polymers consists in adding them before, during or directly after the
polymerisation of the corresponding monomers or before crosslinking. The compounds of
formula I can also be added in encapsulated form (e.g. in waxes, oils or polymers). If the
compounds of formula I are added before or during polymerisation, they can also act as
regulators for the chain length of the polymers (chain terminators). The compounds of
formula I or mixtures thereof can also be added in the form of a masterbatch which
contains these compounds to the synthetic resins to be stabilised, typically in a
concentration of 2.5 to 25 % by weight.
The compounds of formula I can conveniently be incorporated by the following
techniques:
- as emulsion or dispersion (e.g. to lattices or emulsion polymers)
- as dry mixture while blending additional components or polymer mixtures
- by direct addition to the processing apparatus (e.g. extruder, internal
mixer and the like)
- as solution or melt.
Polymer compositions of this invention can be used in different form and processed to
different products, for example sheets, filaments, ribbons, moulded articles, profiles or as
binders for paints and vamishes, adhesives or putties.
- 9 -
The organic materials to be protected are preferably natural, semi-synthetic or, especially,
synthetic polymers. It is especially useful to protect thermoplastic resins, preferably
polyolefins. In this connection, the excellent effect of the compounds of formula I as
processing stabilisers (heat stabilisers) is to be singled out for special mention. To this end
they are conveniently added before or during the processing of the polymer. It is, however,
also possible to stabilise other polymers (e.g. elastomers) or lubricants and hydraulic
fluids against degradation, such as light-induced andlor thermal oxidative degradation.
Examples of elastomers will be found among the above list of possible organic materials.
The suitable lubricants and hydraulic fluids are based, for example, on mineral or
synthetic oils or mixtures thereof. The lubricants are known to the skilled person and
described in the pertinent technical literature, for example in Dieter Klamann, "Schmier-
stoffe und verwandte Produkte" (Lubricants and Related Products), Verlag Chemie,Weinheim, 1982, in Schewe-Kobek, "Das Schmiermittel-Taschenbuch" (The Lubricant
Handbook), Dr. Alfred Huthig-Verlag, Heidelberg, 1974 and in "Ullmanns Enzyklopadie
der technischen Chemie" (Encyclopedia of Industrial Chemistry), Vol. 13, pages 85-94
(Verlag Chemie, Weinheim, 1977).
The invention further relates to a process for protecting organic material against oxidative,
thermal and/or actinic degradation, which comprises incorporating in, or applying to, said
material compounds of formula I as stabilisers.
In addition to the novel compounds, the compositions of this invention, especially if they
contain organic, preferably synthetic, polymers, may contain other conventional additives.
Illustrative examples of such further additives are:
1. Antioxidants
1.1. Alkvlated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-
butyl-4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-
butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol,
2-(a-methylcyclohexyl)-4,6-dimethylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tri-
cyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, 2,6-di-nonyl-4-methyl-
phenol.
2~gl~
- 10-
1.2. ALIcvlated hvdroquinones, for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-
tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octadecyloxy-
phenol.
1.3. Hvdroxvlated thiodiphenvl ethers, for example 2,2'-thiobis(6-tert-butyl-4-methyl-
phenol), 2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol),4,4'-thiobis(6-tert-butyl-2-methylphenol~.
1.4. AL~cylidenebis~henols, for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol),
2,2'-methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(a-methyl-
cyclohexyl)phenol], 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylene-
bis(6-nonyl-4-methylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-ethylidene-
bis(4,6-di-tert-butylphenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-
methylenebis[6-(a-methylbenzyl)-4-nonylphenol], 2,2'-methylenebis[6-(a,a-dimethyl-
benzyl)-4-nonylphenol], 4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylene-
bis(6-tert-butyl-2-methylphenol), 1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)butane,
2,6-bis(3-tert-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol, 1,1,3-tris(5-tert-
butyl-4-hydroxy-2-methylphenyl)butane, 1,1-bis(5-tert-butyl-4-hydroxy-2-methyl-
phenyl)-3-n-dodecylmercaptobutane, ethylene glycol bis[3,3-bis(3'-tert-butyl-4'-hydroxy-
phenyl)butyrate], bis(3-tert-butyl-4-hydroxy-5-methylphenyl)dicyclopentadiene, bis[2
(3'-tert-butyl-2'-hydroxy-5'-methylbenzyl)-6-tert-butyl-4methylphenyl] terephthalate.
1.5. Benzyl compounds, for example 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-
trimethylbenzene, bis(3,5-di-tert-butyl-4-hydroxybenzyl) sulfide, isooctyl 3,5-di-ten-
butyl-4-hydroxybenzylmercaptoacetate, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)
dithiolterephthalate, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate,
1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate, dioctadecyl 3,5-di-
tert-butyl-4-hydroxybenzylphosphonate, calcium salt of monoethyl 3,5-di-tert-butyl-
4-hydroxybenzylphosphonate, 1,3,5-tris(3,5-dlcyclohexyl-4-hydroxybenzyl)isocyanurate.
1.6. Acylaminophenols, for example 4-hydroxylauranilide, 4-hydroxystearanilide, 2,4-
bis(octylmercapto)-6-(3,5-di-tert-butyl-4-hydroxyanilino)-s-triazine, octyl N-(3,5-di-tert-
butyl-4-hydroxyphenyl)carbamate.
1.7. Esters of ~-(3,5-di-tert-butvl-4-hydroxvphenyl)propionic acid with mono- or
2 ~
polyhydric alcoho]s, e.g. with methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
triethylene glycol, pentaerythritol, ~s(hydroxyethyl) isocyanurate, thiodiethylene glycol,
N,N' -bis(hydroxyelhyl)oxalodiamide.
1.8. Esters of ~-~5-tert-butvl-4-hvdroxY-3-methYlphenYI)propionic acid with mono- or
polyhydric alcohols, e.g. with methanol, diethylene glycol, octadecanol, triethylene glycol,
1,~-hexanediol, pentaerythritol, neopentyl glycol, tris(hydroxyethyl) isocyanurate,
thiodiethylene glycol, N,N'-bis(hydroxyethyl)oxalodiamide.
1.9. Esters of ~-(3,5-dicvclohexyl-4-hydroxvphenvl)proPiorlic acid with mono- or poly-
hydric alcohols, e.g. with methanol, diethylene glycol, octadecanol, triethylene glycol,
1,6-hexanediol, pentaerythritol, neopentyl glycol, tris(hydroxyethyl) isocyanurate,
thiodiethylene glycol, N,N'-bis(hydroxyethyl)oxalodiamide.
1.10. Amides of B-(3,5-di-tert-butvl-4-hvdroxyphenvl)propionic acid e.g. N,N'-
bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexamethylenediamine, N,N'-bis(3,5-
di-tert-butyl-4-hydroxyphenylpropionyl)trimethylenediarnine, N,N'-bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hydrazine.
2. UV absorbers and li ht stabilisers
2.1. 2-(2'-Hydroxvphenyl)benzotriazoles, for example the 5'-methyl, 3',5'-di-ter~-butyl,
S'-tert-butyl, 5'-(1,1,3,3-tetramethylbutyl), 5-chloro-3',5'-di-tert-butyl, 5-chloro-
3'-tert-butyl-S'-methyl, 3'-sec- butyl-S'-tert-butyl, 4'-octoxy, 3',5'-di-tert-amyl and
3',5'-bis(~,oc-dimethylbenzyl) derivative.
2.2. 2-Hvdroxybenzophenones, for example the 4-hydroxy, 4-methoxy, 4-octoxy,
4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-
dimethoxy derivative.
2.3. Esters of substituted and unsubstituted benzoic ds. for example, 4-tert-butylphenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoylresorcinol,
bis(4-tert-butylbenzoyl)-resorcinol, benzoylresorcinol, 2,4-di-tert-butylphenyl
3,5-di-tert-butyl-4-hydroxy- benzoate and hexadecyl 3,5-di-tert-bu~yl-4-hydroxybenzoate.
2.4. .~crylates, for example ethyl a-cyano-~"e-diphenylacrylate, isooctyl c~-cyano-
- 12-
,~"B-diphenylacrylate, methyl o~-carbomethoxycinnamate, methyl a-cyano-,~-methyl-p-
methoxy-cinnamate, butyl ~-cyano-~-methyl-p-methoxy- cinnamate, methyl a-carbo-
methoxy-p-methoxycinnamate and N-(~-carbomethoxy-~-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds. for example nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex, with or without additional ligands
such as n-butylarnine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyl-
dithiocarbamate, nickel salts of 4-hydroxy-3,5-di-tert-butylbenzyl-phosphonic acid
monoaLIcyl esters, e~g. of the methyl or ethyl ester, nickel complexes of ketoximes, e.g. of
2-hydroxy-4-me~hyl-phenyl undecyl ketoneoxime, nickel complexes of 1-phenyl-4-
lauroyl-5-hydroxypyrazole, with or without additional ligands.
2.6. StericallY hindered amines. for example bis(2,2,6,6-tetramethylpiperidyl) sebacate,
bis(1,2,2,6,6-pentamethylpiperidyl) sebacate, bis(1,2,2,6,6-pentamethylpiperidyl)
n-butyl-3,5-di-tert-butyl-4-hydroxy-benzylmalonate, the condensation product of
1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensation product of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine
and 4-tert-octylamino-2,6-dichloro- 1,3 ,S-triazine, tetrakis(2,2,6,6-tetramethyl-4-
piperidyl)-I,2,3,4-butanetetraoate, 1,1'-(1,2-ethanediyl)bis-(3,3,5,5-tetramethyl-
piperazinone), bis(2,2,6,6-tetramethylpiperidyl)succinate, bis(l-octyloxy-2,2,6,6-
tetramethylpiperidyl)sebacate.
2.7. Oxalyl diamides. for example 4,4'-dioctyloxyoxanilide, 2,2'-dioctyloxy-S,S'-
di-tert-butoxanilide, 2,2'-didodecyloxy-S,S'-di-tert-butoxanilide, 2-ethoxy-2'-
ethyloxanilide, N,N'-bis(3-dimethylaminopropyl)oxalamide, 2-ethoxy-S-tert-butyl-2'-ethyloxanilide and its mixture with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide and
mixtures of ortho- and para-methoxy-disubstituted oxanilides and mixtures of o- and
p-ethoxydisubstituted oxanilides.
2.8. 2-(2-HydroxyphenYI)- 1~3 S-triazines, for example 2,4,6-tris(2-hydroxy-4-octyloxy-
phenyl)- 1 ,3,5-triazine~ 2-(2-hydroxy-4-octyloxy-phenyl)-4,6-bis(2,4-dimethylphenyl)-
1,3,5-triazine, 2-(2,4-dihydroxy-phenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine,
2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dirnethylphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(4-methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-
4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1 ,3,5-triazine.
2 Q ~ 6
- 13-
3. Metal deactivators, for example N,N'-diphenyloxalyl diamide, N-salicylal-
N'-salicyloylhydrazine, N,N'-bis(salicyloyl)hydrazine, N,N'-bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hydrazine, 3-salicyloyl- amino-1,2,4-triazole,
bis(benzylidene)oxalic dihydrazide.
4. Phosphites and phosPhonites, for example triphenyl phosphite, diphenylalkyl
phosphites, phenyldiaLkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite,
trioctadecyl phosphite, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl)
phosphite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tertbutylphenyl) penta-
erythritol diphosphite, tristearyl sorbitol triphosphite, tetrakis-(2,4-di-tert-butylphenyl)
4,4'-biphenylene diphosphonite, 3,9-bis(2,4-di-tert-butylphenoxy)-2,4,8,10-
tetraoxa-3,9-diphosphaspiro[S.5]undecane.
5. Compounds which decomPose per(~,xide~ for example esters of ~B-thiodipropionic acid,
for example the lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the
zinc salt of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide,
pentaerythritol tetrakis(~-dodecylmercapto)propionate.
6. Polvamide stabilisers. for example, copper salts in conjunction with iodides and/or
phosphorus compounds and salts of divalent manganese.
7. Basic co-stabilisers. for example, melamine, polyvinylpyrrolidone, dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids for
example calcium stearate, zinc stearate, magnesium stearate, sodium ricinoleate and
potassium palmitate, antimony pyrocatecholate or zinc pyrocatecholate.
8. Nucleating a~ents, for example, 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid.
9. Fillers and reinforcing agents. for example calcium carbonate, silicates, glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxydes, carbon black,
graphite.
10. Other additives, for example, plasticisers, lubricants, emulsifiers, pigments,
fluorescent whitening agents, flameproofing agents, antistatic agents and blowing agents.
cJ
- 14-
It is preferred to use the compounds of forrnula I with specific co-stabilisers.Co-stabilisers to be singled out for special mention are phenolic antioxidants (e.g. those
listed above in items 1.1 to 1.10), stearates (e.g. those listed in item 7 above), phosphites
and phosphonites (e.g. those listed in item 4 above) and benzofuran-2-one derivatives, in
particular suitably those disclosed in US-A-4 325 863 and US-A-4 338 244, preferably
dlose of foi~nula ~ 0 , wherein
R91 is phenyl or phenyl which is substituted by I to 3 alkyl radicals together containing
not more than 18 carbon atoms, alkoxy of I to 12 carbon atoms, alkoxycarbonyl of 2 to
18 carbon atoms or chloro,
R'2 and R'4 are each independently of the other alkyl of 1 to 12 carbon atoms,
cyclopentyl, cyclohexyl or chloro,
R'3 has the meaning of R'2 or R'4 or is a radical of formula -(CH23~-OR'6,
O Ol O
-(CH2~C-N(R 7)2~--tCH2~C-O-A-O-~ff~H2 x~E,
CH2 ~ -NR'8-A-NR'8-C~CH2X~ E~ ~ cH2~ c-NR~8-A-o-c~cH2~ E~
~CH2~C--N 1~1--C~CH23~ E, -CH2-S-R'g, -CH(C6Hs)-C-QR 6 or
-D-E, wherein
R'6 is hydrogen, alkyl of I to 18 carbon atoms, alkyl of 1 to 18 carbon atoms which is
interrupted by oxygen or sulfur, dialkylaminoalkyl containing a total of 3 to 16 carbon
atoms, cyclopentyl, cyclohexyl, phenyl or phenyl which is substituted by 1 to 3 alkyl
radicals together containing not more than 18 carbon atorns,
xisO, 1 or2,
the substituents R'7 are each independently of the other hydrogen, alkyl of 1 io lX carbon
atoms, cyclopentyl, cyclohexyl, phenyl, phenyl which is substituted by 1 or 2 alkyl
2 ~ 5 ~
- 15-
radicals together containing not more than 16 carbon atoms, a radical of formula
-C2H40H. ~C2H4~0~CmH2m+1 or -C2H4-O-C-R',o, or, together wilh the linking nitrogen
atom, form a piperidino or morpholino radical,
m is 1 to 18,
R'lo is hydrogen, alkyl of 1 to 22 carbon atoms or cycloalkyl of 5 to 12 carbon atoms,
A is aL~cylene of 2 too 22 carbon atoms which may be interrupted by nitrogen, oxygen or
sulfur,
R'8 is hydrogen, aL~cyl of 1 to 18 carbon atoms, cyclopentyl, cyclohexyl, phenyl, phenyl
which is substituted by 1 or 2 aL~yl radicals which together contain not more than
16 carbon atoms or ben~yl,
R'9 is alkyl of 1 to 18 carbon atoms,
D is -O-, -S-, -SO-, -SO2- or-C(R 11)2-
~the substituents R'll are each independently of the other alkyl of together not more than
16 carbon atoms, phenyl or a radical of formula ~CH2~C-OR'6 or
~CH2~C-N(R',)2, wherein x, R'6 and R'7 have the given meanings,
E is a radical of formula
~ ~C--
R'2 H R'1
wherein R'l, R'2 and R'4 have the given meanings, and
R's is hydrogen, alkyl of I to 20 carbon atoms, cyclopentyl, cyclohexyl, chloro or a radical
O O
of formula -CH2-C-OR'6 or -CH2-C-N(R'7)2, wherein R'6 and R'7 have the given
meanings, or R's together with R'4 forms a tetramethylene radical.
The compounds of formula I can be prepared by standard methods employed in chemistry,
2~581~
- 16-
for example by nucleophilic substitution of the chlorine atom in cyclic diaryl
phosphorochlorodites of formula II with organometallic reagents:
~0
n R3-( p Cl + A(M)n -- (I) + n MCI
~'
~R1
2 (Il)
where M is preferably Li, MgX or ZnX (X = Cl, Br, I).
The substitution can be carried out in the temperature range from -60C to +150C,
preferably from -10C to +70C. Sui~able solvents are typically ethers such as diethyl
ether, dibutyl ether, tetrahydrofuran (1~), dimethoxyethane as well as mixtures of ethers
and aliphatic and aromatic hydrocarbons, such as benzene, toluene, xylene, and aliphatic
hydrocarbons such as hexane and petroleum ether fractions and the like. The
diarylphosphorochlorodite and the organometallic reagent are conveniently used in
equivalent amounts. A small excess of organometallic reagent can also be used, for
example a 1.05- to l.1-fold excess.
The compounds of formula II are prepared by methods which are known per se, for
~OH
example by reacting 2,2'-alkylidene bisphenols of formula III R3~ (III)
~H
R2 R1
with phosphorus trichloride in an inert aprotic solvent in the temperature range from 20 to
200C.
Bisphenols of formula III can also be used direct for the preparation of compounds of
formula I by reacting them with phosphorus compounds of formula A-PC12 or A(PCI2)2 in
the presence of an organic base. The phenols of formula III are known or can be readily
obtained by standard methods.
21~5~
- 17-
The invention is illustrated in more detail by the following Examples in which, and also
throughout the description, unless otherwise indicated, all parts and percentages are by
weight.
Example 1: Preparation of 2,10-dimethvl-4,8-di-tert-butyl-6-n-octyl-12H-dibenzo-rd~glll .3,21dioxaphosphocin
a) A 1.5 I sulfonation flask equipped with condenser, thermometer and stirrer is charged
with 340.2 g of 2,2'-methylenebis-(4-methyl-6-tert-butylphenol), 96 ml of phosphorus
trichloride, 170 ml of o-dichlorobenzene and 7.8 g of triphenylphosphine and the mixture
is heated to 50C, whereupon HCI evolves. After 1.5 h at this temperature, the reaction
mixture is heated to 170C and stirred for about 1 h while introducing a weak stream of
nitrogen. After cooling to 50C, 200 ml of hexane are added, whereupon the product starts
to crystallise. The batch is cooled to room temperature and the precipitate is collected by
sucdon filtration, washed with 2 x 50 ml of hexane and dried at 50C under vacuum.
Yield: 307 g (76 % of theory) of 2,10-dimethyl-4,8-di-tert-butyl-6-chloro-12H-di-
benzo[d,gl[l,3,2~dioxaphosphocin, m.p. 163-165C.
b) Under nitrogen, 2.95 g of magnesium turnings are placed in a 250 ml 3-necked flask
equipped with thermometer, condenser, dropping funnel and nitrogen inlet. The Grignard
reaction is initiated by heating briefly with about S ml of tetrahydrofuran (THF) and 1 ml
of l-octyl chloride. After dilution with 30 ml of THF, another 19.7 g of l-octyl chloride in
20 ml of THF are added dropwise such that a gentle reflux is maintained. When the
addition is complete, the brown solution is stirred for about 1 hour at room temperature.
The concentration of the Grignard compound is about 1.45 mol/l (determined by titration).
c) Under nitrogen, 47 g of 2,10-dimethyl-4,8-di-tert-butyl-6-chloro-12H-dibenzo[d,gl-
[1,3,2]dioxaphosphocin (a) are suspended in 120 ml THF in a 500 ml 3-necked flask
equipped with thermometer, magnetic stirrer and septum. After cooling to about 5C, the
solution obtained in b) is added under nitrogen pressure by means of a double-ended
injection needle. The cooling bath is removed and the reaction mixture is stired for 15
minutes at room temperature. Then about 6 ml of an aqueous solution of sodium sulfate
are added and the grey suspension is clarified by filtration over fuller's earth. The resultant
so!ution is concentrated by evaporation and the residue is recrystallised from 70 ml of
isopropanol, affording 50.1 g (89 % of theory) of the title compound in the form of a white
- ~ , . - .
, ~ .
,
2 ~ 3
- 18-
powder of m.p: 72-74C.
Examples 2- 15
The compounds listed in Tables 1, 2 and 3 are prepared in accordance with the general
procedure described in Example 1.
Table 1
R2~ C(CH3)3
~ O\
CH2 P A
~0
~ C(CH3)3
R2
_ Elemental analysis
Example R2 A Yieid m p [C] p cal. ~%) P ~ound (%) . _ . ....... ,,_
(1) CH3 n~CsH17 89% 72-4 6.42 5.90
2 CH3 CH3 62% 205-7 8.0S 8.08
3 CH3 n-C4Hg 61 % 130-2 7.26 7.20
4 CH3 n-C6H13 66% 95-9 6.81 6.77
CH3 n-C12H25 85% 54 7 5.75 5.53
6 CH3 n~C14Hzs 85% 53-8 5.46 5.36
7 CH3 n~C1sH37 70% 65 4.97 4.95
8 CH3 -(CH2)3-Ph 90% 160-4 6.34 6.24
9 C(CH3)3 n~C12H2s 91 % 105-9 4.97 4.89
C(CH3)3 n-C14H2g 72% ~ 110-6 4.76 4 73
Ph denotes phenyl
20581~
- 19-
Table 2
C~CH3)3
\~C(CH3)3
~0
CH3--( p--A
)~C(CH3)3
C(CH3)3
Elemental analysis
Example Yield m.p.l Cl Pcd.(56) 1'io=Id(9~)
11 n-C8H17 42% 145-9 5.33 5.26
12 n~C12H2s 65% 170-2 4.86 4.72
13 n~C1sH37 46% 70-2 4.30 4.38
Table 3
P2~o~C(CH3)3
~0
[ I~P ~2A
R2 C(CH3)3
. Elemental analvsis
Ex. R2 R3 A Yield m.p.[ C] Pcal.(%) P found (%)
14 CH3 H -(CH2)6- 84% 241-4 7.60 7.29
C(CH3)3 CH3 -(CH2)6- 86% 290-4 ~ ~ 5.75
Example 1~ Stabilisation of polvpropYlene
20581~
- 20 -
1.3 kg of polypropylene powder (melt index 3.2 g/10 min, measured at 230C12.16 kg) are
blended with 0.05 % of calcium stearate, 0.05 % of tetrakis[3,5-di-tert-butyl-4-hydroxy-
phenylpropionyloxymethyl]methane and 0.05 % of stabiliser listed in Table 4. This blend
is extruded at 100 rpm in an extruder having a cylinder diameter of 20 mm and a length of
400 mm, the three heating zones being adjusted to 260C, 270C and 280C respectively.
The extrudate is cooled by drawing it through a water bath and then granulated. The
granulate is extruded a second and a third time. After these three extrusions, the melt
index is measured at 230C/2.16 kg. The results are reported in Table 4.
Table 4:
Compound of Melt index
Example 17.8
1 3.2
2 35 8
4 4 1
6 5.1
78 4 1
9 4.0
5.9
11 4.2
13 s4 7
14 4.0
4.6
Example 18: Stabilisation of polvethvlene
100 parts of unstabilised high-density polyethylene in powder form having a molecular
weight of about 500 000 are dry blended with 0.05 part of tetrakis[3,5-di-tert-butyl-4-
hydroxyphenylpropionyloxymethyl]methane and 0.1 part of stabiliser listed in Table 5.
The blends are kneaded for 50 minutes in a Brabender plastograph at 220C and 50 rpm.
During this time the kneading resistance is registered continuously as torque. The
crosslinking of the polymer causes a rapid increase in the torque during the kneading time
after initial constancy. The effectiveness of the stabilisers is expressed in a lengthening of
the constancy time. The values obtained are reported in Table 5.
2~81~6
- 21 -
Table S
Time in min.
Example until increase in
torque
_ S
1 33
2 32
3 27
4 21.5
S 18.5
6 21
7 18
8 19
9 23.5
10 18
11 18
12 15
13 14.5
14 19.5
.
~ .