Note: Descriptions are shown in the official language in which they were submitted.
2~82~
PROCESS FOR THE PRODUCTION OE HIGHLY PURE ~IYDROGEN (~12)
AT HIGH OUTPUT PRESSURES
The invention relates to a process for the production of highly
pure hydrogen (H2) at high output pressures for use in high pressure
synthesis, for example, ammonia (NH3) synthesis, included in synthesis
gas producing operations. More particu]arly, the invention relates to
the use of molecular sieves under hlgh pressure in pressure ~swing
adsorption ~PSA) systems of such synthe~lis gas producing operations.
The use of PSA technology for the purification of hydrogen is
well known in the art. PSA systems are generally integrated into
operations for the production of synthesis gases for high pressure
syntheses, such as ammonia synthesis gas. The PSA process is a highly
desirable process for the purification of hydrogen, since a ~ery high
purity level (e.g. in excess of 99%) is achievable and there is only
little pressure drop between the feed gas stream and the product gas
stream. However, it is suggested in the art that PSA systems
includlng molecular sieves cannot be run efficiently at the same
pressures as the subsequent high pressure synthesis in which the
purified feed gas is used.
British patents 2,154,566 and 2,191,185 describe process and
apparatus for ammon~a synthesis gas production with typical input
pressures into the PSA system of 5 or 6 bar and disclose the use of
the highly pure hydrogen produced at such pressures in an ammonia
synthesisO
In an improved process for the production of ammonia described in
published European application EP O 115 752, the pressures used for
the operation of a PSA system are 300 to 1,000 psia (20.4 to 68 bar)
pressure adsorption pressure and 60 to 100 psia (4.1 to ~.8 bar) of
purge gas or desorbate pressure.
It is disclosed in European published application 0 092 969 that,
despite the advantages of the PSA system, certain disadvantages result
when the PSA process is used under high adsorption pressures, for
example above about 600 psig (40.8 bar). For the solution of the
problems encountered at higher pressures, the application teaches
sub~ecting a high pressure input stream which has a hydrogen content
of up to 90 mol % to a membrane separation process before feeding it
'
' ' ',
.
-
`
2 ~
to the PSA syste~, which membrane separation proces~ ~electively
retains impurities. The hydrogen-rich permeate gas is subject to an
appreciable pressure drop in passing through the membrane 90 that it
can be directly fed to a PSA system at lower pressure.
Thus, in prior art systems, the purified hydrogen gas is obtained
at relatively low pressures and must be compressed before it may be
used in a high pressure synthesis, whlch significantly increases the
operation costs of the overall synthesis process.
It is now an a~pect of the present invention to provide highly
pure nitrogen more energy efficiently than before for use in high
pressure syntheses or for the sale to consumers.
Applicants performed high pressure tests with molecular sieves
and have surprisingly found that molecular sieves may be used in PSA
systems at pressures much higher than the possible pressures suggested
in the art.
Accordingly, the invention now provides a process for the
production of highly pure hydrogen at high output pressures, which
includes the use of molecular sieves in PSA systems under input
pressure~ of 100 to 220, preferably 150 to 200 bar.
In a preferred embodiment, a crude synthesis gas is produced and
purified in a synthesis gas producing operation by a process in
accordance with the invention, which crude synthesis gas is produced
by thermal cracking of hydrocarbon containing materials with oxygen
and/or oxygen enxiched air and in the presence of steam in an
autotherm non-catalytic reaction, by a subsequent first hydrogen
sulfide scrubbing with cold methanol, a pressure conversion of the
sulfur free gas, a second scrubbing with cold methanol, a third
scrubbing with liquid nitrogen, and compression of the purified
synthesis gas obtained by way of a turbo compressor to a pressure of
30 100 to 220, the turbo compressor being driven by high pressure steam,
the synthesis gas being fed to the high pressure PSA process for the
production of highly pure hydrogen gas at high pressure. The
hydrocarbon containing materials are prPferably selected from the
group of crude oil, light or heavy fuel oil, residue oils, tar oils,
heavy gasoline, light gasoline, liquid gas, refinery gas and natural
gas.
:
:~:
, . ~ .. : .,
,
2~2~
-- 3 ~
In a synthesis gases producing operation including integrated
ammonia and methanol (MeOH) syntheses as well as hydrocracking
operations, a series of economical advantages may be shown when a
process ln accordance with the inventlon is used. For example, the
compression cost are reduced, since the purified hydrogen is obtained
from the PSA system at much higher pressures than in prior art
processes. Furthermore, logistic advantages may be shown. For
example, load changes in the ammonia and methanol syntheses of such an
operation may be achieved more easily without falling below the
minimal output limits or the pump limits of turbo compressors used for
the compression of the synthesis gases.
The use of a process in accordance with the invention in the
production of synthesis gases for high pressure syntheses is further
described in the following b~ way of an exemplary process and with
reference to the drawings, wherein
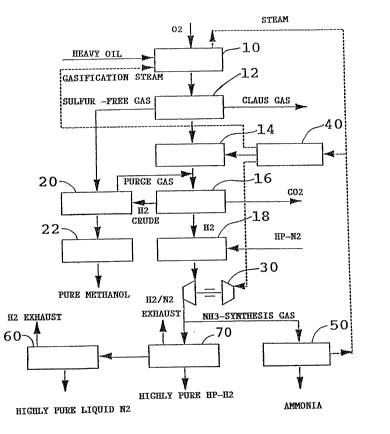
Figure 1 illustrates a flow diagram of a preferred process in
accordance with the invention; and
Figure 2 illustrates schematically the PSA system included in the
proce3s illustrated in Figure 1.
Pressure stability tests were performed with appropriate
molecular sieves of type 4 A grade lot number 135-1-9-110 at between
lO and 40C ambient temperature and in 500 ml autoclaves in order to
gain experience with molecular sieves at pressures above 65 bar. The
tests consisted of pressure load experiments including five phases per
cycle and 10 cycles overall as follows:
1st Phase: Pressurization with nitrogen to 50 bar within 4 to 5
minutes,
2nd Phase: Pressurization with hydrogen to 150 bar within
further 8 to 10 minutes,
3rd Phase: Maintaining the pressure at 150 bar for about 5
minutes,
4th Phase: Depressurization to ambient pressure within 12 to 15
minutes, and
5th Pha~e: 5 minute pause.
Subsequent examinations of the mechanical properties of the
molecular sieve bodies surprisingly showed that the molecular sieves
are able to mechanically withstand the higher input pressures so that
2~2~6
their applicability in high pressure PS~ systems may be assumed
ensured.
In a preferred embodiment of the process in accordance with the
invention for the production of highly pure hydrogen gas at high
output pressures for use in subsequent high pressure syntheses as
illustrated in Figure l, a crude synthesis gas is produced under
pressure by thermal cracking of hydrocarbons with oxygen in an
autotherm non-catalysed reaction step lO. The heat required for this
reaction is obtained from gasification steam which is generated in a
steam superheater 40 and recycled thereto after passage through the
hydrocarbon crac~ing reaction setup. The pressure gasification gas
obtained is subsequently scrubbed under pressure with cold methanol in
a first scrubbing step 12 to bring the contained hydrogen gas to
synthesis purity for use in ammonia and methanol syntheses in
hydrocracking processes. The first scrubbing step 12 is preferably
subdivided so that in a primary hvdrogen sulfide (~12S) scrubbing a
sulfur free gas is produced, which may be directly used in a methanol
synthesis step 20 followed by a methanol distillation step 22 for the
production of pure methanol. The removed hydrogen sulfide rich gas is
transported, for example, to a Claus installation for further
processing. A conventional carbon monoxide pressure conversion step
14 is in~erted after the first scrubbing step 12 for the production of
input gas for ammonia synthesis and for the production of purified
hydrogen gas. The carbon dioxide of the converted crude gas is either
removed as lye by hot potash scrubbing or by ethanolamine scrubbing
with monoethanolamine in a second scrubbing step 16, or the converted
crude gas is directly adjusted in an additional step (not illustrated)
with cold methanol to the synthesis purity required for methanol
synthesis. The pre-purified hydrogen obtained is cleaned of remaining
carbon monoxide and methane (CH4) with high pressure liquid nitrogen
(HP-N2) at -180C by cryogen scrubbing in a third scrubbing step 18.
Simultaneously, the nitrogen and hydrogen contents required for the
ammonia synthesis are ad~usted to 26% nitrogen and 74~/0 hydrogen. The
hydrogen/nitrogen mixture resulting from the cryogenic scrubbing is
used in an ammonia synthesis step 50 after compression to 200 bar by a
turbo compressor/turbine combination 30, for example. In the
alternative, the hydrogen/nitrogen mixture is selectively fed at the
:
2~5~2~
-- 5 --
same input preSs~lre to the ammonia synthesis step 50 and to a high
pressure PSA system 70. Turning now to Figure 2, a PSA system is
preferably used in a process in accordance with the invention inlcudes
5 parallel operating adsorbers 72 which include appropriate high
pressure molecular sieves 74 (type 4 A grade from ~nlon Carbide).
Molecular sleves 74 are poured solid bed sieves. Synthesis gas is
supplied through conduit 74 to all absorbers 72 and highly pure high
pressure hydrogen is removed from the PSA system through line 76.
Depending on the degree of purity, highly pure hydrogen is produced by
the PSA system 70 at a discharge pressure of about 150 bar. The feed
of synthesis gas to adsorbers 72 is controlled by valves 78 which
permit the selective feeding of an excess supply of synthesis gas to a
nitrogen liquifaction installation 60 (see Fig. l). Thus, a
hydrogen/nitrogen mixture is supplied at a ratio of 70:30 volume ~O and
at a pres3ure of for example 50 bar to the nitrogen liquifaction
installation 60. Finally, a waste gas is produced at about 2 bar
which contains only little hydrogen and may be used, for example for
the heating of the steam superheater 40.
Returning to Figure 1, the turbo compressor/turbine combination
30 which is used to compress the hydrogen/nitrogen gas mixture to the
reaction pressure required ln the ammonia synthesis step 50, is driven
by superheated steam generated in steam superheater ~0. The
throughput of the nitrogen scrubbing step 18 must be controlled, in
this embodiment, to remain within the throughput limits of step 18 and
turbo compressor 30 when the input of the ammonia synthesis changes.
The throughput must be especially controlled to remain above the lower
throughput limit of scrubbing step 18 and to avoid a lowering of the
turbo compressor output below the minimum output limit or below the
minimum pump limit. If the throughput in the nitrogen scrwbbing step
18 fell below the lower throughput limit, the beds would dry up, which
would lead to a breakthrough of carbon monoxide to the ammonia
synthesis resulting in irreversible catalyst damage. Thus, in order
to avoid these difficulties, the throughput of nitrogen scrubbing step
18 is maintained above a lower limit, which may lead to the production
of excess compressed ammonia synthesis gas, when the throughput of the
ammonia synthesis step 50 is reduced. This excess gas, however, is
advantageously fed at pressures of 100 to 220 bar to the PSA system 70
. .
20~2~
-- 6 --
which includes high pressure molecular sleves (not shown) for the
production of highly pure high pressure hydrogen tHP-H2).
The highly pure high pressure hydrogen may be used without
additional compression costs for example, in hydrocracking processes
commonly used in refineries. However, ~he highly pure high pressure
hydrogen may also be directly used in high pressure hydrocracking
processes at, for example, 170 bar without additiona]. compression in
contrast to hydrogen obtained from reformer installations which is
produced at about 15 bar and must be compressed.
As discussed above, a nitrogen/hydrogen mixture may be supplied
to nitrogen liquifaction step 60 whereby this mixture is
advantageously passed through a depressurization turbine (not shown)
for the recovery of compression energy.
Also, the highly pure high pressure hydrogen may be
advantageously used for other hydrations such as white oil hydration,
or naphtha hydration, or may be directly filled into pressurized tanks
for sale.
Thus, it i9 apparent that synthesis gas producing installations
may be operated more flexibly and with more efficiency with a process
in accordance with the invention in that the synthesis gas may be very
energy efficiently provided to an additional production step for the
production of high pressure highly pure hydrogen.