Note: Descriptions are shown in the official language in which they were submitted.
$ 8 3 ~ ~
AUTOMATIC ELECTROPHORESIS APPARATUS AND METHOD
This application is related to Canadian patent number
1,337,692 issued December 5, 1995 and Canadian patent number
1,328,845 issued April 26, 1994 both entitled AUTOMATIC
ELECTROPHORESIS APPARATUS AND METHOD.
BAC~GROUN-D OF THE lNv~NLlON
Field of the Invention
This invention is related in general to the field of
electrophoretic analysis of liquid samples. In particular, the
invention relates to an apparatus and method for completely
automating the electrophoresis process beginning with the step of
applying liquid samples to an electrophoresis support media and
without moving the support media further including the steps of
electrophoresing, stA;n;ng~ incubating, drying, scanning and
performing densitometry measurements on the scanned samples.
2a ~8 3 ~ ~
- 2 -
Electrophoresis is the science of moving charged particles in an electric field
through a solid or semi-solid media. The technique is most commonly used in medical
research in medical laboratories for analyzing various blood proteins.
Descri,clion of the Prior Art
In the diagnosing of ailments of human beings and animals, it is known that muchinformation can be provided by an analysis of certain biological fluids such as blood
serum proteins, lippo proteins, hemogiobin and isoenzymes. It is well known thatelectrophoresis is an effective method of separating the respective components of such
fluids for a microscopic analysis or for employing optical densitometry techniques in
analyzing the samples.
In the basic method of electrophoresis, charged molecules of the sample fluids are
separated under the influence of an electrical field wherein the liquid sample to be
examined is applied to a support medium having a buffer moistened porous surface.
Because the various components of the fluid move at different rates through the support
medium, the liquid sample may be separated into its respective components. Subsequent
staining of the fractional components in the support medium may then be subjected to
optical densilo,netry or other methods for examination.
The electrophoresis process has been performed through a series of manual steps
for many years. The manual process typically has started with the operator prepa, ing an
2 0 electrophoresis chamber by filling appropriate cavities of the chamber with buffer solution.
Buffer solution is a liquid used in the electrophoresis process to ,nain~ain the support
medium surface in a moist condition and to provide an electrical interface to a power
source applied to the chamber so that an electric field may be applied to the support
medium. The support medium is typically a piece of MYLAR (trademark) backing which
~
-- ~ 2~ 5~3~
-3 -
has been coated with a gel substance such as cellulose acetate or agarose. The liquid
sample to be examined is typically a blood serum, but of course may be other liquids, the
components of which may be moved through an electric field.
After the operator has prepared the electrophoresis chamber, he then applies as
precisely as he can, consistent volumes of the samples to precise locations on the
support medium. The operator then places the support medium into the electrophoresis
chamber so that the edges of the support medium are immersed in two buffer cavities at
each of its longitudinal ends. Electrophoresis is then performed using a precise and
consistent high voltage applied for a precise and consistent interval of time across the
buffer cavities.
After electrophoresis has been completed, the operator applies a uniform coatingof staining reagent or stain to the surface of the support medium allowing a precise and
consistent interval of time for the reagent and sample to chemically combine. The staining
reagent is a liquid used af~er electrophoresis to chemically combine with the separated
components of the fluid sample, causing its components to exhibit optical characteristics.
Next, the operator places the support medium into a temperature controlled oven
and incubates it using a precise and consistent temperature and time interval. Incubation
is the process of controlling the chemical reaction between the components of the liquid
sample and the staining reagent by means of applying heat for a fixed interval of time.
Next, the operator dries the sample plate by increasing the oven temperature for
a second precise and consistent temperature and time interval. The drying process stops
the reaction between the sample plate and the reagent by removing water from thesupport medium.
One of the problems associated with the manual support medium preparation is
r _~
~ 4 ~
that the liquid samples to be analyzed are multiply applied to the support medium which
is to be subjected to electrophoresis. The samples may be applied to the support
medium one at a time in serial fashion with a hand pipettor, but the hand pipettor must
be rinsed with a cleansing agent and blotted before a new sample is aspirated and then
5 applied to the strip. Applicators have been designed to apply fluid samples
simultaneously or in "parallel" to the strips. Such applicators are described at page 61 of
the General Products Catalog for 1984-1985 of Heiena Laboratories with offices in
Beaumont, Texas. Such applicators may apply eight, twelve or more samples to a
microporous support medium and have the advantage of making the electrophoresis
technique easier and more reproducible.
Such applicators however have been essentially non-automatic and have required
cleaning of the applicator tips after each application to the support medium. A
disadvantage of the prior art applicators is that there has been no means for automatically
washing and cleaning the barrels of the pipettes during each cycle time so as to prevent
15 contamination of each of the barrels during application of a new plurality of fluid samples
to a new support medium. Another disadvantage of the prior art applicators is that there
has been no means for precisely automatically applying a very small amount - of the order
of one microliter - of sample liquid to a support medium. Another disadvantage of the
prior art is that there has been no means for precisely automatically diluting a very small
20 amount of the order of one microliter of sample fluid with a diluting liquid and precisely
applying a very small amount of the diluted sample to a sùpport medium.
There have been prior art apparatus and methods available for automatically
performing electrophoresis and staining of the plurality of samples applied to a support
medium. For example, U.S. Patent No. 4,360,418 to Golias and U.S. Patent No. 4,391,689
3 4 ~
- 5 -
to Golias describe an automated electrophoresis and staining apparatus and method.
Such apparatus includes an electrophoresis chamber and a series of vats mounted
upon a platform and arranged in a row where the vats are adapted to contain respectively
a liquid stain and a series of plate processing solutions. The plate holder rack, having a
5 horizontal open frame, supports an upright electrophoresis plate or support medium onto
which has been applied a sample for electrophoretic fractionization. Such electrophoresis
plate had to have been previously prepared by applying liquid samples either manually
or by using one of the parallel applicators described above. The plate is nested within the
charnber within an electrophoretic circuit for a predetermined time period. A power
operated lift and transfer assembly is provided on the base and is adapted to lift, transfer
and lower the plate holder rack and plate from the chamber progressively into each of the
underlying vats for a predetermined period in a linear stepping motion maintaining the
plate in an upright position at all times. It is noted that the staining process relies on
chemical procedures for the staining process rather than the manual system described
15 above where incubation and drying are used. Although the apparatus described above
has many desirable features, it has a practical disadvantageous feature in that it requires
providing a plurality of chemicals and wash solutions in the unit which must be maintained
periodically.
Prior art apparatus and methods for optically scanning support media which have
2 o been subjected to electrophoresis and staining have used devices such as photomultiplier
tubes, photodiodes or similar devices which produce an electric current or voltage output
proportional to the light falling on such device. These devices are generally referred to
as detectors. Prior art instruments employing these detectors are used for determining
various physical properties of the samples which have been prepared by electrophoresis.
- i~ 2n 5834~
The properties of interest concerning the separated bands of the sample are size and
optical density or intensity of emitted light which is of a wave length different from that of
the excitation light source. Separated bands of each sample which have been subjected
to electrophoresis are known components of the sample under test and it is desirous that
they be quantified for the purpose of aiding in medical diagnosis or research.
The known instruments which use the detectors referred to abov¦e generally find
it necessary to use a blocking optical slit. The purpose of the slit is to allow the detector
to "instantaneously view" a portion of the sample plate which is the same relative size and
shape as the slit. The detector then produces an electrical current or voltage which is
10 proportional in amplitude to the magnitude of the light detected. The current or voltage
produced is then converted by means of an analog to digital converter and the resultant
digital representation of the light magnitude is stored in an organized format in a digital
computer memory.
- - - Although an aiternative embodiment of the invention described below uses prior art
15 detectors in combination with other automatic electrophoresis apparatus, a preferred
embodiment of the invention includes the use of video electronic scanning of the samples
on the support medium which have been prepared by electrophoresis. Video electronic
scanning is preferred in recognition of well known problems of using such prior art
scanning detectors. One of the problems of using such prior art instruments is that the
2 o blocking slit requires a very precise width and length. If the length is too great, some of
the detected light may actually be the result from an adjacent sample. If the length is too
small, all of the light from the sample currently being scanned may not be detected. With
a plurality of samples on a plate it may be necessary to change the physical slit size from
sample to sample.
-7-
If the slit physical width is too great, it is possible that
the light from adjacent bands of the plurality of samples being
scanned could be detected causing the boundaries to be difficult,
if not impossible, to determine. If the width is too small, it is
possible that the detector output will be erratic and not yield
correct proportional results.
Another disadvantage of the prior art slit/detector system is
that in order that the entire sample be observed, it is necessary
that each sample be mechanically scanned by moving either the
detector or the sample plate. The movement must be at a very
constant speed and free of vibration in order that the digital
data being collected by the A to D converter is an accurate
representation of both the optical density and physical size of
the components of the sample.
In order that a plurality of samples may be scanned, it is
necessary that the detector or sample plate be moved in yet
another axis such that the scanner may scan a sample and then step
over to the next sample and continue the sc~nn; ng process. The
step over movement must be accurate and repeatable to insure that
the detector is truly seeing the entire sample and only desired
sample.
Certain separation procedures, called isoelectrofocusing
techniques, apply chemicals to the electrophoresis support media
to alter its pH. Such pH altering chemicals act to focus charged
proteins which move longitudinally in the support media under the
force of a current sheet or electric field. The combination of
the pH altering chemicals and the current sheet modifies the
electric field at the vicinity of the separated protein.
Prior art machine~ have not had the capability to perform
accurately stAn~Ard electrophoresis separations and alternatively
2 0 ~
--8--
isoelectrofocusing separations for at least two lateral rows of
samples on the electrophoresis support media.
Summary of the Invention
Accordingly the invention generally provides a single
apparatus means for automatically applying a plurality of liquid
samples to a support medium, automatically subjecting such samples
on the support medium to the electrophoresis process,
automatically staining, incubating and drying the support medium
on which the component~ of the liquid samples have been separated
into longitudinal bands, automatically electronically scanning
such bands and automatically performing a densitometric analysis
to the data which results from such scans, thereby providing an
analysis of each liquid sample.
-The invention in a broad aspect provides electrophoresis
apparatus comprising a base, an application plate longitudinally
disposed on the base, an electrophoresis support medium removably
disposed on the application plate having longitudinal and lateral
~;~en~ions with raised first, second and third lateral reservoir
strips of electrically conductive material disposed respectively
at one longitll~;nAl end, at the other longitl]~;nAl end and at an
intermediate position. A cathode electrode pair have first two
electrodes di~posed on opposite lateral sides of the support
medium at a first longitll~;nAl end of the application plate, each
of the first two electrodes being adapted for connection to a
negative terminal of a source of d.c. electricity. An anode
electrode pair have second two electrodes disposed on opposite
lateral sides of the support medium at a second longitudinal end
of the application plate, each of the anode electrodes being
adapted for connection to a positive terminal of a source of d.c.
electricity. A first conductive electrode bar is laterally
~P"
,~
2 n ~ ~ 3 ~ ~
-8A-
disposed across the support medium in electrical contact with the
first lateral reservoir strip and a second conductive electrode
bar is laterally disposed across the support medium in electrical
contact with the second lateral reservoir strip. A third
conductive electrode bar is laterally disposed across the support
medium in electrical contact with the third lateral reservoir
strip and cathode connecting means electrically connects the
cathode electrode pair to the first and ~econd conductive
electrode bars and anode connecting means electrically connects
the anode electrode pair to the third electrode bar.
More particularly, the invention provides an automatic
electrophoresis machine which automates electrophoresis testing of
liquid samples. The machine includes a base on which an
application plate is supported. A microporous support strip is
placed on the application
~ ,~
2 ~ 5 8 3 ~ 6
plate. A scanning box encloses the application plate.
A liquid sample plate is supported by the base at a position longHudinally separated
from the application plate. The sample plate includes a plurality of liquid sample wells in
one or more lateral rows. Prior to operation of the machine, liquid samples to be tested
are placed in the wells. A robotic frame is provided for translating between the sample
plate and the application plate through and opening in a side wall of the scanning box.
The robotic frame carries a row of pipettes, one or more staining reagent bottles and one
or more solenoids with associated plungers.
Under computer control, liquid samp!es from the sample plate are applied in a
o lateral row to the surface of the support medium strip. Electrode bars cooperating with
vertically magnetized posts provide a lateral sheet of electrical current through the support
medium strip for electrophoretically longitudinally displacing components of the liquid
samples while the application plate is simultaneously cooled.
Adapter apparatus is provided to be used with the apparatus descrlbed above to
conduct certain types of sample separations including isoelectrofocusing. The adapter
apparatus includes an anode assembly and a cathode assembly which are adapted to fit
between up-standing pairs of cathode magnetized posts and anode magnetized posts.
A modified electrophoresis plate (or microporous support medium) with raised first,
second and third reservoir strips of electrically conductive material is adapted to be
2 o properly aligned when placed on the application plate. Three conductive electrode bars
are provided to be placed across the electrophoresis plate in contact with a respective
lateral reservoir strip. Electrical apparatus of the anode assembly provides posHive
voltage potential from the up-standing anode magnetized posts to the third electrode bar
when placed across the middle reservoir strip. Electrical apparatus of the cathode
~ 2 ~ 3 ~ e~ f ~, ~
~ .d J -'
- 10-
assembly provides negative voltage potential from the vertical cathode magnetized posts
to first and second electrode bars placed across lateral reservoir strips at opposite ends
of the electrophoresis plate. One lateral sample row is placed between the first and third
raised reservoir strips. A second lateral sample row is placed between the third and
5 second reservoir strips.
In operation, a current sheet moves in the electrophoresis plate between the third
and first reservoir strips, thereby separating components of samples in the first row. Such
current sheet moves from the middle or "third" reservoir strip toward the first reservoir
strip. A current sheet of substantially identical intensity moves in the electrophoresis plate
10 between the third and second reservoir strips thereby separating components of samples
in the second row with substantially the same separating force, but in an opposite
longitudinal direction.
BRIEF DESCRIPTION OF THE DRAWINGS
The objects, advantages and features of the invention will become more apparent
15 by reference to the drawings which are appended hereto and wherein like numerals
indicate like parts and wherein an illustrative embodiment of the invention is shown, of
which:
hgure 1 is a perspective view of an automatic electrophoresis machine according
to the invention having a robotic assembly between a sample plate unit and a
2 o microporous support medium in a scanning box wherein the front door of the scanning
box has been removed to show its interior;
hgure 1A shows the automatic electrophoresis machine with an associated
computer which provides command and control signals to digital control circuitry of the
2 o 5 8 3 4 6
machine and which performs densitometric analysis of the sample plates after electronic
scanning;
Figure 2 is a side view of the automatic electrophoresis machine, partially in
section, showing the robotic crane assembly, the sample/wash/blotting plate unit, the
electrophoresis application plate, the microporous support medium, the scanning box and
a video camera mounted atop the scanning box and further shows mechanical aligning
apparatus for precisely aligning pipettes with the support medium;
Figure 3 is a plan view of the automatic electrophoresis machine according to the
invention taken along lines 3-3 of Figure 2 and showing a downward looking view of the
sample/wash/blotting plate unit, the microporous support medium, electrode and
spreader bar apparatus associated with it, and the robotic frame;
Figure 3A is an electrical schematic diagram showing an electrophoresis voltage
source placed across electrode post pairs at longitudinal ends of the support medium and
illustrating the electrophoresis current flowing in a lateral sheet across the longitudinal
dimension of the support medium;
Figure 3B illustrates the simultaneous application of current to a cooling/heating
device disposed beneath the application plate on which the support medium is placed
during the electrophoresis current application to the support medium and shows that
current may be applied in the opposite direction to the device for heating and also shows
mechanical aligning apparatus whereby pipettes of a pipette assembly are precisely
aligned mechanically with application wells of the support means;
Figures 3C and 3D illustrate the combination electrode/spreader bars according
to the invention;
Figure 3E illustrates the displacement of components of the samples applied to the
2 ~ 5 8 3 b~
support medium after the electrophoresis step has been performed;
hgure 3F illustrates an alternative arrangement for applying electrophoresis current
to a support medium such that electrophoresis current is caused to flow in a lateral sheet
across the longitudinal dimension of the support medium;
Figure 4 is an end view taken along lines 44 of Figure 2 and shows the robotic
crane assembly in more detail and shows the construction of a heat sink associated with
the cooling of the plate on which the microporous support medium is secured and shows
a drying duct system by which the microporous support medium is dried after the reagent
is provided to it and after it has been incubated;
o Figure 5 is a downward looking view along lines 5-5 of Figure 2 and shows the duct
pattern by which the application plate and the microporous support medium removably
secured thereto is dried and the duct means by which cooling air is brought from outside
the machine across the heat sink to carry heat away from the plate during the
electrophoresisprocess; as shown with Figure l;
hgure 6 is a schematic illustration of digital control circuitry and its interfaces with
robotic assembly circuits and devices and with electrophoresis chamber circuits and
devices;
Figure 7 is a schematic illustration of a computer associated with the automatic
electrophoresis machine of the invention showing interfaces with the digital control
20 circuitry of the machine, with the scanning camera and with peripheral devices for
input/output communication with the computer;
hgures 8 - 13 are schematic representations of the various steps by which the
robotic crane assembly applies samples to the microporous support medium, applies
reagent to it after electrophoresis has been conducted, and by which ths reagent is
-- ~ 2 ~ 5 8 3 4 6 ~ . A
spread across the surface of the microporous support medium and illustrate the electronic
scanning of the support medium after incubation and drying of it;
Figures 14A-14F illustrate a flow chart of logic blocks of the computer program
stored in the digital computer and digital control circuitry for automatically controlling the
electrophoresis process;
Figure 15A illustrates a uniform support medium used to calibrate the camera lens
system and shows computer templates established about scanning tracks corresponding
to sample tracks or rows of an actual electrophoresis support medium;
Figure 15B illustrates electronic templates established about scanning tracks
corresponding to sample tracks or rows of an actual electrophoresis support medium;
Figure 15B illustrates electronic templates created under program control for
automatically creating pixel boundaries about each of the electronic images of the
electrophoretic patterns after automatically performing the electrophoresis process of a
pluralityofsamples; as shown with Figure lA;
Figure 16 illustrates an alternative embodiment of the invention where mechanically
driven scanning apparatus is disposed on the robotic crane assembly thereby as an
alternative to the electronic video scanning apparatus of Figures 1-15;
Figures 17 and 18 illustrate an alternative embodiment of the electrophoresis
apparatus of the invention where separate electrode bars and spreader bars are
2 0 employed;
hgures 19 and 20 illustrate an alternative support medium where application wells
of rectangular shape are formed in the gel of the support medium;
Figure 21 is a top view of a portion of the electrophoresis apparatus which
illustrates an adapter designed for use with the machine of Figures 1-20 for performing
~ 2 ~ ~ 8 3 4 ~ ?
- 14-
certain isoelectrofocusing operations;
Figure 22 is a cross-section view of a portion of the apparatus as viewed from lines
22-22 of Figure 21, the cross-section illustrating the structure of a new microporous
support medium especially adapted for isoelectrofocusing operations;
Figure 23 is a cross-section view of a portion of the apparatus as viewed from lines
23-23 of Figure 21, the cross-section illustrating an iron striker and a magnetic electrode
post of the machine of Figures 1-20; and
Figure 24 is a cross-section view of a portion of the apparatus as viewed from lines
24-24 of Fi~ure 21, the cross-section illustrating the connection of an electrical lead to a
magnet of a cathode assembly.
DESCRIPTION OF THE INVENTION
Description of the robotic crane assembly
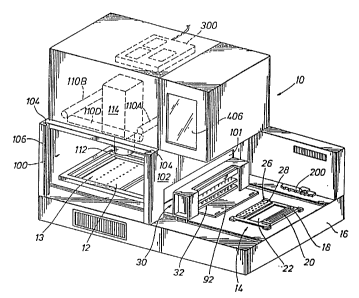
Figures 1 and 1A show the automatic electrophoresis machine 10 and its
associated digital computer 400. As shown in Figure 1, the automatic electrophoresis
machine 10 includes a base 16 on which is mounted a sample plate unit 14 and an
electrophoresis chamber 13 for mounting a microporous support medium 12. The
support media which may be used in the electrophoresis process preferably includes a
MYLAR backing on which a coating of cellulose acetate, agarose, or agar gel is
deposited. The particular construction of the support medium according to the invention
2 0 iS described in detail below.
The automatic electrophoresis machine 10 includes a robotic crane assembly 30
adapted to move longitudinally between the sample plate unit 14 and the electrophoresis
chamber 13. The automatic electrophoresis machine 10 includes a scanning box 100
having a side wall 106 and an entry wall 102 and a back wall. The
front of the sc~nn;ng box includes slots 104 in which a door (not
shown) may be mounted for providing access to the sc~nn;ng box 100
and for closing the scanning box 100 during electrophoresis
processing and st~;n;ng and electronic sc~nn;ng of samples applied
to the support medium 12. The door may include an interlock
safety device in circuit with the electrophoresis high voltage
supply such that when the door is in the open position,
electrophoresis voltage within the chamber 13 is prevented. Such
safety device prevents inadvertent operator shock from voltages as
high as 2000 - 3000 volts in electrophoresis chamber 13. A cover
92, shown in its open position, may be slid longitudinally to open
and close the electrophoresis ch~her 13.
Fluorescent lights llOA - llOD are provided in the top of the
scAnn;ng-box for fluorescently lighting the support medium 12
during electronic sc~nn;ng by the camera 114/lens 112 system under
control of the computer 400. Digital control circuitry 300 used
to control the robotic assembly 30 and the electrophoresis process
will be discussed in detail below. A videographics cathode ray
tube 406 is mounted on the automatic electrophoresis machine and
under computer 400 control provides monitoring information to the
operator.
Turning now to Figure 2, a front sectional view of the
machine 10 shows details of the sample plate unit 14, the robotic
crane assembly 30, the electrophoresis chamber 13 and the camera
114/lens 112 system within the sc~nn;ng box 100. The machine 10
includes a base 16 on which a horizontal mounting plate 15 is
provided for carrying the sample plate unit 14. The sample plate
unit 14 is similar to that described in Canadian Patent No.
1,286,389 issued July 16, 1991 in the name of the assignee of this
2 ~
-16-
invention. Such patent may be referred to for further details of
the operations of automatically applying samples from a sample
plate to a remotely disposed support medium.
The application plate 14, which may be manually supplied with
liquid samples prior to placement on the machine 10, includes two
lateral rows 26 and 28 of sample chambers in which liquid samples
are provided which are to be automatically applied to the support
medium 12. A blotting space 22 is provided on which blotting
paper may be placed. Of course multiple blotting spaces, each
with its own blotting paper may be provided if desired. A waste
well and wash well is provided on the sample plate 14 by which the
pipettes (including barrels and plungers) carried by the robotic
crane assembly are cleansed and excess fluid dumped during the
automatic application of samples. The pipette assembly 32 carried
by the robotic assembly 30 is similar in construction and function
as described in the abovementioned patent for its showing of the
construction and function of the automatic application of samples
from the sample wells of rows 26 and 28 to application on the
support medium 12 in the electrophoresis chamber 13.
As best shown in Figures 2 and 4, the robotic crane assembly
includes a frame 40 which is mounted for translation on robotic
travel tracks 34 by means of rollers 36. The tracks 34 are
supported by base 16. As shown in Figure 4, the rollers 36, 36'
are attached to the frame 40 by means of shafts 38. The rollers
36, 36' have grooves into which lateral projections of the tracks
34 extend, thereby allowing the robotic crane assembly 30 to be
longit--~;n~lly moved between the sample plate unit 14 and the
electrophoresis chamber 13. The tracks 34 are carried by
horizontal members 4 secured to vertical members 3 which are
supported by the base 16.
~' !!?'
'f'--3 , ,;
3 4 ~
As shown in Figure 4, a motor 208 mounted on base 16 has an output shaR 209
secured to a drive wheel 210. As shown in Figure 3, a take-up wheel 210A is provided
at the longitudinally opposite end of the machine. A continuous belt 212 driven by drive
wheel 210 and looped about take-up wheel 210A is secured to an extension 214 of shaft
38 of the frame 40. Thus, by actuation of motor 208 the roller 210 drives belt 212 about
take-up wheel 21 OA thereby translating the robotic crane assembly 30 with respect to the
base 16.
Shown in Figures 2, 3 and 4, the robotic assembly 30 includes a vertical member
56 carried by frame member 40. Ho~izontal plates 58, secured to vertical members 56,
support shafts 52 of bottle support member 50 as illustrated in Figure 4. Two reagent
bottles 48 are secured to bottle support member 50 by means of set screws 61. A
reagent spread motor 60 mounted with respect to frame 40 is provided having its output
shaft connected to shaft 52 of the bottle support member. Actuation of the motor 60
- causes the bottle support member 50 to rotate until staining reagent provided in the
bottles 48 is dumped onto the support member 12 when the robotic crane assembly 30
has been moved to a position over the electrophoresis chamber 13.
As illustrated in Figures 2 and 3, a vertical bar 46 extends upwardly from the frame
40 of the robotic crane assembly 30 and has attached thereto two solenoids 42. Each
of the solenoids has a slotted arm 44 attached to its output shaft. Each of the slotted
2 o arms includes a slot 44A which is adapted to fit about the electrode/spreader bars 74 and
76 of the electrophoresis chamber to be described below. The slotted arms 44 are also
adapted to fit within holes 93 of the electrophoresis cover 92 shown in Figure 3.
From the foregoing it is seen that the robotic crane assembly of the invention is
adapted to move longitudinally between the sample plate 14 and the electrophoresis
2058346
- 18 -
chamber 13 and includes a pipette assembly 32, a pair of solenoids 42 and a pair of
reagent bottles 48. The control of the pipette assembly for applying liquid samples from
wells 26 and 28 to the electrophoresis chamber, the solenoids with their slotted arms 44
for spreading reagent and for closing the electrophoresis cover and the reagent bottles
48 for applying reagent to the support medium 12 is described below with respect to
Figure 6.
Another feature of the robotic crane assembly is described here in conjunction with
Figures 1, 2 and 4. The robotic crane assembly 30 is adapted to move from outside the
scanning box 100 through opening 101 in entry wall 102 of the scanning box 100. It can
o be seen that the top 321 of the pipette assembly has a lateral profile that fits relatively
closely within the opening 101 as the robotic assembly 30 is passing into the scanning
box. During the electronic scanning of the support medium 12 by the camera 114/lens
112 system, external light from outside the scanning box 100 is substantially prevented
from entering into it by virtue of the dimensions of the exterior profile of the robotic
assembly 30 fitting within the opening 101 of the entry wall 102.
Description of the Electrophoresis Chamber
As best shown in Figures 2, 3 and 4, the mounting plate 15 supports application
plate 80 which is disposed laterally between the robotic travel tracks 34. The robotic
crane assembly 30 is free to move on tracks 34 longitudinally above the application plate
2 o 80. As illustrated in Figure 3, the application plate 80 includes one or more guide pins 68
for aligning and removably securing a support medium 12 such as an agarose strip. The
agarose strip (support medium 12) includes two fluid reservoirs 64A, 64B at its longitudinal
ends. The fluid reservoirs are each a raised gelatinous strip constructed of the same
material as the top layer of the support strip, for example, agarose. The support medium
~-- ~0~834~
- 19-
12 preferably includes two lateral rows of wells or indentions 62, 63 in the agarose
material for accepting samples which are to be electrophoresed.
The electrophoresis chamber 13 has a first pair of electrode posts 94 extending
vertically to a level substantially the same as the vertical level of the support medium 12.
A second pair of electrode posts 96 are longitudinally separated from the first pair 94 and
likewise extend above the support medium 12.
The first pair of posts 94 and the second pair of posts 96 are preferably
constructed of a permanently magnetized material such as iron, yet are adapted also to
conduct electrophoresing current. A first combination electrode/spreader bar 74 is
disposed at one longitudinal end and a second combination electrode/spreader bar 76
is disposed at the other longitudinal end of the chamber 13.
The bar 74 and the bar 76 are preferably constructed of a ferro-magnetic material
such as iron or steel. Thus when the electrode/spreader bar 74 and the bar 76 are
disposed as illustrated in Figure 3, they are held in place to electrode post pair 94 and
electrode post pair 96 by the force of magnetism of the magnetic posts snd the ferro-
magnetic material of the bar. Figure 3A illustrates that the posts 94 are connected to the
positive terminal of a source of electrophoresis potential VE and the electrode post pair 96
is connected to its negative terminal.
The bar 74 in cooperation with the post 94 distributes the electrophoresing current
2 o laterally to the reservoir strip 64A and then across the support medium t2. The current
moves longitudinally in a lateral sheet through the support medium 12 until it reaches the
raised reservoir portion 64B of the support medium 12 where it then passes through the
bar 76 to the posts 96 completing the electrophoresing circuit.
Figures 3C and 3D illustrate that the bars 74, 76 may be constructed either entirely
- 20 -
of ferro-magnetic material such as iron as in Figure 3D or it may have its end portions
constructed of a ferro-magnetic material having an intermediate portion constructed of
graphite or stainless steel. Under the influence of the electrophoresing current flowing
through the support medium 12, the components of the liquid samples in the sample
5 indentions or wells of row 62 and row 63 are electrophoresed longitudinally. hgure 3E
illustrates the displacement of components of the material in the support medium 12 in
lateral bands 62A, 62B for example with respect to sample row 62 and in bands 63A, 63B
for example with respect to fluid samples in sample row 63.
Other means for establishing current laterally across support medium 12 from
10raised portion 64A to raised portion 64B are of course possible. For example, Figure 3F
illustrates conductive hinges 75, 77 connected respectively to potential source VE. The
hinges fold outwardly to open plate 80 for placing support medium 12 on it. W~th the
medium 12 in place, the hinges may be folded downwardly establishing electrical contact
with raised portions 64A and 64B respectively.
15Although the components of the liquid samples in rows 62 and 63 have been
longitudinally displaced as shown in Figures 3A and 3E, the support medium 12 must be
stained through a reagent application, incubation and drying process before they may be
optically scanned with illuminated fluorescent light as will be explained below.
So that the electrophoresing step may be accomplished more rapidly by the
2 o application of a higher electrophoresing current (which results in resistance heating of the
support medium 12 and the application plate 80), two thermo-electric cooling/heating
devices 70 (preferably six of them arranged as shown in hgure 3) are provided beneath
the application plate 80. The thermo-electric devices 70 are preferably Peltier devices
which function to carry heat away from its top surface to its bottom surface on the
2 Q 5 1~ 3 6, ~ ~
application of electric current in one direction to the device. When current is apptied in
the opposite direction to the Peltier device, heat is applied to the applic~tion plate 80. An
electrical schematic diagram of FTgure 3B illustrates that the application of current to
devices 70 forces heat from the application plate 80 to a heat sink 84 thermally connected
5 to its lower side. Current in the opposite direction forces heat from the heat sink 84 to the
application plate 80.
Figure 4 shows more clearly the placement of the Peltier devices 70 beneath the
application plate 80 and illustrates that metallic conductors 82 are provided at the
underside of the cooling devices and carry a finned heat sink 84 device beneath their
lower side. Insulation 78 fills the spaces between and to the sides of the cooling devices
70.
As shown in the cross-sectional view of Figure 5, the finned heat sink 84 extends
into an inlet cooling duct 206. Cooling air is brought into the duct 206 by means of fans
204 and passed over the fins of the heat sink 84 and pass out~ardly to the rear of the
automatic electrophoresis machine 10 via exit duct 208. When the circuits as illustrated
in Figures 3A and 3B are actuated, that is during the electrophoresing process, current
is applied to the Peltier cooling devices 70 in one direction by which the heat generated
in the electrophoresing process is carried away and out of the machine by the cooling air
from the duct 206 and out duct 208 as forced by cooling fans 204. The cooling apparatus
20 illustrated is advantageous in the machine 10 in that a higher electrophoresing current
may be applied, thereby reducing the time required for the electrophoresing step. The
additional heat created by such higher current Ts effectively disposed of by the Peltier
cooling devices.
After the electrophoresing step has been accomplished and reagent applied to the
n ~ 8 3 4 6 '~
support medium 12 surface and spreading has been accomplished, all of which steps will
be described in more detail below, it is necessary to incubate the support medium 12 with
the staining reagent spread across its surface. Such incubation is accomplished by first
closing the cover 92 to form a closed chamber about the electrophoresing chamber 13.
As illustrated in hgures 2, 3 and 4, a pair of vertically extending horizontal chamber
bars 88 extend vertically from the plate 80. Longitudinal slots 90 are provided inwardly
of the vertical bars in which the cover 92 may be slid longitudinally thereby covering and
uncovering the electrophoresis plate 80. Figure 3 illustrates the cover 92 in its open
position and shows holes 93 in its end which are provided to cooperate with the slotted
arms 44 of the solenoids 42 for opening and closing the cover.
As discussed above the Peltier devices 70 are provided beneath the application
plate 80 with current provided to them in a direction opposite that for cooling when they
are used during the incubation step (and the drying step). On actuation of an electric
current (see Figures 3B and 6) in the opposite direction to Peltier devices 70, heat is
applied directly to plate 80 which transfers that heat to the support medium 12 for
incubating the reagent stain on the support medium.
Figures 2, 3 and 4 show the means by which drying air is applied across the
surface of the support medium 12 after the incubation step has been completed.
Longitudinally extending slots 86 are provided on lateral sides in the plate 80 outwardly
2 o of the space where support medium 12 is placed. Such slots are illustrated for example
in hgure 3 and may also be seen in cross-section on lateral sides of the end view of the
application plate in Figure 4. The righthand side of the slot 86 communicates with an inlet
drying duct 98 while the leRhand rectangular slot in plate 80 communicates with the outlet
drying duct 99.
5~346~D'?'
- 23 -
A heater element 202 is provided in the inlet drying duct 98 as well as a dryer fan
200. The inlet drying duct is mounted by means of a bracket 2t8 affixed to the metallic
heat sink 84. The outlet duct is mounted by means of a bracket 216 likewise secured to
the metallic heat sink 84. During the drying step, air is brought in from the front of the
machine by means of the dryer fans 200 through inlet drying duct 98 and across the
heating element 202, thereby applying drying heat to the surface of the support member
12.
Scannina Box
As best illustrated in Figures 2 and 4, the scanning box 100 includes four
fluorescent bulbs 110A-110D mounted in a rectangular pattern near the top of the box.
A camera 114 having a lens 112 is mounted in the top wall 109 of the box and views
downwardly toward the surface of the electrophoresis support member 12. Of course,
in order for the scanning of the camera 104 to be effective, the cover 92 must be moved
or displaced longitudinally outwardly so as to reveal the plate 12 to the camera 114/lens
112 system. As previously described of course, during the scanning of the
electrophoresed and stained support member 12, exterior light is essentially blocked by
means of the front cover (not shown) in slots 104 (Figure 1) and by virtue of the robotic
crane assembly 30 essentially filling the opening 101 in the entry wall 102.
Control Circuitry and Interfaces
2 o Figure 6 illustrates in block diagram form the interconnection between the digital
control circuitry 300 as illustrated schematically in Figure 1 and the robotic assembly
elements 30 which control the movement of the robotic crane assembly. Various
information and control circuits disposed in the electrophoresis chamber 13 are also
illustrated. The digital control circuitry 300 is connected to the associated computer 400
~0 ~346
- 24 -
by means of a bus 410. The connection of the computer400 to the digital control circuitry
300 is shown physically in hgure 1A and sche",alically in Figures 6 and 7 which will be
disclJssed in detail below.
The digital control circuitry 300 includes a CPU 301 which is preferably a
5 microprocessor chip commercially available as Motorol~F~lC 6802. A read only memory
302 is provided for storing so~tware control program instructions. A random ~ccess
memory 303 is provided to store temporary data. Input/output inter~aces (VIA) 304
includes programmable input/output interfaces and a system timer which is used to
provide output control and input communication or monitoring functions and
10 programmable time intervals. A digital to analog (D/A) converter 305 is used to provide
analog output voltages to analog circuits in the electrophoresis chamber 13. Analog to
digital converter (A/D) 306 is used to provide monitoring voltages from circuits in the
electrophoresis chamber. A serial input/output interface 328 is used to interface input
co""nands and output signals between the computer 400 and circuitry 300 via bus 410.
A databus 329 is provided as a bi-directional digital connection between the CPU
ROM, RAM, VIA, D/A, A/D circuits and the serial input/output interface circuit. A bus 330
is an address bus used as a uni-directional digital connection from the CPU to the ROM,
RAM, VIA, D/A, A/D circuits and the serial input/output interface. The address bus 330
is used by the CPU to uniquely select a device from or to which digital data is being
2 0 l,~ns~erled.
An output interface bus 331 is connected from circuit 304 and is used to connectthe digital output of the CPU to the digital input of circuits being conlrolled by the CPU.
Similarly, the input interface bus 332 is used to connect the monitor and detector circuits
to the digital input of the CPU via input interface circuit 304.
,r ~ ~ ~,
-25- 2~34~ ~
Turning now to the robotic assembly 30, five separate
elements are controlled: the gantry base 40, the pipette barrel
and pipette plunger of the pipette assembly 32, the reagent
support member 50 and the solenoids 42. A detailed description of
the pipette barrel and the pipette plunger control is not
described here, but their control is as described in detail in the
previously referred to Canadian Patent 1,286,389 of Messrs.
Sarrine and Garsee. The previously mentioned patent provides a
complete description of the operation and control of the pipette
barrels and pipette plungers as they are used in applying liquid
samples from wells 26 and 28 to the support member 12.
Gantry base or frame 40 control is by means of a motor driver
and braking circuit 307 for controlling motor 208. The position
detector 316 schematically illustrated in Figure 6 is physically
embodied by means of the sample cam plate 201, the application cam
plate 203 and limit switches 205 and 207 illustrated in Figure 3.
The position detector functions by counting interruptions of the
switches 205 and 207 as they pass the cams on the ~ample cam plate
201 and the application cam plate 203.
The motor driver and braking circuit 308 of the pipette
barrel and its position detector 317 as well as the motor driver
and braking circuit 309 of the pipette plunger and its position
detector 318 are as described in the above referenced patent.
With respect to the reagent support member 50, a motor driver
circuit 310 is provided under control of the CPU 301 and VIA
circuit 304 to turn motor 60 in one of two directions when the
gantry base 40 is in position over the support medium 12. A limit
switch (not illustrated) serves as a position detector 319
associated with the shaft 52 of the bottle support member 50 as
shown in Figure 4.
A solenoid driver circuit 311 is provided in association with
solenoids 42 80 as to
.
d~
2~ ~8~6
- 26 -
extend the slotted arm 44 to its downward position when a signal is applied to it.
An interlock circuit 323 is provided beneath the sample/wash/blotting plate 14 so
as to signal the CPU via the input/output interface 304 that the sample plate 14 is in
position and that the machine is ready to receive a start command from personal
computer 400.
Turning now to the circuits of the eiectrophoresis chamber 13, a high voltage circuit
325 and a high voltage monitor circuit 326 are used to provide electrophoresis current to
the support medium 12 as illustrated in Figure 3A. The high voltage circuit 325 is
responsive to a command from the CPU 301 via the D/A converter 305, D/A bus 333 and
scanning box door interlock circuit 373. The monitoring signal from the high voltage
monitor 326 is applied to the A/D converter 306 via bus 334.
Similarly, a temperature monitor circuit 327 applies its analog signal to the A/D
converter 306 via bus 334 for recognition by the CPU 301. The temperature sensor or
transducer 327 may be seen within the electrophoresis chamber 13 in Figure 2. Digital
control signals to Cooier/Heater (Peltier devices) 70 are applied from output bus 331 to
a heater incubation circuit 313. Similarly, digital control signals to Peltier devices 70 are
applied to a cooler circuit 314 from output databus 331.
~amp driver circuit 315 responds to digital commands via bus 331 for controllinglamps 110A-110D in the scanning box 100.
2 o Turning now to Figure 7, a schematic description of the elements of the computer
400 indicates its connection to the digital control circuitry 300 via the serial input/output
interface circuit 328 and bus 410. A serial input/output interface 401 provides the
interface via the bus 410 for the computer 400. Preferably the computer 400 is a~Personal Computer" such as the Compaq Desk Pro Model (Trademark). The computer
~~ 2~34~ ~~
- 27 -
400 is used to communicate commands from the operator of the system of Figure 1A to
the automatic electrophoresis machine 10 and to report data from it to the operator,
anayze the digital data stored in the computer and to produce both graphic and text
reports to the operator by means of output devices.
S Input from the operator to the computer is by means of a keyboard 407 while
printed output is by means of a printer 408. A videotext cathode ray tube 405 may be
associated directly with the personal computer 400 while a videographics CRT 406 may
be provided directly in conjunction with the automatic electrophoresis machine 10 as
illustrated in Figure 1A.
o Electronic Scanning and Calibration
The video camera 114 is preferably a VIDIaCNIM T.V. tube w~ich E~mduces a seri~lanalog voltage representation of its viewing area. A frame grabber 403 interfaces w~ith
camera 114 to convert the camera's serial analog output to a digital data represenl~ion.
The frame grabber 403 stores such digital representation in the memory 409 of the frame
grabber which subsequently produces both graphic and text reports of the analysis.
In operation, the camera 114 sees a viewing surface somewhat similar to Figure
3E after the electrophoresis and staining processes have been aulomalically performed
under control of the computer 400 and the digital control circuitry 300. When the
fluorescent lights 11OA-11OD are turned on, the camera scans the entire support medium
2 0 12. The camera produces analog video and synchroni~a~ion signals. The in~tanlaneous
video vo tage amplitude is a representation of the magnitude of the light emitted from ~the
surface of the support medium. This analog output voltage is then converted as indicated
above to a digital representation of a matrix of 512 columns by 512 rows of ~pixeis~ by
means of frame grabber 403. The synchronization signals are used to correlate the video
c
~' ' ' .
~ 4~
~*~
2058346
- 28 -
analog data to precise locations on the sample plate 12.
Before the sample plate unit 14 and the automatic electrophoresis operation begins
with the automatic electrophoresis machine 10, calibration of the camera 114 lens/112
system is performed. Calibration corrects for non-linear parabolic effects which may
produce a non-uniform response of the intensity level of the individual pixels of the matrix
of 512 columns by 512 rows as sensed by frame grabber 403.
In order to calibrate the camera 114/lens 112 system, a uniform test support
medium 12', as illustrated in Figure 15A is placed in scan box 100 on application plate 80.
The test support medium 12 is one that has had no'samples applied to it, and of course
has not been electrophoresed, incubated or stained. The front door to the scan box is
closed and the robotic crane assembly 30 is moved into the opening 101 so as to
simulate actual scanning conditions where substantially all outside light is blocked from
entry into the scan box 100~ Next, the ultraviolet lamps 110A-1 10D are turned on, and the
camera 114/lens 112 system is turned on. The frame grabber 403 (Figure 7) receives a
"snap shot~ of the plate; that is, the intensities of each of the pixels of the 512 by 512
matrix are stored in memory 409.
Next, templates 801, 802, . . . 815 are electronically defined under program control
about the fifteen scan tracks corresponding to the 15 sample tracks or rows of an actual
support medium 12 when placed on application plate 80. The "heighta or "y~ direction of
each track is about 1/15th of the memory's 409 height (approximately 34 pixels). The
width or "x~ direction of each track is equal to the total width, 512 pixels, of the total width
of the image memory. These 15 tracks correspond to the location of the sample tracks
of the electrophoresis plates to be scanned later.
~Ithin each of the 15 tracks, the two dimensional array of pixel intensity data is
2 ~ ~ ~ 3 4 6
- 29
converted into a singls dimensional array of data by summing and averaging the pixel
values in each of the 512 vertical columns within each of the approximately 34 pixel rows.
That is for each track, at each vertical column, the intensities of the 34 pixels in that track
are summed and divided by the number of pixel rows, e.g., 34. As a result, each of the
5 15 tracks is represented by a row vector of average intensities as a function of the x
dimension of pixels running from x=1 to x=512. A search of each of the average
intensities in this raveraged intensity" matrix of 15 by 512 intensity values is then made to
determine the greatest value, I,.~,.
Next, each average pixel intensity in the averaged intensity matrix (15 by 512) is
o divided into the value of I ~. Each element in the matrix is replaced by the result of such
division. Thus each element of the matrix becomes a correction factor of a rcorrection
factor matrix" to be applied to an actual support medium during operational scanning after
the support medium 12 has had samples applied to the two sets of fifteen sample wells,
and after steps of electrophoresis, staining, incubation, drying, etc. have been
automatically performed.
Figure 15B illustrates electronic templates such as 601, 612, 616, 623 which are
formed, under computer programming control, to define automatically the analysis area
of each of the 512 x 512 pixels stored by frame grabber 403 for an actual support medium
12 which has been automatically electrophoresed. The "y~ axial dimension of the
20 templates are the same as each of the 15 calibration tracks described above during
- calibration. For example, the template 601 is predetermined to ft about the longitudinal
electrophoresis pattern of the sample placed on support medium 12 at well 701. Because
the medium 12 is physically located at a predetermined location on application plate 80,
and because the camera 114/lens 112 is fixed with respect to application plate 80, the
0 5 ~
- 30 -
ele~tronic template 601 is assured of precisely fitting about the ele.:lro, ~horesis paller" for
the fluid sample at well 701. Programmed electronic te",~lates are provided for each of
the samples.
The data in each of the templates is then averaged over the pixels in the y rows5 inside the template to produce a single representation of density as a function of
electrophoresis spreading distance x for each of the samples. Next, the average intensity
values for each x pixel position in each template is multiplied by a corresponding
correction factor stored in the correction factor matrix described above. Such data is then
stored in an organized format in the digital memory 409 of the computer 400 where a
densitometric analysis may be performed. U.S. patent 4,242,730, assigned to the
assignee of the invention described herein, describes a prior microprocessor - controlled
densitometer. The 4,242,730 patent ~ describes how digital
representations of the scanned samples may be processed to produce an analog display
on a CRT like display 405 illustrated in computer 400 of Figure 1A. An operator may edit
15 the visually displayed density curve.
It is ad~,anlageous to use a video camera or a similar device such as a CCD array
because the entire sample plate may be scanned in one-thirtieth of a second. Such
scanning includes i"ror,l,alion abut all thirty samples for example, as shown in Figure 3E.
llle data may be oryar,i~ed by the computer in a two-dimensional array of data and
2 o therefore allows the computer to not only exactly define individual longitudinal components
of the sample but also to exactly determine sample boundaries in the event that sample
se~a~lions do not occur in a parallel fashion. Additionally, the sample data may be
enl ,anced by removing or reducing noise artifacts by repeating the scan and averaging
the results.
\ ~
3 4 ~
Mechanica1 Scanning
There may be circumstances where advantages of electronic scanning of the in situ
electrophoresed support medium may not bs indicated. Cheaper manufacturing costsmay dictate the use of a prior art mechanical blocking slH and detector assembly 900 as
part of the robotic assembly 301 of Figure 16. The automatic electrophoresis machine
10 ~ of Figure 16 is substantially the same as machine 10 of Figure 1 except that assembly
900 provides mechanically driven electronic scanning as an alternative to the stationary
video electronic scanning of the video camera 114/lens 112 system of Figure 1.
The scanning assembly 900 preferably mounted on the forward side of robotic
o assembly 30 ~, includes a fixed fluorescent tube 901, collimator 903 and photomultiplier
tube 905. The tube 901 is disposed inside the cover 906. The collimator 903 is disposed
in a lateral slit 904 in the cover and faces downward toward the support medium 12 during
scanning. The photomultiplier tube 905 is responsive to light transmitted via collimator
903.
A motor, not shown, under microprocessor control is provided to step the
collimator 903/photomultiplier tube 905 laterally across the electrophoresed support
medium 12 after sample fluid application, staining, incubation and drying as previously
described. An electrical service loop or cable is provided between the photomultiplier
tube 905 and amplifier/analog to digital converter (not shown) for input of scanning
signals to computer 400. Service loop 907 may also be connected to digital control
circuHrv 300 (Figure 6) for controlling fluorescent lamp 901 illumination during scanning.
Longitudinal stepping across support medium 12 during scanning is accomplished by
incrementally translating robotic assembly 301 longitudinally with motor 210 (Figure 3).
2n~8346
- 32 -
As show above, the mechanical blocking slit and detector assembly 900 generates
electrical signals representative of the intensity of longitudinally separated components of
the electrophoresed and stained samples on support medium 12.
Figure 16 illustrates the robotic assembly 30~ before it is moved longitudinally
5 above support medium 12. It should be appreciated that scanning box 909 may be
constructed with less height than scanning box 100 of Figure 1 because there is no need
to have a camera lens system with the scanning apparatus of Figure 16.
Operation of the Electrophoresis Machine
After the operator has installed the sample plate unit 14 in position on the base of
the machine as indicated in Figure 1 and after a support medium strip 12 has been placed
on the application plate 80 as indicated in Figure 3, a door is closed in front of the
scanning box 100 and the operator enters a start command via keyboard 407 of computer
400. It is to be understood that the liquid samples to be analyzed have been placed in
the pair of well rows 26, 28 of the sample plate unit 14. Each well row preferably includes
15 fifteen separate wells. A standard liquid sample may be placed in one of the wells for
analysis comparison. It is also understood that blotting paper may be put into the blotting
space 22 and that wash well 20 has previously been filled with wash water. The
placement of the sample plate unit 14 on the base of the machine sends a signal over bus
332 to the digital control circuitry 300 as shown in Figure 6. The computer 400 then
2 o receives an indication that the automatic processing may proceed under digital control.
hgures 8-13 illustrate significant steps in the automatic processing of the liquid
samples stored in wells of rows 26 and 28 of the sample plate unit. hgure 8 illustrates
that the pipette assembly draws samples and individual pipettes of a predetermined
amount. As indicated above such operation is described fully and completely in the
2~ ~3~
33 -
previously issued Canadian Patent 1,286,389.
Figure 9 shows that the robotic assembly 30 has been longitudinally moved to thearea of the support medium 12 and that the fluid samples are applied in a row on the
surface of the support medium. It is noted that the cover 92 of the electrophoresis
chamber is in its open position.
Figure 10 illustrates the closing of the cover 92 after the slotted arm 44 has been
actuated by solenoids 42 to engage holes 93 in the cover 92. Figure 10 illustrates that
the robotic assembly 30 has been moved longitudinally toward the electrophoresischamber thereby closing the cover 92.
It is assumed that after the cover has been closed, the electrophoresis process of
the samples is performed. Such process has been described above but in summary, it
includes applying an electrophoresis voltage to the support medium by means of the
posts, electrodes and combination electrode/spreader bars as indicated previously.
Simultaneously with application of the electrophoresis current to the support medium 12,
current is applied in one direction to the Peltier heating/cooling devices 70. Cooling of
the support medium 12 allows a higher electrophoresing process to be accomplished with
higher speed.
Figure 11 illustrates the application of reagent 47 from reagent bottles 48. The
reagent of such bottles are dumped onto the surface of the support medium 12 by turning
the bottle support member 50 by means of motor 60. Figure 11 indicates that the cover
92 has been previously brought to the open position by the reverse process of that
indicated in Figure 10.
Figure 12 illustrates the spreading of the reagent across the surface of the support
medium 12. Preferably the spreading is accomplished by actuation of the slotted plunger
- 34 -
arm 44 so that the slots of both spreader arms are about a bar such as 76. The robotic
assembly is then reciprocated longitudinally such that the reagent is spread across the
surface of the support medium 12. The other spreader bar 74 may similarly be used in
addition to the bar 76 illustrated in Figure 12 to further spread the reagent on the surface
of the support medium 12.
Next, the cover 92 is moved to its closed position in an operation similar to that
shown in Figure 11 and incubation and drying steps are performed. The incubation step
calls for the Peltier devices to be operated so as to heat the application plate 80 for a
predetermined length of time. The drying step calls for additional drying air to be brought
through ducts and across the support medium as illustrated more clearly in Figure 4.
After the incubation and drying steps have been performed, the electronic scanning
of the in situ support medium 12 is conducted. As Figure 13 illustrates, the camera
11 4/lens 112 system produces an analog signal representative of the field of view of the
support medium 12 as illuminated by fluorescent lights 110A-110D. An image of such
optical signal may be reproduced on CRT 406 mounted directly on the machine. Figure
13 also illustrates that the robotic assembly 30 is within the opening 101 of the entry side
wall of the scanning box 100 to substantially reduce outside light from entering the
scanning box during optical scanning by the T.V. camera 114.
Description of computer control of machine operations
Figures 14A-14F illustrate in flow diagram form the control of the machine. Figure
14A shows that a signal from computer 400 is passed to digital control circuitry for
automatic control of the electrophoresis process. As indicated by logic box 500, the
pipette assembly is driven to rest position by driving the barrels Up and the plungers
down. Such control is described in the above-mentioned corresponding Canadian
~ f~
4 ~
- 35 -
Patent 1, Z86, 389. The gantry base 40 is driven to home position by applying a control
signal to motor driver and braking circuit 307 and sensing itS position with position
detector 316. Next, the computer waits for a start command via serial input/output
interface 328, as indicated by logic block 501. The computer then determines by means
of logic block 502 if the sample plate t4 has been placed on the base plate 15 of the
machine. If a signal is present form interlock circuit 323, control of the process continues;
if the signal from circuit 323 is not present, an error signal is returned to computer 40~ for
printing or displaying an error message to the operator of the machine.
As shown in Figure 14B, logic blocks 503 apply liquid samples from sample wells
o 62 to support medium 12. As indicated, functions of washing and drying the pipette tips
precede the application of the samples to the support medium and follow such
application. Liquid samples from sample wells 28 are then applied to sample wells 63 of
support medlum 12. The pipette tips are again washed. As indicated above, the washing,
drying, blotting and application steps are similar to those described in Canadian
Patent 1, 286, 389. Next, in logic step 505 the barrels of the pipette assembly 32 are
driven up and its plungers are driven down.
As illustrated in Figure 14C, a series of steps 506 are performed under digital
control circuitry 300 control to close cover 92 over the electrophoresis chamber 13.
These steps begin with logic block 507 where the gantry base 40 is driven to the open
2 0 position of cover 92. Then in step 508, the current is applied to the solenoid drive circuit
311 so that arms 44 are driven downwardly to engage holes 93 in cover 92. Next in logic
block 509, the gantry base 40 is driven toward the electrophoresis chamber 13 to the
closed position of the cover 92. In logic block 510, current is disconnected from solenoid
driven circuit 311 whereby the arms 44 return to their rest position. In block 511, the
2058346
- 36 -
gantry base 40 is driven to its home position.
Logic blocks 512 are performed to apply electrophoresis current to the support
medium 12, while simultaneously cooling it. ~ogic block 513 sets the time length for the
application of electrophoresis current and adjusts and applies high voltage between
electrophoresis post pairs 94 and 96. In logic block 514, cooler circuit 314 is activated
to turn coolers 70 and fans 204 on. Logic blocks 516 monitor the voltage from circuit 325,
monitor the electrophoresis time and then turn off the voltage and coolers.
Turning next to Figure 14D, logic blocks labeled collectively as 517 describe the
steps necessary to open cover 92. Such steps are similar to those of steps 506 to close
cover 92 and consequently are not described in detail. The blocks labeled collectively as
518 provide the control for applying staining reagent to the surface of the support medium
12. In logic block 519, the gantry base 40 is driven to the support medium 12 to a
position approximately mid-way between electrode/spreader bars 74, 76 In logic block
520, the reagent motor drive is actuated thereby rotating the reagent bottles 48 operably
applying reagent to the top surface of the support medium 12. The reagent motor drive
is then driven in the opposite direction to return the reagent bottle support member 50 to
its rest position.
The logic blocks collectively identified by the reference number 521 describe the
steps necessary for spreading the staining reagent across the top surface of support
2 o medium 12. In logic block 522, the gantry base 40 is moved until solenoids 42 are directly
above electrode/spreader bar 74. In block 523, current is applied to solenoid driver
circuit 311 so that arms 44 are extended downwardly such that the slots 44a of the arm
44 "grip~ or partially envelope the spreader bar 74. In logic step 524, the gantry base 40
is driven toward the second wells 63 and then are driven again to the position of electrode
2 ~ ~ ~ 3 4 ~
- 37 -
posts 94. The current to the solenoid driver circuit 311 is disconnected in logic block 525
to return the arms 44 to the rest position. The logic blocks labeled 526, 527, 528, 529,
control the spreading of the reagent by spreading electrode/spreader bar 76 across the
surface of the support medium and returning the solenoid arms 44 to their rest position.
The cover 92 is then closed according to the logic blocks 530, identical to those
labeled 506 performed earlier in automatic process.
Turning next to Figure 14E, the process continues with logic steps labelled
collectively as 531 for reagent soak. The digital control circuitry 300 in these steps allows
a sufficient time to pass between application of the reagent to the surface of the support
medium and the start of the incubation period.
The logic steps labelled collectively as stain incubation 532 begin with step 533 for
setting the incubation time and step 534 for setting-the incubation temperature. The
incubation heater circuit 313 is turned on the logic step 535. Logic steps 536 monitor
incubation temperature from sensor 327 and passes controi to drying steps 538.
The logic steps labelled collectively as drying 538 begin with steps 539 and 540where drying time and drying temperature are set. In logic step 541 dryer circuits 340 are
activated for turning heater on 202 and fans 200. Steps 542 monitor drying temperature
from temperature monitor 327 and monitors the drying time. Step 543 turns off the
incubation heater circuit 313 and the dryer circuit 340.
2 o Figure 14F shows that, next cover 92 is again opened under control of logic steps
labelled collectively as 544. Such steps are identical to those labeled above as steps 517.
Control is then passed to logic step 545 where a determination is made by computer 400
as to whether or not lamps 100A-100D are on. If not, control is passed to computer 400
which then issues a signal to turn them on in logic block 546. On receipt from a signal
8 3 6~
- 38 -
of logic block 547 indicative that the lights have been turned on, control is passed to logic
block 548 where the video image from camera 104 is captured and stored in memory.
The lights 110A-110D are turned off under control of logic block 549.
The computer 400 then performs densitometric processing in logic step 550
according to known methods of determining the relative densities of components of the
samples which have been longitudinally separated as a result of the electrophoresis
process. Graphical outputs are displayed on cathode ray tubes 405 and 406 and printed
reports are output on printer 408 as indicated by logic blocks 551 and 552.
As explained above, the pipette assembly 32 of robotic crane assembly 30 is
translated from the sample plate 14, after aspirating samples into its individual pipettes
from row 26 or row 28 to the application plate 80 for depositing individual samples onto
application wells 62 or 63 of sample medium 12. Such translation under computer control
of a motor 210, drive belt 212, cams 201, 203, limit switches 205, 207, etc. is accurate,
- but it is important that such samples be precisely laid down on such wells 62 or 63 so that
the electrophoresis steps and densitometric analysis may be as accurate as possible.
Description of mechanical sample positioning
Accordingly, as illustrated in Figures 2, 3 and 4, mechanical alignment of pipettes
32 with application wells 62 or 63 is provided by the interaction of tapered pin pairs 450,
452 and 454, 456 secured to application plate 80 and slots 458, 460 in barrel drive
structures 462, 464 of pipette assembly 32. Such structures 462, 464 may be seen in
alignment with pins 450, 452 in Figure 4. When the pipettes 32 are lowered for applying
sample to application wells 62 on support medium 12 (see Figure 3), the slots 458, 460
mate with pins 450 and 452. As a result, the pipettes are precisely aligned withapplication wells 62.
20583~6
- 39 -
The slots 458, 460 are preferably inversely conically shaped to match the conical
shape of the ends of pins 450, 452 and 454, 456. The slots 458f 460 act like funnels for
pins 450, 452 or 454, 456 as the pipettes 32 are lowered, such that initial misalignments
of the pipettes 32 with the application wells 62 or 63 is substantially eliminated.
Description of reduction of friction variation between robotic frame rollers and robotic
travel tracks
As described immediately above, it is important in electrophoretic processing ofliquid samples that such samples be precisely applied to the application wells 62 or 63.
Longitudinal positioning of pipettes 32 is accomplished by motor driving and braking
circuit 307 as indicated in Figure 6. Due to slight variations in the lateral spacing between
tracks 34 as a function of longitudinal distance between sample plate 14 and application
80 (see Figure 3), the friction between rollers 36 ' and tracks 34 may vary as a function
of such longitudinal distance. This variation may result in variable performance of the
motor driver and breaking components 307, which can, in extreme cases prevent accurate
positioning of the pipette barrels over application wells 62 or 63.
To reduce such friction variation as a function of longitudinal distance betweensample plate 14 and application plate 80, spring mounts 37 (see hgure 3) are secured
to the gantry frame or base 40 and extend outwardly from frame 40. Rollers 36 ~ are
carried by mounts 37 by means of springs 39 which force the rollers 36 ~ 1aterally inwardly
2 0 against their track 34. Such inward force of rollers 361 on one track of tracks 34 reduces
variations in gantry or frame drive friction allowing the motor driver and breaking devices
to more accurately position pipettes 32 over application wells 62 or 63.
Alternative provision of separate electrode bars and spreader bars
2 ~ ~ 8 3 4 ~
- 40 -
As explained above and espscially in connection with Fgures 3A and 12, electrodebars 74, 76 may function also as reagent spreader bars. Figure 12 illus~,a~es the
spreading of reagent on the top of support medium 12 by the action of slotted arm 44
moving (e.g., rolling) bar 76 across the surface of support medium 12.
Under certain circumstances, when the carbon or graphite bars 74, 76 spread
reagent across the top surface of support medium 12, a residue may resuH on suchsurface due to chemical reaction of the carbon of the bars, the reagent and/or the gel
composition of the support medium. Accordingly, the alternative embodiment of Figure
17 obviates that possibility. In such embodiment, it is preferred to use the electrode bars
of Figure 3C outside the electrode post pairs 94 and 96. Such electrode bars 741 and
76 ~ have end portions of magnetic n~alerial such as iron so as to be held magnetically
to magnetized posts 94 and 96, but have middle portions of graphite to better conduct
electrical current via conductive strips 64A and 64B of support medium 12. The all iron
electrode bars of Figure 3D may also be used for electrode bars 741 and 761.
The bars are placed outside of imaginary lines between laterally spaced posts 94and 96. The magnetic posts 94, 96 extend vei lically from applica~ion plate 80 above the
support medium 12 and are arranged with respect to support medium 12 such that when
bars 741 and 76 ' are in place, such bars make electrical contact not only with posts 94
96, but they also contact raised strips 64A, 64B of the support medium 12.
Thé spreader bars 75 77'are placed inside the lateral imaginary lines between
posts 94 and between posts 96. r,eferably, bars 75 77~are faL,ri~led of glass but other
inert ,na~eri&ls could be substituted for glass. The spreader bars 75,~77'will remain in
place atop support medium 12 because (1) appli~;on plate 80 (see Fgure 18) is
3 4 ~ ~
- 41 -
maintained in a level condition during operation of the autorna~ic electrophoresis machine
of the invention and (2) the top surface of support medium 12 is of a gel composition
which has a relatively high coefficient of friction. Consequently, spreader bars 75, 77
remain where placed atop support medium 12 ~djacent post 94 or 96 and do not roll
5 away from same in normal operation. Alternatively, spreader bars 75 77~may
advantageously be constructed in a similar manner to the electrode bars 741 and 761;
that is, as illustrated in hgure 3C, end caps of magnetic material such as iron may be
provided to be held to magnetic posts 94 and 96 respectively so as to hold, under the
force of magnetism, glass rods 75~and 77~against posts 94 and 96. Figure 18, similar to
o Figure 12, shows the step of operating the automatic electrophoresis machine where
slotted arm 44 has been lowered to roll spreader bar 7~back and forth across the surface
of support medium after reagent has been dumped there.
Alternative provision of rectangular application wells in support medium
As explained above and especially in connection with Figures 3, 3A and 3D, lateral
15 rows of application wells 62 and 63 are formed on the planar surface of support medium
12. Uquid samples to be subjected to electropl,orelic processing are placed in such
wells, preferably by the automatic pipette apparatus described above. The rows of
~pplicA~ion wells 62 and 63 are "dot" indentations in the gel surface of the support
medium. Such dots are characterized by generally circular pallerQs on the planar surface
20 or haH spherical indentions in the gel itself. If a sample that is placed in such circular
ir,.3en~tion contains multiple co"~ponents of in~eres~, a relatively longer electro,cl,oresis
time is required to clearly separate such co,npol)ents. If an identical sample is placed in
a rectangular incJentaliGn contai,ling such multiple components of inleres~, a relatively
sho, ler electrophoresis time is required to clearly separate such components.
.~ ~, 2058346
- 42 -
The illustration of Figure 20 shows the relative separation times of identical samples
having hypothetical ~A~ components and "B~ components. Each component is assumedto be a protein or the like which has an inherent identifying charge which causes that
component to electrophoretically move longitudinally under the influence of an electric field
or current applied to the gel of the support member.
The difference in charge on the A components and the B components translates
into a different longitudinal velocity between the two components under the influence of
an identical electric field. As illustrated in Figure 20, the components A and B of the
circular indentation 62A re~uire a relatively long time to translate until components A and
B are separated by a distance S because of the distance d or diameter of the circular
indentations. In other words, a sufficient electrophoresing time must be provided to allow
the faster moving B components to translate such that the Bl components at the back of
the circle are sufficiently separated from the A2 components at the front of the circle. The
shorter distance d ' of the rectangular indentation 62A ~ shortens the time for components
B~ at the back of the rectangle to be separated from components A3 at the front of the
rectangle. A shorter electrophoresing time is beneficial in that diffusion of the charged
components, a function of electrophoresing time, is reduced.
Alternative apparatus and method for applying electrophoresis current to electrophoresis
strip simultaneously in opposite direction to two parts of the strip
As indicated in the Background section above of this specification, certain
electrophoretic type operations cannot be conveniently performed using the apparatus as
illustrated in the foregoing description. For example, isoelectrofocusing operations call
for the application of p-h altering chemicals to the electrophoresis plate. Such operations
depend on such p-h chemicals in combination with electrophoresing current to achieve
~ ~834~ ~i
- 43 -
separation. Using a conventional two electrode system allows for the creation of a single
p-h gradient. Therefors it is possible to run only one row of samples. In the invention
embodied herein a three electrode system allows for the creation of two <~iscr~te, yet
identical p-h gradient fields on the same plate allowing two rows of samples to be
separated simultaneously.
Accordingly, the apparatus illustrated in Figure 21 is provided to cooperate with the
machine as illustrated in Figures 1-20 but to provide electrophoretic current sheets,
substantially identical in intensity in longitudinally opposite directions across two sample
rows.
Rather than use the electrophoresis plate (or microporous support medium) 12 andelectrode bars 74, 76 of Figures 3 and 4 (and 17 etc.), the arrangement of Figures 21-
24 is substituted. A new electrophoresis plate 12 ' is provided, according to the invention,
which is properly aligned on application plate 80 by aligning holes in plate 12 ~ with guide
pins 68. Electrophoresis plate 12~ (also called a microporous support medium above)
includes two rows 62 ~, 631 of wells or indentions in which samples are placed with the
pipettes 32 of the apparatus of Figures 1-20 as illustrated above. In ~ddition, three raised
reservoir strips of gel material, 64A ~, 64B I and 64C ~ are formed on the top surface of
plate 12~. Plate 12~ is prererably not as wide as plate 12 of Figures 1-20.
An anode assembly 280 and a cathode assembly 282 are constructed to fit
2 0 ~el-lleen opposite sets of magnetic posts, specifically cathode posts 94 and anode posts
96 of the machine of Figures 1-20. Such anode assembly 280 and cathode assembly 282
are maintained in physical and electrical contact with post sets 94, 96 by means of iron
striker plates 281A~, 281A~, 281C~, 281C~ which are affxed to outer ends of the
assemblies.
44 2 ~ 4 ~
Bars 2831 and 283n of magnetized ",alerial are affixed respectively at the firstends of cathode assembly 282 and anode assemb1y 280 such that a portion of such
magnetized material/faces outwardly toward the first longitudinal end of plate 12~. An
electrode bar 274 ~ is maintained in physical and electrical contact with magnetic bars
283 ~ and 283~1 because bar 274 ~ is constructed in a similar manner to the electrode bars
74 76 of hgures 3C or 3D. That is electrode bar 2741 is constructed of iron which is
attracted to magnetic material of bars 283 yet it readily conducts current, or alternatively
has iron end caps with a graphite middle portion. Such construction also provides
simultaneous magnetic attraction (due to the iron end caps) and current conduction
(because both iron and graphite conduct current).
At the opposite or "second longitudinal end magnetic bars 285 1 and 28511 are
affixed to the cathode assembly 282 and anode assernbly 280. Such bars have a portion
which face toward the second longitudinal end and are adapted to attract ends of a
second bar 27411 preferably of identical construction to that of first bar 2741. Bar 27411
iS placed on raised reservoir strip 64B I and is in simultaneous ele.1, ical contact with such
strip and magnetic bars 285 1, 285-1 and is held in physical contact to such bars by virtue
of the force of magnetism between magnetic bars 285 ~, 285~ and iron end portions of
bar 274~.
In the middle or third~ portion of the anode assembly 280 and the cathode
assembly 282 third magnetic bars 2871 28711 are affixed such that a portion of such
magnetic bars face longitudinally outwardly sufficiently to contact lateral ends of a third
lateral bar 276 which is constructed like bars 274~ and 2741'. Third lateral bar 276 is
placed in electrical contact with third reservoir strip 64C ~ and is in electrical and physical
~
- 45 -
contact with magnetic bars 287 ',287l' in a manner similar to that explained above.
Anode assembly 280 and cathode assembly 282 are each manufactured from a
unitary stamped or formed piece. In other words, cathode assembly 282 and anode
assembly 280 are fabricated from an identical block of insulating material, but each is
turned 180- from each other so as to face each other. For that reason, the additional
Ubar notch~ 293 in the middle or third arm of the assemblies is provided.
An electrical lead 295 is embedded in cathode assembly 282 which runs from iron
striker plate 281 C ~ at the first end of cathode assembly 282 to both the first end magnet
283~ and the second end magnet 285 ~ . Accordingly, the negative potential, (when a
circuit is connected as in Figure 3A) is placed on first cathode electrode bar 274 ~ and on
second cathode electrode bar 274'1, because an electrical path is-provided between
magnetic posts 94, first end iron striker plate 281Ci, electrical lead 295 and first end
magnet 283 ~ and second end magnet 285 ~ .
In a similar way, an electrical lead 297 is embedded in anode assembly 280 and
runs from iron striker plate 281A~ at the second end of the anode assembly 280 to
magnet 287~. Accordingly, the positive potential (when a circuit is connected as in Figure
3A) is placed on anode or middle electrode bar 276, because an electrical path is
provided between anode magnetic posts 96, striker plate 281A~, lead 297, magnet 287
and anode electrode bar 276.
When a circuit is connected, such that a source of d.c. potential has its positive
terminal connected to anode magnetic posts 96 and its negative terminal connected to
cathode magnetic posts 94, a first current sheet is impressed in electrophoresis plate 12 '
from anode or UthirdU bar 276 to ~second~ cathode bar 274 ~, and simultaneously a second
Q ~ 8 ~
- 46 -
current sheet is impressed from anode or ~third~ bar 276 to ~first~ cathode bar 274 ~ . Such
first and second current sheets are substantially of the same intensity or current level.
Figure 22 is a cross-sectional view through the electrophoresis plate 12~ and
application plate 80 taken along lines 22-22 of Figure 21. Raised reservoir strips 64A ',
64B ~ and 64C I are illustrated along with first electrode bar 274 ~, second electrode bar
274~ and third electrode bar 276. Peltier devices 70, heat sink 84, metallic conductors
82 and insulation 78 are, of course, identical to that illustrated in Figures 1-20.
Figure 23 is a cross-sectional view taken along lines 23-23 of Figure 21. Magnetic
post 94 is illustrated adjacent iron striker plate 281 C ~ which is affixed to arm 291 ' . Figure
24 is a cross-sectional view taken along lines 24-24 of Figure 21. Magnet 285~ is
illustrated with lead 295 attached to its side.
Although the apparatus is preferred as illustrated in Figure 21 such that the current
- sheets travel from the middle portion of electrophoresis plate 12 ' to opposite longitudinal
ends, it is within the scope of the invention to reverse the polarities of the magnetic posts
94, 96 such that current sheets flow from the longitudinal ends toward the middle or third
electrode. Of course, the sample rows would be moved toward the ends of plate 12 ' so
that separation components of samples would have space to migrate toward the middle
of the plate.
Although, all the functions of the machine of Figures 1-20 may not be used with the
modified electrophoresis plate 12' and anode and cathode assemblies 280, 282, many
of them can be used. The application of samples into the wells or indentions of rows 62 ~
and 63 ~ is automatically applied in a manner similar to that depicted in hgures 8 and 9.
Simultaneously, applying electrophoresis current to the electrophoresis plate while cooling
5 ~ ?
- 47 -
it, is of course also achieved. Although staining of the plate after electrophoresis may
not be necessary, drying with the machine of Figures 1-20 may be performed.
Various modifications to the automatic electrophoresis machine and methods
described above may be apparent to those skilled in the art which do not depart from the
5 spirit of the invention. The description above is employed for setting forth the preferred
embodiment of the invention and should be interpreted as illustrative, but not limitative.