Note: Descriptions are shown in the official language in which they were submitted.
WO 91/01707 PCT/GB90/01194
ADHESIVE DRESSINGS
This invention relates to adhesive dressings and
particularly to adhesive bandages as would be suitable
for first-aid dressings.
A prime requirement for first-aid dressings is
that they should be highly conformable and pliable
since they have to be used for the dressing of highly
rounded surfaces such as fingers as well as for flexing
surfaces such as knuckles.
Conventionally first-aid dressings are formed as
a relatively bulky but small pad of an absorbent
material such as gauze layers adhered to a larger
backing sheet made of a woven fabric or filmic
material. Usually the pad is covered by layers of
coverstock intermediate the pad material on the wound
to reduce adherence between the wound eschar and the
fibres of the absorbent material.
The manufacturing of such dressings requires need
to maintain accurate registry between the coverstock
and the pad and the pad and backing layer during the
production stage.
Known dressings may also suffer from the
~ ~ ~ ,~, ° . =,.
208421 ~r~~~a 80/01194
' 12 p7 91
_ 1 2 July 1991
disadvantage, that because they are manufacture from
highly porous materials, air-borne bacteria can enter
the dressing and infect the wound. This problem may be
further accentuated where the dressings are not water
proof and water-borne bacteria and viruses can enter or
leave the dressings.
We have now found that the problems associated
with manufacture and bacterial contamination may be
reduced by highly pliable and conformable dressing
comprises a composite of coextensive layers.
According to the present invention there is
provided a conformable wound dressing comprising an
absorbent layer comprising polymeric foam, an adhesive
layer over the edges of the body facing surface of said
absorbent layer and a layer of a liquid impervious
moisture vapour permeable material over the opposed
surface of and substantially coextensive with said
absorbent layer wherein at least one region of said
body facing surface of said absorbent layer is free
of the adhesive layer.
The dressings of the invention may be in the form
of a composite comprising three layers, a top moisture
vapour permeable layer, an intermediate absorbent layer
and a bottom or wound facing adhesive layer.
u;,~vc~i~..~;.__,~~~,,-~:~;;v~ ~~~~TiT~YE 5~°~~~T
,; . ~: ,
_ ,. .;
~;.. ;,.::_i,;!~
WO 91 /01707
~1 PCT/GB90/01194
- 3 -
In an alternative embodiment a fourth or
intermediate layer, coextensive with the absorbent
layer may be positioned intermediate the absorbent and
adhesive layers. The fourth layer should be a
discontinuous layer so as not to impair the performance
of the absorbent layer.
The adhesive layer may be confined to edges of
the absorbent layer or the intermediate discontinuous
layer so that at least 40% of the absorbent layer is
free from adhesive. Preferably the adhesive free layer
is greater than 50%, more preferably at least 85% free.
It is preferred that the adhesive 'free area of
the wound facing layer be a single area.
Typically, a single adhesive free area would be
located centrally on the body facing surface of the
dressing and would constitute the wound contacting
surface. The adhesive surrounding this wound
contacting surface of the absorbent or intermediate
would secure the dressing to the intact skin around the
wound.
Normally, the layers of the dressing are attached
in a contiguous manner so as to form a laminate.
x . ~ 'roe
g /~;
2054 21 PCT/GB 90 /01 19~
O1 9 1
- 4 - 1 2 July 1991
Wound dressings of the invention can suitably
have an moisture vapour transmission rate (MVTR) of at
least 300 and preferably at least 500 grams/m=/24 hrs
at 37.5°C at 100% to 10% relative humidity difference.
Typically the MVTR will be between 300 to 5000 and
preferably 500 to 2000 grams/square metre/24 hours at
37.5°C at 100% to 10% relative huaidity difference when
in contact with water vapour but not liquid water. It
has been found that such moisture vapour transmission
rates will allow the wound under the dressing to heal
under moist conditions without causing the skin
surrounding the wound to macerate.
The moisture vapour transmission rate as
determined in contact With water vapour but not liquid
water and is determined as follows:
Discs of the material under test are clamped over
Payne Permeability Cups (flanged petal cups) using
sealing rings and screw clamps. The a:posed surface
area of the test sample is lOcm'. Each cup contains
approximately lOml of distilled water.
After weighing the cups are placed in a fan
assisted electric oven which is maintained at 37~1°C.
The relative humidity within the oven is maintained at
approximately 10% by placing 1Rg of anhydrous 3-8 mesh
"._ ~,..; n..ivm
~~~:fa ~...:;~~~.. ~ , . .., c~f:~~ SUBSTITUTE S1~F~T
:~W_.~L.wY. v /'l=~VwIJ~
CVO 91/01707 ~ ~ ~ ~ PCT/GB90/01194
calcium chloride on the floor of the oven.
The cups are removed after a predetermined period
0 of time allowed to cool for 20 minutes and re-weighed.
The MVTR of the test material is calculated from the
weight loss and expressed in units of grams of weight
per square metre per 24 hours.
The adhesives employed in the present invention
are suitably those which do not adhere to the moist
surface of the healing wound. The adhesive on the
wound facing surface of dressings of the invention then
allows these dressings to be adhered to the skin around
the wound site.
The adhesive may be applied as a coating on the
absorbent layer or on a intermediate discontinuous
layer. In another embodiment the adhesive may be
formed in situ on the intermediate or absorbent
surfaces, eg by the polymerisation of a non-adhesive
monomeric precursor.
The adhesive may be a discontinuous layer or more
preferably a continuous layer. A discontinuous layer,
may be randomly distributed, for example as spots or as
a layer of a porous adhesive. Alternatively, the
adhesive may be a regularly_patterned discontinuous
WO 91/01707 ~ ~ PCT/GB90/01194
- 6 -
layer in the form of regularly arranged spots or lines
or in a grid arrangement.
Where the adhesive is coated onto the
discontinuous layer, the adhesive~may be a continuous
coating on the surfaces of the intermediate layer. The
adhesive layer will itself be discontinuous, the
discontinuities registering with the discontinuities in
the intermediate layer.
The adhesive layer is not coextensive with the
absorbent layer. It is desirable that adhesive be
present at the edges of the dressing. The adhesive may
be coloured differently from that of the absorbent
layer or intermediate layer to provide a visual
distinction as a guide for locating the non-adhesive
wound contacting surface over the wound. The absorbent
layer or intermediate layer will therefor be the direct
wound-contacting layer; with a peripheral layer of
adhesive for securing the dressing to intact tissue.
It is preferred that at least 10% of the wound
facing surface. of the absorbent or intermediate layer
be covered with adhesive and that the adhesive be
present at all the peripheral rejgions of the wound
i
facing layer.
WO 91/01707
PCT/GB90/01194
_ 7 -
Suitable adhesive layers for the wound facing
layer of dressings of the invention have a thickness of
at least 15 microns and may more aptly be from 15 to 75
microns preferably from 25 to 50 microns. Suitable
adhesives for the wound facing layer can be any of
those pressure sensitive adhesives normally used for
adhesive surgical or medical dressings. Preferred
pressure sensitive adhesives comprises acrylate ester
copolymers and polyvinyl ethyl ether adhesives, such as
those disclosed in United Kingdom Patent Specification
No. 2070631 and European Patent Specification Nos.
0099675 and 0194881.
The material employed for the discontinuous layer
intermediate the adhesive and the foam absorbent layer
may be a conformable, elastomeric film provided with
apertures or.may be a plurality of conformable,
elastomeric polymer strands laid down to a net of
desired shape, size and configuration. In another
embodiment the discontinuous layer may be formed from
a non-woven material.
Aptly the discontinuous layer is formed from a
polymeric film. The film may be perforated, apertured
or cut to provided. Alternatively the film may be
subjected to physical treatments to form a net.
W091/01707 ~~~~'~~'~
PCT/G B90/01194
_ 8 _
The conformable elastomeric discontinuous film or
net allows wound exudate to pass to the absorbent foam
layer but prevents the absorbent layer making direct
contact with the wound surface.
Preferably, the discontinuous layer used in this
invention is an integral net, that is a net with
strands and junctions which have been formed integrally
from a unitary film during manufacture.
Preferably the discontinuous layer is
sufficiently conformable to allow the wound dressing to
conform with the body contours and thereby maintain
overall contact with the wound surface to ensure that
exudate from the wound is absorbed.
It is also desirable that the discontinuous layer
should be sufficiently elastically extensible to adjust
to any dimensional changes in the absorbent layer which
may occur, for example, by expansion on liquid uptake.
Suitable discontinuous layers will have
elongation at break of 100% to 800% desirably 200% to
750% and preferably 300% to 700% when measured as a
2.5cm wide strip at a 30cm/minute strain rate at 20°C.
Normally the discontinuous layer of elastomer is
WO 91/01707 ~ J ~ ~ ~ ~ PC?'/GB90/01194
_ g _
made of a pharmaceutically acceptable water insoluble
elastomer.
Suitable elastomers for use in manufacturing the
discontinuous layer include polyurethanes,
polybutadiene and the like. Such elastomers may be
present as block copolymers With other polymer
constituents.
The preferred material for the nets are
thermoplastic polyurethanes.
Polymer blends having a continuous phase of an
elastomer and a discontinuous or discrete phaseof a
polymer which is an incompatible with the elastomer may
also be used for the discontinuous intermediate layer
eg. net. Suitable polymer blends include blends of
elastomers such as polyurethanes or ethylene-vinyl
acetate copolymers as the continuous phase and
incompatible polymers such as an olefine, for example
polystyrene.
Preferred thermoplastic polyurethanes are linear
polyurethanes containing polyether or polyester groups.
Suitable linear polyester polyurethanes are disclosed
in United States Patent Specification No. 2871218.
Suitable linear polyether polyurethanes are disclosed
CA 02058421 1999-08-31
- WO 91 /01707 PCT/C B90/O1 194
- 10 -
in United States Patent Specification No. 2899411.
Favoured thermoplastic polyurethanes include Estanes'
from B.F. Goodrich Corp. Preferred solution casting
grades are Estane* 5714F1, 5702 and 5703. A preferred
extrusion grade is Estane* 580201.
Suitable polybutadienes are 1,2 polybutadienes.
Favoured 1,2 polybutadienes contain a major amount of
syndiotactic 1,2 polybutadiene, have a crystallinity of
25% to 30% and an average molecular weight in excess of
100,000. Preferred 1,2 polybutadienes are known as RB
810*, RB 820* and RB 830* made by Japan Synthetic Rubber
Company.
The net of the discontinuous layer of the
dressing can have any convenient form depending on the
chosen arrangement of strand, junc~tiflns and aperture
areas and also their shapes and relative size.
The number and size of the apertures in the net
will be sufficient to allow the wound exudate to pass
through the film to the absorbent layer. Most aptly
the net is adapted so that the size of apertures in
combination with the thickness of the film prevent the
absorbent layer contacting the wound surface. The nets
c_an have apertures with a dimension of at-least O.OSmm.
Generally the aperture size can be upto 4mm. Suitable
* Trade-mark
WO 91 /01707
."~~~ ,I~ ~ " PCT/GB90/01194
- 11 -
nets have apertures with a dimension of from 0.05 to
4mm, more aptly from 0.05 to 2.5mm or 0.05 to 2.0, and
preferably from 0.1 to 2.5mm. Thenet thickness may be
greater than O.Olmm. Net thicknesses of upto 2.5mm may
be aptly used. Thus a suitable net can have a
thickness of 0.01 to 2.5mm, typically of 0.01 to 0.25mm
and preferably of 0.05 to 0.5mm. Favoured nets of the
invention have 4 to 40 apertures per cm with dimension
of 0.05 to 2.5mm. The wound face of the net will have
15 to 80% of its area void (the apertures), more
suitably will have 25 to 75% of its area void and most
suitably will have 35 to 65% of its area void.
The net of the wound dressing of the invention
can have any convenient form depending on the chosen
arrangement of strand, juncture and hole areas and also
their shapes. and relative size.
In one preferred form the net consists
essentially of longitudinal and transverse strands
intersecting at right angles to give a square grid hole
pattern.
Suitable nets of this type aptly have 2 to 50
strands per cm, desirably 4 to 40 strands per cm and
preferably 2 to 24 strands per cm in both longitudinal
and transverse directions.
WO 91/01707 PCT/GB90/01194
_ 12 -
Variations on the square grid pattern can give
other desirable forms of the integral net. Unequal
density of strands in either the longitudinal or
transverse directions will give rectangular hole areas.
Continuous parallel strands in one direction with a
staggered arrangement of connecting strands in the
other direction will give a "brick-work" pattern.
Other apt forms of the inegral polymer nets can have
strands at an angle to the longitudinal or transverse
direction (that is diagonal strands). Another
preferred form of the integral polymer net can have a
staggered arrangment of circular or approximately
circular (for example hexagonal) arrangements of
strands and hole areas. The integral polymer net can
be in the form of a mixed pattern of two or more of the
arrangements id desired.
The apertured film or net used in this invention
aptly will have a weight of at least lOgsm and may have
a weight of from lOgsm to SOgsm, preferably from l5gsm
to 50gsm.
The adhesive layer or combination of adhesive and
discontinuous layer is preferably distensible such that
distortion of the dressing does not occur during
dimensional changes in the absorbent layer which may
WO 91/01707 ~ ~ ~, '~ PCT/GB90/01194
13 - .
occur, for example by expansion in liquid uptake or on
body surface movement eg. flexing or stretching over a
knuckle or elbow.
The absorbent employed in the absorbent layer of
the dressings of the present invention is a polymer
foam. The foam is preferably a highly conformable
hydrophilic foam, more preferably an open celled foam.
The conformable hydrophilic polymer open cell
absorbent layer used in dressings of the invention is
capable of absorbing wound exudate. It is desirable
that the hydrophilic polymer form layer absorbs the
wound exudate rapidly as this enhances the low
adherency properties of the absorbent pad. Such rapid
absorption prevents undesirable pooling of exudate
between the.dressing and the wound.
The ability of open cell hydrophilic polymer foam
layers to absorb and retain fluids depends to some
extent on the size of the foam cells, the porosity of
the foam and the thickness of the foam layer.
Suitable open cell hydrophilic foams of dressings
of the invention have a cell size of in excess of 30
. microns. Generally the foams will have cell sizes of
less than 700 microns. Thus foams having a cell
WO 91 /01707 ~ ~ a
PGT/GB90/01194
- 14 -
weight of 30 microns to 700 micron$ may be aptly used.
Preferably the cell size of the foam will be from 50
microns to 500 microns. Apt open cell hydrophilic
foams of dressings of the invention have at least 20%
and aptly from 20% to 70%, preferably 30% to 60% of the
total membrane area of the cells as membrane openings.
Such open cell foams permit transport of fluid and
cellular debris into and within the foam.
Apt foams may be polyurethane, carboxylated
butadiene styrene rubber, polyacrylate or the like
foam. Such foams may be made of hydrophilic materials
per se or may be treated to render them hydrophilic,
for example with surfactants. It is much preferred to
use foams which are made of polymer which is itself
hydrophilic as it has been found the the exudate is
less likely.to coagulate rapidly.
The use of such foams of hydrophilic polymer in
the absorbent pad of dressings of the invention can
allow the wound to be maintained in a moist condition
even when the exudate produced has been absorbed and
removed from the wound surface.
Favoured hydrophilic polymer foams are
hydrophilic polyurethane and especially those which are
made of crosslinked hydrophilic polyurethane.
WO 91/01707 PCT/GB90/01194
- 15 - ~05~~2~
Preferred foams can be made by reacting a hydrophilic
isocyanate terminated polyether prepolymer with water.
Suitable hydrophilic polyurethane foams of this
type include those known as Hypol* foams. Hypol* foams
can be made from Hypol* hydrophilic prepolymers marketed
by W.R. Grace and Co.
The conformable hydrophilic polyurethane foam can
be made by mixing together an isocyanate terminated
polyether having functionality of more than two with a
surfactant and water and casting the mixture onto a
surface. This surface advantageously may be the
intermediate discontinuous layer.
Preferred isocyanate terminated polyethers
include Hypol* FHP 2000, 2001, 3000, 3001, 2002 and
2000HD marketed by W.R. Grace and Co. Hypol* are
described in a booklet published by W.R. Grace and Co.
"Hypol*: foamable hydrophilic polymers - laboratory
procedures and foam formulations". Their preparation
and use are disclosed in British Patent Specifications
Nos. 1929711 and 1507232.
Suitable surfactants for forming conformable
_ hydrophilic polymer foams include non-ionic
surfactants. Favoured non-ionic surfactants are
* Trade-mark
WO 91/01707 PCT/GB90/01194
- 16 - X521
oxypropylene-oxyethylene block copolymers known as
Pluronic* marketed by BASF Wyandotte. Preferred
Pluronics* include L65, F87, P38, P75 and L62. Another
favoured non-ionic surfactant is a polyoxyethylene
stearyl ether known as Brij* 72 marketed by Honywell
Atlas.
To prepare a suitable foam 100 parts by weight of
Hypol* FHP 2000, 2001, 3000, 3001, 2002 or 2000HD is
mixed with 0.3 to 7 parts by weight of surfactant or
' mixtures of surfactans and 30 to 300 parts by weight of
water and the foaming mixture cast onto a surface.
Typical foaming mixtures have a cream time of about 20
sacs., a rise time of about 250 sacs. and a cure time
of about 400 sacs.
A preferred foam for use in the absorbent layer
of the dressings of the invention is disclosed in our
United Kingdom Patent Specification No. 2188055 which
inter alia there is described a hydrophilic
polyurethane foam comprising residues derived from a
polyalkylene glycol mono alkyl or mono alkaryl ether.
Such foams_can be formed by reacting with water the
reaction product of polyisocyanate which has a
functionality of greater than 2 and polyalkylene glycol
mono alkyl or alkaryl ether.
* Trade-mark
WO 91/01707
PCT/GB90/01194
- 17 -
Preferred polyalkylene glycol mono alkaryl ethers
are those in which the alkylene group contains upto 4
carbon atoms. More preferably the alkylene group is
ethylene.
Suitable polyalkylene glycol mono alkyl ethers
for forming the reaction product are those in which the
alkyl group contains 1 to 20 carbon atoms. Alkylene
favoured ethers are those in which the alkyl group is a
methyl group. Another class of preferred polyalkylene
glycol mono alkyl ethers are those in which the alkyl
group contains 10 to 18 carbon atoms, eg. lauryl or
cetyl.
Suitable polyalkylene glycol mono alkaryl ethers
include those in which the aryl moeity is phenyl.
Preferred ethers are those in which the alkyl moeity
contains from 1 to 20 carbon atoms eg. octyl or nonyl.
The polyalkylene glycol mono alkyl or alkaryl
ether can suitably have an average molecular weight of
180 to 6000. Suitable ethers for forming reaction
products used to prepare flexible foams of the
invention have an average molecular weight of 180 to
1300 and preferably have an average molecular weight of
350 to 1000.
WO 91/01707 PCT/GB90/01194
- 18 - ~05~4 21
Suitable ethers for forming reaction products
used to prepare stiff foams of the invention have an
average molecular weith of 1500 to 6000 and preferably
have an average weight of 3000 to 5000.
Apt ethers are polyethylene glycol mono lauryl
ethers having an average molecular weight of
approximately 1090 and 360 known as Brij* 35 and Brij* 30
respectively available from Honeywell Atlas and
polyethylene glycol mono methyl ethers having an
'average molecular weight of approximately 500 and 5000
known as PEG* monomethylether molecular weight 550 and
5000 respectively available from Aldrich Chemicals.
Suitable polyethylene glycol mono nonyl phenyl
ethers are commercially available under the Trade-marks
Antarox CO-320 and Antarox CO-990. Apt polyethylene
glycol mono nonyl phenyl ethers, having an average
molecular weight of approximately 440 and known as
Antarox* CO-520 and CO-990 respectively available from
GAF (Great Britain) Co. Limited.
The polyethylene glycol mono alkyl or alkaryl
ether used in the invention will normally contain
water. It is preferred however, that the ether
contains less than 1% by weight of water to limit the
number of urea groups formed in the reaction with the
* Trade-mark
WO 91 /01707 PCT/G B90/O1 I 94
'' - 19 - 205421
polyisocyanate.
The polyisocyanate used for forming the reaction
product will have a functionality greater than 2 for
example 2 to 5 and will preferably have a functionality
of 2.2 to 3.5. Suitable polyisocyanates include
aliphatic and aromatic polyisocyanates. Preferred
polyisocyanates are aliphatic polyisocyanate.
Aliphatic polyisocyanates are usually liquid at ambient
room temperature and therefore are convenient to use in
a liquid reaction mixture. An apt aliphatic
polyisocyanate for use in the invention is a biuret of
1,6 hexamethylene diisocyanate which has a
functionality of 2.6 known as Desmodur* N100 available
from Bayer A.G.
Favoured aromatic polyisocyanates for forming the
reaction product are polymeric methylene diisocyanates.
Polymeric methylene diisocyanates comprise a mixture of
4,4'-diphenyl methane diisocyanates and one or more of
polymeric homologues. Apt polymeric methylene
diisocyanates are known as suprasec* VM 20, VM 50, DND
and VM 90.=ava~.lable from ICI and have a functionality
of 2.13, 2.49, 2.70 and 2.90 respectively.
The reaction product suitable for use in the
invention can be a reaction product of one or more
* Trade-mark
~~205421 PCT/G~ 90/0119~
12 07 91
- 2 0 - ~ 2 ~~Y 1991
polyisocyanates and one or more polyalkylene glycol
mono alkyl or aryl alkyl ethers, including mixed alkyl
and alkaryl ethers. The reaction product may
advantageously be formed using a chain extender.
Suitable chain extenders for use in forming the
reaction product include ethane diol, 1.3 propane diol
and 1.4 butane diol.
The conformable moisture vapour transmitting
outer layer of dressings of the invention when present
can be continuous or discontinuous.
A preferred moisture vapour transaitting outer
layer is a continuous conformable fila. The continuous
moisture vapour transmitting conformable film outer
layer of the wound dressing of the invention may be
used to regulate the moisture loss frog the wound area
under the dressing and also to act as a barrier to
bacteria so that bacteria on the outside surface of the
dressing cannot penetrate to the wound area.
Suitable continuous conformable films will have a
moisture vapour transmission rate of at least 300,
favourably at least 500 and aptly 300 to 5000 grams
preferably 500 to 2000 grams/square metre/24 hrs at
37.5°C at 100% to 10% relative humidity difference. It
has been found that such moisture vapour transmission
'.I..iy~ f,~'..., ~~ ,. I n~~,._' n... ~, .~'1A ~T~TE
__ . .. . ., . ~ ;~J
.... ,. '. _.._.... ... . ..__.__..7
WO 91/01707 ~ ~ ~~ PCT/GB90/01194
- 21 -
rate of the continuous film allow the wound under the
dressing to heal under moist conditions without causing
the skin surrounding the wound to macerate.
Suitable moisture vapour transmitting continuous
films can be made of polyurethane or copolymers of
alkoxy alkyl acrylates or methacrylates such as those
disclosed in British Patent No. 1280631: Apt
polyurethanes and polyurethane films, particularly
highly moisture vapour permeable polymers and foams are
also disclosed in European Patent Specification No.
0091800.
The continuous moisture vapour transmitting film
can be a conformable polyurethane incompatible polymer
blend film containing voids. Suitable conformable
polyurethane blend films are disclosed in United
Kingdom Patent Application GB 2081721A. A preferred
film is formed from a blend of polyurethane and high
impact polystyrene.
An apt conformable moisture vapour transmitting
outer layer comprises a microporous film. The
conformable film microporous outer layer of the wound
dressing of the invention may be used to regulate the
moisture loss from the wound area under the dressing
and also to act as a barrier to bacteria to delay or
22 _ ~ ~ ~ G
prevent bacteria on the outside surface of the
dresssing penetrating to the wound area.
Suitable conformable microporous films will have
a moisture vapour transmission rate of at least 300
favourably at least 500 and aptly fro= 300 to 5000
grams, preferably 500 to 4000 grass/square metre/24 hrs
at 37.5°C at 100% to 10% relative humidity difference.
Suitable conformable microporous films have pore
diameter of less than 2 microns desirably less than 0.6
microns and preferably less 0.1 microns. Such
microporous films should have pore diaseter of greater
than 0.01 microns.
Suitable conformable microporous films may have a
thickness of greater than 25p. Apt files may have a
thickness of less than 400p. Thus suitably the
thickness of the film will be frog 25 to 400 microns,
preferably 50 to 300 microns. Generally, the.
conformable microporous film will be wade of a polymer.
Suitable polymers include polether-polyamide
copolymers such as those marketed under the trade-mark
PEBAX (CATCOCHEM SA) containing a particulate filler,
-eg chalk, plasticised polyvinyl chloride, polyurethane
elastomers and ethylene vinyl acetate copolymer
WO 91/01707 ~ ~ PCT/GB90/01194
/_
- 23 -
elastomers.
A favoured conformable microporous film comprises
a microporous plasticised polyvinyl chloride film
having an average pore diameter of less than 2 microns,
a thickness of 250 to 300 microns and a moisture vapour
transmission rate of 3000 to 5000 g/mz/24 hours at
37.5°C at a relative humidity difference of 100 to 10~
relative humidity.
The conformable moisture transmitting outer layer
of wound dressings of the invention may also comprise a
moisture vapour transmitting adhesive layer to bond the
outer layer to the layer of open cell foam. The
adhesive layers can be continuous or discontinuous.
Suitable adhesive which are moisture vapour
transmitting as a continuous layer include various
acrylate ester copolymer and polyvinyl ether pressure
sensitive adhesives for example as disclosed in British
Patent No. 1280631. Favoured pressure sensitive
adhesives comprise copolymers of an acrylate ester with
acrylic acid for example as disclosed in United Kingdom
Application GB 2070631.
The wound dressing of the invention can contain a
topically effective medicament. Most suitably the
WO 91 /01707 ~ ~ Q '~f
PCT/GB90/Ol 194
- 24 -
medicament is an antibacterial agent. Preferably the
antibacterial agent is a broad spectrum antibacterial
agent such as silver salt for example silver
sulphadiazine, an acceptable iodine source such as
povidone iodine (also called polyvinyl pyrrolidone
iodine or PVP/I), chlorhexidine salts such as the
gluconate, acetate, hydrochloride or the like salts or
quaternary antibacterial agents such as bensalkonium
chloride or the like.
The medicament can be located in the foam layer
or in the adhesive coating.
The medicament is preferably located in the foam
layer of the dressing.
Preferred amounts of suitable medicaments for
incorporation into the foam layer of the dressing of
the invention are disclosed in the aforementioned
patent specifications.
The foam in the absorbent layer may also contain
a supersorber. Suitable supersorbers are well known
and can include starch and other cellulosic materials
such as cross-linked methyl cellulose, as well as known
materials containing acrylic unsaturation.
WO 91/01707 e~ ~ ~ ~. PGT/GB90/01194
- 25 -
The wound dressing of this invention may be in
any convenient form of shape or size. In a preferred
form the wound dressing is a pad of rectangular, oval
or circular shape. In another preferred form the wound
dressing can be an elongate strip which may be used as
a bandage or may be used to prepare smaller dressings.
The dressings may also be of irregular shape for use on
flexing or bending surfaces such as knuckles, knees and
elbows.
Although the dressings of the invention are
suitable as first-aid dressings or bandages, they also
have use, as medical or ward dressings.
The dressings of the present invention have a
primary use in the field of first-aid and may employed
in the home .and workplace for the primary dressing of
wounds and contusions which may cause minor bleeding
such as cuts and abrasions, which do not produce large
amounts of wound exudate.
The dressings may be supplied in a variety of
shapes and sizes for the dressing of lesions ranging
from small cuts, for example on the finger to large
skin grazes on, for example, the elbow or knee. The
dressings may be square, rectangular, round, oval or
oblate in shape. The dressings may be of a specialised
WO 91 /01707 ~ ~, I
PCT/GB90/01194
- 26 -
shape, for example having a number, of fingers extending
from a main or central wound contacting region for the
dressing of wound on the knuckle. The central part of
the dressing is placed.over the wound on the knuckle
and the fingers, which have adhesive on the body facing
surface and the finger portion adhered to the adjacent
fingers and back of the hand.
The dressings of the present invention will
generally have a flat profile. However, it may be
desirable for the central region, which will be placed
over the wound, to be thicker than the margins. The
thickness of the marginal regions may be reduced to
ensure maximum conformability. The edges of the
dressing may be chamfered or feathered. This reduces
the possibility that the adhered dressing will 'catch'
and be lifted.
Dressings of this configuration allow the hand to
flex naturally and freely without the risk of the
dressing becoming detached.
The overall dimension of dressings or adhesive
bandages in accordance with the invention may be as
small as 1cm x lcm upto lOcm x lOcm. Alternatively the
dressing may be supplied in roll form, with the
adhesive preferably disposed along the major edges of
WO 91/01707 , PCT/GB90/01194
_~f27 -
the roll and the central area between the adhesive
coated margins free of adhesive.
The dressings of the present invention may also
be employed as wound dressings for the dressing of
wounds and lesions which do not produce large amounts
of exudate. Such dressings have application as
post-operative dressings for the covering of closed
surgical incisions and for wounds treated in hospital
casualty units, which require protection but are
bleeding profusely or producing large amounts of
exudate.
Such wound dressings tend to be of a larger size
than those dressings used for first-aid application in
the home and workplace. Wound dressings may be
required in sizes ranging from that of the larger
first-aid dressing upto for example 50cm x 20cm.
It is desirable that the wound dressing of this
invention is sterile. The wound dressing of the
invention is advantageously provided in bacteria
impervious pouches. Such packed forms can be prepared
under aseptic conditions or alternatively sterilised
after packing by a conventional procedure. A favoured
sterialisation procedure is heat sterilisation, for
example by steam. Other favoured procedures are
WO 91/01707 ~' '~ ~ ~ '~ PCT/GB90/01194
- 28 -
ethylene oxide sterilisation or gamma irradiation.
In another aspect the present invention provides
a process of making a wound dressing of the invention
Which comprises bringing together a layer of a liquid
impervious moisture vapour permeable layer,, an
absorbent layer comprising polymeric foam and wound
facing layer with adhesive on its wound facing surface.
The absorbent layer may be produced by foaming a
suitable polymer into a mould to produce the desired
shape by casting into a block and cutting the desired
shape before combination with the other components or
casting with the other components and then cutting.
Normally the bringing together of the layers will
be a lamination process. Such lamination processes can
also be used to form wound dressings with a conformable
moisture vapour transmitting outer layer.
The adhesive can be coated onto the wound facing
surface of the discontinuous layer before, during or
after the layer has been laminated to the foam layer or
directly onto the wound facing surface of the foam. In
a preferred process the adhesive in a flowable state is
cast into the recesses of a release coated surface
having a pattern of discrete raised areas and
WO 91/01707 ~' ~ ~ PCT/GB90/01194
- 29 -
interconnected recessed areas and the net layer formed
in a similar manner on the adhesive layer.
Preferred casting surfaces are embossed polymer
sheets. Suitable embossed polymer sheets are disclosed
in the aforementioned patent applications.
The adhesive surface of wound dressings of the
invention will usually be provided with a release
coated protector. The release coated protector can be
the embossed sheet carrier used for forming the
adhesive coated net layer. Other suitable release
coated protectors include silicone coated release
papers such as Steralease paper nos. 15 and 67 made by
Sterling Coated Papers Limited.
The dressings of the invention may be readily
manufactured by continuous production techniques. Thus
a moisture vapour permeable film and a polymer net may
be run in together throught the nip of two rollers or a
single roller and a flat bed and a polyurethane foam
injected into the nip between the film and net. The
foam may be formed in situ at the point of injection by
mixing a suitable isocyanate prepolymer as hereinbefore
described with, for example, water. After curing of
the foam is completed, the embossed composite may then
be transfer coated with a suitable adhesive, pre-coated
~l
WO 91/01707 PCT/GB90/01194
3~
on a releasable carrier sheet. Once the adhesive has
been transferred and the carrier sheet removed
protector papers for the adhesive may be run onto the
adhesive surface, according to conventional techniques.
Finally the dressing may be stamped out by cutting
through the composite and the individual dressings
packaged.
If desired the dressings may be sterilised during
or at the completion of the manufacturing and packaging
process.
The present invention will be further described
and illustrated by reference to the accompanying
drawings in which Figures 1 to 5 and 6a are schemtic
representations of sectional elevations of various
embodiments of the dressing of the present invention.
Figures 6b and 6c are, respectively, plan views of the
top side and underside of the dressing shown in Figure
6a.
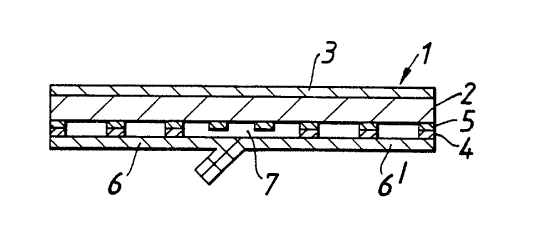
A wound dressing 1 comprises a foam absorbent
layer 2. An adhesive layer 4 may be coated directly
onto the foam layer (Figures 2 and 3) or coated onto an
intermediate discontinuous layer 5 such as a net, which
in turn is bonded or laminated to the foam absorbent
layer 2.
WO 91/01707 PCf/G B90/01194
31 -
Overlying the adhesive 4 are a pair of protectors
5, 61 for the adhesive. Overlying the top surface of
the absorbent layer 2 is a liquid impervious, water
vapour permeable layer 3. In use the release
protectors 6, 61 may be peeled away for the adhesive
face 4 and the adhesive side of the dressing presented
to the skin. The non-adhesive coated region 7 is
presented directly to the wound or lesion.
The invention is now illustrated by the following
Examples:
Example 1
A composite was produced by casting a
polyester-polyamide copolymer (Pebax*) film, a
hydrophilic polyurethane foam, and a polyurethane net.
The polyurethane net was prepared according to
the method described in the Examples of European Patent
No. 0059048 and then crushed to a thickness of l5pm.
The polyurethane foam was prepared by first
producing a prepolymer according to Example 1 of United
_Kingdom Patent Specification No. 2188055 and then
foaming the prepolymer in accordance with the procedure
* Trade-mark
WO 91/01707 ~, ~ PCT/GB90/01194
- 32 -
of Example 7 of United Kingdom 2188055.
The casting was carried out according to the
manner described in European Patent Specification No.
0059048.
An adhesive was prepared according to Example 1
of United Kingdom Patent Specification No. 2070631 and
cast onto a release sheet at a coating weight of 35gm
m-2. Once the adhesive had dried rectangular areas 5cm
x lcm were cut out and the adhesive transfer coated
onto the foam surface.
Oblate shapes measuring 6cm x 2.5cm were cut out,
with the adhesive free areas centrally disposed to give
a first-aid bandage.
The adhesive surface was then covered with
release papers and the product finally cut out of the
composite sheet, through the embossed region, to
produce a first-aid dressing.
Example 2
The procedure of Example 1 was repeated except
that the adhesive was transfer coated directly onto the
foam absorbent layer. Adhesive 'windows' measuring
l~J '~ G ~ ~~1~.
WO 91/01707 PCT/GB90/01194
- 33 -
25mm x 12.5mm were kiss-cut from the coated transfer
sheet.
Once the adhesive had been applied, the
film-foam-adhesive laminate was embossed and cut to
form an oblate first aid dressing measuring 63mm x
22mm. The edges of the dressing were embossed to
provide a thin margin 1.5mm wide around the edge of the
dressing. A representation of the dressing is shown in
Figures 6a, 6b and 6c.