Note: Descriptions are shown in the official language in which they were submitted.
2U58~~~
HOECHST ARTIENGESELLSCHAFT HOE 89/F 153 R Dr. Rlr/Le
Description
New aryl phosphonites, a process for their preparation
and their use for stabilizing plastics, in particular
polyolefin molding compositions
The present invention relates to new diaryl phosphonites
and tetraaryl diphosphonites, a process for their pre-
paration and their use for stabilizing plastics, in
particular polyolefins.
It is known that synthetic polymers have to be protected
against unwanted oxidative, thermal and photochemical
damage during the preparation, the processing and the use
by means of stabilizers or stabilizer systems. Such
stabilizers comprise, for example, a phenolic antioxidant
which should ensure in particular the long-term use
stability of the molded article, and one or more cost-
abilizers, which control the processing stability and in
some cases also reinforce the synergistic effect of the
phenolic component.
Conventional stabilizers include ortho-alkylated aryl
phosphites and phosphonites, the latter being distin-
guished in particular by extensive stability to
hydrolysis.
It is known from European Patent 5,447 (= US Patent
4,406,842 and 4,474,914 = 3apanese Laid-Open Application
79-141,753) that ortho-alkylated phenyl phosphonites of
the formula
R1'P' O~R3
2
R2
can be prepared by reaction of alkyl- or arylphosphonous
~058'~6,
- 2 -
dichlorides with ortho-alkylated phenols in the presence
of at least stoichiometric amounts of a suitable base for
neutralizing the hydrochloric acid formed. Although the
general definitions given in this patent are specified to
the extent that within preferred, relatively narrow
definitions for the radicals R1, RZ and R3, the radical R1
can be phenyl, o-, m-, p-tolyl, o-, m-, p-xylyl, mesityl,
o-cumyl, p-tert-butylphenyl, 2,4-di-tert-butylphenyl,
2,4,6-tri-tert-butylphenyl, or 2,4-di-tert-octylphenyl,
R2 can be tart-butyl, tert-amyl, tert-octyl, tert-octa-
decyl and R3 can be H, methyl, i-propyl, tart-butyl, tert-
amyl, n-hexyl, tart-octyl, tart-dodecyl or n-octadecyl,
a-methylbenzyl or a,a-dimethylbenzyl; of the possible
combinations among these, however, only two embodiment
examgles where R1 is phenyl and R2 and R3 are simul-
taneously tart-butyl or tart-octyl are mentioned, and
furthermore 6 table examples, in three of which Rl is also
phenyl and either RZ is tart-octyl and R3 is methyl or RZ
is tart-butyl and R3 is hydrogen or RZ and R3 simultane-
ously are a,a-dimethylbenzyl. Accordingly, these 5 com-
pounds cannot be deemed new.
However, the process of European Patent 5,447 can be
carried out only to a limited extent because of the
difficult synthesis of the dichlorophosphanes required as
precursors, which is of course a disadvantage if an
industrial preparation is taken into consideration. Thus,
for example, of the aromatic derivatives, only phenyl-
dichlorophosphane is an industrially available product,
which makes derivatives of benzenephosphonous acid only
available, which makes it easy to understand why, of the
compounds in which R1 is an aryl radical, precisely only
those having unsubstituted phenyl are cited.
However, in order to meet the high demands made in
practice on the stability, efficiency, nonvolatility and
migration behavior 'of stabilizers for polymers, it is
desirable that especially the more highly substituted
derivatives of si-ylphosphonous acids be available.
~~~~~~ 3
- 3 - HOE 89/F 153 K
However, they are not accessible by known processes, due
to the fact that until now the preparation of the
precursors has not been economically feasible.
As a result, new stabilizers having improved properties
and processes for their preparation, which do not have
these disadvantages are very desirable.
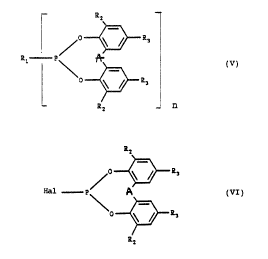
Accordingly, the present invention relates to aryl
phosphonites of the formula V (see formula sheet), i.e.
diaryl phosphonites where n = 1 and tetraaryl diphospho-
nites where n = 2, in which A is non-existent - i.e. the
two rings carry hydrogen - or a divalent hydrocarbon
bridge having 1 to 6 carbon atoms which may be
substituted by groups mentioned further below under R1, or
is a hetero atom such as oxygen or sulfur, cycloalkyli-
dene having 4 to 8 carbon atoms or phenylalkylidene
having 7 to 12 carbon atoms, Rl as monovalent radical is
a phenyl or benzyl radical, each of which carry 1 to 3
substituents, or is a-methylbenzyl, «,a-dimethylbenzyl,
naphthyl or a naphthyl radical carrying 1 to 5 substitu-
ents, in which the substituents are identical or dif-
ferent and are a non-aromatic hydrocarbon radical, an
alkoxy radical, an alkylthio radical or a dialkylamino
radical, in which the alkyl radicals each have 1 to 8
carbon atoms, aryl or aryloxy each having 6 to 10 carbon
atoms or halogen having an atomic number from 9 to 35,
and as divalent radical a naphthylene radical which is
unsubstituted or carries 1 to 4 non-aromatic hydrocarbon
radicals each having 1 to 8 carbon atoms as substituents,
or, if A is non-existent, a phenylene radical which is
~30 unsubstituted or is substituted by up to 2 non-aromatic
hydrocarbon radicals each having 1 to 8 carbon atoms,
RZ is a non-aromatic hydrocarbon radical having 1 to
18 carbon atoms, aryl, arylmethyl, arylethyl or aryliso
propyl, in which the aryl in each case contains 6 to
10 carbon atoms, and
cZ ~ ~ ~ .y r> .3
R3 is hydrogen or a group mentioned under Rz,
where, in the compounds in which n = 1 and R1 is phenyl,
those are excepted in which simultaneously
Rz and R3 are each tart-butyl,
RZ is tart.-butyl and R~ is hydrogen,
RZ is tart-octyl and R3 is methyl,
RZ and R3 are each tart-octyl and
RZ and R3 are each a,a-dimethylbenzyl.
The specific class of compounds according to the
invention in which A is non-existent has the formula I
(see formula sheet) for the preparation of which diaryl
halophosphonites II (see formula sheet) are used. In all
cases, compounds in which R1 is unsubstituted or
substituted naphthyl are particularly preferred.
In the compounds of the formula I according to the
invention, R1 as monovalent radical is, for example, a
phenyl or benzyl radical carrying 1 to 3 substituents,
such as the C1-C8-alkyl, C1-Ce-alkoxy, C1-CB-alkylthio radi-
cal, such as the alkyl radicals having 1 to 8 carbon
atoms and mentioned individually under RZ and the cor-
responding alkoxy and alkylthio radicals, or
CS-C~-cycloalkyl, phenyl, phenoxy and/or halogen.
Individual examples which may be mentioned are the tolyl,
dimethylphenyl, trimethylphenyl, tart-butylphenyl,
anisyl, naphthy.~. radicals which additionally can carry up
to 2 alkyl carbon atoms, and the various biphenyl
radicals, benzy:l, a-methylbenzyl and ca,~-dimethylbenzyl.
Naturally the substituents in R' can only be combined in
such a manner that no steric hindrance results. If R1
contains 3 substituents, more than 5 carbon atoms
together should not be contained in the two o-positions.
Examples of suitable R' as divalent radical are the
various phenylene radicals, which are unsubstituted or
carry 1 to 2 C1-C8, in particular C1-C3-alkyl groups, or
the various naphthylene radicals, which are unsubstituted
- 5 -
or substituted by 1 to 4 C1-CB-, in particular C1-C3-alkyl
groups.
Examples of suitable radicals RZ are non-aromatic hydro-
carbon radicals having 1 to Z8 carbon atoms, such as
alkyl or cycloalkyl, furthermore aromatic radicals which,
including aliphatic groups, have 6 to 18 carbon atoms, in
which not more than 10 carbon atoms are part of an
aromatic ring system. The radicals RZ preferably contain
4 to 12 and in particular ~ to 10 carbon atoms. Indivi-
IO dual examples of non-aromatic hydrocarbon radicals which
may be mentioned are alkyl, such as methyl, ethyl, the
various propyl, butyl, pentyl, hexyl, octyl, decyl,
dodecyl, hexadecyl and octadecyl radicals, and cycloalkyl
having 5 to 10 carbon atoms, such as cyclopentyl, cyclo-
1S hexyl, cycloheptyl and cyclohexylmethyl (i.e. not only
the hydrogenated benzyl radical but also the methylcyclo-
hexyl radical ) ; further examples are Cs-Clo-aryl, -aryl-
methyl, -arylethyl and -arylisopropyl, in which the term
aryl in each case includes alkylaryl, carries not more
20 than three of the substituents mentioned under R1 and
including these has not more than 14 carbon atoms.
If the radical RZ is an alkyl radical, tertiary alkyl
radicals having 4-10 carbon atoms, such as tart-butyl,
2-methyl-2-butyl, 2-methyl-2-pentyl, 2-ethyl-2-butyl are
25 particularly preferred. Othex preferred compounds are
those in which RZ is phenyl, benzyl, a -methylbenzyl or
a,a-dimethylbenzyl.
The invention also relates to a process for the prepara-
tion of aryl phosphonites of the formula V, in which A,
30 additionally to the abovementioned meaning, can also be
a direct bond, R1 additionally to the abovementioned
meaning can also be unsubstituted phenyl or benzyl as
well as a nonaromatic hydrocarbon radical having 1 to
18 carbon atoms, such as C1-ClB-alkyl, and in compounds in
35 which the two phenyl radicaJ.s are linked by A can also be
biphenylene or substituted or unsubstituted phenylene,
- g _
and RZ and R3 have the abovementioned meaning, which
comprises first reacting in a first step a hydrocarbon
halide R1(-Hal)p, in which R1 has the previously mentioned
meaning, n is = 1 or 2 and the halogen has an atomic
weight of at least 35, but is preferably chlorine or
bromine, under Grignard conditions, that is, advant-
ageously with intimate mixing, with at least molar
amounts of magnesium to give the corresponding Grignard
compounds Rl(MgHal)n and reacting these further in a
second step with bisaryl halophosphonites of the formula
VI (see formula sheet), in which R2, R3 and Hal have the
abovementioned meaning, with the formation of bisaryl
phosphonites V. The reaction can be accelerated and the
degree of conversion improved if the magnesium is used in
a small excess. Advantageously, 1.1 to 1.5 equivalents of
magnesium are converted per halogen atom. Exposure to
ultrasound during the Grignard reaction can in some cases
be advantageous. This means that this process is also
suitable for the preparation of the compounds where n = 1
and which fall under the exceptional formulation of the
product claim.
In the compounds of the formula VI, Hal is preferably
chlorine, in particular when compounds are prepared in
which the two phenyl radicals are linked by A. This means
that the process according to the invention is also
suitable for the preparation of those compounds contain-
ing the group A which have been disclosed in European
Pat~nt 337,784, US Patent 4,143,028 and US Patent
4,481,317. The cyclic diaryl chlorophosphites of the
formula VI used as starting compounds are available in a
simple manner from corresponding bisphenols and
phosphorus trichloride, for example by the process
described in European Laid-Open Application 312,915.
The first step of the process according to the invention,
which can be carried out in any conventional manner, is
preferably carried out in an aprotic, organic solvent,
such as an ether, for example diethyl, dipropyl or
~~5~'~~
_ 7 _
diisopropyl ether, ethylene glycol dimethyl or -diethyl
ether, diethylene glycol dimethyl or -diethyl ether,
methyl tert-butyl ether, dioxane or tetrahydrofuran.
Since the Grignard compounds are sensitive to hydrolysis
and oxidation, it may be advantageous to work under an
inert gas atmosphere. However, such a procedure is not
absolutely necessary for the reaction to succeed. Par-
ticularly suitable inert gases are nitrogen and argon.
The reaction temperature is in general between 20 and
125°C, but preferably between 30 and 70°C.
To prepare the compounds I or V, the solution or suspen-
sion of the Grignard compound is metered in the second
step to the diaryl halophosphonite II or VI, which
advantageously is diluted with an inert, aprotic solvent,
for example hexane, toluene, xylene or one of the above-
mentioned ethers. The reactants in this step are combined
slowly, in general between -30°C and +30°C, _but
preferably between -20°C and 20°C. As a rule, the
reaction is exothermic; it can therefore be advantageous
to control the course of the reaction by cooling. The
most favorable results are obtained by using the
reactants in stoichiometric amounts. However, it is also
possible to use one reactant in excess; however, this is
in general not associated with particular advantages.
Advantageously, the mixture is stirred until the reaction
is complete, which is promoted by heating to 0 to 30°C
and then the precipitated magnesium halide is separated
off. The solvents can be removed from the filtrate in the
usual manner, advantageously by distillation, in
particular under reduced pressure.
The ester halides II or VI which are required as starting
materials can be prepared in a simple manner from phos-
phorus trichloride and the corresponding phenols (for
example US Patent 4,739,000). The purity of the starting
materials thus obtained is about 85-90% (according to
- 8 - 2~5~'~~'~?
Sip-~) .
The products V can be separated from the crude products
by any desired method, but preferably by crystallization.
The synthesis of phosphorous esters by reaction of
organometallic compounds with chlorophosphonous diesters
has been described by various authors. They point out in
particular that high-purity chlorophosphonous esters must
be used as starting materials (Houben-Weyl, Methoden der
Organischen Chemie [Methods of Organic Chemistry), volume
12/1, 329 (1963)). Nevertheless, the yields of diaryl
phosphonites were only between about 33 and 75%. Since it
is known that ester groups bound to the phosphorus atom
basically display the same behavior towards organo-
metallic compounds as halogen atoms (Iiouben-Weyl,
Methoden der Organischen Chemie [Methods of Organic
Chemistry], volume 12/1,.44 (1963)) it could not be
expected - even if in contrast to customary practice no
extremely pure chloroesters II or VII are used as
starting materials - that the present process would
provide the target compounds in such a satisfactory
manner and largely without any yield-reducing competition
reactions.
It is particularly surprising that the products are
available by the process according to the invention in
such a high yield and purity, since, according to the
details of Japanese Laid-Open Application 57-46,993,
phosphorous esters can in general only be obtained in
economic yields by Grignard reactions if after the actual
Grignard reaction various aftertreatment procedures with
the addition of auxiliaries and under inert gas
atmosphere are carried out.
As a result, the present invention has made it possible
to obtain any desired substituted aryl phosphonites in a
simple manner and with high yield and purity.
'? 0~1 r~ r! '"~1 Ea 'j
[ 1:6
~.5 ~.~ :.~ LJ a SJ ia4
..
The invention finally relates to the use of the compounds
of the formula V by themselves or in combination with a
phenolic antioxidant for stabilizing plastics, such as
polycarbonates, preferably plastics obtained by polymeri-
nation, such as polyolefins, in particular polypropylene.
The compounds of the formula I provide the plastics in
the molding compositions with improved stability to
degradation by light, oxygen and heat. However, the
purity of the resulting crude reaction product (85-93$
according to 31P-NP4R) is in most cases sufficient for this
application. In this case, they do not have to be iso-
lated in pure form.
Accordingly, the present invention also relates to a
plastic molding composition comprising a thermoplastic or
thermoset plastic and an aryl phosphonite of the formula
V in a ratio of (90 to 99.99)x(0.01 to 10), in which n is
1 or 2, A is non-existent .- i.e. the two rings carry
hydrogen - or a divalent hydrocarbon bridge having 1 to
6 carbon atoms which may be substituted by groups
mentioned further below under R1, or is a hetero atom such
as oxygen or sulfur, cycloalkylidene having 4 to 8 carbon
atoms or phenylalkylidene having 7 to 12 carbon atoms, R1
as monovalent radical is a phenyl or benzyl radical, each
of which carry 1 to 3 substituents, or is «-methylbenzyl,
a,«-dimethylbenzyl, naphthyl or a naphthyl radical
carrying 1 to 5 substituents, in which the substituents
can be identical or different and are a non-aromatic
hydrocarbon radical, an alkoxy radical, an alkylthio
radical ox a dialkylamino radical, in which the alkyl
radicals each have 1 to 8 carbon atoms, aryl or aryloxy
each having 6 to 10 carbon atoms or halogen having an
atomic number from 9 to 35, and as divalent radical a
naphthylene radical which is unsubstituted or carries 1
to 4 non-aromatic hydrocarbon radicals each having 1 to
8 carbon atoms as substituents, or, if A is non-existent,
a phenyl radical which is unsubstituted or substituted
by up to two non-aromatic hydrocarbon radicals having 1
to 8 carbon atoms,
2~5~'~~
-lo-
RZ is a non-aromatic hydrocarbon radical of 1 to 18 carbon
atoms, aryl, arylmethyl, arylethyl or arylisopropyl, in
which the aryl in each case contains 6 to 10 carbon
atoms,
R3 is hydrogen or a group mentioned under RZ,
where, in the compounds in which n is 1 and Rl is phenyl,
those are excepted in which simultaneously
R2 and R3 are each tart-butyl,
R2 is tart-butyl and R3 is hydrogen,
RZ is tart-octyl and R3 is methyl,
R2 and R' are each tart-octyl and
RZ and R3 are each a,a-dimethylbenzyl.
Compounds of the formula I, in particular those contain-
ing no dialkylamino radicals, are preferred.
The plastic molding compound according to the invention
contains a thermoplastic or thermoset organic polymer,
for example one of the following:
1. Polymers of mono- and diolefins, for example polyethy-
lene of high, medium or low density (which, if desired,
can be crosslinked), polypropylene, polyisobutylene,
poly-1-butane, polymethyl-1-pentane, polyisoprene or
polybutadiene and polymers of cycloolefins, such as
cyclopentene or norbornene.
2. Mixtures of the polymers mentioned under 1), for
example of polypropylene with polyethylene or with
polyisobutylene.
3. Copolymers of mono- and diolefins with one another or
with other vinyl monomers, such as ethylene-propylene
copolymers, propylene-1-butane copolymers, propylene-
isobutylene copolymers, ethylene-1-butane copolymers,
propylene-butadiene copolymers, isobutylene-isoprene
copolymers, ethylene-alkyl acrylate copolymers,
- II -
ethylene-alkyl methacrylate copolymers, ethylene-vinyl
acetate copolymers or ethylene-acrylic acid copolymers
and salts thereof (ionomersj, and terpolymers of ethylene
with propylene and a diene, such as hexadiene, dicyclo-
5: pentadiene or ethylidenenorbornene.
4. Polystyrene.
5. Cogolymers of styrene or a-methylstyrene with dienes
or acrylic derivatives, such as styrene-butadiene,
styrene-malefic anhydride, styrene-acrylonitrile, styrene-
ethyl methacrylate, styrene-butadiene-ethyl acrylate,
styrene-acrylonitrile-methyl acrylate;
mixtures of high impact strength and composed of styrene
copolymers and one other polymer, such as a polyacrylate,
a diene polymer or an ethylene-propylene-diene terpoly-
I5 mer; and block copolymers of styrene, such as styrene-
butadiene-styrene, styrene-isoprene-styrene, styrene-
ethylene/butylene-styrene or styrene-ethylene/propylene-
styrene.
6. Graft copolymers of styrene, such as styrene onto w
polybutadiene, styrene and acrylonitrile onto polybuta-
diene (ABS), styrene and malefic anhydride onto polybuta-
diene, styrene and alkyl acrylates or alkyl methacrylates
onto polybutadiene, styrene and acrylonitrile onto
ethylene-propylene-diene terpolymers, styrene and acrylo-
nitrile onto poly(alkyl acrylates) or poly(alkyl meth-
acrylates), styrene and acrylonitrile onto acrylate-
butadfene copolymers, and mixtures thereof with the
copolymers mentioned under 5), which are known, for
example, as eo-called AHS, 1~5, ASA or AES polymers.
7. Halogen-containing polymers, such as polychloroprene,
chlorinated rubber, chlorinated (CPE) or chlorosulfonated
polyethylene, epichlorohydrin homo- and copolymers, in
particular polymers of halogen-containing vinyl
compounds, such as polyvinyl chloride (PVC), polyvinyli-
done chloride (PVDC), polyvinyl fluoride, polyvinylidene
., ..~ _~~ r~ .Y~ ~x ~,.
e.3 ,tr.) S ~ i ;,~d
- 12 -
fluoride (PVDF); and the copolymers thereof, such as
vinyl chloride-vinylidene chloride, vinyl chloride-vinyl
acetate or vinylidene chloride-vinyl acetate.
8. Polymers derived from a,p-unsaturated carboxylic acids
and derivatives thereof, such as polyacrylates and poly-
methacrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers of the monomers mentioned under 8) with. one
another or with other unsaturated monomers, such as
acrylonitrile-butadiene copolymers, acrylonitrile-alkyl
acrylate copolymers, acrylonitrile-alkoxyacrylate copoly-
mers, acrylonitrile-vinyl halide copolymers or acrylo-
nitrile-alkyl methacrylate-butadiene terpolymers.
10. Polymers derived from unsaturated alcohols and amines
or acyl derivatives or acetals thereof, such as polyvinyl .
I5 alcohol, polyvinyl acetate, stearate, benzoate, maleate,
polyvinylbutyral, polyallyl phthalate, polyallylmelamine.
11. Homo- and copolymers of cyclic ethers, such as
polyethylene glycols, polyethylene oxide, polypropylene
oxide or copolymers thereof with bisglycidyl ethers.
12. Polyacetals, such as polyoxymethylene (POM), and
those polyoxymethylenes containing comonomers, such as
ethylene oxide..
13. Polyphenylene oxides and sulfides.
14. Polyurethanes (PUR) derived on the one hand from
polyethers, polyesters and polybutadienes having terminal
hydroxyl groups and on the other hand from aliphatic or
aromatic polyisocyanates and precursors thereof
(polyisocyanates-polyols prepolymers).
15. Polyamides and copolyamides derived from diamines and
dicarboxylic acids and/or from aminocarboxylic acids or
the corresponding lactams, such as nylon-4, nylon--6,
r~
- 13 -
nylon-6/6, nylon-6/10, nylon-11, nylon-12, poly-2,4,4
trimethylhexamethyleneterephthalamide, poly-m-phenylene
isophthalamide, and copolymers thereof with polyethers,
such as with polyethylene glycol, polypropylene glycol or
polytetramethylene glycol.
16. Polyureas, polyimides and polysmidoimides.
17. Polyesters derived from dicarboxylic acids and diols
and/or from hydroxycarboxylic acids or the corresponding
lactones, such as polyethylene terephthalate, polybuty-
lene terephthalate (PBTP), poly-1,4-dimethylolcyclohexane
terephthalate, poly-(2,2-bis(4-hydroxyphenyl)propane)
terephthalate, polyhydroxybenzoates, and block polyether
esters derived from polyethylene having terminal hydroxyl
groups, dialcohols and dicarboxylic acids.
18. Polycarbonates (PC).
19. Polysulfones and polyether sulfones.
20. Crosslinked polymers derived on the one hand from
aldehydes and on the other hand from phenols, urea or
melamine, such as phenol-formaldehyde, urea-formaldehyde
and melamine-formaldehyde resins.
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins derived from copoly-
esters of saturated and unsaturated dicarboxylic acids
with polyhydric alcohols, and vinyl compounds as cross-
linking agents, as well as the halogen-containing, non-
flammable modifications thereof.
23. Crosslinkable acrylic resins derived from substituted
acrylic esters, such as from epoxy acrylates, urethane
acrylates or polyester acrylates.
24. Alkyd resins, polyester resins and acrylate resins
~~~~~~"''
.a~9~~".
- 14 --
crosslinked with melamine resins, urea resins, polyiso-
cyanates or epoxy resins.
25. Crosslinked epoxy resins derived from polyepoxides,
for example from bis(glycidyl) ethers or from cyclo
aliphatic diepoxides.
26. Natural polymers, such as cellulose, natural rubber,
gelatins and polymer-homologous chemically modified
derivatives thereof, such as cellulose acetates, propio
nates and butyrates, and the cellulose ethers, such as
methylcellulose.
27. Mixtures of the abovementioned polymers, such as, for
example, PP/EPDM, nylon-6/EPDM or ABS, PVC/EVA, PVC/ABS,
PVC/MBS, PC/ABS, PBTP/ABS, PC/ASA, PC/PHT, PVC/CPE,
PVD/acrylate, POM/thermoplastic PUR, POM/acrylate,
POM/MBS, polyphenylene ether/high impact strength poly-
styrene (PPE/HIPS) PPE/nylon-6.6 and copolymers, PA/HDPE,
PA/PP, PA/PPE.
28. Naturally occurring and synthetic organic materials
which are pure monomers or mixtures of monomers, such as
mineral oils, animal and vegetable fats, oils and waxes,
or oils, fats and waxes based on synthetic esters or
mixtures of these materials.
29. Aqueous dispersions of natural or synthetic rubber.
The polymer is preferably a polyolefin, in particular
polypropylene. The amount of the polymer in the molding
compound according to the invention is 90 to 99.99,
preferably 98 to 99.98% by weight.
The molding compound contains as stabilizer an aryl
phosphonite of the formula I and, if necessary, a
phenolic antioxidant.
The phenolic antioxidant is, for example, an ester of
fn 7 ° ~_) 1.1 ,,i F ~ ~ s
-- 15 -
3,3-bis(3'-t-butyl--4'-hydroxyphenyl)butyric acid of the
formula ITI (see formula sheet), in which n is 1 or 2 and
R' is a C1-C12-alkyl radical, if n is 1, and a
C1-C12-alkylene radical, if n is 2. Preferably, R' is a
CZ-C4-alkylene radical, in particular a Cz-alkylene
radical.
However, the phenolic antioxidant can also be an ester of
~9°(3,5-di-t-butyl-4-hydroxyphenyl)propionic acid of the
formula IV (see formula sheet), in which the alcohol
component is a mono- to tetrahydric alcohol, such as
methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
diethylene glycol, triethylene glycol, pentaerythritol,
tris(hydroxyethyl) isocyanurate, thiodiethylene glycol or
dihydroxyethyloxamide.
The new stabilizers are incorporated in the organic
polymers by generally customary methods. They can be
incorporated, for example, by admixing the compounds and,
if necessary, further additives to the melt before or
during the molding. They can also be incorporated by
applying the dissolved or dispersed compounds directly to
the polymer or admixing to a solution, suspension or
emulsion of the polymer, if appropriate subsequently
allowing the so7.vent to evaporate. The amount to be added
to the polymers is 0.01 to 10, preferably 0.025 to 5, in
particular 0.05 to 1.0 ~ by weight, relative to the
material to be astabilized.
The new compounds can also be added in the form of a
master batch containing these compounds, for example, in
a concentration of 1 to 50, preferably 2.5 to 20~ by
weight, to the polymers which are to be stabilized.
In addition, the molding composition according to the
invention can also contain other antioxidants, such as
1. a7.lcylated monophenols, for example 2, 6-di-t-butyl-
4-methylphenol, -4-ethylphen.ol, -4-n-butylphenol,
-4-i-butylphenol, 2-t-butyl.-4,6-dimethylphenol,
_ 16 - 2~~~"~~~
2,6-di-cyclopentyl-4-methylphenol, 2-(a-methylcyclo-
hexyl)-4,6-dimethylphenol, 2,6-di-octadecyl-
4-methylphenol, 2,4,6-tri-cyclohexylphenol,
2,6-di-t-butyl-4-methoxymethylphenol;
2, alkylated hydroquinones, such as 2,5-di-t-butyl- and
2,5-di-t-amylhydroquinone, 2,6-di-t-butyl-4-methoxy-
phenol and 2,6-diphenyl-4-octadecyloxyphenol;
3. hydroxylated thiodiphenyl ethers, such as 2,2'-thio
bis(6-t-butyl-4-methylphenol) and -(4-octylphenol)
and 4,4'-thio-bis(6-t-butyl-3-methylphenol) and
-(6-t-butyl-2-methylphenol);
4. alkylidene bisphenols, such as 2,2'-methylene-
bis(6-t-butyl-4-methylphenol), -(6-t-butyl-4-ethyl-
phenol), -[4-methyl-6-(a-methylcyclohexyl)phenol],
-(4-methyl-6-cyclohexylphenol), -(6-nonyl-4-methyl-
phenol), -(4,6-di-t-butylphenol), -[6-(a-methyl-
benzyl)-4-nonylphenol], -[6-(a, a-dimethylbenzyl)-
4-nonylphenol], 4,4'-methylene-bis(2,6-di-t-butyl-
phenol) and -(6-t-butyl-2-methylphenol), 2,2'-ethy-
lidene-bis(4,6-di-t-butylphenol) and -(6-t-butyl-
4-isobutylphenol), l,l-bis- and 1,1,3-tris(5-
t-butyl-4-hydroxy-2-methylphenyl)butane,
2,6-di(3-t-butyl-5-methyl-2-hydroxybenzyl)-4-methyl-
phenol, 1,1-bis(5-t-butyl-4-hydroxy-2-methylphenyl)-
3-n-dodecylmercaptobutane, di(3-t-butyl-4-hydroxy-
5-methylphenyl)dicyclopentadiene;
5. benzyl compounds, such as di[2-(3'-t-butyl-2'-hyd-
roxy-5'-methylbenzyl)-6-t-butyl-4-methylphenyl]
terephthalate, 1,3,5-tri(3,S-di-t-butyl-4-hydroxy-
benzyl)-2,4,6-trimethylbenzene, di(3,5-di-t-butyl-
4-hydroxybenzyl) sulfide, isooctyl 3,5-di-t-butyl-
4-hydroicybenzylmercapto acetate, bis(4-t-butyl-
3-hydroxy-2,6- dimethylbenzyl) dithiolterephthalate,
1,3,5-tris(3,5-di-t-butyl-4-hydroxybenzyl) isocya-
nurate, 1,3,5-tris(4-t-butyl-3-hydroxy-2,6-dimethyl-
benzyl) isocyanurate, dioctadecyl 3,5-di-t-butyl-
4-hydroxybenzylphosphonate and the calcium salt of
monoethyl 3,5-di-t-butyl-4-hydroxybenzylphosphonate;
6. acylaminophenols, such as 4-hydroxylaur- and
4
- 17 - ~~J~ ~~~7
-stearanilide, 2,4-bis(octylmercapto)-6-(3,5-di-
t-butyl-4-hydroxyanilino)-s-triazine and octyl
N-(3,5-di-t-butyl-4-hydroxyphenyl)carbamate;
7. esters of ~-(5-t-butyl-4-hydrozy-3-methylphenyl)pro
pionic acid with mono- or polyhydric alcohols, such
as with methanol, octadecanol, 1,6-hexanediol,
neopentyl glycol, diethylene glycol, triethylene
glycol, pentaerythritol, tris(hydroxyethyl) isocya
nurate, thiodiethylene glycol or dihydroxyethyl
oxamide;
8. amides of ~-(3,5-di-t-butyl-4-hydroayphenyl)pro-
pionic acid, such as N,N'-di(3,5-di-t-butyl-
4-hydroxyphenylpropionyl)trimethylenediamine,
-hexamethylenediamine and -hydrazine.
In addition, the molding composition according to the
invention can contain further additives, such as
1. W absorbers and light stabilizers, for example
1.1 2-(2'-hydroxymethyl)benzotriazoles, such as the
5'-methyl, 3',5'-di-t-butyl, 5'-t-butyl,
5'-(1,1,3,3-tetramethylbutyl), 5-chloro-3',5'-di-
t-butyl, 5-chloro-3'-t-butyl-5'-methyl, 3'-sec.-
butyl-5'-t-butyl, 4'-octoxy, 3',5'-di-t-amyl,
3',5'-bis(a,a-dimethylbenzyl) derivative;
1.2 2-hydroaybenzophenones, such as the 4-hydroxy,
4-methoxy, 4-octoxy, 4-decyloxy, 4-dodecyloxy,
4-benzyloxy, 4,2',4'-trihydroxy, 2'-hydroxy
4,4'-dimethoxy derivative;
1.3 esters of substituted or unsubstituted benzoic
acids, such as phenyl salicylate, 4-t-butylphenyl
salicylate, octylphenyl salicyalate [sic],
dibenzoylresorcinol, bis(4-t-butylbenzoyl)-
resorcinol, benzoyl resorcinol, 2,4-di-t-butylphenyl
3,5-di-t-butyl-4-hydroxybenzoate, hexadecyl
3,5-di-t-butyl-4-hydroxybenzoate;
1. 4 acrylates, such as ethyl or isooctyl a-cyano-p, ~9-di-
phenylacrylate, methyl a-carbomethoxy- and a-carbo-
methoxy-p-methoxycinnamate, methyl or butyl a-cyano-
p-methyl-p-methoxycinnamate, N-(p-carbomethoxy-
6;;~~.,rK."~J~..;
i.~ '.i a:f 't: f ~.3 ;.~
- 18 -
p-cyano~rinyl)-2-methylindoline;
1.5 nickel compounds, such as nickel complexes of
2,2'-thiobis[4-(1,1,3,3-tetramethylbutyl)phenol],
such as the lal or 1c2 complex, if desired with
additional ligands, such as n-butylamine, trietha
nolamine or N-cyclohexyldiethanolamine, nickel alkyl
dithiocarbamates, nickel salts of monoalkyl 4-hyd-
roxy-3,5-di-t-butylbenzylphosphonates, such as those
of the methyl or ethyl ester, nickel complexes of
ketoximes, such as those of 2-hydroxy-4-methyl-
phenylundecylketonox5.me, nickel complexes of
1-phenyl-4-lauroyl-5-hydroxypyrazole, if desired
with additional ligands;
1.6 sterically hindered amines, such as
1.6.1 bis(2,2,6,6-tetramethylpiperidyl) sebacate,
bis(2,2,6,6-tetramethylpiperidyl) glutarate and
bis(2,2,6,6-tetramethylpiperidyl) succinate,
bis(1,2,2,6,6-pentamethylpiperidyl) sebacate,
bis(1,2,2,6,6-pentamethylpiperidyl)glutarate and
bis(1,2,2,6,6-pentamethylpiperidyl) succinate,
4-stearyloxy- and 4-stearoyloxy-2,2,6,6-tetra-
methylpiperidine, 4-stearyloxy- and 4-stearoyl-
oxy-1,2,2,6,6-pentamethylpiperidine,
2,2,6,6-tetramethylpigeridyl behenate,
1,2,2,6,6-pentamethylpiperidyl behenate,
2,2,4,4~-tetramethyl-7-oxa-3,20-diazadispiro-
[ 5 . 1 . 1 1 . 2 ] h a n a i c o s a n - 2 1 - o n a ,
2,2,3,4,4-pentamethyl-7-oxa-3,20-diazadi-
spiro[5.1.11.2]heneicosan-21-one,
2,2,4,4-tetra-methyl-3-acetyl-7-oxa-3,20-diazadi-
spiro[5.1.11.2Jheneicosan-21-one,
2,2,4,4-tetramethyl-7-oxa-3,20-diaza-20-
(~-lauryloxycarbonylethyl)-21-oxo-dispiro-
[5.1.11.2]heneicosane, 2,2,3,4,4-pentamethyl-7-
oxa-3,20-diaza-20-(p-lauryloxycarbonylethyl)-21-
oxo-dispiro[5.1.11.2]heneicosane,
2,2,4,4-tetra-methyl-3-acetyl-7-oxa-3,20-diaza-
20-(p-lauryloxycarbonylethyl)-21-oxo-dispiro-
[5.1.11.2]heneicosa:ne, 1,1',3,3',5,5'-hexahydro-
- 19 -
2,2',4,4',6,6'-hexaaza-2,2',6,6'-bismethano-
7,8-dioxo-4,4'-bis(1,2,2,6,6-pentamethyl-
4-giperidyl)biphenyl, N,N',N",N"'-tetrakis~2,4-
bis[N-(2,2,6,6-tetramethyl-4-piperidyl)butyl-
amino]-1,3,5-triazin-6-yl}-4,7-diazadecane-1,10-
diamine, N,N',N",N"'-tetrakisi2,4-bis[N-
(1,2,2,6,6-pentamethyl-4-piperidyl)butylamino]-
1,3,5-triazin-6-yl}-4,7-diazadecane-1,10-diamine,
N,N',N",N"'-tetrakisi2,4-bis[N-(2,2,6,6-tetra-
methyl-4-piperidyl)methoxypropylamino]-1,3,5-
triazin-6-yl}-4,7-diazadecane-1,10-diamine,
N,N',N°',N"'-tetrakis~2,4-bis[N-(1,2,2,6,6-penta-
methyl-4-piperidyl)methoxypropylamino]-1,3,5-
triazin-6-yl}-4,7-diazadecane-1,10-diamine,
bis(1,2,2,6,6-pentamethylpiperidyl)-n-butyl-
3,5-di-tert-butyl-4-hydroxybenzyl malonate,
tris(2,2,6,6-tetramethyl-4-piperidyl) nitrilotri-
acetate, tetrakis(2,2,6,6-tetramethyl-4-piperi-
dyl)-1,2,3,4-butanetetracarboxylic acid,
1,1'-(1,2-ethanediyl)bis(3,3,5,5-tetramethyl-
piperazinone);
1.6.2 poly-N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)-
1,8-diazadecylene, condensation product of
1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxy-
piperidine with succinic acid, condensation
product of N,N'-bis(2,2,6,6-tetramethyl-4-piperi-
dyl)hexamethylenediamine with 4-tert-octylamino-
2,6-dichloro-1,3,5-e-triazine, condensation
product of N,N'-bis(2,2,6,6-tetramethyl-4-piperi-
dyl)hexamethylenediamine with 4-morpholino-
2,6-dichloro-1,3,5-triazine.
In many cases, a combination of the compounds
according to the invention with the compounds
mentioned under 1.6.1 proves especially
advantageous.
1.7 oaamides, such as 4,4'-di-octyloxyoxanilide,
2,2'-di-octyloxy-5,5'-di-t-butyloxanilide,
2,2'-didodecyloxy-5,5'-di-t-butyloxanilide,
2-ethoxy-2'-ethyloxanilide, N,N'-bis(3-
~: ~~ ~-, ., vy a~ .y
_ 2 0 _ ~~ .~;:e ;;.' "A
dimethylaminopropyl)oxamide, 2-ethoxy-5-t-butyl-2'-
ethyloxanilide and its mixture with 2-ethoxy-
2'-ethyl-5,4-di-t-butyloxanilide, mixtures of o- and
p-methoxy- and -ethoxy-disubstituted oxanilides;
2. metal deactivators, such as N,N'-diphenyloxamide,
N-salicylal-N'-salacyloylhydrazine, N,N'-bis-
salicyloylhydrazine, N,N'-bis(3,5-di-t-butyl-4-
hydroxyphenylprapionyl)hydrazine, 3-salicyloylamino-
1,2,3-triazole, bis(benzylidene)oxalic dihydrazide;
3. phosphates and phosphonites, for example triphenyl
phosphate, diphenylalkyl phosphates, phenyl dialkyl
phosphates, tris(nonylphenyl) phosphate, trilauryl
phosphate, trioctadecyl phosphate, distearyl penta-
erythrityl diphosphite, tras(2,4-di-t-butylphenyl)
phosphate, diisodecyl pentaerythrityl diphosphite,
bis(2,4-di-t-butylphenyl) pentaerythrityl diphos-
phite, tristearyl sorbityl triphosphite, tetrakis-
(2,4-di-t-butylphenyl)-4,4'-biphenylene
diphosphonite, 3,9-bis(2,4-di-t-butylphenoxy)-
2,4,8,10-tetraoxa-3,9-diphosphasparo[5.5]undecane,
tras(2-t-butyl-4-thin(2'-methenyl-4'-hydroxy-5'-t-
butyl)phenyl-5-methenyl)phenyl phosphate.
4. peroxide-destroying compounds, such as esters of
p-thiodipropionic acid, for example the lauryl,
stearyl, myristyl or tridecyl ester, mercaptobenz
imidazole,the zinc salt of 2-mercaptobenzimidazole,
zinc alkyl dithiocarbamates, dioctadecyl sulfide,
dioctadecyl monosulfide, pentaerythritol tetrakis-
(p-dodecylmercapto)propionate;
5. basic co-stabilizers, such as melamine, polyvinyl-
pyrrolidone, dicyandiamide, triallylcyanurate, urea
derivatives, hydrazine derivatives, amines, poly-
amines, polyurethanes, alkali metal salts and
alkaline earth metal salts of higher fatty acids or
phenolates, for example calcium stearate, zinc
stearate and magnesium stearate, sodium racinoleate,
potassium palmitate, antimony catecholate or tin
catecholate, hydroxides and oxides of alkaline earth
metals ar of aluminum, for example CaO, Mg~, ZnO;
i,. -., r7 ; .~t
21 _ ;% d~ s..
N,; :aP = ~ i) E~ ~7 :. ,
6. nucleating agents, such as 4-t~-butylbenzoic acid,
adipic acid, diphenylacetic acid, dibenzylidene~-
sorbitol;
7, fillers and reinforcing agents, such as calcium
carbonate, silicates, glass fibers, asbestos, talc,
kaolin, mica, barium sulfate, metal oxides and metal
hydroxides, carbon black, graphite;
8. other additives, such as plasticizers, lubricants,
emulsifiers, pigments, optical brighteners, flame
retardants, antistats, blowing agents.
The various additional additives of the abovementioned
groups 1 to 6 are added to the polymers to be stabilized
in an amount of 0.01 to 10, preferably 0.01 to 5, ~ by
weight, relative to the total weight of the molding
composition. The relative amount of the additives from
groups 7 and B is in general 1 to 80, preferably 10 to
50, $ by weight, relative to the total molding
composition.
The organic polymers stabilized according to the
invention can be applied in various forms, for example as
films, fibers, ribbons, profiles or as binders for
paints, adhesives or putties.
In the examples 1 to 24 which follow, the compounds
obtained according to the invention were crystallized
using certain solvent mixtures. The ratios given are by
volume. By changing the mixing ratios, it may be possible
to achieve even better results.
Exaanples for the preparation of diaryl phosphonites
General procedure for compounds of the foranula I
'Under a nitrogen atmosphere and with the exclusion of
moisture, 250 mmol of organobromo compound and 250 mmol
(= 6.1 g) of Mg turnings in 170 ml of tetrahydrofuran
were used to prepare the corresponding Grignard compound.
- 22 -
The solution or suspension obtained was then metered into
the solution of 250 mmol of diaryl chlorophosphonite in
150 ml of n-hexane/tetrahydrofuran (2:1) at an internal
temperature of -20 to -10°C with vigorous stirring over
a period of 30-40 min. The reaction mixture was then
allowed to warm to room temperature, and stirring was
continued for 2.5 hours to complete the reaction. After
the precipitated Mg salt had been filtered off, the
solvent was distilled off first in the vacuum of a water
IO pump and then in a high vacuum, and the colorless or
light beige residue obtained was powdered and dried in a
high vacuum.
The amount of product in the crude materials was deter-
mined by 3'P-NMR spectroscopy. It was in general between
85 and 93% (of the total product) . To characterize the
product, it was crystallized in the cases mentioned from
acetonitrile/acetone mixtures.
1) Bis(2~4~-di-tert-butylphenyl) (2,4,6-trimethyl-1-
phenyl)phosphonites starting from 49.7 g of bromomesity-
lene and 119.3 g of bis(2,4-di-tert-butylphenyl) chloro
phosphonite, 140 g of colorless material of a softening
point of about 60°C containing 90% of the above compound
were obtained. Crystallization from acetonitrile/acetone
(15:1) gave colorless crystals of melting point 95-97°C;
[31P-Nl~t: b~13~168.4 ppm]
C3~Hs3~2P Calculateds 79.24% C, 9.52% H, 5.52% P
(560.8) Found: 78.9% C, 9.7% H, 5.3% P.
2) Bis(2~,4~-di-tert-butylphenyl) (2,4,5-trimethyl-1-
phenyl)phosphonite: starting from 49.7 g of 5-bromo-
1,2,4-trimethylbenzene and 119.3 g of bis(2,4-di-tert-
butylphenyl) chlorophosphonite, 140 g of a yellowish
material containing 93% of the above compound were
obtained. Softening point about 30-35°C; [31P-Nl~t:
a~13=155 . 4 ppm] ~ C3~Iis30zP ( 560 . 8 ) .
- 23 - ~~J~~~D~
3) Bis(2~,4~-di-tert-butylphenyl) (4-tert-butylphenyl)-
phosphonite: starting from 53.3 g of p-bromo-tert-butyl-
benzene and 119.3 g of bis(2,4-di-tert-butylphenyl)
chlorophosphonite, about 140 g of colorless material
containing 90% of the above compound were obtained.
Crystallization of the crude product from acetonitrile/
acetone (15:2) gave colorless crystals of melting point
115-117 °C; [ 31P-rtl~t: a~13=155 . 9 ppm] .
C3aHss~aP Calculated: 79.4% C, 9.64% H, 5.38% P
(574.82) Found: 79.8% C, 9.9% H, 5.0% P.
4) Bis(2~-tent-butylphenyl) (4-tent-butylphenyl)-phos-
phonite: starting from 53.3 g of p-bromo-tert-butylben-
zene and 91.2 g of bis(2-tart-butylphenyl) chlorophos-
phonite, about 115 g of viscous resin containing 85% of
the above compound were obtained. Crystallization of the
crude product from acetonitrile/acetone (2:1) gave
colorless crystals of melting point 95-97°C; [31P-Nl~t:
613=155. 9 ppm] .
CaoHse~aP Calculated: 77.89% C, 8.49% H, 6.69% P
(462.61) Found: 77.5% C, 8.7% H, 6.5% P.
5) Bis(2~,4~-di-tert-butylphenyl) 1-naphthylphosphonite:
starting from 51.8 g of 1-bromonaphthalene and 119.3 g of
bis(2,4-di-tert-butylphenyl) chlorophosphonite, about
142 g of a colorless solid of softening point 50-55°C
containing 91% of the above compound were obtained.
Crystallization of the crude product from acetonitrile/
acetone (5s1) gave colorless crystals of melting point
125-127°C; [31P-Nl~t: d~13=158.1 ppm]
C3eH,e~2P Calculateds 80.24% C, 8.68% H, 5.44% P
(568.77) Found: 80.5% C, 8.5% H, 5.3% P.
'-;~ ;2 ;... ,,~ ; fir" ~%
~_
- 2 4 - ~3 .~ ~ ~i , s> ~a
6) Bis(2',4'-di-tart-butylphenyl) (4-methyl-1-naphthyl)-
phosphonite: starting from 55.27 g of 1-bromo-4-methyl-
naphthalene and 119.3 g of bis(2,4-di-tart-butylphenyl)
chlorophosphonite, about 140 g of a beige material
containing 93~ of the above compound ware obtained.
Crystallization from acetone gave colorless crystals of
melting point 145-146°C; [3iP-D1MR: bcDCl3=159.0 ppm].
C3~Hs102P Calculated: 80.37 C, 8.82$ H, 5.31 P
(582.80) Found: 80.7 C, 9.1$ H, 5.1~ P
7) Bis(2'-tent-butylphenyl) (4-methyl-1-naphthyl)-
phosphonite: starting from 55.27 g of 1-bromo-4-methyl-
naphthalene and 91.2 g of bis(2-tart--butylphenyl) chloro-
phosphonite, about 110 g of a yellow resin containing 90~
of the above compound were obtained; [31P-NMR:
dcDCi3-158 . 4 ppm] . C3lHasCzP ( 470. 6 ) .
8) Bis(2',4'-di-tart-butylphenyl) (2-methyl-1-naphthyl)-
phosphonite: starting from 55.27 g of 1-bromo-2-methyl-
naphthalene and 119.3 g of bis(2,4-di-tart-butylphenyl)
chlorophosphonite, about 140 g of a yellowish material
containing 90$ of the above compound were obtained.
Crystallization from acetone/acetonitrile (2:1j gave
colorless crystedls of melting point 157-159°C; [31P-NMR:
acDCi3-164 .4 ppm] .
C39H51~2P CaICLA~.ated: 80.37 C, 8.82$ H, 5.31$ P
(582.80) Found: 79.9 C, 9.1~ H, 5.1~ P,
9) Bis(2',4'-di-tart-butylphenyl) 2-naphthylphosphonite:
starting from 51.8 g of 2-bromonaphthalene and 119.3 g of
bis(2,4-di-tart-butylphenyl) chlorophosphonite, about
143 g of a colorless solid containing 94$ of the above
compound were obtained. Crystallization of the crude
product from acetonitrile/acetone (9:1) gave colorless
crystals of melting point 133-135°C; [31P-NMR:
scDCi3=155. 0 ppm] .
- 25 -
CsaHas~aP Calculated: 80.24% C, 8.68% H, 5.44% P
(568.7?) Found: 80.4% C, 8.9% H, 5.2% P.
10) Bis(2',4'-di-tert-butylphenyl) (6 methoay-2-naphthyl)-
phosphonite: starting from 59.3 g of 2-bromo-6-methoxy-
naphthalene and 119.3 g of bis(2,4-di-tert-butylphenyl)
chlorophosphonite, 147 g of a colorless solid containing
93% of the above compound were obtained. Crystallization
from acetone gave colorless crystals of melting point
146-148 °C; [31P-NMR: 613=155.9 ppm] .
IO C3sIis1~3P Calculated: 78.22% C, 8.58% H, 5.17% P
(598.80) Found: 78.6% C, 8.3% H, 4.8% P.
11) Bis(2',4'-di-tert-butylphenyl) (4-methoayphenyl)-
phosphonite: starting from 46.75 g of 4-bromoanisole and
119.3 g of bis(2,4-di-tert-butylphenyl) chlorophospho-
nite, about 137 g of a colorless material of softening __
point 50°C containing 93% of the above compound were
obtained; [31P-NMR: d~13=155.8 ppm] . .
CssHas~3P Calculated: 76.60% C, 9.00% H, 5.64% P
(548.74) Founds 76.9% C, 9.2% H, 5.2% P.
12) Bis(2',4'-di-tert-butylphenyl)4-biphenylphosphonite:
starting from 58.3 g of 4-bromobiphenyl and 119.3 g of
bis(2,4-di-tert-butylphenyl) chlorophosphonite, about
148 g of a colorless powder having a softening point of
about 90°C and containing 90% of the above compound were
obtained [31P-Nl~ts 613=154.8 ppm] . Crystallization from
acetonitrile/acetone (10:1) gave colorless crystals of
melting point 103-105°C.
C~oHslOzP Calculated: 80.77% C, 8.64% H, 5.20% P
(594.81) Found: 81.2% C, 8.8% H, 4.9% P
13) Bis(2',4'-di-tert-butylphenyl) 4-bromophenyl-
phosphonite: starting from 59 g of 1,4-dibromobenzene and
119.3 g of bis(2,4-di-tert-butylphenyl)chlorophosphonite,
- 2 ~ - ~d ;.1 ~3 ~ i ~~~ :J ~~.~
about 145 g of an amorphous solid of softening point 80°C
containing about 85$ of the above compound [31P-NMR:
~cnci3 = 152.4 ppm] were obtained. C34H,,6Br~zP (597.61) .
14)Tetra(2',4'-di-tart-butylphenyljl,4-phenylenediphos-
phonite: the abovementioned procedure was modified, and
500 mmol (= 12.2 g) of magnesium and 500 mmol (= 238 g)
of bis(2,4-di-tart-butylphenyl) chlorophosphonite were
used instead of 250 mmol per 250 mmol (= 59 g) of
1,4-dibromobenzene to give about 200 g of the above
I0 compound of melting point 17B-180°C from acetone [31P-NMR:
SCDC13-153. I ppm] .
C62H88~4P2 Calculated: 77.62$ C, 9.24$ H, 6.45$ P
(959.32) Found: 78.0$ C, 9.0$ H, 6.2$ P.
15)Tetra(2',4'-di-tart-butylphenyl)1,3-phenylenediphos-
I5 phonite: Example 14 was repeated, except that 1,3-di-
bromobenzene was used, giving about 240 g of a beige
solid containing about 70$ of the ab~ve compound [31P-:
CDC13 = I54.25 ppm] and a saftening point of 70-75°C.
General procedure for compounds of the formula V in which
20 the two phenyl radicals are linDced by A.
At first the procedure adopted was analagous to that in
the preparation of compounds II, except that 250 mmol of
the respective cyclic chlorophosphonite diester of the
formula VI in 2;00 ml of tetrahydrofuran/n-hexane (2:1)
25 were metered in at an internal temperature of -10 to 0°C.
Stirring at 0°C was then continued for 1 hour and at room
temperature for 2.5 hours. After the precipitated
magnesium halide had been filtered off and washed with
250 ml'of tetrahydrofuran/n-hexane (4:I), the solvent was
30 distilled off first in a vacuum of water pump and then
in a high vacuum. The crude products were purified by
crystallization.
- 27 -
16. 4,8-Di-tert-butyl-2,10-dimethyl-6-(1'-naphthyl)-12H-
dibenzo[d,g][1,3,2]diozaphosphocine: starting from 51.8 g
of 1-bromonaphthalene and 101.2 g of 4,8-di-tert-butyl-
6-chloro-2,10-dimethyl-12H-dibenzo[d,g][1,3,2]dioxaphos-
phocine, 94.3 g (= 76%) of the above compound were
obtained from acetone in the form of colorless crystals
of melting point 251-253°C.
C33H3~~2P Calculated: 79.81% C, 7.50% H, 6.23% P
(496.62) Found: 79.4% C, 7.8% H, 6.0% P.
17. 4,8-Di-tert-butyl-2,10-diethyl-6-(1'-naphthyl)-12H-
dibenzo[d,g][1,3,2]diozaphosphocines starting from 51.8 g
of 1-bromonaphthalene and 108.2 g of 4,8-di-tert-butyl-
6-chloro-2,10-diethyl-12H-dibenzo[d,g][1,3,2]dioxaphos-
phocine, 106.2 g (= 81%) of the above compound were
I5 obtained from acetonitrile in the form of colorless
crystals of melting point 206-208°C.
C3sH~102P Calculated: 80.12% C, 7.87% H, 5.90% P
(524.68) Found: 79.5% C, 7.8% H, 5.7% P.
18. 4,8-Di-tert-butyl-2,10-diethyl-6-(2'-naphthyl)-12H-
dibenzo[d,g][1,3,2]dioaaphosphocine: starting from 51.8 g
of 2-bromonaphthalene and 108.2 g of 4,8-di-tert-butyl-
6-chloro-2,10-diethyl-12H-dibenzo[d,g][1,3,2]dioxaphos-
phocine, 109 g (= 83%) of the above compound were
obtained from acetonitrile in the form of colorless
crystals of melting point 222-224°C.
C35Hd102P Calculated: 80.12% C, 7.87% H, 5.90% P
(524.68) Found: 79.9% C, 8.1% H, 5.7% P.
19. 4,8-Di-tert-butyl-2,10-dimethyl-6-(4'-methyl-1'-
naphthyl)-12H-dibenzo[d, g][1,3,2]diozaphosphocine:
starting from 55.27 g of 1-bromo-4-methylnaphthalene and
101.2 g of 4,8-di-tert-butyl-6-chloro-2,10-dimethyl-12H-
dibenzo[d,g][1,3,2]dioxaphosphocine, 95.7 g (_ ?5%) of
the above compound were obtained from acetone/
i ~' 7 . ~ c~
2
- 28 -
dichloromethane (5s1) in the form of colorless crystals
of melting point 273-276°C.
C3,H390zP Calculated: 79.97% C, 7.70% H, 6.06% P
(510.66) Founds 79.3% C, 7.8% H, 6.1% P.
20. 4,8-Di-tert-butyl-2,10-dfethy-6-(4'-methyl-1'-
naphthyl)-12H-dibenzo[d,g][1,3,2]diozaphosphocine: start-
ing from SS.27 g of 1-bromo-4-methylnaphthalene and
108.2 g of 4,8-di-tert-butyl-6-chloro-2,10-diethyl-12H-
dibenzo[d,g][1,3,2]dioxaphosphocine, 100 g (= 74%) of the
above compound were obtained from acetone in the form of
colorless crystals of melting point 258-260°C.
C38H43~2P C8lCUlated: 80.26% C, 8.04% H, 5.74% P
(538.71) Found: 79.9% C, 8.3% H, 5.4% P.
21. 4,8-Di-tert-butyl-2,10-diethyl-6-(4'-biphenyl)-12H-
dibenzo[d,g][1,3,2]dioxaphosphocine: starting from 58.3 g
of 4-bromobiphenyl and 108.2 g of 4,8-di-tert-butyl-
6-chloro-2,10-diethyl-12H-dibenzo[d,g][1,3,2]dioxa-
phosphocine, 103.3 g (= 75%) of the above compound were
obtained from acetonitrile in the form of colorless
crystals of melting point 170-172°C.
C3~H,302P Calculated: 80.70% C, 7.8?% H, 5.62% P
(550.72) Found: 80.2% C, 7.9% H, 5.4% P.
22. 4,8-Di-tert-butyl-2,10-diethyl-6-(2',4',5'-tri-
methyl-1'-phenyl)-12H-dibenao[d,g][1,3,2]-
dioxaphosphocines starting from 49.7 g of 5-bromo-1,2,4-
trimethylbenzene and 108.2 g of 4,8-di-tert-butyl-
6-chloro-2,10-diethyl-12H-dibenzo[d,g][1,3,2]dioxa-
phosphocine, 113.7 g (= 88%) of the above compound were
obtained from acetonitrile/acetone (1s1) in the form of
colorless crystals of melting point 198-200°C.
C3,,H450zP Calculated: 79.03% C, 8.77% H, 6.0% P
(516.70) Found: 79.3% C, 8.7% H, 5.8% P.
20~.4~'~~w~
- 29 -
23. 4,4'-Biphenylene-bis[4,8-di-tart-butyl-2,10-diethyl-
6-yl-12H-dibenzo[d,g][1,3,2]dioaaphosphocine]s the
general procedure was modified, and 200 mmol (= 62.4 g)
of 4,4'-dibromobiphenyl were subjected to a Grignard
reaction with 600 mmol (= 14.6 g) of magnesium turnings
in 300 ml of tetrahydrofuran with exposure to ultrasound
( 40 kiiz ) and then reacted with 400 mmol (= 173 . 2 g) of
4,8-di-tart-butyl-6-chloro-2,10-diethyl-12H-dibenzo[d,g]-
[1,3,2]dioxaphosphocine in 250 ml of tetrahydrofuran.
After evaporation of the solvent, about 190 g of a solid
remained containing (by 31P-NMR) 73% of the above compound
('~P-NNBt: a~,xl3 = 163. 7 ppm) . Colorless crystals of
melting point 320°C (decomposition) were obtained from
acetone.
ZS CBZH,6O4P2 Calculated: 78.61% C, 8.08% H, 6.54% P
(947.23) Found: 78.3% C, 8.2% H, 6.3% P.
24. 4,4'-Biphenylene-bis[4,8-di-tart-butyl-2,10-
dimethyl-6-yl-12H-dibenzo[d,g][1,3,2]dioxaphosphocine]:
the procedure was analogous to that of the previous
example, except that the Grignard reagent was reacted
with 400 mmol (= 162 g) of 4,8-di-tart-butyl-6-chloro-
2,10-dimethyl-12H-dibenzo[d,g][1,3,2]dioxaphosphocine.
About 180 g of a solid remained, containing (by '1P-Nl~t)
72% of the above compound (3'P-NMR: 013 = 163.9 ppm) .
Colorless crystals of melting point 306-310°C
(decomposition) were obtained from acetone.
CseHseO~Pa Calculated: 78.17% C, 7.69% H, 6.97% P
(891.12) Founds 77.8% C, 7.8% H, 6.7% P.
IT. Working Ezamples
The phosphonites according to the invention listed below
were used for the experiments.
25 and 31s Bis(2',4'-di-tart-butylphenyl) 4-biphenylphos-
phon:ite according to Example 12, contained about 98% by
~~~~v~
- 30 -
siP-~
26 and 3Z: Bis(2',4'-di-tert-butylphenyl) ~-naphthylphos-
phonite according to Example 9, contained about 98% by
siP-~
27 and 33: Tetra(2',4'-di-tert-butylphenyl) 1,4-pheny-
lenediphosphonite according to Example 14, contained
about 9 5 % by 31P-NMR
28 and 34: Bis(2',4'-di-tert-butylphenyl) (4-methyl-
1-naphthyl)phosphonite according to Example 6
29 and 35s Bis(2,4-di-tert-butylphenyl) 2-methylnaphthyl-
phosphonite according to Example 8
30 and 36: Bis(2,4-di-tert-butylphenyl) (2-methoxy-
6-naphthyl)phosphonite according to Example 10
25 to 30 and Comparative Bxamples A to C
100.0 g of unstabilized polypropylene powder (density:
0.903 g/cm'; melt flow index MFI 230/5s 4 g/10 min) were
mixed with 0.1 g of Ca stearate as acid acceptor and the
amounts of phosphorus compound listed in the tables and
extruded several times by means of a laboratory extruder
(short-compression zone screw, diameter of screw 20 mm;
length 400 mm, length of nozzle 30 mm, diameter [lacuna]
mm; speed: 125 rpm; temperature programs 200/230/230°C).
After the 1st, 5th and 10th pass, samples were removed
from the granules and used to measure the melt flow index
according to DIN 53 735 and the yellowness as yellowness
index according to ASTM D 1925-70. Tn addition, the
granules of the 1st pass were used to produce extruded
sheets of the dimensions 60 x 60 x 1 maa, and the
yellowness was measured immediately and after hot storage
(7 days 8t 100°C).
The results are listed in Tables 1, 2 and 5.
31 to 36 and Comparative 8zamples D to F
100.0 g of unstabilized polypropylene powder (density:
0.903 g/cm'; melt flow index MFI 230/5: 4 g/10 min) were
- 31 _ r,'~~u~~~ y~Jr
mixed with 0.1 g of Ca stearate as acid acceptor and
0.05 g of ethylene glycol bis(3,3-bis(3'-t-butyl-
4'-hydroxyphenyl)butyrate and the amounts of phosphorus
compound listed in the tables and extruded several times
by means of a laboratory extruder (short-compression zone
screw, diameter of screw 20 mm; .length 400 mm, length of
nozzle 30 mm, 2 mm diameter; speed: 125 rpm; temperature
program: 200/230/230°C). After the 1st, 5th and 10th
pass, samples were removed from the granules and used to
measure the melt flow index according to DIN 53 '735 and
the yellowness as yellowness index according to ASTM D
1925-70. In addition, the granules of the 1st pass were
used to produce extruded sheets of the dimensions
60 x 60 x 1 mm, and the yellowness was measured
immediately and after hot storage (7 days at 100°C).
The results are listed in Tables 3, 4 and 5.
.> .
Ge i ~ ~. ._i , ':.'~ o:~
O
O
4~
ro
O ~o
1-I S-i.C,N Sd1 O ritDM ~ 01
~0
6~ N
'Jy d.1O N O Pit~a9r~l01L~
ie
r-i W .-iN Pi r-i .-i
O cT1
H
W C
O -i
M O ODt~M M O O
P
O d~ ttll~ P tpf~16900SO
t17
.Iw1 it7r-1
r1\
.Ll
N p
J.9P4 4C1
(J1 eP' v-1d'N e1t0m'
U7
H a.9
O tD 10406~tpl0lt1
Ifs
....
0
U O .' f~
1
b
~ ~
1 b
~ ~
p pa ,~ N
O .a~
p
rI1r-I r-I O
tV ~T i'~a
O
E ~
~ b
O ",rr S-!
O
U 1-I .N O
.~
p ~ U = _ _ _
,
!D.~.1 ~ U ~1'
tl1
N
~ +
W r
~
. 6'1 .~J ';r~
l
f.~~., 'T~ 'L9 ..1
.-i
0 N O ~ 0
. U . iaa
6~"' 4"
H .F, ~." (!) ,JY
N
O ~
U ~i
~ ' .n
+~x u~ + ~
.~
~
U p ~ +~
O 'd f~d W W 1
W
O O
O
O CL tT bs 1
~
W p
Q1O O ~ .a ,-a
.-i
.4~1 .~ O N
H W W f~ O O
O
P67
.,i
x
~C CG
W
Qa
H
~ u1voP aoa~~
~
x U ~ N N N N N M
~ ~
0
0
+~
ro
a~
f3~ H H
rl G1 ~T
w ~ h ~ t~1O h M O M
ri
ro
O h 0 01h ~Dh N tO
10
N M M N M N M ri
N
O
W a
ro
h o v~~rh M o h
o
o ~
~ anM w o h o0
..~
h tl1 N N M r1N rlN ~-1
N
1
N
h M 1f1O CDCD10 er
Ca ~ OD
i O M -1O tl101O h d
IA N
rl e~lrl N rl N
rl
~ O O
O .~ f~
N ~1.~ tA
~.1 ~, O
.' > O~
n~
b t ..1 b
ro '~'
,..
rl o a
d ~ ~ x
:
~ .4
ai ~ b
m a -
d ~ ~ V ~
p
~ s s s s s -
~
O ~ ~
~ _
.I fl I CI rl
(3~
~
O rl O .O
F
1 N ~
N
~ 0 ~
O p p
V V ~
~
O 4 p, .4
1
O m +~ ~ ~
V
O
W w 1
w
ro o 0 0
0
a ~ ~ ~ ~
er
m
ro ro o a ~ .~
~
U b er
~ p ~ o o
, O
x
~ ~ ttl
U
N
W +~
O O N N N N N M
O
W U U
U
YEA It '. ~ v ~~ Y l ~_rl
ro
a~
s~ o
a~ a
a~ as
0
h .~ cner c ~ N er.-a~
~
0
~ ~ t~ anereW n r.u1
~O
w P, ,-i
o ro
a~
_
H
0
m o reM m ~ro o,
~n
o n co~o sr~.d~.~erui~
ui
w
tn
.r~
roa
(17 M lV r-IM M cN~ 10
C1
H .~
t04ta ~ d'~9et'crd~
e9'
.... ~
O
U O O d.~ f3~
N ~ U1 rl r~l
O O 't!
C ~
~ ~ N
O r~1
~ ~
b i3~ x O
.N ~ ~ Pa
~
~
O
U f,d ~ U
Caa
O ,Li U
w
tl7b~ 1 b d
O
w .I~
,~
_
Z'Iro 1 d
(S~
O .1 a.a
v ~
b I
td
t0O ~ d.
U .~
~
R~ N t
O ~
w .
~ 1
O U ~ Gva
V
+~DC u1 I
U N ~ w w .l.a
w
a~ro >.a o 0 1
0
w s~ o .~1
w .~ .r~ b~ b~ ty
G~
~ d
3 1 N sn m
ua
~'rO O
O
C~w IZ, ~ O O
O
W
.y,
va N C1W
G~1
M ~--I f./
i~
O O M M M M M M
O
W U U
(J
~~;~,.7 ~'
r.: ';7 '~ '~ w
- 35 -
a
0
.'.,
ro
a~ s~
Ca H
.4' 1f1~-1O d'M O d'tffO
Ql O 1t1~O10 M 01v-1N O O
~i M M M 1
N ~ N N M M
N ro
W h-I
ro
h ADV~ N C1r-1~ N O
O i~ N h 0~ M ehIff1GO M
h t!1 M N '-1 N e1ri rlM N
1
N
~i
h 1f1h h N O M h 1I1
A
(0 M 1f1~ G~C1O O ~ N G1
.-a .~ ~ .-r..~~ .a
s~
x
O N W
O
p, O~
b O b
V ~ ~
~
ce ~ N
'n' ",i
i
b x ~ ~ i~
~ ~ x O
m
s s s s s
~Sw ~ x a ~
O H + ~ ~
~
I rl
r-
~
O V ~
N m
~ ~ d ~ ~
U O V " W
.1
~
~ W V ..
O
o ro o 0 0 0
v ~ a ~ rTer b
, ~
.s~H .C 00 ~ O O ~~", N
U !T GL O O O O ~1
m
x
Cj G1 1~7I~ H
,
O O M M M M M M
W
U U U
~i~ ~ ~N
- 36 -
Table 5; Change in color on 1 mm extruded sheets im-
mediately after production and after heat
treatment (7 days at 100°C)
YI immediately YI after 7 days
Comp. A 4.2 10.1
Comp. B 3.4 13.0
Comp. C 4.5 12.5
25 6.2 14.9
26 2.7 9,9
27 3.8 13.1
28 2.7 10.8
,
29 3.3 13.3
30 2.2 10.6
Comp. D 3.5 5.6
Comp. E 4.7 6.6 -
Comp. F 2.9 3.8
31 2.2 3.2
32 2.4 3.4
33 2.5 3.5 -
34 2.6 4.0
35 4.1 6.0
36 3.5 ~ 4.6
29374-127 ca o2oss~62 2000-02-04
37
R2 R2
(II)
O \ / R3 /O \ / R3
Rt p/ (I) Hal-P\ s
\O ~ / R3 O \ / R
R2
R n
OH
t_C4H9
II t-C 4H9 (I V )
O
CH3-C-CH2-C-O R4 HO O CHZ-CH2-C-OH
t C4H9
~ t-C 4H9
H
n
R2 R2
/ R3 (V) O \ / R3
A VI
Rt p Hal P A ( )
R3 3
/ ~ / R
R2 n R2