Note: Descriptions are shown in the official language in which they were submitted.
2~2089
PATENT
EL~CTROLYTIC CELL ANOD~
Back~round of the Invention
Technical Pield
This application is a continuation-in-part of prior
Application Serial No. 425,084, filed October 23, 1989,
assigned to the assignee of the present application.
The present invention relates to anodes for
electrolytic cells, for such applications as
electroplating, electrowinning, electrofinishing and
electromachining, and particularly to anodes having a
dimensionally stable active anode surface.
Description of the Prior Art
Dimensionally stable electrodes are well known. The
term "dimensionally stable" means that the electrodes are
not consumed during use. Typically, a dimensionally stable
electrode comprises a substrate and a coating on surfaces
of the substrate. The substrate and coating have to
withstand the corrosive action of the electrolyte in which
the electrode is immersed. One suitable material for the
substrate is a valve metal, such as titanium, tantalum,
,
2 ~ 8 ~
zirconium, aluminum, niobium and tungsten. These metals
are resistant to electrolytes and to conditions used within
electrolytic cells. A preferred valve metal is titanium.
The valve metals can become oxidized on their surfaces
increasing the resistance of the valve metals to the
passage of current. Therefore, it is customary to apply
electrically conductive, electrocatalytic coatings to the
electrode substrate. The coatings have the capacity to
continue to conduct current to the electrolyte over long
periods of time without becoming passivated. Such coatings
can contain catalytic metals or oxides from the platinum
group metals such as platinum, palladium, iridium,
ruthenium, rhodium and osmium.
The anode for an electrolytic cell such as an
electrowinning cell is u~ually in the shape of a large
surface which conforms generally to the shape of the cell
cathode, but is spaced from the cathode. For instance, in
the case of a radiaL cell, in which the cathode is in the
shape of a relatively large, cylindrical drum, rotatable on
the axis of the drum, the anode will have a cylindrically
shaped concentric surface circumferentially superimposed
over a relatively large part of the cathode.
It is difficult to manufacture such a large, anode, of
dimensionally stable materials, to the tolerances necessary
to achieve a uniform gap between the anode and the cathode.
This is because the valve metals are resilient, and are
difficult to roll to a predetermined curvature with close
2 ~ 8 ~
--3--
tolerance. Coating the valve metals further exaggerates
the problem, since the coatings have to be heat treated,
and the heat treatment can cause further deviation of the
anodes from a desired curvature.
U.S. Patent No. 4,318,794 discloses a radial
electrolytic cell for metal winning. A plurality of
dimensionally stable, elongated, anode strips are
positioned in the cell electrolyte spaced from a
cylindrical cathode. The anode strips extend,
longitudinally, parallel to the axis of the cathode. Each
strip is relatively narrow in width, being co-extensive,
circumferentially, with only a small surface or arc of the
cathode. By employing a plurality of narrow strips, the
tolerances to which each strip is rolled become less
critical. Typically, the strips are about 2-4 inches in
width.
U.S. Patent No. 4,642,173 discloses an electroplating
cell. The cell comprises a dimensionally stable anode for
depositing metal onto an elongated strip drawn
longitudinally past the anode. The anode is immersed in an
electrolytic solution and comprises an active surface which
i8 directed toward the strip. The active surface comprises
a plurality of lamella~ supported 80 that all of the
lamellas lie in a boundary which conforms to but i~ spaced
from the path of the strip. Each lamella is welded to a
support, along an edge, with the opposite edge of the
lamella facing the strip. Being welded to a support, the
2~%08~
lamellas are not readily replaceable. In addition, they
are spaced apart from each other and thus do not present a
continuous or substantially continuous anode surface.
Summary of the Invention
The present invention relates to an electrolytic cell
which comprises a cathode having a cathode surface movable
in said cell, an anode spaced from said cathode, and means
for maintaining an electrolyte solution between the cathode
and the anode. The anode comprises at least one
dimensionally stable elongated anode strip at right angles
to the cathode surface direction of movement. The anode
strip is laterally flexible and has a formed, first
configuration. A support means supports the anode strip
and flexes the anode strip into a second supported
configuration which is different from the formed first
configuration. The anode strip in the second supported
configuration is essentially uniformly spaced from the
cathode. In a radiàl cell, the anode is flexed into a
configuration, which, in cross-section, at right angles to
the longitudinal dimension of the anode, is arcuate.
The present invention also resides, in one embodiment,
in a novel anode configuration. The anode is dimensionally
stable in an electrolyte. The anode is in the shape of an
elongated channel having an active anode surface, and
longitudinally extending flanges along opposite side edges
of the active anode surface. The active anode surface has
a thickness by which it is flexible. Supports engage the
2 ~
anode flanges so as to flex the active anode surface from a
formed, first configuration, into a second supported
configuration which is different from the foLmed, first
configuration and which positions the active anode surface
uniformly spaced from the cathode.
Preferably, the electrolytic cell comprises a
plurality of elongated anodes positioned in side-by-side
relationship, in an arc or plane, uniformly spaced from the
cathode.
The present invention is useful for an electrolytic
cell in which the anode defines an essentially continuous
surface and the electrolyte flow is confined to a path
defined by the anode and the cathode.
The present invention is also useful for an
electrolytic cell in which the anode is i~mersed in the
electrolyte contained within the cell.
Brief DescriDtion of the Drawinas
Further features of the present invention will become
apparent to those skilled in the art to which the present
invention relates from reading the following specification,
with reference to the accompanying drawings, in which:
Fig. 1 is a perspective schematic view of a portion of
; an electrolytic cell in accordance with one em~odiment of
the presant invention;
Fig. 2 is an end elevation schematic view of the
poxtion of the cell of Fig. 1;
2~2~8~
Fig. 3 is an end elevation schematic view of an
electrolytic cell which includes the portion of Fig. l;
Fig. 4 is a plan schematic view of the portion of the
cell of Fig. 1;
Fig. 5 is a side elevation schematic view of the
portion of the cell of Fig. l;
Fig. 6 is an enlarged plan view of a dimensionally
stable anode used in the electrol~tic cell of Fig. l;
Fig. 7 is a further enlarged section view showing a
portion of the anode of Fig. 6 and a part of the support
therefor;
Fig. 7a is a further enlarged view of a portion of the
support of Fig. 7 showing further details of the support;
Fig. 8 is an enlarged section view of a portion of two
ad;acent anodes of Fig. 6 showing further details of the
anode support;
Fig. 8a is a reduced section view showing how the
anode of Fig. 6 is flexed by the support structure of Figs.
7 and 8;
Fig. 9 is an enlarged detail view of a portion of the
cell of Fig. 3 showing means for introducing electrolyte
into the cell;
Fig. 10 is an end elevation view of an electrolytic
cell and anode structure therefor in accordance with an
embodiment of the present invention;
Fig. lOa is an enlarged section view of one anode of
the anode structure of Fig. 10;
2 ~
Fig. 11 is a sectional view of the electrolytic cell
of Fig. 10 taken along line 11-11 of Fig. 10;
Fig. lla is an enlarged detail view of a portion of
the cell of Fig. 11;
Fig. 12 is an enlarged partial ~ection view of a
support for the anode of Fig. lOa;
Fig. 13 is a section view taken along line 13-13 of
Fig. 12;
Fig. 14 is a partial section view of an anode assembly
in accordance with another embodiment of the present
invention; and
Fig. 15 is a partial section view of an anode assembly
in accordance with a still further embodiment of the
present invention.
D~e~cription of the Preferred Embodiments
The electrolytic cells of the present inven~ion are
particularly useful in an electroplating process in which a
deposit of a metal, such as zinc, is made onto a moving
cathode strip. An example of such a process is
electrogalvanizing in which zinc is continuously galvanized
onto a strip fed from a steel coil.
However, the electrolytic cells of the present
invention can also be used in other electrodeposition
processes, for in~tance plating other metals such as
cadmium, nickel, tin and metal alloys such as nickel-zinc,
onto a substrate, or th0 production of electrodeposited
foil, for instance, copper foil used in the production of
2~2~
printed circuits for electronic and electrical equipment. `
The copper foil is electrodeposited from an electrolyte
onto the surface of a rotating cathode. The foil emerges
from the electrolyte and is stripped from the surface of
the cathode, and is wound in the form of a coil onto a
roll, all in a known manner.
Another electrodeposition process is surface treating
foil, for instance copper foil, previously manufactured.
Copper foil, when used for printed circuit boards, is
bonded to a dielectric substrate. Electrodeposited copper
foil has poor adhesion propertie~, because of its
relatively smooth surfaces. It is conventional practice to
surface treat the copper foil to obtain improved mechanical
bonding properties between the substrate and the foil. One
such treatment comprises forming a layer of dendritic
copper or copper oxide particles on the surface of the
foil. Another such treatmant comprises forming a locking
layer over the dendritic layer which adopts the
configuration of the dendritic surface but helps maintain
the dendri~ic layer intact.
The cell of the present invention can be also used in
non-plating processes such as electromachining,
electrofinishing, anodizing, electrophoresis, and
electropickling. The anode of the electrolytic cell of the
present invention can also be used in such applications as
batteries and fuel cells, and in such proce~ses a~ the
electrolytic manufacture of chlorine and caustic soda.
2~208~
Other specific uses for the apparatus of the present
invention will be apparent to those skilled in the art.
In all of the above processes, it is necessary to
carefully adjust the gap between the anode and the cathode.
- 5 For instance, in electroplating, this in part controls the
thickness of the layer which is electroplated. In the
manufacture of electrodeposited foil, this in part controls
the thickness of the foil. The present invention is
concerned primarily with con~rol of the anode/cathode gap.
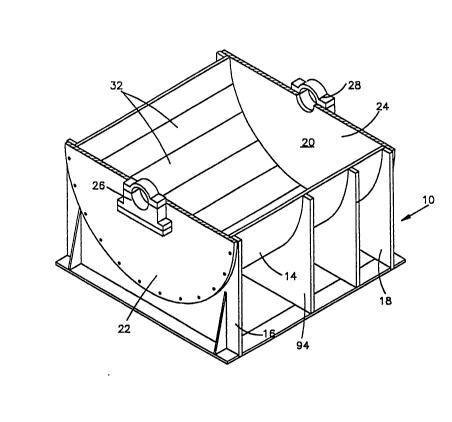
Referring to the embodiment of the invention as
depicted in Fig~ 9, and particularly Figs. 1 and 2,
there is shown a vessel 10 of an electrodeposition
apparatus in accordance with the present invention. For
the purpose of illustration, an electrodeposition apparatus
for the production of electrodeposited foil will be
described. The vessel 10 encompasses a cylindrical cathode
12, shown in phantom lines in Fig. 2. The vessel 10 has a
concave shell 14 which is supported by end stanchions 16,
18. The shell 14 defines a chamber 20 which receives the
cathode 12. The shell 14 is clo~ed at its ends by circular
end plates 22, 24 which are bolted to the stanchions 16,
18. The end plates 22, 24 support pillow blocks 26, 28
which in turn support the cylindrical cathode 12 when the
electrodeposition apparatus is completely assembled. The
cylindrical cathode 12 has an axial supporting shaft which
is rotatably mounted in the pillow blocks 26, 28, in a
known manner. The pillow blocks 26, 28 are positioned on
2~Q8~
--10--
the end plates 22, 24 so that the cathode is partially
encompassed by the shell 14.
The ~essel shell 14 and end plates 22, 24 therefor are
preferably made of a current-conductive metal, having high
tensile strength, such aq steel and copper. The
cylindrical cathode 12 can be of any conventional type.
Typically, for the electrodeposition of copper foil, the
cathode is a metal, e.g., steel or copper, drum having a
surface layer suitable for use in an electrolytic bath.
Examples of suitable metals for the surface layer are
"~astelloy~ (trademark Union Carbide Corp., for a high
strength, nickel-base corrosion resistance alloy) stainless
~teel, titanium, zirconium and tantalum.
It will be understood by those skilled in the art that
although shell 14 i8 shown as substantially a semi-circle,
other shell configurations can be used. For instance, the
shell can be made 80 that it extends circumferentially a
distance more than L80 around the cathode 12, for instance
260. Generally, the shell 14 will be circular so that all
surfaces of the shell 14 are equidistantly spaced from the
cathode 12 which is contained by the shell. The end plates
22, 24 on the shell are removable. This permits the
cathode to be inserted into the shell endwise rather than
from the top. Thîs mode of assembly is particularly useful
if the shell 14 circumscribes the cathode by more than
180
2 ~
--ll--
In operation in metal electrcplating, a thin film of
metal is deposited from electrolyte in the shell 14 onto
the surface of rotating cathode 12. Neans, not shown,
strip the film of metal from the cathode, on emergence of
the film from the electrolyte, and coil the film in the
form of a roll. It will be understood by those skilled in
the art that the apparatus of Figs. 1 and 2 can be used for
other electrolytic processes than manufacture of a metal
film.
Referring back to Figs. 1 and 2, the shell 14 is lined
on it~ inner surface with a plurality of elongated anode
plates 32. Details of the anode plates 32 are shown in
Fig. 6. Essentially, the anode plates 32 are elongated
rectangular members having ends 34, 36, side edges 38, 40
and an active anode surface 42~ The anode plates are
bolted to the shell 14, in a manner to be described. As
shown in Figs. 1, 2 and 4, the entire inner surface of
shell 14 is lined with anode plates 32. The anode plates
are positioned so that the edges 38, 40, Fig. 6, of one
plate are contiguous with edges 38, 40 of ad~acent plates.
Thus, all of the anode plates together lie in an arc which
is coaxial with the axis of the cathode and spaced from the
surface of the cathode, the arc embracing, in the exampla
of Figs. 1-9, about 180 of the circumference of the
cathode.
The anode plates 32 are dimensionally stable
electrodes. The dimensionally stable electrodes have a
2 ~
-12-
substrate which is capable of withstanding the corrosive
action of the electrolyte in which the anode pla~es are
immersed. Preferred materials for the substrate are a
valve metal such as titanium, tantalum, zirconium,
aluminum, niobium, and tungsten. These metals are
resistant to electrolytes ar.d conditions within an
electrolytic cell. A preferred valve metal is titanium.
The valve metals can become oxidized on their surfaces
increasing the resistance of the valve metal to the passage
of current~ thereby passivating the anodes. Therefore, it
is customary to apply electrically conductive
electrocatalytic coatings to the substrate which do not
become passivated. Such coatings can contain catalytic
metals or oxides from the platinum group metals such as
platinum, palladium, iridium, ruthenium, rhodium and
osmium. The coating also preferably contains a binding or
protective agent such as oxides of titanium or tantalum, or
other valve metal, in sufficient amount to bind the
platinum group metal or oxide to the electrode substrate.
An example of one such dimensionally stable anode is a
titanium substrate which has been coated with an
electrocatalytic coating containing ruthenium and titanium.
The Rub~trate can also be a metal such as steel or
copper which is explosively clad or plated with a valve
metal, such as titanium, and then coated with an active
oxide surface.
2 ~ 8 ~
-13-
The anode plates 32 are thin gauge, resilient, rolled,
or otherwise formed plates having sufficient flexibility so
that they can be flexed a small amount using reasonable
bolting force. The plates 32 should have sufficient
thickness to carry current from a current connection
throughout the anode active surface, and sufficient
thickness so that the plates are self-supporting and
capable of re~aining, in the absence of applied force, the
shape imparted to them by rolling or other forming.
Broadly, by way of example, the anode plates 32 have a
thickness of about 0.010 inch to about 0.5 inch. A thin
coated titanium plate rolled, or otherwise formed,
preferably has a thickness of about 0.20 to about 0.25
inch. The thinner the plate, the easier it is to install,
and the lower the material cost.
The specific width dimension of the anode plates 32,
between side edges 38, 40, is not critical. In the example
illustrated in Pig.~6, the anode plates are relatively
wide, about twenty-four inches.
As disclosed in parent Application Serial No. 425,084,
each anode plate 32 can comprise several end-to-end
segments 32a, 32b and 32c, Fig. 6, positloned
longitudinally within the shell 14. The segments are
separated by lines of separation 44 that are biased, e.g.,
biased with respec~ to the direction of travel of a metal
film electrodeposited on cathode 12. This avoids
unevenness of the electrodeposition of metal due to edge
~ , '
2~2~
-14-
effects. The anode plates 32 from end 34 to end 36 can be
relatively long, and dividing the anode plate~ into
segments 32a, 32b and 32c facilitates forming and
installation.
Fig. 7 shows the method of attachment of the anode
plates 32 to the shell 14, and additional details of the
v0ssel 10. Referring to Fig. 7, the vessel shell 14 has an
inner lining 58. The lining 58 covers the entire inner
surface of shell 14, and lies between the plurality of
anode plates 32 and the shell 14. In the embodiment of
Figs. 1-9, the lining 58 can be any suitable lining
material for an electrolytic cell. Preferably, the lining
has a high durometer hardness, for instance a Shore
durometer of about 95 +5, and is machinable. Preferably,
the lining 58 is baked onto the inner surface of the shell
14. One suitable lining material is manufactured natural
rubber. Other suitable materials are neoprene and EPDM (a
terpolymer elastome~ made from ethylene-propylene diene
monomer). The purpose of lining 58 is to protect the shell
14 from corrosion by the electrolyte.
The anode plates 32 on their underside 60, opposit0
the active anode surface 42, have a plurality of spaced-
apart bosses 62 welded thereto. The bosses 62 are shown in
Fig. 6 in phantom lines. The bosses 62 are shown all
aligned on the center-line of the anode plates. Each anode
plate segment 32a, 32b, 32c, preferably has three bo3sQs.
This num~er of bosses can be increased if desired.
2~D~
Preferably, the bosses 62 are made of a valve metal, such
as titanium. The bosRes 62 are drilled and internally
threaded. Bolts 64 extend through holes 66, in shell 14,
aligned with bosses 62 and are threaded into the bosses 62
to secure the anode plates 32 to the shell 14. The bolts
64 can be made of steel or copper alloy, and preferably are
coated with a dielectric, electrolyte resistant coating
such as Teflon (trademark, E. I. DuPont de ~emours & Co.)
for corrosion and galling resistance. The lining 58 ha-q
openings 68 aligned with holes 66 adapted to receive bosses
62. Similarly, the shell 14 i8 undercut on the inside at
70 to receive the bosses 62. The lining 58 is undercut at
72 to receive O-ring seals 74. One suitable seal material,
for seals 74, is Viton (Trademark, E. I. DuPont de Nemours
6 Co.), a fluoroelastomer based on the copolymer of
villylidene fluoride and hexafluoropropylene. The O-ring
seals 74 bear against the underside 60 of the anode plate
32 and prevent leakage of electrolyte from the interior of
vassel 10 around the bolts 64.
As will be de~cribed, the current flow to the anode
plates 32 is from shell 14 through the anode bosses 62.
These anode bosses bear against a contact ring 76 seated at
the bottom of undercut 70. The contact ring conveys
current from the shell 14 to the anode bosses 62. The ring
can be a knurled copper ring, or a compression ring such as
shcwn in Fig. 7a. Referring to Fig. 7a, the contact ring
76 comprises a copper alloy strip 7~. The copper alloy
~2~89
-16-
strip 78 is wound in the shape of a spring coil, and has a
chevron cross-section, as shown. A fiber reinforced
polymeric or rubber filling 80 is positioned between rolls
of the copper strip 78. When an anode plate 32 is pulled
down towards the shell 14, by turning bolt 64, this causes
the boss 62 engaged by the bolt 64 to compress the contact
ring 76 against the seat of undercut 70. The pressure
exerted on the contact ring can be from 0 to about 12,000
pounds compression. ThiR causes the copper alloy strip 78
of the contact ring to compress, as shown in Fig. 7a, and
make good contact with the undercut surface 70 the shell 14
and boss 62, the compression breaking any formed oxide
layer which could reduce contact voltage.
Referring to Fig. 8, the anode plates 32 are sealed at
their edges 38, 40 by meanR of support strips 90. The
support strips 90 are inserted into parallel grooves 92
machined into the exposed surface of the lining 58. The
grooves 92 retain t~e support strips 90. The support
strips 90 have a thickness whereby they protrude slightly
above the exposed surface of lining 58. The opposed edges
38, 40 of ad~acent anode plates 32 seat on the exposed
surfaces of the support strips 90. The support strips 90
can be titanium strips or fiberglass. They are
incompressible and thus are load bearing. The support
strips 90 extend, longitudinally, coextensive with the
anode plates 32, between the end plates 22, 24 of the
electrodRposition apparatus, sealing the opposed edges 38,
2~2~8~
-17-
40 of ad~acent anode plates 32 the full distance between
the end plates 22, 24. The end plates 22, 24 are also
lined, on their inner surfaces, with a lining (not shown) r
similar to the shell lining 58. This seals the anodes 32,
at their ends 34r 36 (Fig. 6) r thereby sealing the vessel
10 against the leakage of electrolyte. Similar sealing can
be employed at bias cuts 44~ if the same are used.
The method of assembling the anode plates 32 into the
electrodeposition apparatus in accordance with the present
inventionr will be apparentr by reference to Figs. 8ar 8,
and 7. Initially, the anode plates 32 are rolled to a
flat, or near flat configuration, as shown by the solid
lines in Fig. 8a. It i~ understood that the anode plates
can be rolled to a convex configuration or a concave
configuration, depending upon the end configuration desired
for the anode plates. The anode plates 32 are then placed
in the shell 14, with the bosses 62 aligned with bolts 64
(Fig. 7). The bolts~ 64 are engaged with the bosse~ 62. At
this pointr the side edges 38, 40 of the anode plate 32
bear against the edge ~upport strips 90, on the inner side
of the lining 58 (Fig. 8). The bolts 64 are turned into
bosses 62. This pulls the bosses 62 toward the shell 14
flexing the anode plate 32 into the configuration shown in
dashed lines in Fig. 8a. When the anode plates 32 at
bosses 62 are against or contiguous with contact rings 76
(Fig. 7), the anode plates 32 have the desired
2~2~
configuration, e.g., the same configuration as the shell
14.
The number of bolts 64, and anode bosses 62, which are
employed for an anode plate 32, is Lmportant. The bolts
64, and anode bosses 62, should be sufficientl~ closely
spaced together so as to pull the anode plate-~ 32 uniformly
towards shell 14, that is, so that there are no waves in
the anode plates 32, in a longitudinal direction with
respect to the plates. Also important i~ the distribution
of current to the anode plates 32. The use of enough
closely spaced bolts 64 and anode bosses 62 to force the
anode plates 32 into non-wavy configuration, is sufficient
to supply current uniformly to the anode plates.
As shown in the drawings, particularly Fig. 8, the
anode plates 32 are spaced from lining 58 by a gap 98. It
will be apparent that this gap, or the radial distance of
the anode plates 32 from the axis of the cathode 12, can be
varied by changing the radial dimensions of support strips
90 and contact rings 76 (Figs. 8 and 7, respectively). ~n
addition, the configuration or arc prescribed by an anode
plate 32, as shown in the dashed lines of Fig. 8a, can be
varied by changing the radial dimension of either the
contact ring 76 or the support strip 90.
Referring to Fig. 2, the shell 14 has at its bottom an
orifice plate 48. The orifice plate 48 is fastened to the
underside of the shell 14. Details of the orifice plate 48
are disclosed in Fig. 9. The orifice plate 48 has a plenum
8 ~
--19--
chamber S0 and an inlet connection 52 for introducing
electrolyte into the plenum chamber 50. The plenum chamber
50 is open at its top in elongated orifice 54 through which
electrolyte flows into the shell 14 and into the
S electrolyte chamber 84, between the cathode 20 and anode
plates 32.
As shown in Fig. 9, the anode plates 32 have at their
edges 38, 40, embracing the orifice plate 48, support
strips 90, sealing the anode plates 32 against leakage of
electrolyte between the plates and shell lining 58. The
support strips 90 are the same as those shown in Fig. 8.
In the embodiment of Figs. 1-9, the flow of
electrolyte into the electrodeposition apparatus is into
the inlet 52 (Fig. 9), into plenum chamber 50 at the bottom
15 of the cell, through orifice 54, and into ths interior of
the vessel 10. Since the anode plates are fully sealed,
along edges 38, 40, and around bosses 62, the anode plates
32, with the cathode 12, define the annular electrolyte
chamber 84 (Figs. 2 and 9) through which the electrolyte
flows. Referring to Fig.3, the electrodeposition apparatus
comprises an upper housing 82. The housing 82 has
discharge plenums 86 along opposite sides. Each plenum 86
has a plurality of discharge ports 88. The electrolyte
flows upwardly along both sides of the cathode 12, spilling
out over edges of the discharge plenums 86 exiting in
di~charge ports 88.
2 ~ 3 ~
-20-
The current flow in the electrodeposition apparatu~ is
establishPd by busses 96 affixed to end stanchions 16, 18
of the cell and ribs 94 (Fig. 1). Only two such busses are
shown, in Figs. 2 and 3. The flow from the end stanchions
and ribs is through the shell 14, contact rings 76 (Fig.
7), and bosses 62 into the anode plates 32. The flow of
current is then through the electrolyte, through the
cathode, and typically to conventional cathode brushes
engaging the cathode support shaft in a known manner.
The apparatus of Figs. 1-9 can be characterized as a
contained-flow apparatus, in which the flow of electrolyte
i8 confined between the anode plates 32 and the cathode.
Wiper seals (not shown) between the end plates 22, 24 and
the cathode 12 prevent the cathode from being immersed in
electrolyte except at the cathode active surface facing
anode plates 32. It will be understood that the anode
support structure disclosed can also be used in the type of
cell in which the a~odes are immersed in the electrolyte.
ln the embodiment shown in Figs. 10-13, the
electrodeposition apparatus comprises a tank 102. The tank
102 is rectangular in cross section, having bottom 104, and
sides 106, 108. The tank is open at its top between sides
106, 108. During operation of the apparatus, the tank 102
i~ filled with a suitable electrolyte (not shown). A
rotatable cylindrical cathode 110, shown in phantom lines,
i8 positioned within the tan~ 102 80 as to be partially
Lmmersed in the electrolyte. The cell of Figs. 10-13 can
2 ~
-21-
be characterized as a flooded design, in which the anode
and part of the cathode are immersed in the electrolyte.
A plurality of supporting rubber coated steel beams
112, positioned near the bottom 104 of the tank, extend
lengthwise in the tank. The beams 112 are supported by the
tank end walls 114 (Fig. 11). The beams 112 in turn
support a plurality of spaced apart supporting ribs 116,
118 (Figs. 10 and 11). Fig. 11 is a section view of Fig.
10, taken so that the side 106 of the tank is remo~ed,
revealing the inside of the tank. As shown in Fig. 11, the
ribs 116 are end ribs positioned near end walls 114 of tank
102, and the ribs 118 are inner ribs, positioned at spaced
intervals between the ribs 116. The end ribs 116 are
supported by inner beams 112a (Fig. 10), and the inner ribs
118 are supported by all of the beams 112 (Fig. 10). As
shown in Fig. 10, the ribs 116, 118 are arrayed in sets
divided by gap 120, one set of ribs being arrayed along one
side 106 of the tank~ 102, the other set of ribs being
arrayed along the other side 108 of tank 102. Each rib
116, 118 along one side of tank 102 has a corresponding rib
oppositely positioned along the other side of the tank.
Each rib has a concave upper edge 122, a lower edge 124
which seats on a beam 112, and a vertical edge 126. The
concave edge 122 of one rib faces the concave edge 122 of
an oppositely positioned rib so that a pair of ribs
together define, by the concave edges 122, a circular
configuration as shown in Fig. 10. Preferably, the
2~2~
--22--
circular configuration is concentric with the circumference
of cathode 110, although this is not essential. In the
tank 102, the concave edges 122 of the ribs along one side
106 of the tank are all aligned lengthwise in the tank,
5 and, similarly, the concave edges 122 of the ribs along the
side 108 of the tank are all aligned lengthwise in the
tank. This also is not critical, and other ~onfigurations
for the ribs will be apparent to those skilled in the art.
To hold the ribs 116, 118 in overall alignment, the
10 ribs are tied together by tie plates 128 welded to the
vertical edges 126 of the ribs. Similar tie plates 128 are
welded to the lower edges 124 of the ribs. The tie plates
128 extend lengthwise iA the apparatus, parallel to beams
112, and are welded to all of the ribs 116, 118. This
15 permits the ribs 116, 118 and other components of the
electrodeposition apparatus to be preassembled outside of
the tank 102, and then set in the tank onto beams 112, a~ a
preassembly.
The electrodeposition apparatus of Fig~. 10 and 11
20 comprises a plurality of anode plates 130, shown in Figs.
lOa and 12. The anode plates 130 are positioned
circumferentially around the cathode 110, generally
concentric with the outer surface of the cathode, similar
to anode plate~ 32 of the embodiment of Figs. 1-9.
Details of the anode plates 130 are shown in Figs. lOa
and 12. The anode plates 130 are dimensionally stable, as
with the anode plates of the embodiment of Fig~. 1-9. The
2 ~
-23-
anode plates 130 are rolled as U-shaped channels 132 as
shown in Fig. 10a. Each channel 132 comprise an elongated
rectangular center portion 134 and longitudinally extending
edge flanges 136, 138. The edge flanges 136, 138 axe
S angled, for instance at generally right angles, with
respect to the center portion 134. As with the embodiment
of Figs. 1-9, the center portion 134, between edge flanges
136, 138 is relatively wide, for instance about 12-24
inches. The center portion has on one side 142, Fig. 10a,
an active anode surface which, as will be described, faces
cathode 110. Only the active anode surface and flanges
136, 13B are coated with a non-passivating coating.
During rolling, the anode plate~ 130 are rolled or
otherwise formed so that the center portion 134 adopts an
essentially flat configuration, as shown by the solid lines
in Fig. 10a. ~owever, it is understood that the channel~
132 can be rolled or otherh~ise formed to a concave or
convex configuration, depending upon the end configuration
of the anode plates which is desired.
Referring to Fig. 12, the flanges 136, 138 of ad~acent
anodes are bolted together by bolts 140. The bolts 140
hold the outer face of one flange 136, of one anode plate
130, against the outer face of another flange 138, of
another anode plate 130. In this way, the bolts 130
connect and hold together the entire array of side-by-side
anode plates. The number of bolts 140, holding one anode
plate to another, is sufficient to maintain an essentially
2~2~
-24-
uniform contact, lengthwise of each plate, between the
connected flanges of adjacent plates.
The anode plates 130 are supported, at each bolt 140,
by a bracke~ assembly 150 (Figs. 12 and 13). Each bracket
assembly 150 comprises a current distribution bar 152,
which extends the full length of the electrodeposition
apparatus. Six such distribution bar 152 are shown in Fig.
11. The distribution bars 152 are affixed, e.g., welded,
along one edge 152a (Figs. 12 and 13), to an ad~ustment
clip 154. Each adjustment clip 154 is bolted to a cell rib
116, 118 by a bolt 156. The adjustment clips are
positioned close to concave edges 122 of the ribs 116, 118.
Each ad~ustment clip 154 has a slot 158 (Fig. 12). The
ad~ustment clips 154 are movable circumferentially, on ribs
116, 118, by engagement of slots 158 with bolts 156. This
permits circumferential adju~tment of each anode plate 130
with respect to cathode 112. A ~ack screw 162 is welded to
each rib and engages~ clip 154. The bolts 156 can be
partially tightened on the clips 154. The ~ack screws 162
can then be turned forcing the clips 154 into a desired
circumferential position. This permits fine positioning of
the anode plates. The bolts 156 can then be fully
tightened on the clips 154 securely holding the anode
plates 112 to the ribs. The distributor bars 152 also have
25 a slot 160 (Figs. 12 and 13). The slots 150 accommodate
bolts 140. The slots 160 are oval-shaped, as shown in Fig.
2 ~
-25-
13, which permits radial adjustment of each anode plate 130
with respect to cathode 110.
In the embodiment of Figs. 10-12, the anode plates are
of titanium or other valve metal. The plates are coated,
as mentioned above, on the acti~e anode surface 142 and
flangee 136, 138 with a non-passivating coating, for
instance a platinum coating. Components of the bracket
assembly 150 are similarly manufactured, clad and/or
coated. For instance, the bolts 140 may be titanium bolts
with a fluorocarbon coating to prevent galling, e.g., a
Teflon coating. The current distribution bars 152
preferably comprise a copper core having a titanium
cladding with a non-passivating coating. Other compononts
Lmmersed in the electrolyte, for in~tance the cell ribs
116, 118 and ad~ustment clips 154 are current carrying, and
thus are made of titanium with a non-passivating coating
such as of platinum at the electrical ~unctions.
In operation, ~urrent is introduced into the cell
through busses 180 (Figs. 10 and 11), attached to inner
ribs 118. The current flows from the busses 180 through
the ribs 116, 118 to each current di~tribution bar 152, as
Qhown in Fig. 11.
Assembly of the apparatus of Figs. 10-13 should now be
apparent. The elongated channels 132 (Fig. 10a) are
sufficiently flexible that they can be flexed laterally, in
the center portion 134 between flanges 136, 138, to the
configuration shown in da~hed lines in Fig. 10a. As
2~2~
-26-
mentioned above, the anode plates 132 are initially rolled
or otherwise formed so that the center portion 134,
thereof, is relatively flat. Flexing the center portion
134 laterally into the concave configuration shown in
dashed lines in Fig. 10a is achieved by spreading the
flanges 136, 138 apart from each other and slightly
truncating them as shown in Fig. 10a. SLmilarly, if a flat
center portion 134 were desired, the anode could be rolled
or otherwise formed to a convex or concave configuration,
and then flexed into a flat configuration by appropriately
manipulating the anode flanges 136, 138.
Referring to Figs. 12 and 13, the anode flanges 136,
138 are movable both circumferentially and radially in the
cell. The anode flanges 136, 138 of the multiple anode
plates 130 can be mo~ed simultaneously radially towards the
cathode, and at the same time spread apart, increasing the
amount of flex or curvature in center portion 134 (Fig.
10a) of each anode plate. Conversely, the anode flanges
136, 138 of the multiple anode plates 130 can be moved
simultaneously away from the cathode, and at the same time
brought closer together, to reduce the amount of flex or
curvature in the anode plates. Once the desired
configuration of the multiple anode plates 130 is achieved,
the plates can then be held in that configuration, by
securely tightening the bracket a~semblies 150 to ribs 116,
118.
-27-
In the apparatus of Figs. 10-13, even though the
apparatus is a flooded design, with anode plates 130 and
cathode 110 Lmmersed in electrolyte, it is desirable to
establish a flow of electrolyte in the gap 168 between the
S anode plates 130 and the cathode 110 (Pig. 10). This is
accomplished by the use of induced flow in gap 120, Fig.
10, between ribs 116, 118 (by conventional means, not
shown), into the gap 168 between the anode plates 130 and
the cathode 110. The flow divides and extends upwardly in
the gap 168, along both sides of the cathode 110, spilling
over into the tank 102 at the upper edges of the uppermost
anode plates. To confine the flow to the gap 168, the
cathode 110 comprises at each end, a circumferential wiper
seal 166 (Fig. lla). The end ribs 116 each support a
flanged seal ring 170. Each seal ring 170 extends all of
the way around the inside of the cell, in an arc which has
an axis coaxial with the cathode 110. Each seal ring 170 ~-~
extends, as shown i~ Fig. 11, from the upper edge of one
rib 116, on one side of the tank 102, to the upper edge of
the rib 116 on the oppo~ite side o~ the tank 102. The seal
rings 170 each have a flange 172 (Fig. lla). Each flange
172 has an annular slot 174. The slots 174 engage the
wiper seals 166 and thereby confine the flow to gap 168.
It will be apparent to those skilled in the art, from
the above, that the embodiment of Fig~. 10-13 can be
employed with a controlled flow apparatus such a~ disclosed
with regard to Pigs. 1-9. Alternatively, the anode design
~2~8~
-28-
of Figs. 1-9 can be employed with a flooded cell design
such as discloQed in Figs. 10-13. To adapt the anode
plates 130 of the embodiment of Figs. 10-13 to controlled
flow, a seal can be provided between contiguous flanges
136, 138 of adjacent anode plates 130. In this way, the
anode plates would define, with the cathode, a completely
confined channel through which the electrolyte would flow.
If desired, the flanges 136, 138 could be undercut to
accommodate a seal sealing the as~embly against the leakag~
of electrolyte between the flange~.
Fig. 14 ~hows an alternative embodiment of the present
invention in which the anode plates 210 can be flexed into
a desired configuration. Each anode plate 210 is provided
with a plurality of spaced apart main supports 230 aligned
lS with the center line of the anode plate, a plurality of
spaced apart edge aligned supports 240 aligned with one
edge 242 of each anode plate, and a plurality of additional
edge aligned supports 250, aligned with an opposite edge
252 of each anode plate. ~y suitably ad~usting the
20 3upports 230, 240 and 250, the anode plate 210 can be
flexed into a desired configuration. Electrolyte is
contained in the apparatus within outer rubber-covered
shell 260. All of the supports protrude through the shell
and are ad~ustable radially with respect to the shell. The
upports are sealed with respect to the shell 260 by seals
262.
2~$~
-29-
~ ~imilar de~ign is shown in Fig. 15. The cell
comprises a plurality of anode plates 310. The anode
plates 310 are provided, on their underside, with a
plurality of bosses 312 similar to the cell of Fiys. l-9.
The bosses protrude into holes 314 of a rubber lined shell
316. The shell 316 is similar to shell 14 of Fig. 1
extending circumferentially around a cathode (not shown).
A rubber lining 317 covers the entire inner surface of the
shell 316. The bosses are movable radially in the holes
314 and are sealed within the holes by seal rings 318. The
bosses 312 are internally bored and threaded. Bolts 320
engage the bosses 312. The bolts 320 are located in holes
314, in a radial direction, by support washers 322 and
spacer rings 324. Shims (not shown) similar to support r
strips 90 (Fig. 8), in shell 316, engage the longitudinally
extending parallel opposite edges (not shown) of the anode
plates 310. By suitably dimensioning the shims, spacer
rings 324, and the amount bolts 320 are turned into bosses
312, the anode plates 310 can be flexed into whatever
arcuate configuration is desired.
In the embodiment of Fig. 15, current is supplied to
the anode plates by buss connections 326. Alternatively,
the spacer rings 324 can function as contact rings, and
current can be supplied to the anode plates 310, through
the shell 316 and the spacer rings 324.
From the above description of preferred embodiments of
the present invention, those skilled in the art will
~2~
-30-
percei~e Lmprovement~, changes and modifications. Such
improvements, changes and modification~ within the skill of
the art are intended to be covered by the appended claLms.