Note: Descriptions are shown in the official language in which they were submitted.
WO~t/1~7~2 PCTtVS91/02~0
20~27~a
ELECTROLUMINESCE~T PHOSPHORS WITX LONG LIFE
AND METHOD FOR ~AKING THE SAME
.;'.",
FIELD OF THE INVENTION
This invention relates to electroluminescent
phcsphors and a method for making such phosphors. In
particular, this invention relates to long life
phosphors, preferably zinc sul~ide based phosphors,
obtained by a specially controlled ~echanical
deformation of an initially fired crystalline zinc
sulfid~ phosphor precursor material. While the
phosphors of the present invention may be controlled to
emit light in a broad spectrum of colors, the present .~;
invention is particularly suited for the manu~acture of
phosphors e~ittin~ in the blue, blue-green, green a~d
yellow-o.range portions o~ the spect~lm.
This invention also relates to electroluminescent .:~
lamps and a method for making such lamps. In ~
particular, the present invention relates to ;; :
electroluminescent lamps having unexpectedly long life -~
and retained high brightness.
, ., . ~,, . :: . :-
BACKGROUND OF TH INVENTION ~ :
;,;,
Electrolu~inescent (EL) phosphors, particularly
.- ;.~.,.
,~
W~9l/16722 P~T/US91/02640
2~5?~7~ ~ '
- 2 -
~inc sulfide phosphors, and method fox their
manufacture are well-known. While such phosphors can
be controlled to emit light in a broad spectrum,
especially common phosphors emit light in the green and
blue portions of the spectrum.
Blue EL phosphors arP Xnown for their poor life.
The half life (time at which the brightness o~ the
phosphor is one-half the initial brightness~ for ~any
blue EL phosphors is in the range of from about 100 to
300 hours when the phosphors are excited at 115 volts
and 400 Hz. The half lives are even shorter when these
phosphors are used at higher voltages and frequencies.
Green EL phosphors have longer half lives than blue
EL phosphors, typically in the range of about lO-1000
hours at 115 volts, 400 Hz; but they also exhibit
shortened half life when sub~ectPd to higher voltages
and/or frequencies. the foregoing data is based upon
typical EL lamp constructions wherein the blue phos?hor
typically emits light in the 15 ft-~ range and the -
green EL phosphor typically emits in the 25 ft-L range.
Blue EL phosphors are normally prepared by firing
zinc sul~ide, a cupper activatar, and one or more
halide ~luxes at high temperatures. Phosphors such as
ZnS:Cu:X (X=Cl or Br) containing mo-e than about 0.05
weight % Cu are typically blue-green rather ~han blue.
It is known that ~s the copper content increases, there
is a gradual shift in ~he light emitted from blue to .
green. Similarly, a yellow-orange shift in emitted -
light can be achieved by adding manganese to
conventional ZnS:Cu phosphor materials. West German ¦
Patent No. 3,011,815 (Fischer) claims a long life blue
- :: . - . , ,. . . .. : .~- : ,: : .. .
WO~ 722 P~T/U~91/02~
20~27~0
- 3 -
phosphor consisting o~ zinc-cadmium sulfide containing
magnesium sulfide "to give a blue shift," however no
absolute values for "long li~e" are provided in the I ~-
patent to veri~y this claim.
For many years, various techniques have been
proposed for improving the brightness and half life of
zinc sulfide phosphors activated with copper. O.ten,
these techniques have involved the addition of one or
more coactivators such as halide (e.g. Cl, Br, I) or
trivalent ion (aluminum, gallium, indium, etc.)
compounds or complexes. See, for example, U.S. Patent
Nos. 3,082,344 (Thornton), 3,152,995 (Strock) and
Peters, et al. (J.-~D1. Phys., 34:2210-2215, tl963~)
which describe various method for manufacturing such
phosphors with the objective of improving their
brightness.
Zinc sulfide electroluminescent phosphors of the
prior art have typically been prepared via the s,eps -
of: (a) preparing a mixture of predominantly zinc
sulfide, about one weight percent of an
electroluminescent activator (e.g., a copper compound
such as CuSO4) and a few weight percent of a halide
coactivator fluxing compound; (b) ~iring this mix~ure, I
e.g., at from about 1000C to 1300C; (c) a~lowin~ ! :
this ~irst-fired material to return to room tempera~ure , !
and washing with water: (d) mechanically stressing ~he
first-fired material te.g., by milling); (e) ~iring
(baking) the de~ormed powder at an elevated ,
temperature, e.g., from about 600C to 950C; and ~
tf) bringing this second-fired`powder material back to
room temperature (e.g., either by removing it from the
over and allowing it to cool slowly, or by cooling
."',:
;,~',.'
, .. ... , . , .. . .. . . , ,, . ,. , . .- - - -
,. :.. .~. ~ : ~ . . - . . -; . . : - - . . - : . - -
.. - ; - - .. - .. . . - .
WO91/16722 PCT/US91/~2~0
. ..~,
~Q~ r,ia
4 --
slowly and then quenching).
In the process described above, the first firing
causes a tran~formation of the initially predominantly
cubic structure of the phosphor precursor material to a
predominantly hexagonal conformation. Upon the
subsequent cooling, therè is some reversion of the
hexagonal conformation to the cubic conformation;
however, such reversion is minimal. The mechanical
stressing step further reverts more phosphor to the
cubic conformation and introduces "faults" within the
phosphor particle. The second firing and cooling cycle
results in the further transformation of the phosphor
particles back to a predominantly cubic structure.
It is generally accepted in the phosphor art that
the reversion of the phosphor back to a predominantly
CUhiC structure or conformation is necessary to attain
brightness and life. In following therewith, it is
recognized that the parameters of a mechanical
stressing step are critical for promoting or attaining
a sufficient level of reversion to the cu~ic struct~re
to obtain a commercially useful phosphor. Such
deformation is typically accomplished by milling or
mulling the first-fired mat~rial for a standard period
of time, i.e., that which by prior experiments and/or
experience has affor~ed the manufacturer with maximum
phosphor brightness.
.. ..
While the art teaches that various stressing steps
may be employed for fracturing the first-fired zinc
sulfide crystals, none of these processes have resulted
in zinc sulfide phosphors having both the high initial
brightness and the long half life with the retained
:.,
.'' ' , ' . - -
W0~1/167~2 PCr/US91/02~0
_ 5 _
high brightness of the phosphors of the present
invention.
SUMMARY OF T~E INVENTION
As described above, the present invention relates
to electroluminescent phosphors and to a method for
making such phosphors. In particular, the present
invention relates to phosphors having long life
obtained by a specially controlled mechanical
deformation or an initially fired crystalline zinc
sulfide phosphor precursor material.
It is known in the phosphor art that duration is a
key parameter of the mechanical stressing step in order
to attain maximum brightness. Generally, it has been ;~
shown that the initial brightness of a phosphor product
increases during the early periods of mechanical i~
stressing to a maximum brightness level and thereafter
decreases with continued stressing. See Peters, et
al., supra. It has now been unexpectedly discovered
that the charac~eristic time to half brightness of the
electroluminescent phosphor product (i.e., the hal~
life) is a positive function of mechanical stressing
time, not only up to the time that brightness peaks,
but beyond that time as well. This phenomenon has been
~ound to exist for all variations of parameters
employed in the processes used ~or making
electroluminescent phosphors thus far expiored.
..~ ~, . . . . .
Furthermore,-it has been discovered that the rate
at which ~he phosphor brightness tends to decrease with
continued stressing after peak brightnes~ has b-en
''`'' ','. '
' ''~ ~"'
W091~167~2 PCT/US91/01~0
~a ~ t~ - 6 -
achleved is substantially slower than the rate at which
brightness increases with stressing before peak
~rightness ls reached. Thus, the present invention is
based upon the discovery that extreme mechanical
stressing of a first-fired phosphor precursor material,
i.e., stressing for a period of time beyond that at
which peak brightness is normally realized, yields an
electroluminescent phosphor product having unexpectedly
superior properties in tarms of both brightness and
useful life (i.e., improved half life with little
sacrifice in briqhtness).
The critical factor for obtaining the improved
properties in electroluminescent phosphors in
accordance with the present invention is the
performance of the mechanical stressing of the
first-fired phosphor precursor material. As noted, it
is critical that this mechanical stressing be per~ormed
for a period of time which exceeds that at which
maximum brightness is attained for the given phosphor
material. The specific parameters chosen for the
mechanical stressing step will depend upon the starting
materials, the type of mechanical stressing imparted
upon the phosphor, the efficiency of the apparatus or
methodology employed in stressing the phosphor
precursor, as well as the brightness and li~e
characteristics de~ired of the ~inal phosphor product.
With resp2ct to tha starting materials, it is found
that the addition o~ high levels of the copper
activator, besides resulting in a more green phosphor,
results in phosphors having lonyer ~ife and higher
brightness as compared to similar phosphors employing
less copper activator. Furthermore, it is also found ~ -
that a small quantity of zinc selenide added to the
WO91t~7~ PCT/VS~I/O~O
_ 7 _ 20~27~
starting materi~l will also contribute in a bene~icial
way to th~ quality o~ the EL phosphor so produced,
particularly with respect to the production of hi~h
brightness and excellent long life green phosphors.
The process of the present invention thus comprises
the following steps:
(a) preparing a mixture of a predominant amount of
zinc sulfide and a minor amount of an activator
compound, preferably a copper-containing activator
compound; ;~
- (b) firing the mixture (first fire) at a
temperature sufficient to form particles having a
predominantly (>50%) hexagonal crystalline structure;
(c) mechanically stressing the first-~ired
mixture, e.g., by mortaring, mulling or milling, for
period of time exceeding that at which maximum
brightness would be achieved;
(d) firing the mechanically stressed material for
a second time (second fire), to complete the reversion
of the crystalline structure to a predominantly cubic -
conformation; and
(e) subsequently cooling the twice-fired material
to yield the desired high brightness, long half life
electroluminescent phosphor products.
'~ ..
The present invention is generally applicable ~o
all elactroluminescent phosphors, regaxdless of color
emittance, but is particularly applicable to phosphor
materials emitting in the blue, green:and yellow-orange
portions of the spectrum. Ultimate coloration of the ,
finished phosphor is dependent, at least in ~art, upon
the starting materials employed. Variations in
coloration ~ay be effected by varying the constituents
'.
WO91/16722 PCT/US91/02~0
~ ~ 8
and relati~e amoun~s o~ the constituents in said
s~arting materials~ as is well-known by those of
ordinary skill in the phosphor arts. For example, Ihe
brightness and color may be controlled by shifting the
copper and/or zinc selenide content.
In any event, many improvements and attributes are
realized from the phosphors produced in accordance with
the practice of the present invention as compared with
typical, comparable phosphors. For example, phospnors
made in accordance with the practice of the present
invention which emit in the yreen portion of the
sDectrum have halr liv~s in excess of 2000 hours to
over lO,000 hours, as compared with its standard
counterparts which typically have half lives of only
about lO00 hours. Similarly, blue electroluminescent
phosphors produced in accordance with the practice of
the present invention typically have half lives 3 to 5
times longer than those of traditional blue phosphors.
In addition to the improved phosphors and improv~d
process for making phosphor as described abovP, the
present invention also relates to electroluminescent
lamp elements having superior lifa and brightness
characteristics. The improved electroluminescent lamps
of the present invention result from tha incorporation
of the phosphors produced in accordance with the
practice of the present invention into typical
electroluminescent lamps. The construction and method
of manufacture of.these EL lamps is well-known and
well-described in the patent literature.
. :
~0~l~l67~? PCT/US91/02~0
.
_ 9 - 2~27~ :
BRIEF DESCRIPTION OF THE DRAWINGS
.
Figure 1 shows a side sectional view of the i--
electroluminescent lamp used for testing phosphors of
this invention.
Figures 2 through 6 are plotted curves,
corresponding to each of a number of different phosphor
compositions which were incorporated into test
electroluminescent lamps, showin~ how the
characteristic brightness and life of each phosphor
product changes as a function of the duration of
~echanical stressing during processing.
Figure 7 shows the brightness and life
characteristics versus stressing time for a phosphor
composition wherein the stressing is brought about by
mulling.
Figure 8 shows the life characteristics of a zinc
sulfide:copper phosphor stressed for 20 minutes by --
~ortaring, wherein the retained Cu content was
sequentially elevate~ from 0.054% through 0.091%, and,
for comparative purposes, the life characteristics of a
comparable commPrcial green EL phosphor. The ~ -
commercial phosphor has a retained Cu content of 0~08%
and showed shorter life than the 0.054~ Cu content
phosphor ~rom the present invention~
.
, . _ . .
. .
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The process of the present invention is generally
applicable to any electroluminescent phosphor precursor
w~ gl/167~2 Pcr/ussl/n26~0
1 0
~aterial or co~position. Such compositions are
~ell-known and readily recognizable by those of
ordinary skill in the art.
Typical phosphor precursor materials generally
comprise zinc sulfide, alone or in combination with
zinc selenide, an activator therefore, and one or more
coactivators. In preferred phosphor precursor
compositionsr a combination of zinc sulfide-and zinc
selenide is employed. This zinc sulfoselenide mixture
generally comprises at least 80~, preferably go~, and
~ost preferably, at least 96% by weight zinc sulfide,
-~ith the remainder comprising the zinc selenide.
Activator compounds which may be used in the
phosphor precursor compositions of the instant
invention include, but are not limited to, copper,
silver, gold, phosphorus, arsenic, vanadium, antimony,
lead, tin, manganese, iron, sodium, lithium, gallium,
tellurium, scandium, indium, and rare earth compounds.
The preferred activator comprises a copper compound,
alone or in combination with one or more of the other
aforementioned ackivator compounds. Preferred copper
co~pounds include copper sulfate, copper nitrate,
copper chloride, copper bromi~e, copper acetate, copper
fluoride, copp~r iodide and copper carbonate: with
copper sulfate being most preferred. Gene~ally, the
activator will be present in an amount of less than ~%,
pre~erably less than or equal to about 1~ by weight,
based upon the aforementioned zinc sulfide or zinc
sulfoselenide materials.
'' :' ' '
Coactivators that may be employed in the practice
of the present invention include compounds or complexes ~ -~
',''" .
':
WO 9 1 / ] 67 ? 2 PCT/US91/0~40
2~27~
o~ halide (Cl, Br, I) and trivalent ions (Al, Ga, In,
etc.)~ Prefe~red coactivators include complexes based
on halides, most preferably halide fluxes. Suitable
coactivators are well-known to those of ordinary skill
in the art. Exemplary of suitable coactivators there
may be given barium bromide, magnesium bromide, sodium
bromide, magnesium chloride, ammonium bromide, sodium
chloride, barium chloride, magnesium iodide, ammonium
iodide, barium iodide, sodium iodide, and the like.
Generally, the coactivator is pr~sent in iks hydrated
form, e.g., magnesium chloride (hydrated)
(MgC12.6H2O). The coactivator component may
comprise one or more coactivators and is generally
e~.ployed in an amount of 20% by weight or less,
preferably 10~ by weight or less, and, most preferably,
about 8~ by weight or less, based upon the weight of ~-
the zinc sulfide or zinc sulfoselenide material.
As noted, additional constituents may be added cr :
incorporated into the phosphor precursor mixture or
composition as desired. Such additional constituents
include zinc sulfate, zinc oxide, sulfur and the like,
and will be readily known to those sXilled in the art.
While the exact reasons for the improved results
obtained by Applicant's process are not fully
undexstQod, the results are quite clear. The present
invention provides a zinc sulfide phosphor which, when
emitting in the green portion o~ the spectrum, has a
half li~e in excess of 2000 hours and,-when emittins in
the blue portion of the spectrum,- has a half life in
excess of 1000 hours~ both of which represen~ drama~ic
improvements over existing comparable emitting
phosphors.
. J~
WO91/167~ PCT/US~1!02~0
1~ _
Briefly, the present invention starts with the
aforedescribed phosphor precursor materials or
compositions. Preferably, said mixture comprises a
mixture of cubic or predominantly cubi^ zinc sulfide
and a copper activator,-e.g., copper sulfate, and at
least one coacti~ator such as a halide flux (e.g., a
chloride or bromide flux). This precursor composition
is fired at a temperature and for a period of time
sufficient to convert the cubic conformation of the
zinc sulfide ~o a predominantly (> 50~) hexagonal
conformation. Appropriate temperatures and time for
this firing will be readily apparent to those of
ordinary sXill in the art. Typically, the first firing
will be conducted at a temperature of at least about
1000C, preferably of at least 1100C, most
prererably of at least about 1200C. However, the
maximum firing temperature will generally not exceed
1400C. The period of time for which the phosphor
precurso_ material is subject to said firing will
depend upon the precursor materials and the temperature
of the firing itself. Generally, the first firing ~ill
be for a period of time of from about 1 hours to 6
hours, preferably from about 2 hours to about 4 hours.
It should be noted that all reference within this
specification, including the examples, to ~iring time,
is to ~e understood as the time of the actual firing of
the phosphor precursor or phosphor material ~rom when
the temperature of the material achieves the ~iring
temperature until the firing is completed. Thus, the
actual residence tim in the furnace will be somewhat~;
longer than the firing times so as to allow the
phosphor matarials to attain the desired firing
temperature. ~ ;
. ~.
...
WO~1~167~2 PCT/VS91/02~0
- 13 _ 2 ~ ~2 ~
~ fter firing, the ~irst-ired materials are allowed
to cool to allaw the same to be further processed.
During said cooling cycle, which may be an ambient
cooling or a Eorced coo}ing, some of the crystalline
zinc sulfide will revert from the hexagonal ,'
conformation to the cubic conformation.
The first-fired materials are then milled or '',,'~
mortared in a mortar-grinder, for example in a Model
~o mortar-grinder made by Retsch GmbH of West Germany,
f~r a period of time in excess of that at which maximum
~rightness is achieved (Tb). Tb is easily
aetermined through simple and undue experimentation for
any given phosphor composition and/or is well-known in ~
the ~rt for standard phosphor compositions. The, ,','
purpose of the extended milling process is to produce a
highly fractured zinc sulfide crystal.
. !
While it is known that milling will induce faults ,
or fractures into the crystalline structure of the zinc
sulfide particles, because phosphors havP typically
been rated on krightness, it was believed to be
detrimental to mill for exten~ed periods of time and ~;-
thus induce a degree of faulting or fracturing beyond
that at which maximum brightness was attained. ,i
Unexpectedly, however, it has now been found that
continued milling and, thus, the inducement of higher ' ~''
levels or degrees of ~aulking or fracturing in the zinc ',
sul~ide crystals results in phosphors having far
superior lives with only minimal loss in brightness. , ~,
- ~ .
In addition,to produoing a more highly fractured or
faulted ~inc sulfide crystal, the milling also causes l
the conversion of some of the ~inc sulfide crystals '~ ',
:
WOgl~l67~' PCT/~S91/02~0
~7J~ 14 -
from the he~agonal conformation to the cubic
conformation and, perhaps more importantly, app~ars to
~acilitate the subsequent and more extensive
crystalline phase transition ~rom hexagonal to cubic
conformation caused by the second firing step. It has
also been found that continued milling may lead to a
more uniform and smaller particle size, which from the
standpoint of phosphors useful in the manufacture of
electroluminescent lamps may be desirable, but is not
otherwise believed to be critical.
The specific length of time for which the first-
fired materials are mechanically stressed is depe~dent
upon the materials themselves, the desired final
properties and characteristics, and the mechanical
stressing technique or methodology employed. Exemplary
of the method by which-stressing can be accomplished
include mortaring, mix-mulling, ball milling,
fluid-energy milling, jet pulverization, grinding,
vibratory milling, cen~rifugal milling, hydrostatic
pressure, uniaxial or biaxial pressure and the like.
Furthermore,this invention is also intended to
encompa s mechanical stressing by means other than
directly applied external forces as exemplified above.
Although not yet attempted, such other methods include
subjecting the material to thermal shocks or other
shocks that would most c~rtain~y entail a mechanical
stressing o~ the .individual particles o~ the in-process
phosphor. -
.
Concurrent with or subsequent to the mechanicalstressing steps, additional activators, coactivators,
or other co-constituents may be added to the in-process
phosphor mixture. Such additives or dopants, as they
-
' .
wn ~I/1fi7~ PCT/US91/02~0
.
1 S ~ L~ ~ ~
are sometimes referred, are as previously defined. In
general, it is preferred to add additional activators,
preferably copper activakors, alone or in combination
with other co-constituents. A preferred combination of
dopants to be used at this point include copper sulfate
and zinc sulfate. Generally, the amount of activator
added to the in-process phosphor will be from about
0.5~ to about 5~, preferably from about 1% to about 3%,
by weight based upon the total weight of the in-process
phosphor. While lower amounts may be employed, such
amounts are less likely to provide as much improvement
in brightness and life in the final compositionO
However, said compositions will still have properties
superior to con~entional phosphoxs. Similarly, while `
higher amounts of the activator may be employed, it is
belie~ed that amounts of the activator may be empioyed,
it is believed that amounts in excess of the 5~ by
weight are beyond the saturation point for the
composition and, thus, generally do not provide any
further improvement in brightness and/or life. -
With respect to the co-constituent, the
co-constituent is preferably employed in an amount of
from about 1% to about 20~, preferably from about 1~ to
about 16~, by weight base~ upon the total weight of the
in-process phosphor. A~ noted previously, the
preferred combination of dopants added during or
following the ~irst firing comprises copper sulfate and
zinc sulfate.
The mixture is then refired at a temperature lower
than the initial firing, generally less than about
1050C, preferably less than about 900C. The most
preferred second firing temperatures range from about
. .
.
. : - : , , . ~ :. :. ~ . : . . - ,
-: - : . . ~ , . ...... .
W~ 67~2 P~l/US~1/02~0
16 ~
7~0C to about 800c. The length of time for which
~he sacond firing is c~nducted will generally depend
upon the ~iring te~perature, the materials themselves
and the desired characteristics and properties of the
final phosphor materials. Generally, the time of
firing is that time which is sufficient to complete
reversion of the conformation of the crystalline
structure from a hexagonal conformation to a
predominantly cubic conformation. This time is
generally on the order of one-half hour to 3 hours or
more. Preferxed firing tim~s for the second firi~g are -
on the order of one-half hour to 2 hours, more
specifically 1 hour to about 2 hours.
Following the second firing, the phosphor materials
are allowed to cool, either atmospherically or by
induced cooling or quenching. Thereafter, the phosphor
particles are treated or washed to remove excess
activato-, e.g., copper compounds, from the surface of
the zinc sulfide crystals. Such washings may be
accomplished by any methods known in the art.
Exemplary of such methods include a multi-step wasning -~
employing, in sequence, hydrochloric acid (4N), water
and a 4~ sodium cyanide solution. Following washing,
the phosphor materials are dried,
.
After drying, the phosphor is preferably sieved
through a 325 mesh screen so as to remove all
axcessively large phosphor particles. Although the -
unsieved phosphors do provide the attributes of long
life with high brightness, they are less desirable for
use in the manufacture of electroluminescent lamps -
which have preferred parti~le si~e tolerances for
insuring a good phosphor layer in its construction.
wn 91/lfi722 PCT/VS91/02~0
- 17 - 2~ ~2 ~ ~
In addition to the ~oregoing steps, other steps or
pr~cesses may be conducted at various times in the
process as described above. For example, following ~he
initial firing, it may be desirable to wash the
in-process phosphor materials with water so as to
remove the excess coactivators, including any ions
thereof that may have formed, especially any excess
halide ions. Such washing may be accomplish~d with ;
repeated treatments with hot, deionized water until the
conductivity of the wash water is below certain levels,
preferably less than about 50 micromho cm-l
Additionally, it may be desirable to sieve the
first-fired materials to remove any excessively large~ -
particles. Generally, at this stage, it is preferred
to employ a lO0 mesh screen for sieving purposes.
. , .
Although not intending to be bound by theory, it is
believed that in the practice ~f the instant process
the initial high temperature firing, combined with the
excessively long period of mechanical stressing, leads
to phosphor particles having a higher degree of faults
or fractures in the crystalline structure as well as to
phosphor compositions having a higher level or
percentage of particles having the cubic as opposed to
the hexagonal conformation. It is believed that the
increased number of fractuxes or faults in the zinc
sulfide particle provides additional sites for
activator, copper compol~nd, deposition during the
second firing.
. . ....- . . : . ~ . ............................ -
,
The doping of the ~irst-fired material with-
additional copper activator tends to increase the
likelihood of saturation of the numerous faults or
fractures in the crystalline structures. Obviously,
-- .. . . ... ..... . .
WO91~167~ PCT/US91/02640
~ 18 -
with very low levels of copper activator in the
in-p~ocess mixture, the opportunity or likelihood o~ -
the copper compound to infiltrate th~ fracture or fault
is less likely than in instances where high levels of
copper activator are employed. In general, the amount
of initial copper and added copper is maintained
sufficiently high during the two firing steps such that
the total retained copper content of the final product
after washing is at least 0.02~, preferably at least
0.06%, by weight. In general, the total retained
copper content will preferably be in the range of 0.0~%
to about 0.25%, m~st preferably about 0.06~ to about
0.2%, by weight of the phosphor mixture. Compositions
within the foregoing limitation and manufactured in
accordance with practice of the present invention
generally provide phosphors having high initial
brightness and a long h~lf life.
To determine the effect of mortaring time on
phosphor performance, several series of ~-
elPctroluminescent phosphors were manufactured and
electroluminescent test lamps produced with said
phosphors. Each series differs from one another by
some variation in the method ~or making
electroluminescent phosphor and/or the composltional
makeup of the phosphor precursor materials. Within
each series, the phosphor and its method of
manufacturing were held constant while the phosphor
mortaring time was varied. Each phosphor sample was
then incorporated into five electroluminescent lamps
~nd the lamps tested.
.. : . . . .
The EL test lamps (illustrated in Figure l~ were-
prepared as follow~. A paste was formed by dispersing
. .
WO91~1~722 PCT/US91/02~0
.
-- 19 -- '
~e electroluminescent phosphor powder in a A7elQctric ~
resin. A layer of the phosphor powder paste 30 and a . .-
layer of a barium titanate paste 34 are sand~iched
between an opaque metallic foil (aluminum) servicing as
a rear electrode 20 and a transparen~ ~ron~ electrode
(indium tin oxide) 22. A layer 24 of a desiccant is : ::
formed over the front transparent electrodP 22. A pair .
of lead wires 26 and 28 are attached, respectively, to
the front and rear electrodes 22 and 20. A polymeric
protective film 32 encapsulat~s this assembly, but with :
the lead wires 26 and 28 extending away therefrom, ~
provide physical support and a moisture barrier. The : .
phosphor layer 30 has a thickness of 0.04 millimeters. .
The barium titanate layer 34 is 0.015 millimeters
thick.
A more detailed description of the construction of
an electroluminescent lamp is provided in U.S. Patent - :
No. 4,104,555 (Fleming), the disclosure of which is
hereby incorporated herein by reference.
The general meihod for making the phosphor as
àescribed above will be referred to hereinafter as the
reference method.
In the ~ollowing examples, the brightness of each
test lamp was measured with excitation at 115 volts and
400 H7. The brightness and life date points for each
phosphor.produced were determined by averaging the
measured brightness and li~e for each of the five test
lamps per.group. By convention, the half life o~ an
electroluminescent phosphor.(or-lamp-containing the -
same). is defined as the time re~uired for the initial
brightness (Bo) to decrease to one-half of its
,, ., .. - , . , ,, . ... - - , , . ,, , ; , ~ . . . . ~ . . - .
~V~91/167~ PCT/~S91/02~10
- ~0 -
initial ~alue. S.ince this time may be several thousand
~o~rs, an accelerated li~e test is a near necessity.
One such test was devised by British Aerospace and
consists o~ operating la~ps at elevated temperature and
fre~uency. This method is described in R.H. Marion et
al. "Analysis of the Lifetime and Powder
Electroluminescent Phosphors" in
Shionoya, S. and Kobayashi, H., Ed., Springer-Verlag,
P. 332, 1939.
The accompanying figures represent the brightness
and life data realized from evaluations of various ~-
phosphors made in accordance wîth the practice of the
present invention as set forth in the following
examples. Figures 2 through 6 are graphic
representations of the life and brightness data versus
mortaring time for compositions made in accordance with
the practice of the present invention. ~igure 7
illustra~es the effect on brightness and life of
mulling rather than mortaring. Figure 8 represents
data demonstrating the effect on life of various levels
of retained copper in the phosphor. Figures 2, 3 and 6
are based on actual life test data. All other figures
represent life data which are based on accelerated life
test results. Figure 2 sets forth the actual data
points arrived at, whereas Figures 3 throu~h 7 present
the brightness and life data in a normalized fashion,
i.e., the data points representing the greatest level
of brightness and life, as measured, are each set to a
value of 1.0~ ~ -
''' - :
The following examples are-intended to aid in the
understanding of the present invention, but are not to
be construed as limitations thereof. Unless otherwise
: :'.
.
.
W~91~67~ P~T/~ /02~0
- 21 - 2 ~2 ~.
indicated, all a~ounts with respect to the various
constituents o~ the phosphor precursor materials and
the in-process additives are in parts by weight~ All
temperatures are expressed in degrees Celsius.
EXAMPLE 1
:
~ series of phosphor compositions emitting in the
green portion of the visible light spectrum were made
according to the following procedure:
,
98 parts zinc sulfide, 2 parts zinc selenide,
0.3 parts copper sulfate (anhydrous~, 3 parts
barium bromide (hydrated), 3 parts magnesium
bromide (hydrated) and 2 parts sodium bromide were
dry blended together in a V-blender for at least
one-half hour. The blended materials were then
placed in a quartz crucible, covered and fired at
1200C for 3 hours. After 3 hours firing, the
crucible was brought out of the furnace and cooled
rapidly with cold air blowing on the bottom of the
cruciDle. Once the phosphor was cooled, it was
washed free of ions, especially bromide ions, with
hot deionized water at least s.ix times, or until
the conductivity of the wash water was less than 50
micromho cm l. The phosphor was then filtered,
dried and sieved through a lOO mesh scre~n. This
step of the process yields a material that is
referred to as the first-fired material.
.. . .
, . . ... . . .
Individual 200 gram aliquots~ of the first- ~-
fired material were then mortared for various -
lengths of time to demonstrate the effect thereof.
.. . . . . . . ......... . . . . . ........ ... . . . . .. .. ..
.. . . . . . ,, : . ., ; : . , : - ~ - . ..
- . . ,~. ~ : - ~: ., . . :
. - - . . , . ~ . . . .. .. . .. .. . . .
WOg~/~672~ PCr/US91!02~0
- 22 -
In this example, mortaring times of 0, 5, 10, 20,
30 and 40 minutes were employedO
Each ~ortared sample was then doped with 5
grams of copper sulfat2 (CuSO4) and 32 grams of
zinc sulfate (ZnSO4), blended and fired at
800C for 1 hour in a covered quartz crucible.
Following this second firing, the phosphor was then
cooled and sequenti~lly washed with hydrochloric
acid (4N), water, and finally a 4% NaCN solution to
remoYe any excess copper found on the surface o~
the phosphor particles. The phosphor was then
dried, sieved through a 400 mesh screen and
processed into electroluminescent lamps.
The data representing the brightness levels and
half lives attained for the various samples made in
accordance with this example are depicted in Figure 2
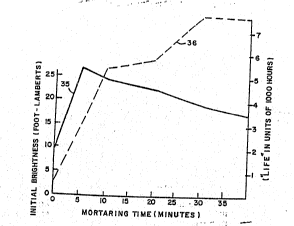
above. As shown, brightness (curve 35) peaked at a
mortaring time of about 5 minutest whereas the half
life (curYe 36) continues to increase even up to 30 ~ ;
minutes. Furthermore, although brightness diminishes
with continued mortaring beyond the time at which peak
brightness is attained, its rate of diminution is much
slower as compared to its rate of enhancement in the
early stages of milling. Based on ~his data, it is
clearly advant~geous to mortar the phosphor beyond the
5 minutes at which peak brightness is obtained, thereby
obtaining a signi~icant increase in life at the expense
of a minimal loss in brightness. These same plots are
depicted in a normalized fashion in Figure 3 as per
~~~ curves 41 and 42, respectively.
~ ' ~ ''~ ':' ' :
. .,, . 1- .
- : - - .. ---.-: . , , . . .. ; , . . .-- , . . . .
W~9~/167~2 PCTtUS~I/02~0
. .
- 23 - 2052~
EX~MPLE 2
A second phosphor composition was made in
accordance with the procedure of Example 1 with the
exception that the second firing was at 750c instead
of 800C. This phosphor composition was slightly
different from that set forth in -Example 1 in that zinc
selenide was eliminated from the formulation and that
magnesium chloride (hydrated) was substituted on a
weight for weight basis for the magnesium bromide of
Example 1. The brightness and life data for this
sample of phosphor is depicted in a normalized fasAion
in curves 45 and 46, respectively, of Figure 3. .
EXAMPLE 3
A phosphor composition was made in accordance with
the procedure of Example 1 with the exception that the
amount of copper su}fate starting material was
increased from 0.30 parts by weight to 0.40 parts by
weight. Brightness and life data, as a function of
mortaring time, for this phosphor composition is
presented in Figure 4 by curves 51 and 52,
respectively. ~ :
:'
~XAMP~
.,
A phosphor composition emittlng in the blue portion
- of the spectrum was made in accordance with the
procedure of Example 1 except that the level of ccppër
sulfate was reduced-from 0.30 parts by weight-to Q;15
parts by weight, 1 part by weight of ammonium bromide
,.. . . . .. . .... . . . .
~O~1/1672 PCT/US~/0~0
~ Q - 24 -
was substituted for the 1 ~luxes (barium bromide
(hydrated), ~agnesium bromide (hydrated) and sodium
bromide)), and 1 part by wei~ht of sulfur was added as
a co-constituent. The brightness and life data for the
phosphor so made is depicted in Figure 4 by curves 55
and 56, respectively.
EXAMPT ~ 5
An additional series or phosphors was made in
accordance with the procedure of Example 1 except that
the starting materials were blended through a wet
mixing effect2d by the addition of water, which was
subsequently removed by heating and drying, and the
second firing was conducted at a temperature of
750C. Additionally, the composition of Example 1
was modified in that the magnesium chloride (hydrated)
was substituted on a weight for weight basis for the
magnesium bromide. The data points detDrmined for the
brightness and life of the phosphor so produced are
depicted by curves 61 and 62 7 respectively, in Figure
5.
~!
. .
EXAMPLE 6
A series a phosphor compositions were manu~actured
in accordance with the general procedure ~f Example 1
with the exception that the firing time-was varied as
set ~orth below and that a magnesium chloride
(hydrated) flux was substituted for the magnesium
bromide (hydrated) flux on a weight for weight basisO
The first series of phosphors as represented by curves
I ' ..
~... i . -
~V~ 91/16722 PCr/VS9~/02640
2~6~7~
71 and 72 (~igure 6), for brightness and life,
xespectively, were ~ired for 2 hours in both the first
and second firings. On the other hand, curves 75 and
7~ correspond to the brightness and life behavior,
respectively, of similar series of phosphors with the
exception that the first firing was co~ducted for a
period of 2 hours and the second firing for a period of
4 hours. Once again, as shown in ~igure 6, the
advantages of mortaring beyond the time at which
brightness becomes greatest is clearly evident for all
o~ these changes in the method or making the phosphor.
':
EXAMPLE 7
A series of phosphor compositions as described in
Example 1 were prepared in accordance with the method -:;
of Example 1 with the exception that the starting
materials, following the first firing, were mulled,
rather than mortared, and the second firing was
conducted at 750C. The mulling machine employed was
a Simpson Mix-Muller, Model LF, Style UD, made by the :;
National Engineering Company o~ Chicago, Illinois.
This device consists of two heavy crusher wheels
mounted at the ends of a horizontal axle that rotates
in a horizontal plane about the axle center. Sweepers
direct the material being mulled under the wheels.
As shown in Figure 7, the phosphors produced in
accordance with the above teachiny mani~ested a maximum
brightness after 30 minutes of mulling (curve 813.
Continued mulling of the phosphor composition beyond
the 30 minute peak period resulted in the development
o~ phosphors having a longer life perfo~mance with a
~ ~.
~ ' ' ' ' '' ' '' ' ' .. ' '~ ' ... . . . .
WO~1/167~? PCT/US9l/02~0
~ 26 -
minor loss in brightness (curve 82). This example
demonstrates the fact that alternative methods for
mechanically stressing the phosphors may be emploved in
the practice of the present invention. It is
recognized that certain mechanical stressing
methodologies may require longer periods of stressing
ln order to achieve the same result. Nevertheless, the
practice of the present invention still provides
improved phosphor compositions and, subsequently,
electroluminescent lamps having enhanced brightness and
longevity.
EXAMPLE 8
A series of phosphor compositions was prepared in ;;,;
accordance with the methodology of Example 1 and of the
formulation of Example 1 with the exception that the
amount of copper added initially was varied so as to
provide phosphor compositions having a formulated
initial copper content of 0.06%, 0.08%, 3.10% and 0.12%
by weight, respectively. The actual lamp brightness
and half life data, as function of copper content and
mortaring time, is depicted in Table I.
;:
-: ~ ' ' . - ~ ' ' , .
- . . -
. : .. , . . - -~ - . -
.:. , .
W~91~167~'
Pcr/ uss ~ !02b40
- 27 - 20~27~3
~ABLE I
Relationship of Brightness and Life
to Initial CooDer Content and Mortarinq_Tlme
Mortaring Initial Half Life
CuTime fMin.)Brightness (Ft-LltHours) : :
~ ' -.
0.06 0 18.9 150
23.2 1900
0.08 0 9.6 200 :
20.1 4gO0
0.10 0 9.4 250
24.6 6150
- 0.12 0 9.5 . 500
27.5 3200
25.3 5300
22.5 5650 : .
19.3 7700
17~5 7600
.:
! :`~
I
,
i~
WO 91/1~727 PCI/US91/0264~)
2 ~ -
The mortared samples always showed higher initial
brightness and better half life than the unmortared
samples. The hal~ life also improved with increasing
copper content. The 30 minute mortared sample, for the
0.12% Cu, showed a slight decrease in brightness, but
continued to show very good half life.
The relationship o~ brightness and life to copper i'
content is shown in Table II and depicted in Pigure 8.
Also shown in Figure 8 is the plot of a typical
commercial green phosphor which has a retained copper
content of 0.08%. After 600 hours operation, the :
brightness of the EL lamp was only 66~ of its initial .
brightness level.
`.
.
;::
;' '
'`~'" '
`"'' '
'
., .
, -: :- - .. . . . . . . . . ,, , . . " .. , . ,, , . , . . .. , . - - : -
WO 91/167~2 PfCr/US91/02f640
- 29 -
~ f~ ~2 ~
~'
J ~
l m
f~l¦ Ul t` N 0 f~
O
~1 ~ o n o~ I f f f ~1
~ ~ ~7 0 t~
O, t~ f~7 ~1 1~ 7
. f t~ ~ 1~ 7 ~ Clf O f~l ~7
~ ~ O ~ f7 ~ f
f~
~3 ~ f ~ -
n ~ ~¦ . ~ n f~ f~ f~
U3 f~ I C:~ 0 C~ a~
H f_ ~ _ ' f~ 7 f7
~ ~ l o ~ 0 O e ~ ~ '` '` f`
~ o o o o ~ ~ r 1~ ~
~_~ O f ,1 ,. ,_f _1 :
~, C ~¦ ". fC ~ frf .
O O O O f '
U -I ~
f5
f3~ ~ ~ 7 f,~
~-~1 r` ~ fn cl:~ .o f~ f.~7. frl
r~ S 01 o ~7
.~J . r~ . ..... ... ... ... ... '
~ ~1 :
ul o o ~ o 7 0 ~ ~
o~ O O O 0 3 d~ O f ~ O O : :
' ~' ~
,.
' ;,~
WO91/1~72~ PCr/US~ 2~10
The present invention has been described in detall,
including the preferred embodiments thereof. However,
it will be appreciated that those skilled in the 2-~,
upon consideration of the present disclosure, may make ~:
modiflcations and/or improvements of this invention and
still be within the scope and spirit of this invention :
as set forth in the following claims.
'`'.''"~'" '
-