Note: Descriptions are shown in the official language in which they were submitted.
2 ~ 3
CASE 100-7677
NE~ ADENOSI~ DERIV~T~ T9BIR PRODUCTIO~ ~ND US~
The invention relates to new adenosine-2',3'-carbonates,
processes for their production and their use, among other thing~,
for ~he treatment of raised blood pressure.
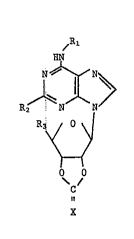
The invention relates in particular to compounds of formula
~R,~
HtJ
R3 ~ \J
/
~1
o o
c
wherein
Rl signifies (C1_6)alkyl, (C3_8)cycloalkyl, phenyl or
phenyl(C1_6)alkyl? whereby the phenyl rings, independe~tly of
one another, may be optionally mono- or di-substituted by
halogen with an atomic number of 9 to 35, (Cl_4)alkyl,
(Cl_4)alkoxy and/or CF3, and in the case of phenylalkyl, the
. ` , ' :
.`: ; ' ' '. '
. -`` 2 ~
- 2 - 100-7677
alkylene ch~in is straight-chain or branched,
Rz denotes hydrogen, (C1_4)alkyl, halogen with an ato~ic number
of 9 to 35, or (C3_8)cycloalkyl,
R3 denotes groups of formulae -C~zO~ or -CONHR4, wherein
R4 signifies hydrogen, (C1_6)alkyl or (C3_8)cycloalkyl, and
X is O or S
,with the proviso when
R2 signifies hydrogen or halogen with an atomic number of 9 to
R3 signifies the group of formula -CON~ (Cl_6)alkyl and
X - O
then
R1 doe~ not signify (C1_6)alkyl
Of the compounds of formula I, preferred compounds possess the
formula ~ R~ `
~N
N ~ I~
~ ;'
O O
wherein X
R1~ signifies p-methoxyphenyl, p-chlorophenyl,
R2R signifies hydrogen or methyl and
R3a signifies CONH (Cl_4)alkyl, and
X signifies O or S.
.: - - ~ : . . .
: ` , - . ,
~ 1 0 ~ 9
- 3 - 100-7677
In formula I~ halogen with an atomic number of 9-35 is fluorine,
chlorine or bromine, preferably fluorine or chlorine, a (Cl_4)-
alkyl group is methyl, ethyl, n propyl, i-propyl9 n-butyl,
i-butyl, tert.-butyl, and where the alkyl group has up to 6
carbon atoms, it is also n-pentyl, i-pentyl, 3-pentyl, n-hexyl,
i-hexyl, etc., especially methyl, ethyl, isopropyl or 3-pentyl, a
(Cl_4)alkoxy group i3 methoxy, ethoxy, n-propoxy, i-propoxy,
n-buto~y, i-butoxy, tert.-butoxy, especially methoxy, (C3_a)-
cycloalkyl signifies cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl or cyclooctyl, especially cyclopsntyl or
cyclohexyl. Substitution of the phenyl ring may be made in o-, m-
or p-position, ~hereby in the case of disubstitution,
substitution is preferably made in m- and p-position and in the
case of monosubstitution, ~t is made in m- or p-position, but
preferably in p-position. In phenylalkyl~ the alkylene chain and
the phenyl substitution are as discussed above.
Certain adenosine carbonatec are already disclosed in US-Patent
4167565. The instant application is limited therefrom. The known
adenosine carbonates are of a different use however they serve
for controlling undesired ani~als including rodents, coyotes and
birds.
Production of compounds of formula I, wherein R3 is a group of
formula -C~20H, may be effected for example, whereby the
protecting group is cleaved from compounds of formula
2 ~
_ 4 _ 100-7677
. HN
y ~ N~
R~L N N
D~0 ~ II
O O :~:
y ~.
wherein Rl, R2 and X are defined as above, and D~T is the 4,4'-
dimethoxytrityl protecting group.
Cleavage of the 4,4'-dimethoxytrityl protectin~ group
conveniently takes place in an acidic medium, for example in 80% ~.
acetic acid, at room temperature for 6 hours whilst stirring. ~`! `
Further agents which can be used instead of acetic acid are
aqueous hydrochloric acid or aqueou9 for~ic acid.
Production of compound~ of ~ormula I9 wherein R3 is the group ;
-CONHR4 ~ iS effected by introducing the group
C ~ X uherein X is defined as above, into compound of formula
,
.1 .
.~ '' . .
; ' `: '
. -- .
~,:
.
2 ~
- 5 - 100-7677
hN
N~
o ~/ III
H0 o~
wherein Rl, R2 and R4 are defined as aboYe.
\
Introduction of ~he C - X
group into compounds of formula III conveniently takes place by
reacting them with for example 1,1'-carbonyl- resp. 1,1'-thio-
carbonyl-diimidazole in a solvent such a~ dimethylformamide
whilst stirring for 5 hours at room temperature.
The compounds of formula III are known, i.or example irom German
published application 3810551.
Production of the compounds of formula II may be effected for
example whereby the 4,4'-dimethoxytrityl protectin~ group i9
introduced into compounds of formula
a
R~ N~) IV
HO--~
. . . ~ . . .. . .
'. ' ~ : ' ~ - ' :
. ~' ,' . . ' ...
-, . ': , , : :.
' ' ' ' . ,
2 ~
- 6 - 100-7677
wherein R~ is defined a~ above. This is effected by means of a
reaction with 4,4'-dimethoxytrityl chloride in dimethylforma~ide
at room temperaeure. The compounds of formula
Cl
R~
H O CN4
thereby formed, wherein R~ and DMT are defined as above, are
reacted with a compound of formula Rl-N~29 wherein Rl is defined
as above, in a solvent such as dioxane at: boiling temperature of
the reaction mixture, and then the group C Y X, wherein X is
defined as above, is introduced into the compounds of formula
~aO
R~J~N ~ N~ VI
~<~ y ~:
\~ " ~
: H O ~ ~ ~
' ~ . . ! . .
''`'''~. ' ~ "' ' ~' ' ,' '' -
-: . , . ~ , ' ': '
' ~
'
. ' ~, . , , . " . ' '
2 ~
- 7 - 100-7677
thereby formed, wherein R1, R2 and DMT are defined as above.
Introduction of the C = X group into compound
of formula VI conveniently takes place by m2ans of a reaction
with for example 1,1'-carbonyl- resp. 1~1'-thiocarbonyl-
diimidazole in a solvent such as dimethylformamide whilst
stirring for 5 hours at room temperature.
Insofar as the production of the required ~tarting materialQ is
not described, these are known or may be produced by known
processes resp. analogously to the processes described here or
analogously to known processes.
In the following examples, all temperatures are given in degrees
celsius and are uncorrected.
~ .
:
. ~ ~ . ,
.: .
~.. . ..
2 ~ g ~
- 8 - 100-7677
~xample 1
1'-de~oxy-1'-(6-p-methoxyphenylamino-2-meth~l-9-~rinyl)-~-D-
ribofuranuronic acid-N-ethylamide-2',3'-carbonate
2.14 g of 1'-desoxy-1'-(6-p-methoxyphenylamino-2-methyl-9-
purinyl~ D-ribofuranuronic acld-N-ethylamide and 3.0 g of 1,1'-
carbonyldiimidazole are dissolved in 15 ml of dimethylformamide
and stirred for 5 hours at room temperature. The solution is
subsequently concentrated at reduced pressure and the residue is
partitioned ~etween ethyl acetate and water. After drying over
sodium sulphate, the organic phase is purified on silica gel. The
compound named in the title is crystallized from ethyl acetate
and diethyl ether. M.p. 168-170~.
Examplc 2
6-cyclohexy~adenosine-2',3'-carbonate
4.2 g of 6-cyclohexyl-5'-(4,4'-dimethoxy~rityl)-adenosine-2',3'-
carbonate are dissolved in 21 ml of 80% acetic acid and stirred
for 6 hours at room temperature. The solution is subsequently
concentrated at 40 in a high vacuum, the residue i9 taken up in
ethyl acetate and ad~usted to pa 8 with a~ lOX sodlum hydrogen
carbonate solution whilst cooling. The aqueous phase i shaken
out three times with ethyl acetate, and the combined organic `
extracts are dried over sodium sulphate. The product i9 purified
by elution on silica gel wlth a mixture of ethyl acetate/n-hexane
8:2.
The compound named in the title is crystallized from ethyl
acetate~diethyl ester. M.p. 152-154~.
The 6-cyclohexy1-5'-(4,4'-dimethoxytrityl)-adenosine-2'93'-
carbonate used as starting material may be produced e.g. as
~ollows:
:` .
,
::; ' '' ' . :. :
: ~
_ 9 _ 105-7677
a) To a solution of 9.17 e of 6-chloropurine-9-~-ribofuranoside
and 5.6 ml of triethylamine in 96 ml of dimethylformamide i~
added in drops at room tempe~aeure a solution of 14 g of
4,4'-dimethoxytrityl chloride in 96 ml of dimethylformamide,
and the mixeure is stirred for 1 hour at room temperature.
The solvent is then distilled off in a high vacuum at 35 and
the residue is partitioned between ethyl aceeate and ice
water. The organic extracts are dried over sodium sulphate,
concentrated and the residue is eluted on silica gel with a
mixture of ethyl acetate~hexane 8:2.
The purified 6-chloro-5'-(4,4'-dimethoxytrityl)-adenosine is
obtained as a foam and in ethyl acetate has a Rf-valu2 of
0.6.
b) 5 g of 6-chloro-5'-(4,4'-dlmethoxytrityl)-adenosine and 2.95
ml of cyclohexylamine in 30 ml of dioxane are stirred for
2 hours in an oil bath o~ 105. It is subsequently cooled,
filtered, and the filtrate i9 concentrated at reduced
pressure. The residue is dissolved in ethyl acetate and
shaken out with cold 0.1 N hydrochloric acid. Afeer washing
with water and drying over sodium sulphate, the organic phase
is concentrated and eluted on silica gel with ethyl acet~te/-
; hexane 8:2. The resultlng 6-cyclohexyl-5'-(4,4'-dimethoxy-
trityl)-adenosine is a foam, and in ethyl acetate ha~ a
RF-value of 0.5.
c) 4.6 g of 6-cyclohexy1-5'-(4,4'-dimethoxytrityl)-adenosine and
4.46 g of 1,1'-carbonyldiimidazole in 30 ml of dimethyl-
formamide are stirred for 1 hour at room temperature. The
solvent is subsequently distilled off at 35 in a high
vacuum, and the residue is partitioned bet~een ethyl acetate
and ice water. The aqueou~ phase is extracted three times
with ethyl acetate a~d the organic extracts are dried over
sodium sulphate. After concentration, the residue is
., :
':
~.
'-
., : ,
-
:'
~ 3
- 10 - 100-7677
chromatographed on silica gel with a m~xture of e~hyl
ac~tate/hexane 8:2. The 6-cyclohexyl-5'-~4,4'-dimethoxy-
trityl)-adenosine-2',3'-carbonate is a foam, and in ethyl
acetate has a R~-value of 0.8.
The following compounds of formula I may be produced using
the process described in examples 1 (R3 _ CONHR4 ) or 2 (R3 =
CH2-OH):
Exa~ple R~ R2 R3 ~ ~.p.
- :,
3 cyclopentyl Me CON~B~ O amorph.
4 cyclopentyl H CON~Bt O 106-110
; 5 cyclohexyl H CON~Et O 112-115
6 p-methoxyphenyl ~ CONHBt O 120-124
7 phenyl H CON~E~ O 126-130
8 p-fluorophenyl H CON~Et O 122-126
9 p-chlorophenyl 8 CONHEt O 130-135
cyclohexyl Me CON~Bt O 185-187 :
11 phenyl Me CON~Xt O 116-121
12 p-fluorophenyl Me CON B t O 115-118
13 p-chlorophenyl Me CON~t O 119-124
14 p-meeho~yphenyl Me CON~t S 149-151 `
p-methoxyphenyl H CONH~t S 125-128
16 p-chlorophenyl Me CONHRt S 131-136
17 p-fluorophenyl Me CON~t S 12S-130
18 p-methoxyphenyl ~ CH20H 0 121-123
19 p-chlorophenyl H CH20H 0 110-114
20 3-pentyl H CH20~ 0 156-159
. ~. .
:
2 ~
~ 100-7677
Th~ compounds according to the invention are notable for their
interesting pharamacological properties. They may therefore be
used as medicaments.
In particular, the compounds according to the invention possess
anti-hypertensive activity, as can be deduced from the results of
the following trials:
Measurement of the binding to adenosine A1 and A2 receptor~ in
membranes from the rat's cortex or from the cortex or striatum of
the pig's brain, using the method of R.F. BRUNS, G.~. LU and
T.A. PUGSL~Y, which is described in MOL~C. PHAR~ACQL. 29, 331-346
(1986). Further testing of the activity of the compounds
according to the invention on the isolated, perfused rat's
kidneys for the following parmeters:
- renin secretion,
- renal haemodynamics
- Inhibition of the release of noradrenaline from the nerve
endings following electro-stimulatioln of the renal nerves
accordin to the method of ~.J. Schurek, J.P. ~recht~
H. Lohfert and K. ~ierholzer, described in CO~MUNICATION a la
R~UNION de l'ASSOCIATION DES P~ARMACOLOGISTES LO W AI~ UCL 4th
June 1977, as well as P.M. Vanhoutte, D. Browning, ~. Coen,
T.J. Verbeuren, L. Zonnekeyen and M.G. Collins described in
~YPERTENSION 4, 251-256 (1982).
- Measurement of blood pr~ssure, heart rate, urine production
; and renin activity in plasma of wake, NaCl-depleted and
repleted, normotensive or spontaneously hyperten~ive rats
with catheters implanted in the abdominal aorta and in the
vena cava, followlng i.v. administration or administration of
:
~, ' ' ' ~
~ ~ ;
- 12 - 100-7677
the compounds according to the invention as an infusion or
bolus according to the method of J.F.M. Smits and J.M. Brody
described in Am. J. Physiol. 247, Rl 003-R1 008 (1984).
It can be deduced from the results of the examinations that both
the inhibition of renin s~cr~tion and release of noradrenaline
from nerve endings, and the direct vasodilation, take part in the
anti-hypertensive activity of the compound~ according to the
invention. It results from this that the compounds according to
the invention can not only bc used as antihypertensive agents but
they also possess protective effect against cardiac
insufficiency. They are reducin~ the work load of the heart by
reduction of the afterload due to inhibition of renin secretion
and release of neurotransmitters. Further the cardiac metabolism ~
is improved by coronary vasodilation. ~oth the quality and the -
duration of life are increased by treatment with compounds of the
invention. Being peripheral vasodilators the compounds of the
invention are widening the blood vessels directly,they are
increasing the supply of blood and consequently the oxygen supply
of the tissue. Especially this increase of the nutrive blood
supply has consequences on the lschaemic skeleton muscle. For the
compounds of the invention there is a potential for the treatmen~
of peripheral vaseular diseases like claudicatio intermittens and
the Raynaud's disease. Further the coronary vasodilation leads to
the suppression or improvement of myocardial lschae~ia and
consequently of angina pectoris. The compounds of the invention
have activity in the treatment of arrhythmia.
Being adenosine A1-receptor agonists they lead to a normal sinus
rhythm at supraventricular and ~-adrenergic stimulated
ventricular tachycardias. The co~pounds of the invention possess
fureher a neuroprotective activity. Accordingly they can be used
for the prophylaxis of peripheral vascular diseases ~hich are
accompanied by neuronal degeneration. They reduce further the
.... .
:
- 13 - 100-7677
formation of thrombi by inhibition of the aggre ation of
thrombocytes and protect the vascular endo~helium by inhibition
of the activation of leucocytes. The compounds of the invention
are lowering the plasma insulin level ~1thout influencing the
glucose tolerance. They are potentiating the uptake of glucose in
the fatty tlssue. This insulin saving ~ctivity can be used for
the treatment of typ II diabetes. They further reduce the blood
lipid level, an activity that may influence positively
arteriosclerosis, the reason o~ many cardiovascular diseases.
For the above indications, of the compoundc according to the
invention, the compounds of examples 1, 6, 9, 12 and 14,
especially the compound of example 1, are preferred.
For the above application, the dosage to be used varies according
to the substance used, the type of admini9tration and the desired
treatment. In general, however, satisfactory results are obtained
with a daily dosage of approximately 0.01 to 10 mg per kg body
weight; if necessary, administration may be effected in 2 to 4
portions or even in sustained release form. For larger mammals,
the daily dosage lies ln the range of approximately 1 to 500 mg;
suitable dosage forms for e.g. oral or non-oral administration
generally ~ontain about 5 to 2S0 mg of a compound of formula I
toge~her with solid or liquid carrier substances.
The compounds according to the invention may be administered
alone or in suitable dosaging forms. The medicinal form, e.g. a
solution or a tablet, can be produced analogously to known
methods.
- .
, ~ ' , . .:
.
~ 53
- 14 - 100-7677
The invention therefore also relates to medicaments which contain
the compounds according to the invention in free form or in the
form of ~heir physiologically acceptable salts, as well as the
production of these medicaments in known manner. The assistants
and carrier substances which are usual in pharmacy may be used
for their preparation.
. . '' ..' i . ,
,~
', ' ' :