Note: Descriptions are shown in the official language in which they were submitted.
" ~ cj~/~ S'~
i~ IP~A/US 2 2 NOV l991
BACKGROUND OF_?HE INVENTION
1. Field of the Invention
This invention is directed toward novel alpha
olefin copol~mers having a narrow molecular weight
distribution (MWD) and a broad compositional
distribution (CD) , and a process for making same.
2. Descri~tion of_Related Art
Ethylene-propylene copolymers, particularIy
elastomer~, are important commercial products. Two
basic type~ of elastomeric ethylene-propylene
copolymers are commercially available. Ethylene-
propylene copolymers (EPM) are saturated compounds
requiring vulcanization with free radical generators
such as organic peroxides. Ethylene-propylene
terpolymers (EPD~) contain a small amount of non-
conjugated diolefin, such as dicyclopentadiene, 1,4-
hexadiene or ethylidene norbornene, which provides
suf~icient unsatura~ion to permit vulcanization with
sulfur~ Such polymers that include at least two
monomers, i.e. EPM and EPDM, will hereinafter be
collectively re~erred to as copolymers.
Thes~ elastomeric copolymers have outstanding
resistance to weathering, good heat a~ing properties
and the ability to be compounded with large quantities
o~ ~illers and pla~ticizers resulting in low cost
compounds whiah are particularly useful in automotive
.
and industrial mechanical~goods applications. Typical -
automotive uses àre tire sidewalls, inner tubes,
radiator and heater hose, vacuum tubing, weather
stripping and sponge doorseals and Viscosity Index
V.I.) improvers for lubricating oil compositions.
~SU~STI~UIE ~HEE~
IPEAIUS
. :. . : . . ,... .. - . ,: . .. . ..... . ... . . . .. . . .
,. . . , . ~ . ~ . . .. .. . .. ... .. .. ...
q c~ /
2 - Ip~/US 2 2 NO\1 193'
Typical mechanical goods uses are for appliance,
industrial and garden hoses, both molded and axtruded
sponge parts, gaskets and seals and conveyor belt
covers. These copolymers also find use in adhesives,
appliance parts as in hoses and gaskets, wires and
cable and plastics blending.
As can be seen from th~ above, based on their
respective properties, elastomeric EPM and EPDM
copolymers find many and varied uses. It is known that
the properties of such copol~mers which make them
useful in a particular application are, in turn,
determined by their composition and structure. For
example, the ultimate properties o~ an EPM or EPDM
copolymer are determined by such factors as
composition, compositional distribution, sequenc~
distribution, molecular weight, and molecular weight
distribution (MWD).
The efficiency of peroxide curing depends on
composition. As the ethylene level increases, it can
be shown that the "chemical" cross-links per peroxide
molecule increase. Ethylene content also influences
the rheological and processing properties, because
crystallinity can be introduced. The crystallinity
present at very high ethylene contents may hinder
proces~ibility and may make the cured product too
"hard" at temperatures ~elow the crystalline melting
point to be useful as a rubber.
: . .
The properties of EPM and EPDM copolymers are
a func~ion o~ the catalyst system and polymerization
process used to produce them. Elastomeric olefin :
Copolymers may be produced at relatively low
polymerization temperatures and pressures by means of
. the so called Ziegler catalysts which comprise a
, transition metal compound used in combination with a
; metal alkyl. Mor~ specifically, catalyst systems based
`J~T,TUT~ S'l~.
~? ~ ~P~A/US
Y ~ ::
~, . - , . . . . . . . .. . .
~c ~ q v/(~ t ~
; ; - 3 ~ IP ~/~S 22NOVl991
.
on a combina~ion of a vanadium compound, an aluminum
alkyl or aluminum alkyl halide and, in some cases, a
halogen-containing organic compound which serves as a
polymerization promoter are known in the art.
For example, UtS. Patent 4/540~753 relates to
ethylene copolymers with narrow molecular weight
distribution (MWD) and a narrow intermolecular
composition distribution (Inter-CD) . The catalyst
system used in this refer~nc comprises a hydrocarbon-
soluble vanadium compound having the formula:
VClX(O~)3-x
and ~n organo-aluminum compound. In the polymerization
process, the catalyst components are premixed in the
premixing device and aged for 1-50 seconds. The inlet
temperature of the reaction mixture is about -50 to
150 C.
As pointed out in the above mentioned U.S.
Patent 4,540,753, Inter-CD defines the compositional
variation, in terms of ethylene content, among polymer
chains. It is expressed as the mi~imum deviation
(analogous to a standard deviation3 in terms of wei~ht
percent ethylene from the averase ethylene composition
for a given aopolymer sample needed to include a given
weight percent of the total copolymer sample which is
obtained by excluding equal weight fractions from both
ends o~ ~he distribution. The deviation need not be
symmetrical, When expressed as a single number for
example 15% Inter-C~, it shall mean the larger of the
positive or negative deviations. For example, for a
Gaussian compositional distribution, 95.5% of the
polym~r is within 20 wt.% ethylene o~ the mean if the
standard deviation is 10%. The Inter-~D for 95O5 wt.%
of the polymer is 20 wt.% ethylene for such a sample.
SU8STI~UTE SHE~
IpF~!lJS
- ~ : . . . ..
, :: . . ~'. . .. .. ... . .. . .
:: :. . ,-, : ,: :. ,.-
.. ~, . . . .. . . . .;
p C ~ qc/~
/;~ ~ 4 ~ IPEA/US 22NOVl991
.
G.B. Patent 902,385 teaches a process of
preparing a copolymer of ethylene and higher 1-olefin -
that is essentially homogeneous as to its compfsition
using a catalyst system based on VYn and ALR3 where Y
is alkoxide or acetyacetonate group, and n is 2 or 3,
and R i5 a hydrocarbon radical. A mixture of carbon
tetrachloride and an inert organic liquid solvent or
carbon tetrachlorida alone is used as the solvent for
the copolymerization reaction. Temperature is within
the range of from OC to 125C, more preferably from
250C to 80~C. A molar ratio of Al/V is from 3 to 8.
U.S. Patent 3,000,866 teaches ethylene
copolymers with about 20% ethylene units by weight and
at least 25% alpha-olefin units by weight and about
0.5% to 10% of dicyclopentadiene units by weight. The
catalyst system used in this disclosure is made by
mixing vanadium tetrachloride or vanadium
oxytrichIoride with (R)3Al or (R)2AlX. The
polymerization is conducted by contacting ethylene and
dicyclopentadiene in a solution of tetrachloro ethylene
with the said catalyst system at temperatures between
about 20C to lOO-C.
: ' :
GB Patent 1,005,282 relates to a catalyst
syste~ such as vanadium or chromium acylacetonate,
vanadyl diacylacetonate, and vanadyl alkyl
orthovanadate and a halogen-free metal organic compound .:
such as aluminum trialkyl or aluminum alkenyl for the
preparation o~ linear head-to-tail high molecular
weight homopolymers of alpha-ole~in having the general
formula R-CH=CH2 The polymerization is carried out in
the presence of a halogenated hydrocarbon compound such
as chloroform, methylene chloride or a mixture thereof.
The polymerization iB carried out at temperatures of
from -80~C to +125~C.
. . .
STITl~T~ SH~E~
~pF~ s
.
.
` - . ~ - ~ . ^ - ~ . .
:~
.. . .
~c ~ /v s G ~ ~
~ - 5 - IPEAIUS 22NOVl99l
U.S. Patent 3,301,834 relates ~o a process
for the polymerization of ethyl~n~ and for the
copolymerization of ethylene with other ethylenically
unsaturated hydrocarbons. The catalyst system
comprising vanadium compounds (VOC13 or VC14) and
organoaluminum compounds is ~ormed in the presence of a
halogenated compound such as benzotrichloride. The
ratio of halogenated compound to vanadium compound is
preferably from 10l to 100:1. The molar ratio of V/Al
generally is 1:3 to 1:30, but higher ratios up to
1:3000 are disclosed to be operable. The polymerization
temperature range is from room temperature to about
150C.
U.S. Patent 3,349,064 also relates the same
catalyst cystem as that defined in U.S. Patent
3,301,834 except that the halogenated promoter is a
group of unsaturated carboxylic compounds containing at
least 4 halogen atoms, at least 2 of which are attached
to doubly bonded carbon atoms and at least 1 of which
is attached to a singly bonded carbon atom alpha to the
double bond (e.g., 2, 2, 3, 4, 5, 5-
hexachlorocyclopentene) . The use o~ VC14, TEA and
hexachlorocylocpentadiene is described in example 6
The molar ratio o~ promoter to vanadium compound is
preferably from 10:1 to 100:1. The molar ratio of V/Al
3 to 1:30; 1:60 is disclosed to be operable.
U.S. Patent 3,4i~9,729 rela~es to a method of
making EPDM polymar by using the catalyst system
compri~ing R3~1 organo-aluminum compounds and vanadium
compounds having ~ormula VOYnr toge~her with halogen-
containing compounds such as hydrogen chloride,
elemental chlorine, benzyl chloride, or t-butyl
chloride. The temperature for the polymerization is -
100 to 200'F. The molar ratio of organoaluminum
compound to vanadium compound is in the range o~ 3/1 to
20/1~ The a~ount of active halogen-containing compound
, .
i' SU8ST!TUTE SHEEf
!P~A /IJS
. . ' . . , -
.
` ' ~G l ~V' ~ q c /G~
f~ - 6 - IPEA/US 22NOVl99~ ~
based on vanadium compound is 1 to 30 mols per mol of
vanadium compound.
BE Patent 592,247 teaches a process for
preparing copolymer of ethylene with alpha-olefins
whose molecular weight depends on the amount of
halogenated alXanes used. Triisobu~yl aluminum
([(CH3)2CH2]3Al), vanadium tetrachloride (VC14) and
carbon tetrachloride (CC14) may be used as a catalyst
system.
GB Patent 1,059,865 relates to the
polymerization of ethylene, or ethylen~ togeth~r with
one or more ole~in monomers. TE~, CC14, CHC13, an~
vanadium di-isopropyl salicylate are used as the
catalys~ system.
The breadth of the intermolecular
compositional distribution (CD) and molecular weight
distribution (MWD) of polymers such as prepared by the
above referenced disclosures are largely a function of
the particular catalyst system employed to prepare the
polymer. Such catalyst systems generally yield polymers
with either narrow CD and narrow NWD or broad CD and
broad NWD. Until the present invention, ethylene
copolymers (EPN or EPDM) having a broad CD and at the
same time a narrow ~WD are not believed to have been
disclosed in the art. Such elastomers are especially
useful in that they posse~s novel combinations of
propertie~ 9uch as excellent green streng~h and
processa~ility which lead~ to superior performance in a
number of applications.
SUM~ARY OF q; HE INVENTION
The present invention is directed toward
novel elastomeric copolymers of ethylene and at least
. .
S~8STIIUTE SH~
JS
.
. .
', , . . ~ ' , .. . . . . . .
. .
... . . . . .. ` .. .. . . .
p Gl /~ jC /~iS ~ ~ ~
~_ ~ 7 ~ IP~A/US 2 2 NOV 1991 .,
one other alpha-olefin monomer which copolymers have an
intermolecular compositional distribution (CD), as
hereinafter defined, such that at least about 25% by
weight of the polymer differs from the mean ethylene
content of the polymer by at least plus or minus 5 wt %
e~hylene, and have a relatively narrow molecular weight
distribution (MWD) such that the weight average
molecular weight (Mw) of the polymer divided by the
number average molecular weight ~Mn) of the polymer is
not greater than about 5Ø
The copolymers of this invention which are
characterized by exceptional green strength and good
processing properties, may be prepared using a catalyst
system comprising:
a) a vanadium compound with a valence of 3
or more;
b) a triorgano aluminum compound: and
c) a specific halogenated organic promoter
used in catalytic amounts.
The catalyst system allows for efficient
polymerization of high quality polymer products and at
generally higher polymerization temperatures of up to
about 140C to yield higher molecular weight polymer
products which ar~ essentially free of gel, and which .
possess excellent green strength and processability.
BRI~F DESCRIPTION OF THE DRAWING
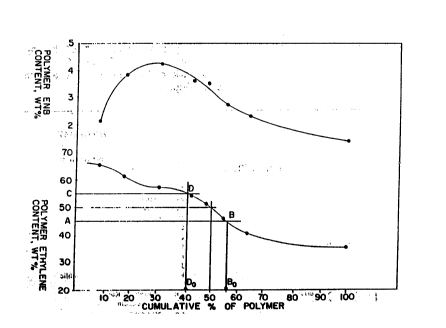
Figure 1 is a graph showing pol~mer
compositional dis~ribution based on mean polymer
content of polymerized ethylene. : .
DET~ILED DESCRI~TION OF THE INVENTION
_ ~ .", :) ' ;~ L `;
~ '~? F ~
; .
':, . ' ' ' ': . - - . ' :. ' . ~ , . : .. . . '. ., , :: , - ,, ' ,':'
. ' ,., ' , ., ' . , '' ' : " ' . ' ' '. ' :' ' ~ ' : '' ' " ' . ~ ' :.
': ,' ` ' ' ' ' ' . ~ ' :. ':: ', '. ' , ' :
pc ~ q~l/c~
~ - 8 ~ lP ~/Us 22NOVl991
The term intermolecular compositional
distribution (CD) as used herein defines the
compositional variation among polymer chains in terms
of ethylene content as compared with the mean ethylene
conten~ of the copolymer as a whole. The CD is
expres~ed by first determining the mean ethylene
content of the copolymer sample by a suitable test such
as described in ASTM D-3900. Next, the copolymer
sample is dissolved in solvent such as hexane and a
number o~ ~ractions o~ differing composition are
precipitated by ~ihe addition of incremental amounts o~
a liquid such as isopropanol in which the copolymer is
insoluble. Generally, from about 4-6 fractions are
precipitated in this way and the weight and ethylene
content of each fraction are determined after removing
the solvent. From the weight of each fraction and its
ethylene content, a plot is prepared of weight percent
composition vs. cumulative weight percent of polymer
which is shown in Figure 1, and a smooth curve is drawn
through the points. The ethylene composition
corresponding to 50% by weight o~ the polymer is
locat~d as shown by th~ construction in the figure, and
horizontal lines A-B and C-D are drawn at 1 5 weight
percent ekhylene from the mean composition. Vertical
lines through points B and D are drawn to locate points
Bo and Do respectively at the horizontal base axis, and
i~ the cumulative percent of polymer represented by Bo
~inus Do is 75% or less, then the polymer falls within
the scope o~ this invention. For example, with the
data shown in Flgure l, the mean composition o~ the
copolymer a~ a whole is 50 weight percent ethylene, Do
is about 41 cumulative %, Bo is about 56 c~mulative %,
and Bo minus ~O is therefore about 15%. Thus, with
respect to a copolymer as represented in Figure 1,
about 85% og the copolymer has an ethylene content
which is greater by plu5 or minus 5% than the mean
ethylene content of 50~ of the copolymer as a whole.
SU~S~l~lltE SH~
IPrA/US
. . , . . - ` .. . . , . . ... , . . . .
. ~, . . . . .
: .: . . . - . .. . . .
- . . . .- ~ ::
~ ~l /v'~ 9 G /~-v~ ~
g J~IU~ ~ 2 I~OV i~
This exemplifies a broad compositional distribution
within the scope of this invention.
Molecular weight distribution (MWD~ is a
measure of the range of molecular weights within a
given copolymer sample. It is characterized as a ratio
of weight average to number average molecular weight,
i.e. Mw/Mn. MWD can be measured by gel permeation
chromotography (GPC), for instance, using a waters 150
gel permeation chromatograph equipped with a Chromatix
KM-6 on-line light scattering photometer. The system
is used at 135C with 1,2~4 trichlorobenzene as mobile
phase. Showdex (Showa-Denko America, Inc.) polystyrene
gel columns ~02, 803 804 and 805 are used. This
technique is discussed in "Liquid Chromatography of
Polymers and Related ~aterials III", J. Cazes editor,
Marcel Dekker, 1981, p. 207, which is incorporated
herein by reference. No corrections for column
spreading are employed; however, dat~ on generally
accepted standards, e.g., National Bureau of Standards
Polyethene 1484 and anionically produced hydrogenated
polyisoprene~ (an alternating ethylene-propylene
copolymer) demonstrate that such corrections on Mw/Mn
or Nz/Mw are less than .05 unit. Mw/Mh is calculated
from an elution time-molecular weight relationship
wherea-~ M~/Mw is evaluated~using the light scattering
photometer. ~ The numerical analyses can be performed
using the commercially available computer so~tware
GPC2, MOLWT2 available from LDC/Milton Roy-Riviera
Beach, Florida. The low molecular weight cut off for
the calculation is 1500-2000. ~
.:
The elastomeric polymer o~ this invention
comprises ethylene-containing elastomerlc polymers that
have been copolymerized with one or more higher alpha
ole~ins and optionally a diene monomer. As applied to
polymers of thi~ invention, the terms 'lelastomeric" or
"elastomer" are defined to mean that when they are
~ .: . :.
o)'v~TlTuTE S13EET
IP~A/US
. .
. . .. .. ..
- . , . ... ... . ~ . .. .. ~ .. - . . ... .... ..
C1 c/~
- 10 - 3PEAlUS 22NOV199t
crosslinked, they are capable of recovering from large
deformations quickly and forcibly. Free from diluents,
the crosslinked polymers retract within one minute to
less than 1.5 times their original lengths after being
stretched at 18C-29C to twice their lengths and held
for one minute before release.
.
Typically elastomers are "sub~tantially
amorphous", and when that term is used to defin~ the
ethylene containing elastomaric polymers o this
invention, it is to be taken to mean having a degree of
crystallinity less than 25~, pre~erably less than about
15%, and more preferably less than about 10% as
measured by means known in the art. The three major
known methods o~ determining crystallinity are based on
specific volume, x-ray diffraction, and infrared
sp~ctroscopy. Another well-established method, based
on measurement of heat content as a function of
temperature through the fusion range, is differential
scanning calorimetry. It is known that these
independent techniques lead to good experimental
agreement.
Additionally, it i5 known in the art that the
tendency of a particular combination o~ catalyst system
and monomers to produce blocky, random, or alterna~-ing
monom~r sequence distribution in the polymer can be
characterized by the product of the reactivity ratios
defined ~or the given monomers under the specific
reaction conditions encountered. If this product is
egual to 1.0~ th~ sequence distribution will be
per~ectly random; the more the product is less than
1.0, the moxe the monomers will approach alternating
sequence; and, the more the product is greater than
1.0, th~ more the monomers will tend to have a blocky
sqquence distribution. Generally speaXing, the
segments of a polymer which crystallize are linear
segments which have a number of identical (both by
, p
'
.
- ., , .' . , , . , . . . I ,' . . ' .
,: . : . ' . ' ' ' ' ' :, , ' : , ' ' ' . ' ' '
, , ' . ' ' ' ' , ' ' '
. ~ ' ' ,.' ~ . . ' ' .'- " ". ' '. ' , ' .' '' ' ~ ',' ':
.. . . .. . . . .
~C~ 5 /~
22NOV1991 7
chemical make-up and stereo-specific orientation) units
in a row. A combination of such segments are said to
yield blocky polymer. If there is little or no such
sequential order within the segments makinq up a
polymer chain, that chain will be very unlikely to
conform itself into the correct shape to ~it into the
spatial ordPr of a crystal and will accordingly exhibit
a low degree of crystallinity. The ethylene-containing
elastomeric polymers of this invention, accordingly,
have a reactivity ratio product less than 2.0,
preferably l~ss than about 1.5, and more preferably
less than about 1.25, and are substantially amorphous.
As alraady noted, copoly~er~ in accordance
with the present invention are compri~ed o~ ethylene
and at least one other copolymerizable alpha-olefin~
Such alpha-olefins include tho~e containing 3 to 18
carbon atoms, e.g., propylene, butene-1, pentene-l,
hexene 1, etc. Alpha-ole~ins of 3 to 6 carbons are
preferred due to economic considerations, and they are
generally pre~ent in the copolymer within the range o~
about 10 to so percent by weight, more pxeferably from
about 15 to about 70 percent by weight most preferably
20 to about 70 percent by wei~ht. The most preferred
copolymers in accordance with the present invention are
those comprised of ethylene and propylene or ethylene,
propylene and a diene.
As i~ well known to those skilled in the art,
copolymers of ethylene and higher alpha-ole~ins such as
propylene often include othQr copolym~rizable monomers.
Typical of these other monomers may be non-con~ugated
dienes such as the following non-limiting examples:
.
a straight chain acyclic dienes such as
1,4-hexadiene; 1,6-octadiene;
b branched chain acyclic dienes such as 5-
me~hyl-l, 4-hexadiene; 3,7-dimethyl~
':
SUBST~TUTE SHEET
IPEA/US
~ ~. ; ' . . ... ,.. ~ . .. , ..... ; .
c ~ q~ s
f ` ~
`- ` IPEA/US 2 2 NOV 1991
6-octadiene; 3j7-dimethyl -1,7-octadiene
and the mixed isomers of dihydro-myrcene
and dihydroocinene:
c single ring alicyclic dienes such as:
1,4-cyclohexadiene: 1,5-cyclooctadiene;
and 1,5-cyclododecadiene;
d multi ring alicyclic fused and bridyed
ring dienes such as: tetrahydroindene;
methyltetrahydroindene;
dicyclopentadiene; bicyclo-(2,2,1)-
hepta2,5-diene: alkenyl, ~lkylidene,
cycloalkenyl and cycloalkylidene
norbornenes such as S-methylene-2-
norbornene (MNB), 5-ethylidene-2-
norbornene (ENB~, 5-propyl-2-norbornene,
5-isopropylidene-2-norbornene, 5-(4-
cyclopentenyl) -2- norborne~e: 5-
cyclohexylidene 2-norbornene.
Of the non~conjugated dienes typically used
I to prepare these copolymers, dienes containing at least
I one of the double bonds in a strained ring are
preferred. The~most preferred diene is 5-ethylidene 2-
norbornene (ENB). The amount of diene (wt. basis) in
I the copolymer may be ~rom about 0% to 20~ with 0% to
i 15% being~preferred~ The most preferred range is 0% to
10%. Where the diene is present, it is generally
present at a min~mum level of about 1 weight percent.
. . . . ..
As already noted, the most preferred
copolymer in accordance with the present invention is
ethylenepropylene or ethylene-propylene-diene. In
e~ther event, the ~average ~ethylene content o~ the
copolymer may be~as low as~abou~ 10% on a weight basis.
The preferred minimum is about ~5%. A more preferred
minim~m is about 30%.~ The maximum ethylene con~ent may
be about 90% on a weight basis. The preferrad maximum
is about 85%, with the most preferred being about 80%.
TUT~ S~IEF~
IPEA/US
; :
.' .. . : ` - :' '.. : '. '. . .: .: ':. . , ~ -. . , `. , ,, . . ~ ' . ' : ' , : ,
pc ~ ~ q ~/~ ~ ' C ,2 ~
~ 13 ~ IP~/US 22NOVl991
A further unexpected characteristic of the
copolymer of this invention is the broad compositional
distribution of the optionally included non-conjugated
diene. As is known in the art, copoly~er having
typically narrow MWD will also be expected to have a
narrow compositional distribution of non-conjugated
diene. The copolymer of this invention containing non~
conjugated diene will thus exhibit a compositional
distribution such that the diene content of at least
about plus or minus 20% of the polymer differs from the
mean value of incorporated non-conjugated diene by at
least plus or minus 0.5 weight percent diene. A
typical curve representing this compositional
distribution appears in the upper portion o~ Figure 1.
The molecular weight of copol~mer made in
accordance with the prçsent invention can vary over a
wide range. The preferred minimum is about lO,OoO
The most preferred minimum is about 20,000. The
maximum weight average molecular weight may be as high
as about 12,000,000~ The preferred maximum is about
1,000,000, with the most prefarred maximum being about
750,000.
Copolymers prepared in accordance with the
present invention exhibit a broad CD with at least
about 25% of the polymer differing from the mean
ethylene content by ~ 5% e~hylene, and a relatively
narrow molecular weight distribution within the range
of from about 2 to 5, evaluated as described above.
Copolymers having the most.superior green strength and
processing properties are those having a CD breadth
ranging from about 40% to about 80% of the polymer
differing from the mean ethylene content by + 5% and
(MW/Mn) of ~rom greater than about 2.0 up to about 4.5.
.
. .
SU~STl~UTE SHEEl~
IPEA/US
~ ` , . ~
.
. ' , ' ~
~G ~ -q o/a ~) o ~ ~
~ 14 ~ IPEA/uS 2 2 NOV1991
As indicated above, the novel copolymers o~
this invention are produced using a catalyst system
comprising:
a) a hydrocarbon soluble, non-supported
vanadium compound with a valence of
three or more;
b) a triorgano aluminum compound: and
c) a specific halogenated organic promoter
used in catalytic amounts.
The vanadium component of the catalyst system
may have the genera~ formulas
':
O
VXaYb or VXcYdr
wherein X is halogen, preferably chlorine, and Y is an
organic substituent selected from the group consisting
of an alcoholate, carboxylate, ketonate or diketonate
having up to 10 carbon atoms, a and b may range from O
to 3 with the proviso that the sum of a and b is 2 or
3, and c and d may range from O to 4 with the proviso
that the sum of c and d is 3 or 4. Preferred vanadium
compounds for the purposes of this invention include:
~OC13,
14, v- E O-C-R~3, and
VO~OR)3/ ¦1
Vocl2(oR)i Cl V-CO C-R]2
wherein R is a hydrocarbon radical preferably having
~rom about l to 10 carbon atoms. R preferably
represents ~an ~ aliphatic, alicycllc or aromatic
hydrocarbon :radical ~such as ethyl (Et) , phenyl,
isopropyl, butyl (Bu) , propyl, n-butyl, i-butyl, t~
butyl, hexyl, cyc}ohexyl, octyl, naphthyl and so forth.
Non limiting and illustrative examples of preferred
iT~ SI~E~
IPtA/lJS
Pc I /~" ~~ q c/C ~~ c ~ ~
15 - IPEA/US 22NOV1991 ~
vanadium compounds are vanadyl tetrahalides and
trihalides, ~lkoxy halides and alkoxides, such as VC14,
VOC13, vocl2 (OEt), vOC12 (OBu), VO (OBu)3 and
vo(oc2Hs)3 The most pref~rred vanadium compounds are
the chlorinated compound~ such as VOC13, VC14 and
vocl2(R)
The triorgano aluminum component of the
catalyst system has the ~ormula AlR3 wherein R is a
hydrocarbon radical having one & to ten carbon atoms as
defined above with respect to the vanadium compounds.
Examples of suitable R groups include methyl, ethyl, i-
butyl, hexyl and phenyl. Preferred compounds are
trialkyl aluminum compounds, including triethyl, tri
isobutyl and tri n-hexyl aluminum. It is important for
the purposes o~ this invention that the aluminum
compound is free o~ halogen, i.e., that aluminum alkyl
halides not be used. Catalytic activity is markedly
reduced using these latter compounds.
The selection of the proper halogenated
polymerization promoter is a key feature of the present
invention. A certaln range of halogen substituent
reactivity toward the catalyst is required to give the
proper balance of ca~alyst activity and properties of
the ~opolymer product. If the reactivity is too low,
catalyst e~ficiency ls reduced, while if it is too
high, undesirable side reac~ions occur which are
deleterious to catalyst performance. Cooper (T.A
Cooper, Journ. ~n. C~m. Soc., 95, 4158 (1973), the
di~alosure o~ which is incorpora~ed herein by
reference, has de~ined in Table 1 an organic halide
activity index based an the ability of the halide to
oxidize VC12 (py)4 to V(III) under standard conditions.
For example, CCl~ is assigned a reactivity of l in
tetrahydrofuran at 200-C.,`ànd other listed halogenated
organic compounds have reactivitie5 of from about 0.02
to greater than 200 relative to CC14.
:
~ SU~S~I~UTE 5HEEI
lp~/us
.... . . . . . ... 1 . `~ . ; ... ` ~ - `
~c // ~ q c/~ c ~ ~
~ 16 IPEA/US 2 2 NOV 1991
It has been found that organic halides as
defined in the above referenced article with a Cooper
Index ranging from about 0.01 up to about 30 are
suitable promoters for the purposes o~ this invention.
Most preferred promoters meeting this criteria are
selected ~rom the group consisting of carbon
tetrachloride, hexachloroethylene, benzyl bromide,
benzyl chloride and 2,3-or 1,3-dichloropropylene.
It is important that ~he vanadium component
of the catalyst system is both not hydrocarbon
insoluble and not supported on an inert or hydrocarbon
insoluble support. Vanadium Catalyst systems that are
hydrocarbon insoluble or deposited on inert supports
are not typically useful for the preparation of
elastomeric copolymers of ethylene according to the
procedures o~ this invention.
The polymerization in accordance with this
invention may be carried out either in ~olution or in
suspension, but solution polymerization is preferred to
avoid problems of reactor fouling. The process may be
carried out as a batch process or a continuous process,
although continuous flow stirred tank reactors are
pre~erred, and at normal atmospheric pressure or under
elevated or reduced pressures. The polymeri2ation may
also be carried out u~ing a series o~ two or more
conkinuou~ flow atirred tank reactors or equivalents
thereof. Normally, pressures of 1-10 atmospher2s are
pre~erred. The polymerization may be aarried out at
temperatures in the range of about 60 to about 140~C,
as well as the more common temperatures within the
range o~ about 10 to 60 Q C. When the polymerization i5
conducted at the higher range o~ about 55C to 140
there is additional process advantage in that the
energy requirements ~or both recovering the polymer
from solution and cooling the reactor during
CU8ST~TUTE S~EET
IPEAtUS
.
,
- ,
.. . . .. . . . ~
~ c ~ q c/~ ~~ c~ ~
~ J ~ h ~ J V !J~ ~
poly~erization are reduced. Preferred polymerization
temperatures for the purposes of this invention lie in
the range of from about 40 to 120C, more pre~erably
from about 55 to 100~C. Solvents used in the process
include one or a mixture of hydrocarbons such as
pentane, hexane, benzene, toluene, xylene, cyclo hexane
and the like. Diluents useful for a suspension process
are propane, butane or a mixture of the liquefied
monomers use~ul in accordance with this invention.
PrePerably, but not necessarily, the solvent will al~o
be a solvent for the vanadium catalyst compound. The
po~lymerization reaction should be conducted in the
absence of oxygen, carbon dioxide, water and other
materials which have a deleterious effect on the
catalyst activity.
The catalyst and halogenated promoter may be
combined prior to contact with the monomers, or dilute
solutions of these components may be introduced
separately into the reactor. It is pre~erred ~or the
purposes o~ this invention that the vanadium catalyst
and alkyl aluminum are introduced separately into the
reactor and allowed to react to form the active
catalyst in the presence of the monomers, since
catalyst activity may suffer if the catalyst components
are prem~xed. Also, it is preferable not to premix the
promoter and alkyl aluminum since undesirable side
xeactions might occur.
. .
Polymer molecular weight may be controlled by
the introduction of known chain trans~er agents such as
hydrogen gas or diethyl zinc. In general, the quantity
o~ chain trans~er agent in~roduced into the reactor
ranges ~rom about 0.1 to about 100 moles per mole of
vanadium catalyst. In some cases it may also be
desirable to add known chain branching suppressors,
including Lewis Bases such as NH3, pyridl~e and
Si(oEt)4 to the reactor along with the catalyst
~'..
.;~,r-i3~^~
. :
.. . .. . . . . .. .. .. . .. . . .
; . ., : ~ . . .- .. . . :
.,, : . . : :-. . ~ ., . . .. . :,. ~ .. . ,, . , . , -, . ., . ... ,,, ,, .. ,. .... . .. , . . . : .
~c ~ -q c~ c ~ S
~ ~<- 18 ~lp ~/~S 22NOV1991
components. The preferred molar ratio of such
suppressors ranges from about 1:2 to 10:1 with respect
to the quantity of the vanadium catalyst employed.
The average residence time o~ the reactants
in the reactor generally ranges from about 5 minutes to
about 2 hours or more.
Following polymeri2ation, the polymer product
can be conventionally recovered from the effluent by
coagulation with a nonsolvent such as isopropyl alcohol
or ~-butyl alcohol, acetone, or the polymer can be
recovered by stripping the solvent with heat or steam.
An antioxidant can be incorporated in the polymer
during the recovery procedure, such as phenyl-beta-
naphthylamine, di-tert-butylhydroquinone, triphenyl
phosphite, heptylated diphenylamine, 2,2'-methylene-
bis(4-methyl-6-tert-butyl)phenol, and 2,2,4-trimethyl-
5-phenyl-1,2-dihydroquinoline.
The amount of the vanadium catalyst employed
in the present invention is relatively low as compared
~I with prior art processes not employing a promoter. In
qeneral, the amount of vanadium catalyst ranges from
about 0.02 to about 0.5 millimoles per liter of solvent
solution, with~ levels of from about 0.05 to about 0.5
millimoles being most preferred.
- For best catalytic per~ormance, tha molar
amounts of vanadium catalyst and aluminum compound
added to the reaction medium should provide a molar
ratio of aluminum to vana~ium ~Al/V) o~ at least about
10 and not greater than about 250. Pre~erred such
ratio~ rang~ from about 15 to 50. The amount of
b ,halogenated~organic~promoter used with respect to the
vanadium oompound may generally range in the promoter/V
molar ratio of ~etween about 5 to about 250. It is
; desirable that the promo~er/V ratio be not
'. :
: SU8STI~UTE SHEEI
lPEA/U~
. .. .. . . .~ .. . .. .. , , , . ~ , . . . . .. . . .. .. ... .
.: . -:` ., :.-. , .... . - . : .: : . ~ . . . .... . ..
,, . ,, ~ . . .. .. . . ...
.. . . - .. . . . . . : . . . . : ... .
9 IPEA/US 22NOVl991
substantially higher than the Al/V ratio, and preferred
ratios range from about 5 to about 50.
This invention is further illustrated by the
following examples. In Examples 1-14, polymerizations
were conducted in a 1 liter volume continuous flow,
stirred tank reactor. Feeds to the reactor were
purified to remove water and other polar impurities
that could act as catalyst poisons. Ethylene and
propylene were metered through calibrated rotameters
into a stream o~ hexane solvent which was pumped at a
known rate with a metering pump. This mixture then
entered a heat exchanger which lowered the temperature
to about -20 to -10C to prechill the feed prior to
reaction and to dissolve the monomers in the hexane.
The cold stream leaving the heat exchanger then entered
the reactor. Catalyst, cocatalyst, promoter, and diene
monomer were prepared as dilute solutions in hexane and
each was pumped separately into the reactor via
metering pumps. H2 was also added to the reactor to
control polymer molecular weight, and in some cases NH3
was added to suppress any tendency for long chain
branchlng. Temperature in the reactor was controlled by
circulating water through a jacket. Iso-propyl alcohol
was added to the polymer solution exiting the reactor
to terminate poly~erization, and the solution was added
to boilinq water to remove solvent and monomers. The
wet polymer was then dried on a hot rubber mill to
yield the final product. Polymerization rate was
measured by determining the weight of polymer produced
in a fixed perlod of time.
Polymer ethylene content was determined by
ASTM D3900. ~Ethylidene norbornene content was measured
by infrared based on the haight of the 1690 cm~1 band.
Mooney viscosity~ was determined by AS~M D1646. The
compositional distribution (CD) and molecular weight
, ,'
.
: , .
ST'IllT SllE~
IPEA/US
.
l ' )-q ~ s G ;~ ~
~ - 20 - IPEA/U~; 2 2 Nav 199~ -
distribution (MWD) of the polymer were determined as
described above.
Specifically, the CD was determined by
cutting a,sample of the finished copolymer into small
pieces and adding the pieces to hexane to give a
concentration of about lg/lOOcc. This mixture was then
stirred gently at 22~C for 48 hours, or until
equilibrium is reached. The mixture i5 then poured
through a fine me~h stainless steel screen to recover
any insolubLe polymer, which is dried, weighed and
analyzed for composition. Isopropanol is then slowly
added to the solution until precipitated polymer first
appears. This polymer is recovered on a screen, dried,
weighed and also analyzed for composition. Additional
isopropanol is adde~ incrementally to the remaining
solution to precipitate four to six fractions in total,
all of which are recovered as described previously. The
final isopropanol~hexane solution is then evaporated to
dryness to yield a final polymer fraction. From the
weight o~ each fraction and its ethylene and termonomer
(i~ any~ composition, a plot is prepared of weight
percent composition vs cumulative weight percent
polymer as described above and as illustrated in Figure
1. '
Exam~le 1
A polymerization was c~nducted by the process
de~crlbed ahove with a VC14- triethyl aluminu~ (TEA)
catalyst system with CC14 as a promoter at a
temperature of 75 C. NH3 was also added to the
reactor at a NH3/V molar ratio of 1:1. Hexane feed
rate was 2500 g/hr. All other polymerization
conditions are given in rrable 1. The po~ymerization
went smoothly giving high monomer conversion and high
catalyst activity.
:
SUBSTITUTE SHEE~
IPEA/US , "
:. .
.
. . . :
. .. - .. .. . ` .. . ` . . . . .. ..... . . . . . . . . . . . . . .
C ~ Gjo ~
21 IPEA/US 2 2 NOV 1991
Polymer fract}onation by the process
described above gave the cumulative compositian curve
shown in Figure 1. Polymer species were pr~sent that
ranged from at least 65.5 wt~ ethylene to at least 35.5
: wt% ethylene. From this figure it can be determined
that about 44% the polymer had an ethylene content 5%
hi~her than the mean of 5Q%, while about 41% of the
; polymer had an ethylene content 5% less than the mean.
Thus, the CD of this polymer is 85%. Mw/Mn for this
polymer was 3Ø
Example 2
This example illustrates the use of the VOCl3/TEA
catalyst system with CC14 promoter at a polymerization
temperature of 75C. NH3 was added to the reactor at
NH3/V molar ratio of 1:1. Hexane flow rate was 2500
g/hr. As shown in Table l., catalyst activity was high
and monomer conversions were good. About 65% of the
polymer has an ethylene content +5% greater than th~
I . mean value. Mw/Mn for this pol~mer was 3.8.
¦ Example 3
I
Polymeriza~ion was attempted with a VOCl3/
diethylaluminum chloride ~DEAC) catalyst system at 75~C
accord~ng to the conditions in Table 1 with a hexane
~eed of 2500 g/hr. Very poor polymerization resulted
giving large amount~ o~ insoluble polymer and low
polymerizatlon rate~. The DEAC feed was replaced by an
equal molar feed of TE~. Polymerization rates began to
improv and the insoluble polymer disappeared. After
allowing th~ reactor to reach steady stat~, high
polymerization rat~ and good monomer conversion was
measured as shown in Table 1.
. . , ' ' ,
,','.,.
~IJCi~ S~!E~
~PEA~US
.
; -- 22 -'~~' J~ 0~
J
Exam~le 4
This run illustrates the use of benzyl
chloride as a promoter with the VC14/TEA catalyst
system. Benzyl chloride has a cooper reactivity index
of .0S. An NH3jV molar ratio of 1:1 was used and the
hexane flow rate was 2500 g/hr. The composition
distributlon of the polymer was such that 64 wt% of the
polymer has an ethylene content + 5~ greater than the
mean value. Mw/Mn for the polymer is 3.1.
Example 5 (A.B.C.D~
This example illustrates the use of various
promoters with the VOC13/TEA catalyst system at 75C
polymerization temperature. The hexane feed rate was
2500 g/hr and the other polymerization conditions are
as shown in Table 1. The promoters used are indicated
below:
Cooper
Example Promoter Reactivitv Index
5A Benzyl bromide .1
5B 2,3 Dicholoropropylene .02
5C 1,3 Dichloropropylene .02
5D Hexachloroethylene 5
A~ shown by the results in Table l all of
these promoters gave good catalyst activity and monomer
conversion.
.
,
:~
~: . . . . .. .. : . .. ~ . .. . . . . .. , , ~
p C i/U ~- q C~
~ 23 _ 3P ~/~S 22NOV~91
Comparative ExamPle 6
An attempt was made to conduct a -
pol~merization with the VOC13-TEA catalyst system and
benzoyl chloride as the
' '"
SU~STlTUTE SHEE~
IPEA~US
/~C ~ q Q/~ G ~ ~
~: ~`i IP~A/US 2 2 NoV~991
__ o ~ T ELE L Irl . ¦~ _ _ o o l
-"u ' U- ~ ~ l .- . . .. _ ~ ql ~
. ~v~ c~ o ~ t ~ ~ ,. ~ I~ID . ~ ~
__ ._ ~ ~ _ _ _ _ __ ~ _
o 26 r~ I ~ I l l l l l ~
vOI '~, ~. ~, ~n, o. ~, ~. o. _ r~ ~, ~,
3~ . U~ ~ ~D ~ U~ U~ ~. ff
_ .. .~ . _ _ _ _ _ _ _ _
ZJ~ . P n _ __ I _ _ _ r~ ,
__ ~ '~ ~ ~ ~ ~'I d _ ~'q ~ ~ .~ .
.' . tJ U !i ~ ~ u~ ~ n u- ~ u~ 1 ~
_ _ _ _ _ _ _ _ _ I_ _
rl r~ ~ .~ ~ ~ ~ r~ O ~D .~ . '
~ ~ r~ ~ ~ ~ ~ ~> ~ o o
.: ~ qoww/a~ ~ ~r ~ ~ ~ r~ ~ ~ r~ ~ ~ ~ . , .
_ _ . ... r~ ~D n _ _ 2~--o _ ~ ~ _
~'IO~O~ ~0~/~ ~ _, ~ ~ o~ ~ _, ~ ~ ~o '.''
_ _ _ _ _ _ _ _ _ _ _ _ _
, ~ OWli~ ff ~ _~ O 1~ ~ 1~ r e) o o
R ~ _l ~ ~ ~ ~ ~ ~ . .
_ _ _. _ . _ _ . _ _ _
OS;I~Y~ o o r~ t~ o o o ~ ~ o ~ -.:
;!W~O~I ~lr~i .~ rl ~ ~o rl I ~ . .
~1 _ _ __ _ _ 1~, ._ _ _ ~l. _ __ Ir~ a- ..
.; 0~ Yq'O ~ O _ ~ O O O ~ 01 d ~
~/~O~O~OUd
~ :_ __ _
:1 . ----~ ~ i ~~ ~I ~ O n 1~ o _ r~ ~1
~IATCIYM' . . . . . _ . _ _ .
. . a/~ 2 ~ 0 n O O O O O
. Q _ _ _ __ _ _ _ _ _ __ _
. ~ a~do~ ~ 3 o ~ 3 ~ u~ o~ m In ~n
~ .. ~ ~ _ _ C ~ _ _ _ ~ _ _
: 31~ Haa In 1 ~ 1~1 ~1 U~ 1~1 111 P In Itl
. _ . ._ _ _ _ _ _ _ _ ~_ _ _ _
. . SNIW ~S5 5~1 d ~ ~ ~ ~ ~1 C~
.. ~ .,,. _ _ _ _ ~ _ _ _ ._ _ .~ .
: ~ ~3S ~n r. ~ ~ #~ ~ U~ h~ :~ U- n ~ . .
'~X3 ~ . ~ i e ~ It~ ~ e D U ~ ~ ,
. ,,: :
.'
, ~
.
SU8STITUT~ S~EE~ :
PEA/US
.~ , . ~ -
.
Pc ~ q ~/G ~- ~ ~
~ 25 - IP~/US 22NOV1991 J
amounts of in~oluble polymer were produced and steady
state operating data could not be obtained.
Comparative Example 7 ~A, B, C)
In this example, trichlorotoluene was used as
a promoter with the VOCl3-TEA catalyst system.
TrichlorotoIuene has a Coopex Index of 40.
Polymerization conditions and result~ are shown in
Table 1. Hexane feed .rate was 2500 g/hr, In the
copol~meri%ation runs, 7A and 7~, catalyst activity was
very high. GPC analysis of the copolymer produced in
run 7B indicated a broad, bimodal MWD, with a low MW
mode shifted to a Mn of 251 and a high MW mode with an
Mn of 31,000. Mw/Mn ~or polymer 7B was 80.
Introduction of ENB into the polymerization in run 7C,
which is at conditions otherwise similar to 7A, caused
catalyst activity and monomer conversion to drop
substantially re}ative. to 7A. GPC analysis of this
polymer showed a single.broad peak and Mw/Mn was 8Ø
The compositional distribution for the polymer mode in
Example 7C is broad with 37% of the polymer greater
than.~ 5~ ethylene of the mean~
:
; ExamQle 8 fA.B.C,D~ ~
.
A series o~ terpolymerization runs were
carried out with the VOCl3-triisobutyl aluminum (TIBA~ .
catalyst system at di~erent CCl4/V and Al/V ratios. A ..
NH3/V ratio o~ 1.0 wa~ usQd and the hexane ~eed rate
was 2500 g/hr. As shown by the result in Table 2,
catalyst` activity wa~ good over the entire range of
conditions tested. ~ -
: ~ . ...
':
; ~ . ; ' '
~ ,? t ~ rr ~
~ ,'F~
: : ~
~C /~ qG/C~ G~ ~
~26 - ~PEA/U~ 2 2 NOV 1991
~ Example 9
Vanadium tris~hexanoate was prepared by
reacting VC13 with kexanoic acid. A terpolymerization
was carried out with this catalyst and TEA cocatalyst
with CC14 as the promoter. Polymerization results are
shown in Table 2. H~xane feed rate was 3030 g/hr and a
NH3/V ratio of 0.65 was used. The compositional
distribution was broad with 77% of the pol~mer greater
than +5 wt% ethylene from the mean. Mw/Mn was 4.3.
Exam~le 10 (A.B.C)
This run was made with varying levels of CC14 promoter
and the VC14-TEA catalyst system. The hexane flow rate
was 2~00 g/hr and the NH3/V ratio was 1.}. In examples
lOA through lOC in Table 2, the CC14/V ratio was
reduced from 20/1 to 1.67/1. ~s shown by the results
in the Table, polymerization rates are reduced
consid~rably when insufficient CC14 is present and
Mw/Mn goes up as CC14/V is reduced. The compositional
distributions for polymers lOB and lOC are broad. For
polymer lOB, 90% of the polymer has a composition
greater than +5 wt% ethylene from the mean, while for
polymer lOC, 87% o~ the polymer differs from the mean
by great2r than +5 wt~ ethylene.
. .":
~21~ ,
.'..
This run was made to demonstrate
¦polymerization with the VC14/trinormal hexyl aluminum
catalyst sy~tem and CC14 promoter. Hexane ~eed rate
was 3030 ~/hr. As shown by the results in Table 2,
high cataly~t activity was obtained. The polymer had
an Mw/~n value o~ 4.5 and the CD was broad with 79% of
. ..
.
S~STIIUT~ SHEEl
IPEAIU~:
f. - 27 - 22NOV1991
the polymer differing from the mean ethylene content by
greater than 5 wt~ ethylene.
Example 12_ ~A.B.C.D~
.
This run was made to investigate the effect
of NH3 on the polymerization with the VCl 4/TEA
catalyst system and CC14 as a promoter. Hexane ~eed
rate was 2500 g/hr. In runs A through D, increasing
amounts of NH3 were added to the reactor. After each
change in NH3 feed, the reactor was allowed to reach
steady state and a sample was tak2n to determine
polymerization rate and polymex properties. As shown
by the results in Table 2, NH3 has no effect on polymer
composition or Mooney viscosity until a level of 13.6 :~:
mole/mole V was reached in run D at which point a
decrease in catalyst activity occurred. Mw/Mn stayed
constant at about 3.4 for this series of runs. 70% of
the polymer differed from the mean ethylene content by .. .
at least +5 wt% ethylene in Run C, and 66% of the
polymer differed from the mean ethylene content by at
least i5 wt% ethylene in Run D. The lack of effect of
NH3, a long chain branching suppressor, on Mooney
viscosity or MW/Mn indioates that the catalyst system
o~ this invention produces polymers with a low
ranching lev~l. .
: , .
' '
Example 13
Vanadium chloride bis hexanoate was prepared
by the reaction of VC13 with 2 moles of hexanoic acid.
A~polymerizat~ion was~carried out with this catalyst and
~EA coca~alyst with ~C14 as the promoter. As shown by
the result~ in Table 2, catalyst activity was good.
The polymer had~an Mw/Mn of 4.0 and 72% of the polymer
IJ' S I iT~JTE SHEE~
IPEA/US
. .... ... .... .. .. . . . . . , ~ . .
~ l/u~ q~/c~
NO\/~'
_ 28 --
T~BLE 2
~ ~, ~ ~
- I __ _ _ _ _ _ _
C ~ _ ~ ~ _ ~ _ ll _ l ' _ ~ ~ l
~o ~n ~ fl ~ ~ _ ~. ;) i` ~g ~ ~ ~ ~. .,
o~ ~ v ~ ~ ~ ~ r~ q ~- r~ ~ ~1 1 ~
_ r ~ ~ O Q ~ ~ 1~ O ~ 'O ........ !`
Q ~ ~ ~ lo ~ ~ ~n Y ~ ~ ~ In o o- ~n ~
2 ~ ~ 1- ~ ~ r~ o ~ o~ r- o~ I _ _ 7
Y ~ ~ 1 ~ r~ ~ ~ ~ ~ 1~ ~ 1! ~_~ U~
C~ ' _ _ _ __ _ _ _ _ _ _ _ _ _ _
3~ ~ ~ ~ ~ _ ~ .~ 1~ ~ ~ _ r~ ~ ~ ~
8 ~ ~ ~ ~ ~ ~ ~ ~ ~ n ~ c r o
~ ~ r~ lo ~ o 1~ I ~ ~, o _ o~ _ ,.
__ _ ~ _ . _ _ _ _ _ _
10~ q ~ O ~ ~ 1~ ~ ~ o~ ~ a ~ O
~1~# ~ ~ t- r 1~ O ¦ O _ o r o O
a~ a ., n ~ ~ ~ _ I ~ ~ _ ~ ~ _ r~
_ _ _ _ _ _ _ _
YrSO~OA.~ ell ~ ~ _ O a ~ ~ O r ~ e~ ~ o
OS W wqC~ Il~ ~ ~ O O n ~ O ~ ~ ~ ~ o .. ..
. ___ _ _ _ _ . _ _ _ _ _
1 r~ n ~ o o o o a ~- .- ,.
. ~Ul; ~YY5t~ n ~ n n o, n n ~ ~ ~ , ~ ~ ~
. YIIJ6 _ _ ~ _ _ _ _ _ . _
~ ~ ~ " ~. ~ ~ a~ ~ _ ~ ~111 ~ D ~1
e~ ' _ __ _ __ _ _ _ _ _ _ _ .
- ~ a~o~a ~0 c 3 n 0 ~ cl e~ a n ~ n ~o ~
bl ' ~_ _ _ _ _ _ _ _, . _ _ _ _
_ YIV 5 n r n n o n ~ n o n ~ ~ ~ o~
~ISS~ Y ~ ~ ' ~ ~ r~ ~ ~ ~ ~ ~ ~ .~ . ~ ~ ; ' , . . . _ _ _ _ _ ~ _ . _ _ _ _ .
. ~0 ~S ~ ~n d~ n n I~ ~ r n ~ ~ ~ ~ ~n _
~ __ _ _ I_ _ ~0 ~01 ~ rl ~C ~ ~ Q ~ o
:
SUBSTITUTE SHEE~
IPEA!US
.~
- . : . .. . ... . .. . ~ ~ ... .. . .... .
r-~ p C / /~ ) q O /~
`'`3 - 29 IP~ 22NOV1991 J
Example 14 (A,B.C)
This run was made to investigate the effect
of reaction temperature on ethylene-propylene
copolymerization with the VOC13/TEA catalyst system and
CC14 as promoter. Hexane feed rate was 2500g/hr.
Other reaction conditions are shown in Table 3. As
indicated by the results in this Table, catalyst
activity remains almost constant at polymerization
temperatures from 65 to 97C.
; Example 15 fA,B,C)
This run was made to investigate the effect
of reaction temperature on ENB terpolymerization with
VOC13/TEA catalyst and CC14 promoter. ~exane fPed rate
was 2500 gjhr and other conditions are shown in Table
; 3. The results in Table 3 indicate that catalyst
performance was una~fected by temperature over the
i range o~ 55 to 75~C.
~ll Exam~ 16 (A.B~
. .
The polymerization procedure described in
Example 15 was used except that the polymerization was
carried out in two 7.6 liter volume stirred tank
reactor~ connected in series. Cataly~t, solvent and
mono~ers were fed to the first reactor, and the pr~duct
stream entered the second raac~or to whlch additional
monomers dissolved in hexane solvent were added~
Poly~erization~conditions are given in Table 3. The
catalyst system was VC14jTEA with CCL4 as promoter.
Hexane ~eed to the two reaotors was 29.9 and 4.45 kg/hr
respectively. Runs A and B are es~entially similar
except that additional ethylene was fed to the second
reactor in Example 16B.
.'' ' ' ' ' '
'''.,"S,I~IJ~E SHEFI
~, IpF,Q./I~
.. .. . . . . ... . . . '
- . : .
~ , - , : :.:
~C l/c/S-q/~ Q
-- 30 --
IPEA/US 2 2 NOYl991 i
o U ~ _ _ rABL ' 3 . _ _ _: . . .
S ~ ~ ~ r~ ~ ~ ~ e~ al eo
~_ _ ,, _ _ _ _ _
o 8 ~ I l l ~ ~ o r ~
~ ~ - .~. _ _ _ __ ~ _
o
~ ~ ~ ~ ~D a~ O O O O ~r ~Ir ~ ~
8~ ~ ~ ~ u~ u~ u~ ~ u~ ~ u~ u~
. . _ _ _ _ , ~ . _
~ l l ~ ~D ~ ~ ~ ~ ~ D~
Z ! ! l ~ ~_ ~ ~ ~ . .~ r~
O U~l'
~:~ cl:l cn ,~ w ~o ~ ~o ~9 ~o r~
~:~~r ~ ~r 1~ ~ t~ r~ ~lr r~ \1-
~ l_ __ _ _ ._ _
8 !u ~ N O O O O~1 r u~
~ r~ ~ O ~ ~ ~ ~ ~ C~
oww/a~ ~ ~ ~ O 0 ~D ~ I~ ~ ~ r
;LYa ~ ~- ~r ~ ~ ~ ~~r r~ In ., ' .
~ ~ ~ ~ O 0~ O ~r ~O
W~qO-10;~ ~0 :a.l;Y~I _~ _l _ _~ ,~ ~ u~ c In
, ~ _ . .............. _ _ _ .. _
XH~'IOWW ~ r~ ~ r' ~` r~
a~ Z~ ., ~ r. ~ ~. ~ O O O O
.- ~ _ _ _ _
OI~lnI o o o o o o o , o
~Y-iO~/~
OI~ 2~IOW ,~ _ _~ r~ ~ o t o l
.Ow~a .,
~1~ 'ID~' ~ o o o o o o o _ o
l;S~ . ~. ~. . . ~ ~ ~ r`
WnIaNYA . . . . . . . .
,_ _ _ _ __ . _ I
r~ a~ P~ o~ ~,
~N~ o o o r` ~_ r` o. o~
__ ._ _ _ _ _ _ _
` ~'6 o o o o o o ~ o ~ o
N~ ao~la u~ ~ ~ o O O ~ _ O _
UH/ 0 . ~ ~ u~
~tN: I'IX~3 1~ r~ 1~ ,~ ,~ ~ o o o
,, _ ,_ __ ___ _ _~ __ -'_ .~ .,
SNIW ~tI~ S:~ . ~-r ~ ~ ~ '1 ,~ ~ ,~
. . .__ , _ . _ . _
aO a~ In ~ ~ ~ U~ In ~D U~ 10 U . ~ .
~ -- ------ ~----- ~-
~ e ~ r~ ~ 6~ t~ ~ ~ ~ 3 ~~~ ~ ~
~ ,
SU~S~I~ul~ SHEE~
IpEAlus ;
~r f c 1/(~ /O ~~C ~
31 Lp~A/us 2 2 NOV l99~ -
The results of the polymerization are also shown in
Table 3. In this Table, the conversions, catalyst
efficiency~ polymerization rate, and polymer
composition shown for reactor 2 are the cumulative
results for operating both reactors in series. As
indicated by the data, series reactor operation gives
increased catalyst efficiency and ethylene and
propylene conversion.
Reasonable variations or modifications of
this invention can be made or followed, in view of the
foregoing, without d~parting from the spirit or scope
thereof.
~I'.,S, ) ~ U','c SHE
pF,4illS
-: .
.
. . ., ; : ,
,