Note: Descriptions are shown in the official language in which they were submitted.
9 l ~(~3--- I Pcr/US90/051 3~)
PRODUCIlON OF GROWTH HORMONE IN l~ANSGENIC ANIMAL MILK
Background of the Invention
S This generally relates to the production of growth honnone
in the milk of transgenic mammals.
Human growth hormone (hGH) is one member of the
cascade of hormones responsible for normal growth in vertebrates.
The cascade is initiated when, in response to neurological stimulation,
the hypothalmus is induced to release either a positive growth factor
called growth hormone releasing factor (GHRF~, or a negative factor,
called somatostatin. GH~F stimulates the pituitary to release growth
hormone (GH), which in turn acts on the liver to produce insulin-like
growth &ctor I. This in turn binds to receptors on the cells of
peripheral tissue to modulate growth. Somatostatin acts on the
pituitary to inhibit release of growth horomone.
In normal humans this cascade effectively modulates
growth during childhood, usually resulting in adults of normal stature.
However, there at least are two cases in which nonnal statures are not
attained. In one case, the short children are deficient in endogenous
GH, probably as a result of some genetic defect. Administration of
exogenous GH is effective in overcoming this deficiency in most of
these individuals. In the other case, children of short stature have
normal levels of endogenous GH, and thus are probably somewhat
resistant to the effects of exogenous GH. Although one might expect
treatment in-volving adrninistering exogenous hGH to be useless in
these individuals, a study done at Emory University, reported by
Shiner, G., Research Resources Reporter, U.S. Dept. Health and
Human Selvices, vol. IV, pg 1-5 (1980), demonstrated that about 30~o
of these children are responsive to exogenous hGH treatment. After
this study was conducted, Rudman, et al, reported in Journal of
Clinical Endocrinology and Metabolism, 49, 92-99 (1979), that the
endogenous GH in the subset of short stature children who were
responsive to the exogenous GH was defective in its ability to bind
GH receptors. This study effectively enlarged the population of short
stature children who could be helped by hGH treatment.
,. ,- : . .
. ' . ~
~ 91/03~1 PCr/VS90/OS130
Z~ 9 -2-
Besides its use as a treatment for short stature in some
children, new evidence has emerged which suggests a role for GH in
imrnunoregulation. Kelsey, et al, reported in N~usleic Acids Research
15, 1459-1474 (1987), that GH can stimulate macrophages to produce
5 more than double the normal amounts of superoxide anion (o2-~ in
rats. Superox~de anion is one of the intermediates responsible for
intracellular killing of pathogenic rnicrobes by macrophages, a function
that is also carried out by interferons. Since macrophages are central
to the induction and expression of many immune responses, the
10 discovery that GH acts on these macrophages in this way could lead
to the discovery of other important macrophage activating properties
of GH.
Other potential clinical applications of hGH include use in
enhanced healing of wounds, cartilage darnage and fractures, and
15 treatment of burn trauma, stress ulcers, hypercholesterolerrua and
osteoporosis.
Because growth horrnone is species specific, hGH has been
available only in limited quantities as a purification product from the
pituitaries of human cadavers. Although the recent cloning and the
20 expression of this cloned gene in bacteria has increased availability of
hGH, as first reported by Martial, et al., Science 205, 602-606 (1979),
the relatively low yield and purification difficulties have caused the
price of hGH treatment to be between $8,000 and $30,000 per patient
per year. Clearly a cheaper, more efficient way o-f producing hGH
25 with higher yield would be beneficial both for patients and for use in
the initiation of new studies to test for additional properties of hGH.
A proposed alternative method of production of GH is
through expression in transgenic animals. Unfortunately, expression of ;the hormone in transgenic animals incorporating the gene for growth
30 hormone has had a number of unexpected side effects. Por example,
in pigs containing the gene for bGH in combination with an inducible
metallothionein promoter, as described by Ramabhadran, et al., in
. - . - . .. . . . . ~ .
.... - ~ ., ~ , : .
., .- ~ . - ,: ~ .
-. ~ ~ . -
. . . : , . .
. .
- Pcr/ussn/0s
r 2
3; ~ ~
Gene 38, 111-118 (1985), the animals suffered from severe early onset
rheumatoid arthritis. Transgenic mice having the gene for hS:~H fused
with mouse metallothionein I promoter were infertile, as reported by
~ Bartke, et al., J,Experimental Zoo. 248, 121-124 (1988). See also
Kyung-Kwang, et al., Korean J. Anim. Sci. 31(3), 139-147 (1989).
One way to avoid the systernic effects and increase
purification yield is to create transgenic animals incorporating the
gene for GH in combination with a tissue specific promoter, for
example, a cost effective alternative to production of recombinant
hGH in bacteria would be its production in the rnilk of transger~ic
farm animals. By attaching the gene of interest to a tissue specific
promoter for a highly expressed gene product, one can achieve specific
expression of the gene of interest in tissues appropriate to the
regulatory sequences. Some of the methodologies for making tissue
specific sequences, and the problems associated with it, such as the
lack of correlation between expression in cell culture in vitro and in
vivo expression and the effect of regulatory proteins normally
expressed by the targeted tissues, are discussed by S.~ Camper in
Biotechniques 5(7), 638, 641-643 (1987).
Despite the problems, the production of foreign proteins in
transgenic animals is an attractive alternative to bacterial or tissue
culture fermentation as a means of producing large arnounts of
recombinant proteins. Successes have been reported, including the
production of human alpha-1-anti-trypsin in mouse and sheep serum
by Kelsey, et al. (1987), as well as the production of sheep beta-
lactoglobulin and human t-PA in mouse milk by Simons, et al., Nature
328, 53~533 (1987) and ~ordon, et al., Biotechnolo~y 5, 1183-1187
(1987). Some proteins are present in milk at concentrations as high
as 16 grarns per liter, as reported by Clark, et al., Trends in
Biotechnology, 5, 2~24 (1987).
It is impossible to predict whether it is possible to minuc
these high levels by placing the hGH gene under control regions for
.
~'~ PCr/US90/05130
4-
2~
milk proteins which are selectively expressed in mammary tissues.
However, even at 10% efficiency the expression levels could be as
high as 1.6 grarns per liter, which is significantly higher than current
production levels in either bacteria or marnrnalian cell systerns.
S It is therefore an object of the present invention to provide
transgenic animals capable of tissue specific expression of grouth
hormone, especially human growth hormone.
It is a further object of the invention to provide transgenic
animals which stably transmit the gene for expression of growth
hormone in their milk.
It is a still further obect of the invention to provide vectors
and regulatory sequences for expression of growth hormone, especially
human growth hormone, for use in creating transgenic ar~imals capable
of tissue specific expression of the growth ho~none.
Summary of the Invention
DNA coding for human growth hormone (hGH) was linked
to mouse whey acid protein promoter fragment and microinjected into
fertilized mouse ova. Females of the resulting transgenic rnice were
mated. After completion of gestation and birth of the litter, the milk
from the mothers was assayed and found to contain hGH protein.
Brief Description of ~he Drawings
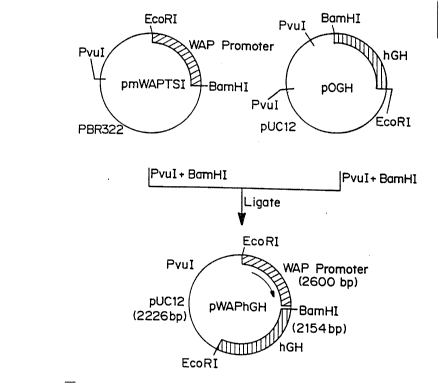
Figure 1 is a schematic of the construction of the
pWAPhGH fusion vector, containing the WAP tissue specific
promoter in combination with the gene for hGh.
Detailed Description of the Invention
The construction of transgenic mice expressing human
growth hormone in their mammary glands which can be isolated and
purified for use as a pharrnaceutical is described in detail below can,
with rninor variations, be used to incorporate the same genes and
~- . , . :
.. . , , . ' - .: -
: , :
., ,., . :
: .
.: : : . . ~. .. ...
:
~o 9l/03~ Pcr/ussn/o5l3o
~'
tissue specific promoters into animals of others species, such as rats,
rabbits, pigs, sheep, and cows, for expression and purification of
human growth hormone. Similarly, genes for growth horrnones of
other origin, such as bovine or porcine growth hormone, can be
5 incorporated into similar vectors and inserted into the genome of the
desired species.
The production of the growth hormone in the transgenic
animals has a number of advantages, including normal glycosylation
and absence of bacterial contarninants, unlike recombinant growth
lû hormone produced by bacterial fermentation processes.
Experimental Design ~nd Methods:
Vector Construction:
pmWAPI`SI, containing EcoRI-BarnHI fragment of mouse
15 WAP promoter obtained from Dr. Lothar Henr~ighausen and
described by Pittices, et al., in Proc.Natl,Acad.Sçi, 85, 5874-5878
(1988), was cut with ~I and BarnHI and ligated to pOGH cleaved
with uI and BamHI. pOGH contains the DNA sequences coding
for hGH and its polyadenylation signal. The resulting plasmid
20 pWAPhGH was isolated after transformation into E. coli using the
method of Maniatis, et al., Molecular Cloning: A Laboratory Manual
(Cold Spring Harborj NY 1982) and screening with appropriate
en~ymes such as EcoRI, BarnHI, SmaI, SphI, and XhoI. The results
are shown in Figure 1.
Preparation of DNA for mieroinJection:
pWAPhGH was digested with EcoRI and the 4754 bp
~agment containing the WAPhGH fusion gene was isolated on l~o -
agarose gel followed by electroelution in a dialysis bag, as described
30 by Maniatis, et al. (1982). The eluted DNA was precipitated,
redissolved in water and puri~led by passing through an elutip-D
column as per the instructions of the manufacturer (Schleicher and
.: . ` : '
~VO(?1/~ 1 I'Cr/US90/05130
-6-
Schuell, Inc., Keene, NH). The purified DNA was dissolved in SmM
Tris (pH 7.4) and 0.1 mM EDTA at 3 ~g/ml concentration for
microinjection.
S ~nimals and emblyos:
Mice were obtained from Charles River Laboratories,
Boston, MA and Jackson Laboratories, Maine. Reagents such as
bovine serum alburr~in, gelatin, and pronase were obtained from Sigma
Chen~ical Co., St. Louis, MO. Hormones for superovulation, PMS and
hCG, were obtained from Organon, Inc., NJ. Hyaluronidase was
purchased from Sigma. Restriction enzymes were obtained from
Biolabs, Bever]y, MA The micromanipulator made by Nara Shige,
USA, Inc., Rairun Instruments Co., Woburn, MA, was used to
rnicroinject DNA into the pronuciei. DMEM, ~etal bovine serurn, and
DPBS were supplied by GIBCO Laboratories, Gaithersville, MD.
Procedures for embryo manipulation and rnicroinjection are
described in "Manipulating the Mouse Emb3~o" by B. Hogan, F.
Costantini and E. Lacy (Cold Spring Harbor Laboratory, 1986). - -
Mouse zygotes were collected from six week old females that have
been superovulated with pregnant mares serum (PMS) follwed 48
hours later with human chorionic gonadotropin. PAmed females were
placed with males and checked for vaginal plugs on the follow~ng - -
morning. Pseudopregnant females were selected for estrus, placed
with proven sterile vasectomized males and used as recipients.
Zygotes were collected and cumulus cells removed by treatment with ~;
hyaluronidase (1 mg/ml).
Pronuclear embryos were recovered from B6D2 female
rnice mated to CDI males. Females were treated with pregnant mare
serum, PMS, (5 IU) to induce follicular growth and human chorionic
gonadotropin, hCG (51 U) to induce ovulation. Embryos were
recovered in a Dulbecco's modified phosphate buffered saline (DPBS)
.. . .
.
,
.
:
.
~V() 91/(~ 51 PCI`/US90/05130
.? 2 ~ J ~3 ~ S ' ;
and maintained in Dulbecco's modified essential medium (DMEM)
supplemented with 10% fetal bovine serum.
Microiruection:
Microinjections were performed using Narishige
micromanipulators attached to a Nikon diaphot microscope. Embryos
were held in 100 microliter drops of DPBS under oil while being
microinjected. DNA solution was microinjected into the largest visible
male pronucleus. Successful injection was monitored by swelling of
10 the pronucleus.
Embryo transfer:
Immediately after injection embryos were transferred to
recipient females, mature CDI rmice mated to vasectomized male CD
15 mice. Recipient females were anesthetized using 2,2,2-
tribromoethanol. Paralumbar females were made to expose the
oviducts and the emblyos were transformed into the ampullary region
of the oviducts. The body wall was sutured and the skin closed with
wound clips. Recipients were appropriately ear notched for
20 identification and maintained until parturition.
Sampling for DNA integration:
At three weeks of age about 2-3 cm long tail samples were
excised for DNA analysis. The tail samples were digested by
incubating overnight at 55C nutator in the presence of 0.7 ml S0 mM
Tris, pH 8.0, 100 mM EDTA, 0.5% SDS and 350 ~g of prokienase K
The digested material was extracted once with equal volume of phenol
and once with equal volume of phenol:chloroform (1:1 mixture). The
supernatants were mixed with 70 I l 3 M sodium acetate (pH 6.0) and
the DNAs were precipitated by adding equal volume of 100% ethanol.
The DNAs were spun down in a microfuge5 washed once with 70~o
ethanol, dried and dissolved in 100 ~L TE buffer (10 mM Tris, pH 8.0
and 1 mM EDTA). 10 to 20 ~1 of DNAs were cut with BamHI and
.... . . .. .
. .. .. .. .
..
:
WO 91/113.~1 PCr/US90/05130
2~ 35~j
-8-
B~111 or EcoRI, electrophoresed on 1% agarose gels, blotted onto
nitrocellulose paper and hybridized with 32P-labeled hGH DNA
sequences. Transgen~c animals were identified by autoradiography.
S Propagation of ~ransgenic mice:
At five weeks of age transgenic female mice were mated to
CDI males. At five days following parturition nulk samples were
taken and assayed for hGH. At six to seven weeks of age transgenic
males were mated to two CDI females. The Fl litters were analyzed
for transgene. Four positive females were kept and mated at five
weeks of age. At five days following parturition rlulk samples were
assayed for hGH.
Collection of milk:
Milk samples (5~200 ~1) were collected from anesthetized
mice injected with 0.05 units of oxytocin, an inducer of lactation. The -
milk was collected in a glass capillary with the aid of mammary
palpation.
R~dioimmunoassay:
Human growth hormone produced in the mouse milk was
assayed by an RL~ kit available commercially from Nichols Institute
Diagnostics, SanJuan Capistrano, C~
After successful microinjection of DNA into 720 embryos
69 live offspring were born. Fourteen of these, four males and ten
females, were found to be transgenic and carrying different number of
copies of WAPhGH. The females were mated and, after parturition,
their milk samples were collected and assayed for hGH. The assay
results are tabulated as follows:
,~. . .. .. . . . .
- - , . - ~-
- - ,
,
- . . .
', .. . , .: . ', : .. ' .. ~ .. : ~ . :: .
: -: - .: :. .
PCT/~JS 90/05130
IP~A/US 08 OCT199l
Table 1: Expression of hGH in milk of Transganic Mice.
Transgenlc female hGH in milk
(nq/ml)
Control nontransgenic mouse o
<1
11 o
14 <1
525
26 <l
27 970
<5
-~
Mouse #27 is producing hGH at the rate of 970 ng/ml
(970 ~g/liter) in its milk. Embryos of transgenic mouse
embryo WAP-hGH(1-49) were deposited on September 11, 1991 with
the American Type Culture Collection, Rockville, MD., as ATCC
72007.
Stable lines of transgenic animals expression hGH in
their milk are produced by mating the females expressing the
- gGH in their milk at the highest levels and by mating the
offspring of the transgenic males. Despite the relatively
high cost of generating these transgenic animals, scale-up
costs are relatively low. In addition to conventional
breeding as a means of proliferating these production animals,
artificial insemination and embryo transfer techniques can be
employed to increase the number available for production
purposes.
Modifications and variations of the present
invention will be obvious to those skilled in the art from the
foregoing detailed description of the invention. Such
modifications and variations are intended to come within the
scope of the appended claims.
We claim.
SU~SlllUT Sl~
IPEA/U~
.~
.. .. . , ,, . .. , . . :
. . .-
... . .
.