Note: Descriptions are shown in the official language in which they were submitted.
.~ECHST AKrlENGESELLSCHAFT HOE 91/F 11~K Dr. WN/fe
2 ~ 8 ~
Description
5 Quinoxalines, processes for their preparation, and their use
The present invention relates to quinoxalines, to processes for their preparation, and
to their use.
Quinoxalines are a well-known class of compound (O. Hinsberg, J. Liebi~s Ann.
Chem. 237, 327 (1986)).
Quinoxaline derivatives have been described in the patent literature for use in various
applications in medicine.
Austrian Patent 284,848 (19.12.67) mentions 1-N-dialkylaminoalkyl-
3,4-dihydroquinoxalin-2(1H)-ones as spasmolytic agents. A series of patent
applications by the Japanese company Sumitomo Chem. Co. Ltd. describe 4-N-aroyl-,
arylacyl- and arylsulfonyl-3,4-dihydroquinoxalin-2(1H)-ones which have an
antiinflammatory action (JA 17,137t69 (11.4.66), JA 17,136/69 (8.4.66), ~IA 7,008/422
(9.8.66), BE 706,623 (16.11.66)). 3,4-Dihydroquinoxalin-2(1H)-one-3-carboxamides ars
contained in US Patent US 3,654,275 (4.4.72). They, too, have an antiinflamma~ory
action. In US Applications US 4,203,987 (21.5.79) and 4,032,639 (22.3.76), pyridinyl-
alkyltetrahydropyrazino[1,2-a]quinoxalinone derivatives are described by Am0rican
Horne Prod. Corp. as antihypertensive and antisecretory reagents. A European Patent
Application by Pfizer Inc. (EP 266,102 A (30.10.86)) includes 4-N-benzenesulfonyl-
3,4-dihydroquinoxalin-2(1H)-one-1-alkylcarboxylic acids as aldose reductase inhibitors.
However, an antiviral activity has not been demonstrated to date.
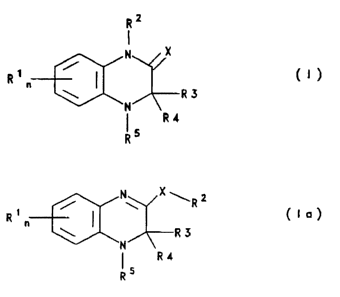
Surprisingly, it has now been found that quinoxalines of the formulae I and la
2 2 ~ 8 ~
R 2
I
R 1 ~N~R 3 ( I )
R 4
R 5
and their tautomeric forms of the formula la
N X
R ~ R 2 ( I a )
~N/~
1 5 R4
R
and physiologically acceptable salts or prodrugs thereof have an antiviral action, in
particular against retroviruses, for example against the human immunodeficiency virus
(H IV) .
20 In the compounds of the formula I or la according to the invention,
1) n is ~ero,
one,
twl
three
or four,
the individual substituents R' independently of ons another are
fluorine, chlorine, bromine, iodine, trifluoromethyl, trifluoromethoxy~ hydroxyl,
C1-C8-alkyl, C5-C8-cycloalkyl, C1-C~-alkoxy, (C:1-C6-alkoxy)-(C1-C4-alkoxy3,
2~g8~
C,-C6-alkylthio, C,-C6-alkylsulfinyl, C,-C6-alkylsulfonyl, ni~ro, amino, azido,
C,-C6-alkylamino, di(C,-C6-alkyl)amino, piperidino, morpholino, 1-pyrrolidinyl,
4-methylpiperazinyl, thiomorpholino, irnidazolyl, triazolyl, tetrazolyl, C,-C6-acyl,
C,-C6-acyloxy, C,-C6-acylamino, cyano, carbamoyl, carbo~
(C,-C6-alkyl)oxycarbonyl, hydroxysulfonyl, sulfamoyl
or
a phenyl, phenoxy, phenoxycarbonyl, phenylthio, phenylsulfinyl, phenylsulfonyl,
phenoxysulfonyl, phenylsulfonyloxy, anilinosulfonyl, phenylsulfonylamino,
benzoyl, 2-pyridyl, 3-pyridyl or 4-pyridyl radical which is substituted by up to five
radicals R6 which are independent o~ one another,
where R6 can be
t5
fluorine, chlorine, bromine, iodine, cyano, trifluoromethyl, trifluoromethoxy, nitro,
amino, azido, C1-C6-alkyl, C3-C9-cycloalkyl, C1-C6-alkoxy, C,-C6-alkylthio,
C1-C6-alkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-alkylamino, di(C,-C6-alkyl)amino,
(C1-C6-alkyl)oxycarbonyl, phenyl, phenoxy, 2-, 3- or 4-pyridyl,
R2 is hydrogen, C,-C6-alkoxy, hydroxyl, picolyl, cyclopropyl or isopropenyloxycarbonyl
and R5 is
hydrogen, hydroxyl, C1-C6-alkoxy, aryloxy, C,-C6-acyloxy, cyano, amino,
2~ C,-C6-alkylamino, di(C,-C6-alkyl~amino, arylamino, Ct-C6-acylamino, I:~,-C8-alkyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C6-alkoxy, C,-C6-alkylamino,
di(C1-(::6-alkyl~amino, C,-C6-alkylthio, C,-C:6-alkylsulfonyl, phenylsulfonyl, oxo,
thioxo, carboxyl or carbamoyl;
2~9~
C2-C~-alkenyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C,-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C6-alkoxy, C1-C6-alkylamino,
di~Cl-C8-alkyl)amino, C1-C6-alkylthio, C1-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl and carbamoyl;
C3-Ca-allenyl, optionally substituted by fluorine, chlorine or hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C3-CB-alkynyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
Cl-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C8-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, C1-C6-alkylthio, Cl-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
C3-C8-cycloalkyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-G6-alkoxy, C1-C6-alkylamino,
di(C1-C6-alkyl)amino, C,-C6-alkylthio, C,-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbarnoyl;
C3-CB-cycloalkenyl,
2~ optionally ~ubstituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, Cl-C6-alkoxy, C,-C6-alkylamino,
di~Cl-C6-alkyl)amino, Cl-C6-alkylthio, C,-S:~6-alkylsulfonyl, phenylsulfonyl, oxo,
thioxo, carboxyl or carbamoyl;
2 ~ 8 ~
(C3-C8-cycloalkyl~-(C,-C4-alkyl),
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, Cl-C6-alkoxy, C1-C6-alkylamino,
di(C1-C6-alkyl)amino, C1-C6-alkylthio, Cl-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
(C3-C8-cycloalkenyl)-(C, -C4-alkyl),
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, rnercapto, hydroxyl,
C,-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C6-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, C,-C6-alkylthio, C,-C6 alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
1 5 C,-C6-alkylcarbonyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C6-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, C,-C6-alkylthio, C,-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
C2-C8-alkenylcarbonyl, optionally substituted by fluorine, chlorine or hydroxyl,C1-C4-alkoxy, oxo, phenyl;
2~ (C3-C6-cycloalkyl)carbonyl, optionally substituted by fluorine, chlorine or
hya'roxyl, C,-C4-alkoxy, oxo, phenyl;
(C5-C~-cycloalkenyl)carbonyl, optionally substituted by fluorine, chlorine or
hydroxyl, C,-C4-alkoxy, oxo, phenyl;
- :
2~6~
(C3-C~-cycloalkyl)-(C1-C~-alkyl)carbonyl, optionally substituted by fluorine,
chlorine or hydroxyl, C1-C4-aikoxy, oxo, phenyl;
(C5-C6-cycloalkenyl)-(Cl-c3-alkyl)carbonyl~ optionally substituted by fluorine,
chlorine or hydroxyl, C1-C4-alkoxy, oxo, phenyl;
C,-C8-alkyloxycarbonyl, optionally substituted by fluorine, chlorine, bromine,
hydroxyl, C1-C4-alkoxy, Cl-C4-alkylarnino, di(Cl-C4-alkyl)amino, C1-C4-alkylthio;
C2-C8-alkenyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, ph~nyl;
C2-C5-alkynyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C1-CB-alkylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,Cl-C4-alkoxy, oxo, phenyl;
C2-C~-alkenylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C1-CB-alkylamino- and di(C1-C5-alkyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydro)~yl, Cl-C4-alkoxy, oxo, phenyl;
pyrrolidin-1-yl, morpholino-, piperidino-, piperæinyl-, w 4-methylpiperazin-
1-ylcarbonyl, in each case optionally substituted by ~1-C4-alkyl, C2-C6-alkenyl,C1-C4-acyl, oxo, thioxo, carboxyi, or phenyl;
C2-C5-alkenylamino- and di(C,-C6-alkenyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, C1-C4-alkoxy, oxo, phenyl;
2 ~ 8 5
C1-C6-alkylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C:1-C4-
alkoxy, oxo, phenyl;
C1-C6-alkenylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C1-C4-
alkoxy, oxo, phenyl;
or aryl, arylcarbonyl, aryl(thiocarbonyl), (arylthio)carbonyl, (arylthio~thiocarbonyl,
aryloxycarbonyl, arylaminocarbonyl, ~arylamino)thiocarbonyl,
arylalkylaminocarbonyl, arylsulfonyl, arylalkyl, arylalk~nyl, arylalkynyl, arylalkyl-
carbonyl, arylalkenylcarbonyl, arylalkoxycarbonyl or aryl(alkylthio)carbonyl, each
of which is substituted by up to five radicals R5 which are independent of one
another, it being possible for the alkyl radical to contain in each case 1 to
5 carbon atoms, and R6 being as defined above,
or heteroaryl, heteroarylalkyl, heteroarylalkenyl, heteroarylalkylcarbonyl or
heteroarylalkenylcarbonyl, heteroaryloxycarbonyl, (heteroarylthio)carbonyl,
heteroarylaminocarbonyl, heteroarylalkyloxycarbonyl,
heteroaryl(alkylthio)carbonyl or heteroarylalkylaminocarbonyl, each of which is
substituted by up to three radicals R6 which are independent of one another, it
being possible for the alkyl raclical to contain in each case 1 to 3 carbon atoms,
R3 and R4 are identical or different and, independently of
one another, are hydrogen, C1-C8-alkyl which is optionally substituted by
fluorine, chlorine, hydroxyl, amino, mercapto, Cl-C:4-acyloxy, b~nzoyloxy,
benzyloxy, phenoxy, G1-C4-alkoxy, C1-C4-alkylamino, di(C1-C4-alkyl~amino, C1-C4-alkylthio, C1-C4-alkylsulFonyl, Cl-C4-alkylsulfinyl, carboxyl or carbamoyl;
C2-C8-alkenyl, optionally substituted by fluorine or chlorine, hydroxyl, amino,
mercapto, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, Gl-C"-alkoxy, C1-C4-
.
2 ~ 9 3 ~
alkylamino, di(C1-C4-alkyl)amino, C1-C4 alkylthio, C,-C4-alkylsulfonyl, C;1-C4-
alkylsulfinyl, carboxyi or carbamoyl;
C3-CE,-cycloalkyl, optionally substituted by fluorine, chlorine, hydroxyl, amino,
mercapto, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C"-alkoxy, C,-C4-
alkylamino, di(C1-C4-alkyl)amino, C,-C,-alkylthio, C1-C4-alkyisulfonyl, C,-C4-
alkylsulfinyl, carboxyl or carbamoyl;
C3-C8-cycloalkenyl, optionally substituted by fluorine or chlorine, hydroxyl,
amino, mercapto, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-~4-alkoxy,
C1-C4-alkylamino, di(C,-C4-alkyl)amino, C,-C4-alkylthio, C,-C4-alkylsulfonyl, C1-C4-
alkylsulfinyl, carboxyl or carbamoyl;
aryl, arylalkyl, heteroaryl or heteroarylalkyl, each of which is substituted by up to
five radicals R6 which are independent of one another, it being possible for thealkyl radical to contain 1 to 3 carbon atoms in each case, and R6 being as
defined above,
R3 and R4 can furthermore also be
part of a saturated or unsaturated carbo- or heterocyclic ring which has 3 to 8
carbon atoms and which can optionally be substituted by fluorine, chlorine,
hydroxyl, amino, C,-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C1-C6-acyloxy,
benzoyloxy, C1-C6-alkoxy, oxo, thioxo, carboxyl, carbamoyl or phenyl,
?5
X is oxygen, sulfur~ selenium or substituted nitrogen N-R2, it being possible for R2
to have the abovementioned meanings,
with the exception of those compounds in which R3 and R4 are both hydrogen, and
compounds in wnich R2 and R5 are hydrogen and R3 and/or R4 are/is arylalkyl, andcompounds in which X is oxygen and R2 and Rs are hydrogen.
2 ~
In a preferred group of compounds of the formula I or la,
2) n is zero,
one,
two
or three,
the individual substituents R1 independently of one another are
fluorine, chlorine, bromine, trifluoromethyl, trifluoromethoxy, hydroxyl, Cl-C4-alkyl, C5-C6-cycloalkyl, C1-C4-alkoxy, (C1-C4-alkoxy)-(C1-C4-ali~o~ C~ 4-
alkylthio, C1-C4-alkylsulfinyl, C1-C4-alkylsulfonyl, nitro, amino, C1-C4-alkylamino,
di(C1-C4-alkyl)amino, piperidino, morpholino, 1-pyrrolidinyl, 4-methylpip~razinyl,
thiomorpholino, imidazolyl, C1-C4-acyl, C,-C4-acyloxy, C1-C4-acylamino, cyano,
carbamoyl, carboxyl, (C1-C4-alkyl)oxycarbonyl, hydroxysulfonyl or sulfamoyl
or
a phenyl, phenoxy, phenoxycarbonyl, phenylthio, phenylsulfinyl, phenylsulfonyl,
phenoxysulfonyl, phenylsulfonyloxy, anilinosulfonyl, phenylsulfonylamino,
benzoyl, 2-pyridyl, 3-pyridyl or 4-pyridyl radical which is substituted by up to two
radicals R6 which are independent of one another,
where R6 can be
fluorine, chlorine, bromine, cyano, trifluoromethyl, nltro, amino, C1-C4-alkyl,
C3-C7-cycloalkyi, Cl-C4-alkoxy, C,-C4-alkylthio, Cl-C4-alkylsulfinyl, C,-C4-
alkylsulfonyl, C,-C4-alkylamino, di(C,-C4-alkyl)amino, ~C:l-C4-alkyl)oxycarbonyl,
phenyl or phenoxy,
2~9~ ~
R2 is hydrogen and R5 is
hydrogen, hydroxyl, cyano, amino,
C,-C6-alkyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, rnercapto, hydroxyl, C:l-C4
acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C~-alkoxy, C,-C4-alkylamino,
di(C,-C4-alkyl)amino, C,-C4-alkylthio, oxo, thioxo, oarboxyl or carbamoyl;
C2-C8-alkenyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl, C,-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C4-alkoxy, C1-C4-alkylamino,
di(C1-C4-alkyl)amino, C,-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
t5 C3-Ca-allenyl,
C3-C8-alkynyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl, C1-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C4-alkoxy, C,-C4-alkylamino,
di(C,-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
C3-CB-cycloalkyl,
optiorlally subs~ituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl, C,-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C4-alkoxy, C,-C4-alkylamino,
di(C,-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
C3-C8-cycloalkenyl,
optionally substituted by
11
fluorine, chlorine, bromine, iodine, ~yano, amino, mercapto, hydroxyl, C1-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C4-alkoxy, C1-C4-alkylamino,
di(C1-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
(C3-C~-cycloalkyl)-(C1-C2-alkyl)
optionaliy substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl, C1-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C1~C4-alkoxy, Cl-C4-alkylamino,
di(C1-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
(C3-C~-cycloalkenyl)-(C,-C2-alkyl),
optionally substituted by
fiuorine, chlor;ne, bromine, iodine, cyano, amino, mercapto, hydroxyl, C1-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C4-alkoxy, C~-C4-alkylamino,
di(C,-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
C1-C6-alkylcarbonyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl, C1-C4-
acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C~,-alkoxy, C1-C4-alkylamino,
di(C1-C4-alkyl)amino, C1-C4-alkylthio, oxo, thioxo, carboxyl or carbamoyl;
C2-C6^alkenylcarbonyl, optionally substituted by fluorine, chlorine or hydroxyl,C,-C4-alkoxy, oxo, phenyl;
~C3-C6-cycloalkyl)carbonyl, optionally substituted by fluorine, chlorine or
hydroxyl, C1-C4-alkoxy, oxo, phenyl;
(C5-C6-cycloalkenyl)carbonyl, optionally substituted by fluorine, chlorine or
hydroxyl, C1-C4-alkoxy, oxo, phenyl;
g ~ 5
12
~C3-C6-cycloalkyl)-(C,-C2-alkyl)carbonyl, optionally substituted by fiuorine,
chlorine or hydroxyl, Cl-C4-alkoxy, oxo, phenyl;
(C5-C6-cycloalkenyl)-(C,-C2-alkyl)carbonyl, optionally substituted by fluorine,
chlorine or hydroxyl, C1-C4-alkoxy, oxo, phenyl;
C1-C6-alkyloxycarbonyl, optionally substituted by fluorine, chlorin~, bromine,
hydroxyl, C1 C4-alkoxy, C1-C~,-alkylamino, di(Cl-C4-alkyl)amino, C,-C4-alkylthio;
C2-C6-alkenyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C2-C6-alkynyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
~5
C1-C6-alkylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,C1-C4-alkoxy, oxo, phenyl;
C2-C6-alkenylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C1-C6-alkylamino- and di(C1-C6-alkyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, C1-C4-alkoxy, oxo, phenyl;
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl-, or 4-methylpiperazin-
1-ylcarbonyl;
C2-C6-alkenylamino- and di(C1-C5-alkenyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, C1-C4-alkoxy, oxo, phenyl;
~ ~ 6 ~
13
C1-C4-alkylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C,-C4-
alkoxy, oxo, phenyl;
C1-C4-alkenylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C,-C4-
alkoxy, oxo, phenyl;
or aryl, arylcarbonyl, aryl(thiocarbonyl), (arylthio)carbonyl, (arylthio)thiocarbonyl,
aryloxycarbonyl, arylaminocarbonyl, (arylamino)thiocarbonyl,
arylalkylaminocarbonyl, arylsulfonyl, arylalkyl, arylalkenyl, arylalkynyl, arylalkyl-
carbonyl, arylalkenylcarbonyl, aryl(alkyl~hio)carbonyl or arylalkoxycarbonyl, each
of which îs substituted by up to three radicals R6 which are independsnt of one
another, it being possible for the alkyl radical to contain in each case 1 to 5
carbon atoms and R6 being as defined above,
or 1- or 2-naphthylmethyl, 2-, 3- or 4-picolyl, 2- or 3-furylmethyl, 2- or
3-thienylmethyl, 2- or 3-pyrrolylmethyl, 2-, 3- or 4-pyridylcarbonyl, 2- or
3-furylcarbonyl, 2- or 3-thienylcarbonyl, 2- or 3-thienylacetyl, 2-, 3- or
4-picolyloxycarbonyl, 2- or 3-furylmethyloxycarbonyl, 2- or 3-thienylrnethyl-
oxycarbonyl, each of which is substituted by up to two radicals R6 which are
independent of one another,
and
R3 and R4 are identical or different and independently of
2~ one another are
hydrogen,
C1-C6-alkyl,
optionally substituted by fluorine, chlorine, hydroxyl, amino, mercapto, C1-C4-
acyloxy, benzoyloxy, ben7yloxy, phenoxy, C1-C4-aikoxy, C,-C4-~lkylamino,
2 ~
14
di(C1-C4-alkyl)amino, C,-54-alkylthio, C1-C4-alkylsulfonyl, C,-C4-alkylsulfinyl,carboxyl or carbamoyl;
C2-Ca-alkenyl, optionally substituted by fluorine or chlorine, hydroxyl, amino,
mercapto, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C4-alkoxy, C,-C4-
alkylamino, di(C1-C4-alkyl~amino, Cl-C4-alkylthio, C,-C4-alkylsulfonyl, C,-C4-
alkylsulfinyl, carboxyl or carbamoyl;
C3-Ca-cycloalkyl, optionally substituted by fluorine, ch!orine, hydroxyl, arnino,
mercapto, Cl-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C4-alkoxy, C1-C4-
alkylamino, di(C,-C4-alkyl)amino, C,-C4-alkylthio, C,-C4-alkylsulfonyl, C l-C4-
alkylsulfinyl, carboxyl or carbamoyl;
C3-Ca-cycloalkenyl, optionally substituted by fluorine or chlorine, hydroxyl,
amino, mercapto, Cl-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C4-alkoxy,
C1-C4-alkylamino, di(C1-C4-alkyl)amino, C,-C4-alkylthio, C,-C4-alkylsulfonyl, C,-C4-
alkylsulfinyl, carboxyl or carbamoyl;
aryl, arylalkyl, heteroaryl or heteroarylalkyl, each of which is substituted by up to
three radicals R~ which are independent o~ one another, it being possible for the
alkyl radical to contain in each case 1 to 3 carbon atoms and R6 being as
defined above,
R3 and R4 can furthermore also be
par~ of a saturated or unsaturated carbo- or heterocyclic ring which has 3 tQ 7
carbon atoms and which can optionally be substitut~d by fluorine, chlorine,
hydroxyl, amino, C1-C4-alkyl, C2-C4-alkenyl, C2-C4-alkynyl, C1-C4-acyloxy,
benzoyloxy, C1-C4-alkoxy, oxo, thioxo, carboxyl, carbamoyl or phenyl, and
X is oxygen, sulfur or selenium.
2~6~85
In a yet more preferred group of compounds of the formula I or la,
3) n is zero,
one
or two,
the individual substituents R' independently of one another are
fluorine, chlorine, bromine, trifluoromethyl, hydroxyl, C1-C4-alkyl, C,-C4-alkoxy,
(C1-C4-alkoxy)-(C1-C4-alkoxy), C1-C4-alkylthio, nitro, amino, C1-C4-alkylamino,
di(C,-C4-alkyl)amino, piperidino, morpholino, 1-pyrrolidinyl, 4-methylpiperazinyl,
Cl-C4-acyl, C1-C4-acyloxy, Cl-C4-acylamino, cyano, carbamoyl, carboxyl, (C1-C4-
alkyl)oxycarbonyl, hydroxysulfonyl or sulfamoyl,
or
a phenyl, phenoxy, phenylthio, phenylsulfonyl, phenoxysulfonyl, benzoyl,
2-pyridyl, 3-pyridyl or 4-pyridyl radical which is substituted by up to two radicals
R6 which are independent of one another,
where R6 can be
fluorine, chlorine, bromine, cyano, trifluoromethyl, nitro, amino, C1-C4-alkyl,
C1-C4-alkoxy, (C1-C4-alkyl)oxycarbonyl, phenyl or phenoxy,
R2 is hydrogen and R5 is
C1-C6-alkyl,
optionally substituted by
fluorine, chlorlne, hydroxyl, C1-C4-acyloxy, benzoyloxy, benzylo~y, phenoxy,
:
2 ~
16
C1-C4-alkoxy, C1-C4-alkylamino, di(C,-C4-alkyl)amino, Cl-C4-alkylthio, oxo, thioxo,
carboxyl or carbamoyl;
C2-C6-alkenyl,
optionally substituted by
fluorine, chlorine, hydroxyl, Cl-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy,
C,-C4-alkoxy, C1-C4-alkylamino, di(C1-C4-alkyl)amino, C,-C4-aikylthio, oxo, thioxo,
carboxyl or carbamoyl;
C3-t:~B-allenyl,
C3-C8-alkynyl,
optionally substituted by
fluorine, chlorine, hydroxyl, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy,
C,-C4-alkoxy, C,-C4-alkylamino, di(C,-C4-alkyl)amino, C,-C4-alkylthio, oxo, thioxo,
carboxyl or carbamoyl;
C3-Ca-cycloalkyl,
optionally substituted by
fluorine, chlorine, hydroxyl, C,-C4-alkyl, C,-C4-acyloxy, benzoyloxy, benzyloxy,phenoxy, C,-C4-alkoxy, C,-C4-alkylamino, di(C,-C4-alkyl)amino, C,-C4-alkylthio,
oxo, thioxo, carboxyl or carbamoyl;
C3-C8-cycloalkenyl,
optionally substituted by
fluorine, chlorine, hydroxyl, C,-C4-alkyl, C;,-C4-acyloxy, ben7oyloxy, benzyloxy,
phenoxy, C,-C4-alkoxy, C,-C4-alkylamino, di(C,-C4-alkyl)amino, C,-C4-alkylthio,
oxo, thioxo, carboxyl or carbamoyl;
2~98~
17
(C3-Cs-cycloalkyl)-(C1-C2-alkyl~,
optionally substituted by
fluorine, chlorine, hydroxyl, C1-C4-alkyl, C1-C4-acyloxy, benzoyloxy, benzyloxy,phenoxy, C1-C4-alkoxy, C1-C"-alkylamino, di~C1-C4-alkyl)amino, C1-C4-alkylthio,
oxo, thioxo, carboxyl or carbamoyl;
(C3-C6-cycloalkenyl)-(C,-C2-alkyl),
optionally substituted by
fluorine, chlorine, hydroxyl, C1-C4-alkyl, C1-C4-acyloxy, benzoyloxy, benzyloxy,phenoxy, C1-C4-alkoxy, C1-C~-alkylamino, di(C1-C4-alkyl)amino, C1-C4-alkyithio,
oxo, thioxo, carboxyl or carbamoyl;
C1 -C6-alkylcarbonyl,
optionally substituted by
fluorine, chlorine, hydroxyl, C1-C4-alkyl, C1-C4-acyloxy, benzoyloxy, benzyloxy,pheroxy, C1-C4-alkoxy, C1-C4-alkylamino, C,-C4-alkenylamino, di(C1-C4-
alkyl)amino, 1-pyrrolidinyl, piperidino, morpholino, 4-methylpiperazin-1-yl, Cl-C4-
alkylthio, oxo, thioxo, carboxyl or carbamoyl;
C2-C6-alkenylcarbonyl, optionally substituted by fluorine, chlorine or hydroxyl;
(C3-C6-cycloalkyl)carbonyl,
(C5-C6-cycloalkenyl)carbonyl,
(C3-C6-cycloalkyl)-(C1-C2-alkyl)carbonyl,
(Cs-C6-cycloalkenyl)-(C,-C2-alkyl)carbonyl,
C1-C6-alkyloxycarbonyl, optionally substituted by flvorine, ehiorine, bromine,
hydroxyl, C1-C4-alkoxy, C1-C4-alkylamino, di(C1-C4-alkyl~amino or C1-C4-alkylthio;
2~6~9~
18
C2-C6-alkenyloxycarbonyl, optionally substituted by flusrine, chlorine, hydroxyl,
C,-C4-alkoxy;
C2-C6-alkynyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
Cl-C4-alkoxy;
Cl-C6-alkylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
Cl-C4-alkoxy;
C2-C6-alkenylthiocarbnnyl, optionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy;
C1-C6-alkylamino- and di(C1-C6-alkyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, Cl-G4-alkoxy;
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl-, or 4-methylpiperazin-
1 -ylcarbonyl;
C2-C6-alkenylamino- and di(C1-C~-alkenyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, Cl-C4-alkoxy;
Cl-C4-alkylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C1-C4-
alkoxy;
C1-C4-alkenylsulfonyl;
or aryl, arylcarbonyl, (arSlthio)carbonyl, aryloxycarbonyl, arylaminocarbonyl,
(arylamino~thiocarbonyl, arylsulfonyl, arylalkylaminocarbonyl, arylalkyl,
arylalkenyl, arylalkylcarbonyl, arylalkoxycarbonyl or aryl(alkylthio)carbonyl, each
of which is substituted by up to two radicals R6 which are independent of one
2 ~
19
another, it being possible for the alkyl radical to contain in each oase 1 to 3
carbon atoms, and R6 being as defined above,
or 1- or 2-naphthylmethyl, 2-, 3- or 4-picolyl, 2- or 3-furylmethyl, 2- or
3-thienylmethyl, 2- or 3-pyrrolylmethyl,
2-, 3- or 4-pyridylcarbonyl, 2- or 3-furylcarbonyl, 2- or 3-thienylcarbonyl, 2- or
3-thienylacetyl, 2-, 3- or 4-picolyloxycarbonyl, 2- or 3-furylmethyloxycarbonyl or
2- or 3-thienylmethyloxycarbonyl, sach of which is substituted by up to two
radicals R6 which are independent of one another,
and
R3 and R4 are identical or different and independently of
one another are hydrogen, C1-C4-alkyl, optionally substitu~ed by fluorine,
chlorine, hydroxyl, amino, mercapto, C1-C4-acyloxy, benzoyloxy, phenoxy,
Cl-C4-alkoxy, Cl-C4-alkylamino, di(Cl-C4-alkyl)amino, Cl-C4-alkylthio, Cl-C4-
alkylsulfonyl, C1-C4-alkylsulfinyl, carboxyl or carbamoyl;
C2-C6-alkenyl, optionally substituted by fluorine or chlorine;
C3-C6-cycloalkyl, optionally substituted by fluorine, chlorine, hydroxyi, amino,mercapto, Cl-C4-acyloxy, benzoylo~y, benzyloxy, phenoxy, C1-C4-alkoxy, Cl-C4-
alkylamino, di~C,-C4-alkyl)amino, C1-C4-alkylthio, C1-C4-alkylsulfonyl, C~-C4-
alkylsulfinyl, carboxyl or carbamoyl;
~5
C3-Ca-cycloalkenyl, optionally substituted by fluorine or chlorine;
- aryl, benzyl, heteroaryl or heteroarylmethyl, each of which is su~stituted by up
to two radicals R~ which are independent of one another,
2 ~ 5
2û
R3 and R4 can furthermore also be
part of a saturated or unsaturated carbo- or heterocyclic ring which has 3 to 6
carbon atoms and which can optionally be substituted by fluorine, chlorine,
hydroxyl, amino, C1-C4-acytoxy, benzoyloxy, C,-C"-alkoxy, oxo, thioxo, carboxyl
or carbamoyl, and
X is oxygen or sulfur.
In a yet again preferred group of compounds of the formula I or la,
4) n is zero,
one
or two,
the individual substituents R' independently of one another are
fluorine, chlorine, bromine, trifluoromethyl, hydroxyl, C1-C4-alkyt, C1-C4-alkoxy,
(C1-C4-alkoxy)-(C1-C2-alkoxy), C1-C4-alkylthio, nitro, amino, C1-C4-alkylamino,
di(C,-C4-alkyl)amino, piperidino, morpholino, 1-pyrrolidinyl, 4-methylpiperazinyl,
C1-C4-acyl, C1-C4-acyloxy, C,-C4-acylamino, cyano, carbamoyl, carboxyl, (C1-C4-
alkyl)oxycarbonyl, hydroxysulfonyl or sulfamoyl
or
a phenyl, phenoxy, phenylthio, phenylsulfonyl, phenoxysulfonyl, benzoyl,
2-pyridyl, 3-pyridyl or 4-pyridyl radical, each of whk:h is substituted by up to two
radicals R6 which are independent of one anoth0r,
where R6 can be
~ ~ .
2 ~
21
fluorine, chlorine, bromine, cyano, trifluoromethyl, nitro, amino, C1-C"-alkyl,
C1-C4-alkoxy, (C1-C4-alkyl)o~carbonyl, pheny~ or phenoxy,
R2 is hydrogen and R5 is
C1-C6-alkyl,
optionally substituted by C1-C"-alkoxy or C1-C"-alkylthio;
C2-C6-alkenyl,
optionally substituted by oxo;
C3-C6-allenyl;
C3-CB-alkynyl, in particular 2-butynyl;
C3-C6-cycloalkyl;
Cs-C6-cycloalkenyl;
(C3-C6-cycloalkyl)-(C1-C2-alkyl), in particular cyclopropyimethyl, optionally
substituted by C1-C4-alkyl;
(C3-C6-cycloalkenyl)-(C,-C2-alkyl), in particular cyclohexenylmethyl;
C1-C6-alkylcarbonyl,
optionally substituted by
fluorine, chlorine, hydroxyl, benzyloxy, phenoxy, C,-C4-alkoxy, C1-e;4-alkylamino,
C1-C4-alkenylamino, di(C1-C4-alkyl)amino, 1-pyrrolidinyl, piperidino, morpholino,
4-methylpiperazin-1-yl or C1-C4-alkylthio;
22 2 ~ 8
C2-C6-alkenylcarbonyl;
Cl-C6-alkyloxycarbonyl, optionally substituted by fluorine, chlorine, bromine,
hydroxyl, C1-C4-alkoxy, Ct-C4-alkylamino, di(C1-C4-alkyl)amino or C1-C4-alkylthio;
C2-C6-aikenyloxycarbonyl, in particular vinyloxycarbonyl, allyloxycarbonyl,
isoprnpenyloxycarbonyl, butenyloxycarbonyl or pentenyloxycarbonyl;
C2-C6-alkynyloxycarbonyl, in particular propynyloxycarbonyl or
butynyloxycarbonyl;
C1 -C6-alkylthiocarbonyl;
C2-C6-alkenylthiocarbonyl, in particular allylthiocarbonyl;
C,-C6-alkylamino- and di(C1-C6-alkyl)aminocarbonyl;
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl-, or 4-methylpiperazin-
1-ylcarbonyl;
C2-C6-alkenylamino- and di(C,-C6-alkenyl)aminocarbonyl;
C1 -C4-alkylsulfonyl;
~5 C,-C4-alkenylsulfonyl;
or aryl which is substituted by up to two radicals R6 which are inciependent of
one another, in particular phenyl, aryicarbonyl, in parSicular benzoyl,
(arylthio)carbonyl, aryloxycarbonyl, arylaminocarbonyl, (arylamino~thiocarbonyl,arylalkylaminocarbonyl, arylsulfonyl, arylalkyi, in particular benzyl, phenylethyl,
~$~
23
arylalkenyl, arylalkylcarbonyl, arylalkoxycarbonyl or aryl(alkylthio)carbonyl, it
being possible for the alkyl radical to contain in each case 1 to 3 carbon atomsand R6 being as defined above,
or 1- or 2-naphthylmethyl, 2-, 3- or 4-picolyl, ~- or 3-furylmethyl, 2- or
3-thienylmethyl, 2- or 3-pyrrolylmethyl, 2-, 3- or 4-pyridylcarbonyl, 2- or
3-furylcarbonyl, 2- or 3-thienylcarbonyl, 2- or 3-thienylacetyl, 2-, 3- or
4-picolyloxycarbonyl, 2- or 3-furylmethyloxycarbonyl, or 2- or 3-thienyl-
methyloxycarbonyl, each of which is substituted by up to two radicals R6 which
are independent of one another,
and
R3 and R4 are identical or different and independently of
one another are
hydrogen,
Cl-C4-alkyl,
optionally substituted by hydroxyl, mercapto, C1-C4-alkoxy, C1-C4-alkylthio,
C,-C4-alkylsulfonyl, C,-C4-alkylsulfinyl, carboxyl or carbarnoyl;
C2-C6-alkenyl,
aryl, benzyl, thienyl or thienylmethyl, each of which is substituted by up to two
radicals R6 which are independent of one another, R6 beinç3 as defined above,
R3 and R4 can also be
part o~ a saturated or unsaturated carbo- or heterocyclic ring which has 3 to 6
carbon atoms and can optionally be substituted by oxo or thioxo, an~
2 ~ 8 ~
24
X is oxygen or suifur.
Compounds of the formula I or la as defined above wherein the substituents
mentioned have the following meanings are very particularly important:
n is zero or
one,
the individual substituents R' independently of one another are
fluorine, chlorine, bromine, C1-C2-alkyl, C,-C2-alkoxy, C2-C4-acyl or cyano,
R2 is hydrogen and Rs is
1 5 C2-C6-alkenyl,
C3-C8-alkynyl, in particular 2-butynyl;
(C3-C6-cycloalkyl)-(C1-C2-alkyl), in particular cyclopropylmethyl, optionally
substituted by C1-C4-alkyl;
(C3-C6-cycloalkenyl)-(C1-C2-alkyl), in particular cyclohexenylmethyl;
C2-C6-alkylcarbonyl,
C2-C6-alkenylcarbonyi;
C1 -C6-alkyloxycarbonyl;
2 ~ 5
C2-C6-alkenyloxycarbonyl, in particular vinyloxycarbonyl, allyloxycarbonyl,
isopropenyloxycarbonyl, butenyloxycarbonyl or pentenyloxycarbonyl;
C~-C6-alkynyloxycarbonyl, in particular propynyloxycarbonyl or
butynyloxycarbonyl;
C2-C6-alkenylthiocarbonyl, in particular allylthiocarbonyl;
Cl-C4-alkylsulfonyl;
C1 -C4-alkenylsuHonyl;
or arylalkyl, in particu!ar benzyl or arylalkenyl, which is substituted by up to two
radicals R6 which are independent of one another, it being possible for the alkyl
radical to contain in each case 1 to 3 carbon atoms and for the alkenyl radical
to contain 2-3 carbon atoms,
or 1-naphthylmethyl, 2- or 3-picolyl, 2-furylme~hyl or 2- or 3-thienylmethyl, each
of which is substituted by up to two radicals R6 which are independent of one
another,
where R6 is
fluorine, chlorine, bromine, cyano, C1-C2-alkyl or C1-C2-alkoxy,
~5
and
R3 and R4 are identical or different and independently of
one another are
2 ~
26
hydrogen,
C1-C4-alkyl,
optionally substituted by hydroxyl, mercapto, C,-C4-alkoxy. C1-C2-aikylthio, and
5 X is oxygen or sulfur.
The alkyl groups in the above definitions can be straight-chain or branched. Unless
otherwise defined, they preferably contain 1-8, particularly preferably 1-6, in particular
1-4, carbon atoms. Examples are the methyl, ethyl, propyl, 1-methylethyl, butyl,10 1-methylpropyl, 2-methylpropyl and 1,1-dimethylethyl ~roup, and similar groups.
The alkenyl groups mentioned in the above definitions can be strai~ht-chain or
branched and contain 1 to 3 double bonds. Unless otherwise defined, these groupspreferably contain 2-8, in particular 2-6, carbon atoms. Examples are the 2-propenyl,
1-methylethenyl, 2-butenyl, 3-butenyl, 2-methyl-2-propenyl, 3-methyl-2-butenyl,
2,3-dimethyl-2-butenyl, 3,3-dichloro-2-propenyl and pentadienyl groups and similar
groups.
The alkynyl groups mentioned in the above definitions can be straight-chain or
20 branched and contain 1 to 3 triple bonds. Unless otherwise defined, they contain
preferably 2-8, particularly preferably 3-6, carbon atoms. Examples are the 2-propynyl
and 3-butynyl group and similar groups.
Unless otherwise defined, the cycloalkyl and cycloalkenyl groups mentioned in the
25 above definitions contain ,orefsrably 3-8, particularly preferably 4-6, carbon atoms.
Examples are the cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl or
cyclohexenyl group.
The acyl groups mentioned in the above definitions can be aliphatic, cycloaliphatic or
30 aromatic. Unless otherwise defined, they preferably contain 1-8, particularly preferably
2 ~ 5
27
2-7, carbon atoms. Examples of acyl ~roups are the formyl, acetyl, chloroacetyl,trifiuoroacetyl, hydroxyacetyl, propisnyl, butyryl, isobutyryl, pivaloyl, cyclohexanoyl or
benzoyl group.
5 The aryl groups mentioned in the above definitions are preferably aromatic groups
having 6-14 carbon atoms, in particular 6-10 carbon atoms, for example phenyl ornaphthyl.
Suitable hetero atoms in the abovementioned heterocyclic rings Dr heteroaryi groups
10 are, in particular, oxygen, sulfur and nitrogen, where, in the case of a nitrogen-
containing ring which is saturated in this position, a structure N-Z is present in which Z
is H or R5 w1th the individual above-described definitions.
Unless otherwise defined, the heterocyclic rings preferably have 1-13 carbon atoms
and 1-6 hetero atoms, in particular 3-9 carbon atoms and 1-4 hetero atoms.
Suitable radicals for the heteroaryl groups mentioned in the above definitions are, for
example, heteroaromatic radicals such as 2- or 3-thienyl, 2- or 3-furyl, 2-, 3- or
4-pyridyl, pyrimidyl, indolyl, quinolyl or isoquinolyl.
Examples of the aralkyl groups mentioned in the above definitions are benzyl,
phenylethyl, naphthylmethyl or styryl.
The abovementioned substituents R1 to Rs are preferably trisubstituted, particularly
25 preferably disubstituted, in particular monosubstituted, by the particular substituents
mentioned.
In the case of the particular definitions of composite substituents (such as, for
example, arylalkoxycarbonyl~, the ranges which have been described above as being
30 preferred for the individual substituents are also preferred.
2 ~ 6 ~ 9 8 ~
28
Depending on the various substituents, compounds of the formulae I and la can have
several asymmetric carbon atoms. The invention therefore relates both to the pure
stereoisomers and to mixtures thereof such as, for example, the corresponding
racemate.
The pure stereoisomers of the compounds of the formulae I and la can be prepareddirectly by known methods or analogously to known methods, or they can be resolved
later.
The compounds of the formulae I and la can be prepared by known methods or
10 modifications thereof (seel for example, Rodd's Chemistry of Carbon Compounds, S.
Coffey, M. F. Anse!l (Editor); Elsevier, Amsterdam, 1989; Vol. IV Part IJ, p. 301-311.
Heterocyclic Compounds. R. C. Elderfield (Editor); Wiley, New York, 1957; Vol. 6, p.
491-495)-
The present invention furthermore includes a process for the preparation of
15 compounds of the formulae I and la as explained in 1) - 4) above, which comprises
A) for preparing compounds of the formula I where X is oxy~en and the radicals R1,
R2, R3, R4 and R5 are as defined under 1) - 4), reacting a compound of the formula ll
H
I
R n~N~R 3 ( I I )
R 4
with the definitions mentioned under 1) - 4) applying to R1, R3 and R4, with a
compound of the ~ormula lll
R-Z
;
2 ~
29
where R has the meanings for R5 and R2 which have been mentioned above under 1) -
4) with the exception of hydrogen, hydroxyl, Cl-C6-alkoxy, aryloxy, C1-C8-acyloxy,
amino, C,-C6-alkylamino, di(C1-C;6-alkyl)amino, arylamino and C,-C6-acylamino, and Z is
a leaving group,
5 or
B) preparing compounds of ~he formula I where X is sulfur and R1, R2, R3, R4 and R5
are as defined under 1) - ~) by reacting a compound of the formula I where X is
oxygen and the definitions mentioned under 1) - 4) apply ~o R1, R2, R3, R4 and Rs, with
a sulfurizing reagent,
10 or
C) preparing compounds of the formula la where X and the radicals R1 to Rs are as
defined under 1) - 4), by reacting a compound of the formula IV
H
I
R ~ ~N~R 3 ( I V
R 4
R 5
20 or
R 1 n~N~ ( I V ~ )
R 4
R5
where the definitions mentioned under 1) - 4) apply to R1, R3, R4 and R5, with acompound of the tormula lll
2~3
R2 z (111)
where the definitions described under 1) - 4) for formula I and la apply to R2, with the
exception of hydrogen, hydroxyl, C1-C8-alkoxy, aryloxy, C1-C6-acyloxy, amino,
5 C,-C6-alkylamino, di(C1-C6-alkyl)amino, arylamino or C1-C6-acylamino, and Z is a
leaving group,
or
D) preparing compounds of the formula I where X is oxygsn and the radicals R' to Rs
are as defined under 1) - 4) by cyclizing a compound of the formula V
R2
I
R n ~ J~R 3 V
1 5
R
where R' to Rs are as defined under 1) - 4) and Y is hydroxyl, C1-C4-alkoxy, optionally
halogenated C,-C4-acyloxy, chlorine, bromine or iodine,
or
20 E) preparing compounds of the formula i where X is oxygen, R4 and R5 are hydrogen
and the definitions mentioned under 1) - 4) apply to R1 to R3, from the quinoxalinones
of the formula Xl
R 2
25 R ~N~R 3 X I
where R1 to R3 are as defined under 1) - 4), by addition of hydrogen on the C=N
30 bond,
2 ~
31
or
F) preparing compounds of the formuia I where X is oxygen and Rl to R5 are as
defined under 1) - 4), from compounds of the formula Vl
R 2
R 1 ~N H V I
N H
1 5
where R1, R2 and R5 are as defined under 1) - 4), by reacting them with chloroform or
bromoform and a carbonyl cornpound of the formula Xlll
R3-Co-R4 Q(lll)
where R3 and R4 are as defined under 1) - 4), or with a-(trihalomethyl)alkanols of the
formula XIV
Hal3C-C(OH)-R3R4 (XIV)
where Hal is Cl, Br or 1,
in which R3 and R4 are as defined under 1) - 4),
25 or
G) praparing compounds of the formula I where X is oxygen and R', R2, R3, R4 and R5
are as defined under 1) - 4), by reacting a compound of the formula I where X isoxygen and the definitions mentioned und0r 1) - 4) apply to Rl, R2, ~5 and to R3 and
R4, with the exception that at least one of the radicals R3 or R4 is hydrogen; ~ith an
30 alkylating reagent of the formula XV
2 ~
32
R'-Z (XV)
where R' has the meanings mentioned above for R3 and R4 with the exception of
hydrogen and Z is a leaving group,
or
H) preparing compounds of the formula I where X is oxygen, Rl, R2, R3 and R4 are as
defined under 1) - ~) and R5 is C1-C8-alkyl, optionally substituted by fluorine, chlorine,
bromine, iodine, hydroxyl, C1-C6-acyloxy, benzoyloxy, pheno)~y, C1-C6-alkoxy,
C1-C6-alkylamino, di(C1-C6-alkyl)amino, C1-C6-alkylthio, cyano, carboxyl, carbamoyl,
C3-C8-alkenyl, optionally substitut~d by fluorine, chlorine, bromine, iodine, hydroxyl,
Cl-C6-acyloxy, benzoyloxy, phenoxy, C:l-C6-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, Cl-C6-alkylthio, cyano, carboxyl or carbamoyl, C3-C8-alkynyl,
optionally substituted by fluorine, chlorine, bromine, iodine, hydroxyl, C1-C6-acyloxy,
benzoyloxy, phenoxy, C1-C6-alkoxy, C1-C6-alkylarrlino, di(Cl-C6-alkyl)amino, C1-C6-alkyi-
1~ thio, cyano, carboxyl or carbamoyl, C4-Ca-cycloalkyl, optionally substituted by fluorine,
chlorine, bromine, iodine, hydroxyl, C1-C6-acyloxy, benzoyloxy, phenoxy, Cl-C6-alkoxy,
C1-C6-alkylamino, di(Cl-C6-alkyl)amino, C,-C6-alkylthio, cyano, carboxyl or carbamoyl,
Cs-C6-cycloalkenyl, optionally substituted by fluorine, chlorine, bromine, iodine,
hydroxyl, C1-C6-acyloxy, benzoyloxy, phenoxy, Cl-C6-alkoxy, C,-C;6-alkylamino,
C1-C6-dialkylamino, Cl-C6-alkylthio, cyano, carboxyl or carbamoyl, (C1-C6-alkoxy)-
(C1-C6-alkyl), di(C1-C6-alkylamino)-(C1-C6-alkyl) or (C3-C6-cycloalkyl)alkyl, (C6-C8-cyclo-
alkenyl)alkyl, or arylalkyl, naphthylalkyl or heteroarylalkyl, each of which is substituted
by up to five radicals R6 which are independent of one another, it being possible for
the alkyl radical to contain in each case 1 to 3 oarbon atoms,
by reductive alkylation of a compound of the formula I where R5 is hydrogen and X is
oxygen and the definitions mentioned under 1) - 4) ~pply to R1, F~2, R3 and R4, with a
carbonyl compound of the formula XVI,
2~5~5
33
R''-C(=O)-R''' (XVI)
whsre R" and R~" are identical or different and independently of one another arehydrogen, C,-C7-alkyl, optionally substituted by fluorine, chlnrine, bromine, iodine,
hydroxyl, C,-C6-acyloxy, benzoyloxy, phenoxy, C1-C6-alkoxy, Cl-C6-alkylamino,
ditC.-C6-alkyl)amino, C1-C6-alkylthio, cyano, carboxyl or carbamoyl, C3-C7-alkenyl,
optionally substituted by fluorine, chlorin~, bromine, iodine, hydroxyl, C,-C6-acyloxy,
benzoyloxy, phenoxy, C,-C6-alkoxy, Cl-C5-alkylamino, di(C,-C6-alkyl)amino,
C1-C6-alkylthio, cyano, carboxyl or carbarnoyl, C3-C7-alkynyl, optionally substituted by
fluorine, chlorine, bromine, iodine, hydroxyl, C,-C;6-acyloxy, benzoyloxy, phenoxy,
C,-C6-alkoxy, C,-C6-alkylamino, di(C,-C6-alkyl)amino, C,-C6-alkylthio, cyano, carboxyl or
carbamoyl, C4-C8-cycloalkyl, optionally substituted by fluorine, chlorine, brornine,
iodine, hydroxyl, C1-C6-acyloxy, benzoyioxy, phenoxy, C,-C6-alkoxy, C1-C6-alkylamino,
di(C1-C6-alkyl)arnino, C,-C6-alkylthio, cyano, carboxyl or carbamoyl, C6-cycloalkenyl,
optionally substituted by fluorine, chlorine, bromine, iodine, hydroxyl, C,-C6-acyloxy,
benzoyloxy, phenoxy, C1-C6-alkoxy, Cl-C6-alkylamino, di(C,-C5-alkyl)amino,
Cl-C6-alkylthio, cyano, carboxyl or carbamoyl, (C,-C6-alkoxy)-(C,-C5-alkyl),
[di(C1-C6-alkyl)aminol-(C1-Cs-alkyl) or (C4-C6-cycloalkyl)alkyl, (C6-cyeloalkenyl)alkyl, or
arylalkyl, naphthylalkyl or heteroarylalkyl, each of which is substituted by up to five
radicals R5 which are independent of one another, it being possible for the alkyl radical
to contain in each case 0 to 2 carbon atoms,
and where R" and R"' can be linked to each other to form a 4- to 8-membered ring,
or
1) preparing compounds of the formula I where X is oxygen and R1, R2, R3 and R4 are
as defined under 1) - 4) and R5 is Cl-C8-alkyloxycarbonyl, C1-C8-alkylthiocarbonyl,
C2-C~-alkenyloxycarbonyl, C2-C8-alkenylthiocarbonyl, C2-C8-alkynyloxycarbonyl,
Cl-C6-alkylaminocarbonyl, C3-C6-alkenylaminocarbonyl, di(C,-C6-alkyl)aminocarbonyi,
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl-, 4-methylpiperæin-1-ylcarbonyl,
optionally substituted by fluorine, chlorine, bromine3 iodine, cyano, amino, mercapto,
30 hydroxyl, C1-C6-acyloxy, ben~oyloxy, benzyloxy, phenoxy, C1-G6-alkoxy,
.
2 ~
34
C1-C6-alkylamino, di(Cl-C6-alkyl)amino, C1-C6-alkylthio, C1-C6-alkylsulfonyl, phenylsul-
fonyl, oxo, thioxo, carboxyl or carbamoyl;
or aryloxycarbonyl, arylthio(carbonyl), arylaminocarbonyl, heteroaryloxycarbonyl,
heteroary!thiocarbonyl, hateroarylaminocarbonyl, arylalkyloxycarbonyl, (aryl-
5 alkylthio)carbonyl, arylalkylaminocarbonyl, heteroalkyloxycarbonyl,
(heteroalkylthio)carbonyl or heteroalkylaminocarbonyl, each of which is substituted by
up to five radicals R6 which are independent of one another, it being possible for the
alkyl radical to contain in each case 1 to 3 carbon atoms, by reacting a compound of
the formula X\/ll
R2
n ~ ~R 3 )( V I I
to
( C H 2 ) n
where the definitions mentioned under 1~ - 4) apply to R1, p~2, R3 and R4, n is 0, 1, 2 or
2a 3, X is oxygen and U is a leaving group, with a compound of the formula XVIII
Nu-H ~NIII)
where Nu is Cl-C8-alkoxy, C2-Ca-alkenyloxy, C2-C~-alkynyloxy, C,-CB-alkylthio,
C2-C8-alkenylthio, Cl-C8-alkylamino- and di(C,-Ca-alkyl)amino, C2-C8-alkenylamino- and
di(C1-C6-alkyl)amino, optionally substituted by fluorine, chlorine, bromine, hydroxyl,
C,-C4-alkoxy, C1-C4-alkylamino, di(C1-C4-alkyl)amino, C,-C4-alkylthio,
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl- or 4-methylpipera~in-1-ylcarbonyl,
optionally substituted by C,-C4-alkyl, C2-C~-alkenyl, C,-C4-acyl, oxo, thioxo, carboxyl or
phenyl, or aryloxy, arylthio, arylamino, arylalkyloxy, arylalkylthio, arylalkylamino,
2 ~
heteroaryloxy, heteroarylthio, heteroarylamino, heteroarylalkyloxy, heteroarylalkylthio or
heteroarylalkylamino, each of which is ~ubstituted by up to five radicals R6 (R6 is as
defined at the outset) which are independent of one another, it being possible for the
alkyl radical to contain in aach oase 1 to 3 carbon atoms.
The abovementioned method A preferably proceeds under the following conditions:
The substituent Z in formula lll is a suitable leaving group such as, for example,
chlorine, bromine or iodine, a suitable radical of sulfuric acid, an aliphatic or aromatic
10 sulfonate, or optionally halogenated acyloxy.
The reaction is expediently carried out in an inert sol\lent. Suitable solvents are, for
example, aromatic hydrocarbons such as toluene or xylene, lower alcohols such asmethanol, ethanol or 1-butanol, ethers such as tetrahydrofuran or glycol dimethyl
15 ether, dipolar aprotic solvents such as N,N-dimethylforrnamide, N-methyl-2-pyrro-
lidone, acetonitrile, nitrobenzene, dimethyl sulfoxide, or mixtures of these solvents.
Two-phase systems with aqueous solutions of bases in the presence of a phase
transfer catalyst such as, for example, benzyltriethylammonium chlorid~, are also
possible.
The presence of a suitable base, for example of an alkali metal carbonate, alkali metal
hydrogen carbonate, alkaline earth mstal carbonate or alkaline earth rnetal hydrogen
carbonate such as sodium carbonate, calcium carbonate or sodium bicarbonate, of an
alkali metal nydroxide or alkaline earth metal hydroxide such as potassium hydroxide
25 or barium hydroxide, an alcoholate such as sodium ethanolate or potassium tert.-
butylate, an organolithium compound such as butyllithiurn or lithiumdiisopropylamine,
an alkali metal hydride or alkaline earth rnetal hydride such as sodium hydride or
calcium hydride, an alkali metal fluoride such as potassium fluoride, or an organic
base such as triethylamine or pyridine for scavenging the acid which is liberated
30 during tne reaction, may be expedient.
2 ~
In some cases, the addition of an iodide, for example potassium iodide, is expedicnt.
The reaction is generally carried out at temperaturss between -10 and 160C,
preferably at room ternperature.
5 To carry out this reaction, any nucleophilic substihJents such as, ~or example,
hydroxyl, mercapto or amino groups, with the exGeption o~ the 1- and/or 4-position in
compounds of the formula ll or lll, must, before the reaction is carried out, bederivatized in a suitable manner or provided with conventional protective groups such
as, for example, acetyl or benzyl, which can then be eliminated.
The sulfurizing reagent which is preferably used for the reaction as described above
under B) is 2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide
(Lawesson's reagent), bis(tricyclohexyltin) sulfide, bis(tri-n-butyltin) sulfide,
bis(triphenyltin) sulfide, bis(trimethylsilyl) sulfide or phosphorus pentasulfide. The
15 reaction is carried out expediently in an organic solvent or in a solvent mixture, at
room temperature or above, preferably at the boiling point of the reaction mixture, and,
if possible, under anhydrous conditions. Suitable substances are, for example, carbon
disulfide, toluene, xylene, pyridine and 1,2-dichloroethane. If the tin sulfides or silyl
sulfides which have been mentioned are used, it is advisable to carry out the
20 sulfurization reaction in the presence of a Lewis acid, such as boron trichloride.
In the presence of other carbonyl groups in a compound of the formula 1, for example
in a compound where X is oxygen and one or more radicals p~1 to R6 are acyl, thecarbonyl is to be protected by known methods prior to the sulfurization reaction by a
25 suitable protective group, for example by acetalization; subsequent elimination of the
protective groups results in the desired compound.
For the reaction described above under C, the substituent Z is a suitable leaving
group, preferably chlorine, bromine or iodine, a suitable radical o~ sulfuric acid, an
30 aliphatic or aromatic sulfonate, or optionally halogenated acyloxy.
2~$~
The reaction conditions for this reaction correspond to those of method A.
The cyclization described under D) is effec~ed in a suitabie solvent such as rnethanol,
ethanol, N,N-dimethylformamide or N-methylpyrrolidone, in the pres~nce of a base;
S suitable bases are alkali metal carbonates, alkali nnetal hydrogen carbonates, alkaline
earth metal carbonates or alkaline earth metal hydrogen carbonates such as sodium
carbonate, calcium carbonate or sodium bicarbonate, alkali metal hydroxides or
alkaline earth metal hydroxides such as potassium hydroxide or bariu~ hydroxide,alcoholates such as sodium ethanolate or potassium tert.-butylate, organolithium10 compounds such as butyllithiurn or lithium diisopropyiamine, aikali metal hydrides or
alkaline earth metal hydrides such as sodium hydride or calcium hydride, or an
organic base such as triethylamine or pyridine - the latter substances can also be
used as solvents, or organic or mineral acids such as glacial acetic acid, trifluoroacetic
acid, hydrochloric acid or phosphoric acid. The reaction is preferably earried out at
temperatures between 20 and 120C, particularly preferably at room temperature.
The compounds of the formula V, where R1 to R5 and Y are as defined under 1) - 5),
can be obtained from compounds of the formula Vl
R 2
R n~N H V I
1 5
where R', R2 and R5 are as defined under 1) - 4), by alkylation with a compound of the
formula Vll
~.
.
2~$~
38
C O - Y
kR3 V I I
Z R4
5 where R3, R4 and Y are as defined under 1) - 5) and Z is as defined undar A). The
reaction conditions for this alkylation correspond to those given in method A.
Simultaneous cyclization to give the dihydroquinoxaline of the formula I takes place
under suitable conditions.
10 Compounds of the formula V in which R', R3 to R5 and Y are as defined under 1) - 5)
and R2 is hydrogen can also be prepared from compounds of the formula Vlll
R 1 ~XN/~ ( V I I I )
I R 4
where R', R3 to Rs and Y are as defined under 1) - 5) by reducing ~he nitro group by
known processes to the amino group.
Simultaneous cyclization to give the dihydroquinoxaline of the formula I takes place
under suitable conditions, for example by carrying out the reduction in the presence of
an acid.
The reduction is carried out by standard methods (see, for example, Methoden derOrganischen Chemie [Methods in Organic Chemistry3 (Houben-Weyi), E. Muller
(Editor); G. Thieme Verlag, Stuttgart, 1957; Vol. Xl/1, p. 360-490), for example using
tin(ll) chloride in glacial acetic acid, TIC13 in hydrochloric acid, or by catalytic
hydrogenation, the choice of reagent being determined by the chemical stability of the
various substituents R1 and R3 to R5; if, for example, one of the radicals is alkenyl, the
first method will be selected to obtain the double bond.
;
39
The phenylenediamines of the formula Vl which are required as startin0 materials for
the syntheses described are known from the literature or commercially available or can
be synthesized by methods known from the literature.
5 N-ortho-nitrophenylamino acid derivatives of the formula Vlll, where Rln and R3 to Rs
are as defined under 1) - 4) and Y is oR7, where R7 is hydrogen, C,-C6-alkyl, optionally
in each case for example halo~en-substituted phenyl, benzyl or 9-fiuorenylmethyl, can
be obtained for example by amination of ortho-halonitro aromatic substances of the
formula IX
,~N 2
R n~ t ll I x
~w
where R' is as defined under 1) - 4) and W is fluorine, chlorine, bromine or iodine, with
15 amino acids or their esters of the formula X
C O O R 7
~R~ X
HN R4
R S
where R3, R4, Rs and R7 are as defined under 1) - 5). The reaction can be carried out
in the presence of an inorganic or organic auxiliary base such as, for exampie, sodium
carbonate, potassium carbonate, sodium hydroxide or triethylamine. It is
advantageous to use an inert solvent at temperatures between 0 and 150C,
preferably at reflux temperature. Suitable solvents are open-chain or cyclic ethers, for
example tetrahydrofuran or glycol dimethyl ether, aromatic hydrocarbons, for example
toluene or chlorobenzene, alcohols, for example ethanol, isopropanol or glycol
monomethyl ether, dipolar aprotic solvents, for ~xample N,N-dimethylformamide,
N-methyl-2-pyrrolidone or 1,3-dimethyl-tetrahydro-2~1H)-pyrimidinone.
2 ~
The N-ortho-nitrophenylamino acids of the formula Vlll where Y is hydroxyl can, if
desired or necessary, be converted by well-known standard methods into the acid
derivatives of the formula Vlll where Y is hydroxyl, C:1-C4-alkoxy, optionally halogenated
C,-C4-acylo)ty, chlorine, bromine or iodine.
Ortho-halonitroaromatic compounds of the formula IX and amino acids of the formula
X are known from the literature and comrnercially available or can be prepared by
methods known from the literature.
The reaction described above under E) is preferably effected by means of catalytic
hydrogenation (using hydrogen) or hydrosilylation (using alkylsilanes, for example
diphenylsilane) in the presence of a hydrogenation ca~alyst, for example Raney nickel
or palladium-on-charcoal, at a hydrogen pressure of 1 to 5 bar, or by means of areducing agent from the class of the complex metal hydrides such as sodium
borohydride or sodium cyanoborohydride, or using metals, or metal salts, and acid
such as, for example, zinc/glacial acetic acid or SnCI2/HCI. It is advantageous to carry
out the reaction in an inert solvent such as lower aicohols, for example methanol or
isopropanol, ethers such as tetrahydrofuran or glycol dimethyl ether, dipolar aprotic
solvents such as N,N-dimethylformamide, aromatic hydrocarbons such as toluene orxylene, or mixtures of these solvents, at temperatures between -20 and 100C,
preferably at room temperature.
If a chiral hydrogenation catalyst, for example di-/u-chloro-bis[(cycloocta-1c,5c-diene~-
rhodium(l)]/(~) or (-)-4,5-bis-(diphenylphosphinomethyl)-2,2-dimethyl-1,3-dioxolane, or
a chiral complex metal hydride, for example sodium tris-lN-benzyloxycarbonyl-
L-prolinoyloxy)-borohydride, are used in the above-described reaction, the individual
enantiomers can be prepared selectively.
If, in compounds of the formula Xl, substituents are present which can be
hydrogenated or reduced under the above-described conditions, for example oxo, it is
2 ~
41
necessary to use an intermediate of the formula Xl with substituents which are not
attacked, but which can be derivatized to give the ~roup raquired, for example
hydroxyl. The substituents can also be provided with a customary protective group, for
example an acetal protective group, which can then be removed after th~ above-des-
5 cribed reaction.
Quinoxalinones of the formula Xl where R1 to R3 are as defined uncler 1) - 4) can be
obtained by known processes by condensing a phenylenediamine of the formula Vl,
where R' and R2 are as defined under 1) - 43 and Rs is hydrogen, with an alpha-
10 ketocarboxylic acid of the formula Xll
R3-Co-CooH (Xll)
where R3 is as defined under 1) - 4).
15 The reaction is expediently carried out in an inert solvent in a temperature range of
between 0 and 150C; examples of suitable solvents are alcohols, for exampls ethanol
or isopropanol, open-chain or cyclic ethers, for example glycol dimethyl ether or
tetrahydrofuran, or dipolar aprotic solvents, for example N,N-dimethylformamide or
acetonitrile.
2~
The reaction described above under F) is expediently carried out in a two-phase
system cornposed of an organic solvent or solvent mixture which is not miscible with
water, composed of, for example, halogenated hydrocarbons, for example
dichlorornethane or 1,2-dichloroethane, or aromatic hydrocarbons, for example toluene
25 or xylene, and a concentrated aqueous solution of an alkali metal hydroxide or alkaline
earth metal hydroxide, for example sodium hydroxide or barium hydroxide. The
presence of a phase transfer catalyst such as, for example, benzyltriethylammonium
chloride or tetrabutylammonium ~romide, is advantageous.
The reaction is usuaily carried out at temperatures be~ween 0 and ~0C, preferably at
30 room temperatur~.
2 ~
42
Substituents in compounds of th~ ~ormulae Vl and Xlll, or XIV, which arc not stable
under the reaction conditions must be replaced by those which can be derivatized to
the required group. The substituents can also be provided with a customary protective
group which can then ~e removed a~ter the above-described reaction.
In the reaction described above under G), Z in formula XV is a suitabl~ leaving group
such as, for example, chlorine, bromine or iodine, a suitable sulfuric acid radical, an
aliphatic or aromatic sulfonate, or optionally halogenated acyioxy.
10 The reaction conditions for this reaction correspond to those in method A.
The reaction described under H) is preferably effected by catalytic hydrog0nation
(using hydrogen) in the presence o~ a hydrogena~ion catalyst, for example palladium-
on-charcoal, at a hydrogen pressure of 1 to 5 bar, or by means of a reducing agent
15 from the class of the complex metal hydrides, such as sodium borohydride, sodium
triacetoxyb¢rohydride or sodium cyanoborohydride.
The reaction is expediently carried out in an inert solvent, sur,h as lower alcohols, for
example methanol or isopropanol, ethers, for example tetrahydrofuran or glycol
20 dimethyl ether, halogenated hydrocarbons, ~or example dichloromethane or
dichloroethane, at temperatures between -20 and 100C, preferably at room tempera-
ture. The presence of an acid such as, for example, acetic acid or trifluoroacetic acid,
or of a Lewis acid such as, for example, titanium tetrachloride, is advantageous. If, in
compounds of the formulae I and XVIJ substituents are pres~nt which can be
25 hydrogenated or reduced under the above-described conditions, for example oxo, the
use of an intermediate of the formulae I and XVI with substituents which are notattacked but which can be derivatized to the required group, for example hydroxyl, is
necessary. Acid-labile groups such as, for example, acetals, or groups which react
under the reaction conditions, such as, ~or exarnple, primary amines, are also ~o be
30 avoided or to be provided with a customary protective group.
2 ~
43
The reaction described under 1) is expediently carried ou~ in an inert solvent. Examples
of suitable solvents are aromatic hydrocarbons such as toluene or xylene, lower
alcohols such as methanol, sthanol or 1-butanol, ethers such as tetrahydrofuran or
glycol dirnethyl ether, dipolar aprotic solvents such as N,N-dimethylformamide,
N-methyl-2-pyrrolidone, acetonitrile, nitrobenzene, dimethyl suHoxid~, or mixtures of
these solvents. Two-phase systems wlth aqueous solutions of bases in the presence
of a phase transfer catalyst such as, for example, benzyltriethylamrnonium chloride,
are also possible.
The presence of a suitable base, for example an alkali metal hydroxide or alkaline
earth metal hydroxide such as potassium hydroxide or barium hydroxide, of an
alcoholate such as sodium ethanolate or potassium tert~-butylate, an or~anolithium
compound such as butyllithium or lithium diisopropylamide, an alkali metal hydride or
alkaline earth metal hydride such as sodium hydride or calciurn hydride, an alkali metal
fluoride such as potassium fluoride, or an organic base such as triethylamine orpyridine, may be useful. The reaction is usually carried out at temperatures between
-10 and 160C, preferably at room temperature.
To carry out this reaction, any nucleophilic ~ubstituents in compounds XVII and XVIII
which do not participate in the reaction, such as, for example, hydroxyl, mercapto or
arnino groups, are to be derivatized in a suitable manner or to be provided withcustomary protective groups such as, for example, acetyl or benzyl, which can then
be eliminated.
The compounds XVII which are required for the abovementioned reaction and in which
the definitions described under 1) - 4) apply to R', p~2, R3 and R4, n is 0, 1, 2 or 3, X is
oxygen and U is a suitable leaving group, halogen ~uch as, for example, chlorine,
bromine, iodine, a halogenated aliphatic or aromatic alcoholate such as, for example,
2,2,2-trichloroethoxy, chlorophenoxy, or ~ heterocycle which is linked via nitro~en such
as, for example, imidazolyl, tria~olyl or benzotri~olyl, are prepared by reac~ing a
compound of the formula I where Rs is hydrogen and X is oxygen, and the definitions
2 ~ 8 ~
~4
described under 1) - 4) apply to R', R2, R3 and R4, with a suitable carbonic acid
derivative, for exarnple phosgene, diphosgene, triphosgene, trichloroethyl
chloroformate or carbonyldiimidazole, or with a suitable halo carbonyl halide, for
example bromoacetyl chloride.
The reaction is expediently carried out in an inert solvent. Examples of suitable
solvents are aromatic hydrocarbons such as toluene or xylene, ethers such as
tetrahydrofuran or glycol dimethyl ether, or halogenated hydrocarbons such as
dichloromethane or dichloroethane.
The presence of a suitable base, for exarnple of an alkali metal hydroxide or alkaline
earth metal hydroxide, such as potassium hydroxide or bariurn hydroxide, or an
organic base such as triethylamine or pyridine, may be useful.
15 The reaction is usually carried out at temperatures be~veen -30 and 16ûC, preferably
at room temperature.
The present invention furthermore relates to the compounds as described under
1) to 4) as pharmaceuticals, preferably for treating viral diseases, in particular diseases
20 caused by HIV.
The invention furthermore relates to pharrnaceuticals comprising at least one
compound according to the invention, and to the use of the abovementioned
compounds for the preparation of pharmaceuticals, preferably for the treatment of viral
25 diseases, in particular for the treatment of diseases caused by HIV.
The present invention furthermore relates to the use of compounds of the
abovementioned formula I or IA in which
2 ~ 8 ~
~5
n is zero,
one,
two,
three
or four,
the individual substituents R1 independentiy of one another are
fluorine, chlorine, bromine, iodine, ~rifluoromethyl, trifluoromethoxy, hydroxyl,
C,-C8-alkyl, Cs-C8-cycloalkyl, C1-C6-alkoxy, (C,-C6-alkoxy)-(Cl-C;4-alkXY3,
C1-C6-alkylthio, C1-C6-alkylsulfinyl, C1-C6-alkylsulfonyl, nitro, amino, æido,
C1-C6-alkylamino, di(C,-C6-alkyl)amino, piperidino, morpholino, 1-pyrrolidinyl,
4-methylpiperazinyl, thiomorpholino, imidazolyl, triazolyl, tetrazolyl, C1-C6-acyl,
C1-C6-acyloxy, C1-C6-acylamino, cyano, carbamoyl, carboxyl,
(C,-C6-alkyl)oxycarbonyl, hydroxysulfonyl, sulfamoyl
1~
or
a phenyl, phenoxy, phenoxycarbonyl, phenylthio, phenylsulfinyl, phenylsulfonyl
phenoxysulfonyl, phenylsulfonyloxy, anilinosulfonyl, phenylsulfonylamino,
benzoyl, 2-pyridyl, 3-pyridyl or 4-pyridyl radical which is substituted by up to five
radicals R6 which are independent of one another,
where R6 can be
fluorine, chlorine, bromine, iodine, cyano, trifluoromethyl, trifluoromethoxy, nitro,
amino, azido, C,-C6-alkyl, C3-C8-cycloalkyl, C1-C:6-alkoxy, C1-C6-alkylthio,
C1-Cs-alkylsulfinyl, C;l-C6-alkylsulfonyl, C,-/:~6-alkylamino, di(C,-C6-alkyl)amino,
(Cl-C6-alkyl)oxycarbonyl, phenyl, phenoxy, 2-, 3- or 4-pyridyl,
2~6~5
46
R2 and R5 are identical or different and independently of
one another are
hydrogen, hydroxyl, C,-C6-alkoxy, aryloxy, Cl-C6-acyloxy, cyano, amino,
C,-C6-alkylamino, di(C1-C6-alkyl)amino, arylamino, C,-C~-acylamino, Cl-C8-alkyl,optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyi,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, Cl-C~-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, C,-C6-alkylthio, C,-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
C2-Ca-alkenyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C6-alkoxy, C1-C6-alkylamino,
di(C,-C6-alkyl)amino, C,-C6-alkylthio, C1-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl and carbamoyl;
C3-C~,-allenyl, optionally substituted by fluorine, chlorine or hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C3-CB-alkynyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, m0rcapto, hydroxyl,
C,-Cs-acyloxy, benzoyloxy, ben7yloxy, ph~noxy, Cl-C6-alkoxy, Cl-C6-alkylamino,
di(C1-C6-alkyl)amino, C,-C6-alkylthio, C1-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
C3-CB-cyClOalkyl~
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
2~3~
~7
Cl-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C6-alkoxy, C,-C~-alkylamino,
di(C,-C6-alkyl)amino, C1-C6-alkylthio, Cl-C6-alkylsulfonyl, phenylsuKonyl, oxo,
thioxo, carboxyl or carbamoyl;
C3-C8-cycloalkenyl,
optionally substituted by
fluorine, chiorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C;6-alkoxy, Cl-C6-alkylamino,
di(C1-C6-alkyl)amino, C1-C6-alkylthio, C1-C6-alkylsulfonyl, phenylsul~onyl, oxo,thioxo, carboxyl or carbamoyl;
(C3-C8-cycloalkyl)-(C1-C4-alkyl),
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C6-alkoxy, C1-C6-alkylamino,
di(C1-C6-alkyl)amino, C1 C6-alkylthio, Cl-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
(C3-C8-cycloalkenyl)-(C1-C4-alkyl),
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C:1-C6-alkoxy, C,-C6-alkylamino,
di(C1-C6-alkyl)amino, C1-C6-alkylthio, C1-C6-alkylsuHonyl, phenylsulfonyl, oxo,
thioxo, carboxyl or carbamoyl;
~5
C1 -C6-alkylcarbonyl,
optionally substituted by
fluorine, chlorine, bromine, iodine, cyano, amino, mercapto, hydroxyl,
C1-C;6-acyloxy, benzoyloxy, benzyloxy, phenoxy, C1-C6-alkoxy, C1-C6-alkyiamino,
2 ~
48
di(C1-C6-alkyl)amino, C1~C6-alkylthio, C1-C6-alkylsulfonyl, phenylsulfonyl, oxo,thioxo, carboxyl or carbamoyl;
C2-C8-alkenylcarbonyl, optionally substituted by fluorine, chlorine or hydroxyl,C,-C4-alkoxy, oxo, phenyl;
(C3-C8-cycloatkyl)carbonyl, op~ionally substituted by fluorine, chlorin~ or
hydroxyl, C1-C4-alkoxy, oxo, phenyl;
(C5-C8-cycloalkenyl)carbonyl, optionally substituted by fluorine~ chlorine or
hydroxyl, C1-C4-alkoxy, oxo, pnenyl;
(C3-Ca-cycloalkyl)-(C1-C3-alkyl)carbonyl, optionally substituted by fluorine,
chlorine or hydroxyl, C1-C4-alkoxy, oxo, phenyl;
(C5-C6-cycloalkenyl)-(Cl-C3-alkyl)carbonyl, optionally substituted by fluorine,
chlorine or hydroxyl, C1-C4-alkoxy, oxo, phenyl;
C1-C8-alkyloxycarbonyl, optionally substituted by fluorine, chlorine, bromine,
hydroxyl, C1-C4-alkoxy, C1-C4-alkylamino, di(Cl-C4-alkyl)amino, C1-C4-alkylthio;
C2-Ca-alkenyloxycarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
C,-C4-alkoxy, oxo, phenyl;
C2-Ca-alkynyloxycarbonyl, op~ionally substituted by fluorine, chlorine, hydroxyl,
C1-C4-alkoxy, oxo, phenyl;
C1-C8-alkylthiocarbonyl, optionally substituted by ~luorine, chlorine, hydroxyl,C1-C4-alkoxy, oxo, phenyl;
2 ~
49
C2-C0-alkenylthiocarbonyl, optionally substituted by fluorine, chlorine, hydroxyl,
Cl-C4-alkoxy, oxo, phenyl;
C1-C~-alkylarnino- and di(C1-Ca-alkyl)aminocarbonyl, in each cas~ optionally
substituted by fluorine, chlorine, hydroxyl, Cl-Cs-alkoxy, oxo, phenyl;
pyrrolidin-1-yl, morpholino-, piperidino-, piperazinyl-, or 4-methylpiperazin-
1-ylcarbonyl, in each case optionally substituted by C1-C4-alkyl, C2-~6-alkenyl,C,-C4-acyl, oxo, thioxo, carboxyl, or phenyl;
C2-C8-alkenylamino- and di(C1-C6-alkenyl)aminocarbonyl, in each case optionally
substituted by fluorine, chlorine, hydroxyl, C1-C,,-alkoxy, oxo, phcnyl;
C1-C6-alkylsulfonyl, optionally substituted by fluorine, chlorine, hydroxyl, C~-C4-
alkoxy, oxo, phenyl;
C1-C6-alkenylslilfonyl, optionally substituted by fluorine, chlorine, hydroxyl, Cl-C4-
alkoxy, oxo, phenyl;
or aryl, arylcarbonyl, aryl(thiocarbonyl), (arylthio)carbonyl, (arylthio)thiocarbonyl,
aryloxycarbonyl, arylaminocarbonyl, (arylamino)thiocarbonyl,
arylalkylaminocarbonyl, arylsulfonyl, arylalkyl, arylalkenyl, arylalkynyl, arylalkyl-
carbonyl, arylalkenylcarbonyl, ary1alkoxycarbonyl or aryl~alkylthio)carbonyl, each
of which is substituted by up to five radioals R6 which are independent ~f one
another, it being possible for the alkyl radical to contain in each case 1 to 5
carbon atoms, and R6 being as defined above,
or heteroaryl, heteroarylalkyl, heteroarylalkenyl, heteroarylalkylcarbonyi or
heteroarylalkenylcarbonyl, heteroaryloxycarbonyl, (het~roarylthio)carbonyl,
heteroarylaminocarbonyl, heteroarylalkyloxycarbonyl,
2 ~
heteroaryl(alkylthio)carbonyl or heteroarylalkylaminocarbonyl, each of which is
substituted by up to three radicals R6 which are independent of one another, it
being possible for the alkyl radical to contain in each case 1 to 3 carbon atoms,
R3 and R4 are identical or different and, independently of
one another~ are hydrogen, C,-C8-alkyl which is optionally substituted by
fluorine, chlorine, hydroxyl, amino, mercapto, C,-C~-acyloxy. benzoyloxy,
benzyloxy, phenoxy, C,-C4-alkoxy, C1-C4-alkylamino, di~C1-C4-alkyl)amino, Cl-C"-alkylthio, Cl-C4-alkylsulfonyl, Cl-C4-alkylsulfinyl, carboxyl or carbarnoyl;
C2-C8-alkenyl, optionaliy substituted by fluorine or chlorine, hydroxyl, amino,
mercapto, C1-C4-acyloxy, benzoyloxy, ben~yloxy, phenoxy, Cl-C4-alkoxy, Cl-C4-
alkylamino, di(Cl-C4-alkyl)amino, C1-C4-alkylthio, C,-C4-alkylsulfonyl, Cl-C4-
alkylsulfinyl, carboxyl or carbamoyl;
C3-Ca-cycloalkyl, optionally substituted by fluorine, chlorine, hydroxyl, amino,mercapto, C,-C4-acyloxy, benzoyloxy, benzyloxy, phenoxy, C,-C4-alkoxy, C,-C4^
alkylamino, di(C,-C4-alkyl)amino, Cl-C4-alkylthio, C,-C4-alkylsulfonyl, C,-C4-
alkylsulfinyl, carboxyl or carbamoyl
C3-CB-cycloalkenyl, optionally substituted by fluorine or chlorine, hydroxyl,
amino, mercapto, C,-C~,-acyloxy, benzoyloxy, bsnzyloxy, phenoxy, C,-C4-alkoxy,
C,-C,,-alkylamino, di(Cl-C4-alkyl)amino, S:~l-C4-alkylthio, Cl-C4-alkylsulfonyl, Cl-C4-
alkylsulfinyl, carboxyl or carbamoyl;
aryl, arylalkyl, heteroaryl or heteroarylalkyl, each of which is substituted by u,p to
five radicals R6 which are independent of one another, it being possibi~ for thealkyl radical to contain 1 to 3 carbon atoms in eaeh case, and R6 bein0 as
defined above,
2 ~
R3 and R4 or R3 and R5 can furthermore aiso be
part of a saturated or unsaturated carbo- or heterocyclic ring which has 3 to 8
carbon atoms and which can optionally be substituted by fluorine, chlorine,
hydroxyl, amino, C1-C6-alkyl, C2-CB-alkenyl, C2-C6-alkynyl, C,-C6-acyloxy,
benzoyloxy, C1-C6-alkoxy, oxo, thioxo, carboxyl, carbamoyl or phenyl,
X is oxy~en, sulfur, selenium or substituted nitrogen N-P~2, it being possible for R2
to have the abovementioned meanings,
for the preparation of pharmaceuticals ~or the treatment of viral diseases.
The compounds mentioned and elucidated above under 1)-4) are preferred for this
use.
The pharmaceuticals according to the invention can be aclministered enterally (orally),
parenterally (intravenously), rectally, subcutaneously, intramuscularly or locally
(topically).
They can be administered in the form of solutions, powders, (tablets, capsules
including microcapsules), ointments (creams or gels) or suppositories. Suitable
ad~uvants for such formulations are the liquid or solid fillers and extenders, solvents,
emulsifiers, glidants, flavorings, colorings and/or buffer substances which are
customary in pharmacology.
0.1 - 10, pr~erably 0.2 - 8 mg/kg of body weight are administered on~e or several
times daily as an expedient dosage. The dosage units used depend expediently on the
specific pharmacokinetics of the substanc~ usecl, or on the pharmaceutical formulation
used.
2 ~
For example, the dosage unit of the compounds according to the invantion is
1 - 1500 mg, preferably 50 - ~00 mg.
The compounds according ~o th0 invention can also be administered as a combination
with other antiviral agents such as, for example, nucleoside analogs, protease
inhibitors or adsorption inhibitors, immunostimulants, interferons, interleukins and
colony-stimulating factors ffor example (àM-CSF, G-CSF, M-CSF).
Activity tests
Test of preparations against HIV in cell culture
Description of method
Medium:
RMPI pH 6.8
Complete medium additionally contains 20% fetal calf serum and 40 IU/ml
recombinant interleukin 2.
Cells:
Lymphocytes which have been isolated frorn fresh donor blood by means of Ficoll~gradient centrifugation are cultured for 36 hours in complete medium with an addition
of 2 ,L~g/ml phytohemagglutinin ~IVellcome) at 37C under 5% of CO2. A~ter 10% of
DMSO has been added, the cells are frozen at a density of 5 106 and stored in liquid
nitrogen. For the test, the cells are defrosted, washed in RPMI medium and cul~ured
for 3 - 4 days in the complete medium.
Mixture:
The test preparations were dissolved in DMSO at a concentration of 16.7 mg/ml and
diluted in complete medium to 1 mg/ml.
0.4 ml of medium was introduced into 24-multiwell dishes. 0.1 ml of ~he dissolvsd
2~6~9~
53
preparation was ad~ed to the upper row of the dish, and, by transferring 0.1 ml
portions, a geometric dilution series was established. Controls without preparation
always contained 0.4 ml of complete medium containing 0.5% of DMSO. Lymphocyte
cultures with a cell density of 5 ~ 105 cells/ml were infected by adding 1/50 volume
5 supernatant from HlV-infected Iymphocyte cultures. The titer of these culture
supernatants was determined by end-point titration as 1 5 106 infectious units/ml.
After 30 minutes' incubation at 37C, the infected Iymphocytes were removed by
centrifugation and taken up in an equal volume of medium. From this cell suspension,
0.6 ml aliquots were transferred into all wells of the test plate. The mixtures were
10 incubated for 3 days at 37C.
Evaluation:
The infected cell cultures were examined under the microscope for the presence of
giant cells, which indicate active virus multiplication in the culture. The lowest
15 concentration of preparation where no giant cells were observed was de~ermined as
inhibitory concentration against HIV. As a control, the supernatants from the culture
plates were tested for the presence of HIV antigen with the aid of an HIV antigen test
following the manufacturer's instructions (Organon).
20 Results:
The results from this test are shown in Table 1.
Compound ofT-cell oulture assay
Examp e No. MIC (ug/ml)
~ .
IV >0,8
_ _
Vl-A 0,16
2~$~85
54
_ _
Compound of T-cell culture assay
Example No. MIC ~ug/ml)
Vl-B 20 ¦
Vl-C < 0,8
Vll < 0,16
11
X 0,8
Xll < 0,8
Xlll < 0,16
. . _ ^11
XIV <0,16
3-7 0,08
~1
3-21 0,16
. 11
3-23 0,08
3-24 0,08
3-25 0,4
3-26 0,4
._
3-29 < 9,4
3-30 . _
3-32 < 0,4
3-33 -- 0,4
3-36 < 2,0
3-44 ' __
2 ~ 5
_ ~ I
Compound of T-cell culture assay
¦ Example No. MIC Cug/ml)
3-48 <0,8
3-49 0,8
3-52 >0,8 __
1 3-53 > 0,8
3-57 < 0,8_
3-62 ~4,0
3-64 > 0,8
3-66 > 0,08
3-67 < 0,8
3-73 > 0,4
3-75 <0,8
3-76 ~ 0,08
3-80 ~,4
¦ 3-81 0,08
3-87 > D B
3-88 - 0,8
XX <4,0
6-1 0,4
6-16 0,3
56
Compound of T-cell cul~ure assay
Example No. ~ MIC ~g/ml)
6-17 < 0,8
6-19 < 0,8
6-20 <0,8
6-22 > 0,8
_
6-27 < 0,4
6-32 c 0,08
6-33 > 0,8
6-34 ` - < 0,4
¦ 6-35 < D,08
6 33 0,8
L 6-39 0,4
6-41 20
6-50 < 0,01
I
XXIII 0,01 l
7-1 < 0,16
7-2 < 0,01
_ I
7-3 ~ 0,01
I
1 7-7 0,04
¦ 7-1 D ~ 0,04
~7 2~p~
_._ . .
Compourld of T-cell culture assay
Example No. MIC ~ug/ml)
7-11 < 0,01
7-12 < 0,8 ¦
7-13 < 0,08
7-14 < 0,08
7-16 0,4
7-21 < 0,01
... . .. . _ ~
7-22 < 0,01
¦ 7-23 < 0,01
10-4 0,4
10^5 < 0,8
10-9 < 0,8 . .
_ . .. _ .. . . . _
10-10 0,08
10-13 0,08
10-14 < 0,8
10-17 0,~
10-18 ~ 0,8
10-20 _ ~_0,8
~0-2~ < 0,~ _
10-27 0,8
2~9~
58
. .__
Compound of T-cell culture assay
Example No. MIC Gug/ml)
10-28 < 0,8
11-1 < 0,8
11-2 > 0,8
11 4 < 0,8
._
11 -11 0,01
Assay of the substances for HIV reverse transcriptase inhibition
The activity of reverse transcriptase (RT) was determined with the aid of a scintillation
proximity assay (SPA).
The reagent kit for the RT-SPA was obtained from Amersham/Buchler (Braunschweig).
The enzyme RT (from HIV cloned in E. coli) originated from HT-Biotechnology Ltd,15 Cambridge, UK.
Mixture
The assay was carried out using the manufacturer's (Amersham) protocol manual,
20 with the following modifications:
- bovine serum albumin was added to the assay buffer to give an end
concentration of 0.5 mg/ml
2 9 ~
59
- the assay was carried out in Eppendorf reaction vessels, using 100 ~JI volume
per batch
- the manufacturer's RT concentrate (5000 U/ml) was diluted in Tris-HCI buffer
20 mM; pH 7.2; 30% of glycerol, to an activity of 15 U per ml
- the incubation time for ~he mixtures was 60 minutes (37C)
- after stopping ~he reaction and ~developing~ with the bead suspension, 130 ,ul of
mixture were transferred to 4.5 ml of Tris-HCI buffer, 10 mM; pH 7.4; 0.15 M
NaCI, and the tritium activity was measured by means of a -counter.
Assay
For a pre-assay for inhibitory activity, the substances were dissolved in DMSO (stock
solution c = 1 mg/ml), and tested as a 101, 102, 103, etc., dilution in DMSO.
To determine IC50 values, the inhibitor stock solutions were diluted further in Tris-HCI
buffer, 50 mM, pH 8, and tested in suitable concentrations.
The concentration corresponding to a 50% enzyme inhibition was determined from aplot of RT activity versus log Cjnh.
The test results are shown in Table 1a.
.
:
2 ~
Table 1a
Compound of Reverse Transcriptase Assay
Example No. ICso ~u9/ml)
V - ~ 7,5~
Vl-A 0,08
Vl-C 0,8
Vll 0,1
Xlll 0,04
I
XIV 0,16
3-23 0,1 -1
I _ _ _ I
3-24 0,1 - 1
3-25 0, 1 -1
I
1 3-29 0,1-1
3-30 0,025
3-32 approx. 0,1
_ _
3-36 0,1 -1
_ _
3-49 approx. 1
. _ _ _ _
3-57 approx. 1
3-75 ~ -
I _
1 3-76 0,~18
¦ 3-~1 approx. 1
. ~
2~98~
61
Compound ofReverse Transeriptase Assay
¦Example No.IG50 ~ug/ml)
6-1 approx. 1
I . . . .. I
6-8 0l1 -1
6-9 approx. 1
~_
6-16 approx. 1
6-1 7 0,1 -1
.. . .... . . ___
6-27 approx. 1
6-35 0,1 - 1
6-50 0,01-0,1
XXIII 0,025
7-1 0,08
7-2- --~-~ 0,07 ~
7-3 0,07
_ . _
7-7 0,1
7-10 0,1 1
7-11 0,01
. .. _ .
7-12 approx. 1
_ . e _ r
7-13 0,1 -1
_ .
7-16 appro)t. 1
10-9 _pprox.
2 ~ 5
62
_ ..... _ _ _
Compound of Reverse Transcriptase Assay
Example No. ICso ~ug/ml)
. .
10-10 approx. 1
. ............. _
10-13 approx. 1
10-17 approx. 1
.. _ I
10-18 0,1 -1
1 0-20 0, 1 -1
. .
1 0-21 0, 1 -1
10-27 0,1 -1
10-28 0,1 - 1
11-11 0,1 - 1
10-34 0,1 -1
11-6 0,1 - 1
0,1 - 1
11-7 approx. 1
11-13 approx. 1
1 5 7-20 0, 1 -1
7-14 0,01 - 0,1
. ...
7-15 0,01 - 0,1
7-17 0,01 - 0,1
7-18 0,01 - V,1
2 ~ 8 ~ .
63
¦ Compound of Reverse Transcriptase Assay
lExample No ICso ~ug/ml)
¦ 7-19 0,01 - 0,1
I
¦ 7-21 0,01 - 0, l
I
¦ 7-22 0,01 - 0,1
I _
¦ 7-23 0,01 - 0,1
¦ 3-34 0, 1 -1
I
¦ 3-35 0, 1 -1
¦ 3-37 0, 1 -1
3-7 0,08
I _
¦ 3-127 0,01 - 0,1
3-128 0,01 - o,1
3-129 0,01 - 0,1
7-24 < 0,01
7-25 < 0,01
7-26 0,01 0,1
1 5 7-27 0, 1 -1
7-28 < 0,01
_ _
7-29 0,01 - 0,1
7-30 < 0,01
. _ _
7-31 ~ ~,Ot
20 ICsO = 0.08 ~ug/ml
. , ~
2 ~
64
The examples which follow and the content of the patent claims illustrate the present
invention in greater detail.
5 Example I
(3S)-6-Chloro-3-methyl-3,4-dihydroquinoxalin-2(1 H)-one
A) (S)-N-(3-Chloro-6-nitrophenyl)alanine
2,4-Dichloronitrobenzene ~21.0 y, 0.109 mol) and 23.0 g (0.258 mol) of L-alanine were
refluxed for 48 hours in 400 ml of 2-methoxyethanol with an addition o~ 120 rnl of 2N
sodium hydroxide solution. The mixture was subsequently concentrated in vacuo, and
the residue was taken up in aqueous sodium hydrogen carbonate solution. the mixture
was extracted three times using ethyl acetate, the extract was then acidified wlth 6N
15 hydrochloric acid, and the yellow product was extracted using ethyl acetate. The
organic phase was washed once with saturated aqueous sodium chloride solution and
dried (magnesium sulfate), and the solvent was removed under reduced pressure.
14.7 g (55%) of a yellow solid of melting point 167-169C remained (after
crystallization from ethyl acetate).
1H NMR (270 MHz, d6-DMSO): ~ = 1.47 (d, J = 7 Hz, 3 tl), 4.57 (quintet, J = 7 Hz,
1 H), 6.77 (dd, J = 9, 2 Hz, 1 H), 7.11 (d, J = 2 Hz, 1 H), 8.12 (d, J = 9 Hz, 2 H),
8.41 (br. d, J = 7 Hz, 1 H), 13.2 pprn ~br., 1 H).
MS: (M + H)~ - 245
B) (3S)-6-Chloro-3-methyl-3,4-dihydroquinoxalin-~1H)-one
The product of Example IA (14.0 g, 0.057 mol) was dissolved in 400 ml of methanot
and hydrogenated with Raney nickel catalysis at room temperature, using 1 atm
hydrogen. After the calculated amount of hydrogen had been taken up, the catalyst
30 was removed by filtration with suction, and the reaction solution was concentra~ed in
2 ~
vacuo. The residue was puri~ied by silica gel chromatography using ethyl
acetate/heptane = 1:2 and 1:1 as the eluent. The yield was 6.0 g (53%) of a brownish
solid of melting point 122-123C ~after reerystallization from isopropanol/heptane).
'H NMR (60 MHz, d6-DMSO): ~ = 1.23 (d, J = 11 H~, 3 H), 3.~1 (dq, J = 11, 4 Hz
1 H), 6.27 (br., 1 H), 6.3 - ~.9 (m, 3 H), 10.3 ppm (br., 1 H).
MS: (M + H)~ = 197
[~]D23 = +77.3 (c = 1, MeOH)
C) ~3R)-6-Chloro-3-methyl-3,4-dihydroquinoxalin 2(1H)-on~
The compound was prepared from D-alanine by the methods described under
Example IA and IB. Melting point 123-124C (after recrystallization frorn
isopropanol/heptane)
The NMR data agree with those of the compound described in Example IB.
[a]D23 = -81.0 (c = 1, MeOH)
D) (3RS)-6-Chloro-3-methyl-3,4-dihydroquinoxalin-2(1H)-one
The compound was prepared starting from D,L-alanine by the methods described in
20 Examples IA and IB. Melting point 110C (after recrystallizati~n from
isopropanol/heptane)
The NMR data agree with those of the compound described in Example IB.
The fsllowing compounds of the formula i were synthesized analogously using the
25 corresponding haloaromatic compounds and ~rnino acid derivatives:
2~$~
66
Example ll
(3S)-3-Benzyl-7-chloro-3l4-dihydroquinoxalin-2(1 H)-one
A) (S)-N-(4-chloro-2-nitrophenyl)-phenylalanine
L-Phenylalanine (8.3 9, 0.05 mol) and 4.8 g (0.025 mol) of 2,5-dichioronitrobenzene
were dissolved in 40 ml o~ anhydrous dimethyl sulfoxide (DMSO), and the stirred
solution was heated to 80C under an argon atmospher Potassium tert.-butylate
(4.2 9, 0.025 mol), dissolved in 30 ml of DMSO, was added dropwise in the course of
40 minutes. Stirrina was continued for 3 hours at 8û to 90C, the mixture was allowed
to cool, and unreacted phenylalanine was removed by filtration with suction and
washed with water. The collected alkaline filtrates were extracted twice using diethyl
ether to remove unreacted dichloronitrobenzene. The mixture was then acidified using
glacial acetic acid and extracted several times using cthyl acetate, and the extracts
were dried over magnesium sulfate and evaporated.
The product was obtained in the form of a red oil (6.7 9, ~4%), which was further
reacted without purification.
B) (3S)-3-Benzyl-7-chloro-3,4-dihydroquinoxalin-2(1H)-one
The product of Example IIA (12 g) was dissolved in 300 ml of anhydrous methanol and
hydrogenated at room temperature with palladium/charcoal catalysis, using 1 atm
hydrogen. When the reaction had ended, solids were filtered off with su~tion, the liquid
25 was concentrated, and the concentrate was ehromatographed on silica gel usingdiisopropyl ether as the eluent. This gave 1.32 ~ of the desired product which
crystallized from isopropanol, melting point 185~.
2 ~ 8 ~
67
1H NMR (270 Mtlz, d6-DMSO): ~ = 2.9 (m, 2 H), 4.08 (m, 1 H), 6.09 (d, 1 H), 6.7 (m,
2 H), 6.78 (m, 1 H), 7.2 (m, 5 H), 10.34 pprn (br. s, 1 H).
MS: (M + H)~ = 273, (M - 92)' = 181.
5 The compounds in Table 2 were prepared as described in the ~bove examples.
Table 2
H
I
R l n~ ~ H
R 5
. ~ ~
Nr. R~n R3 Rs M.P. C
1 ~-CI CH3 H Wax
6-CI C2Hs 120
3 6-CI C2H4COOH H
. _
_ 6-CI -CH2cH2~O-
6-CI (CH3)2CH H
_ .
6-CI ~CH3)2CH( ~H2 H Oil
6-Ci C2Hs(CH3)CH Oil
6-C;1 C6HsCH2 _ _ _156-157
2~6~9~3
- -
Nr. Rln R3 Rs M.P. C
_
9 6-CI CH3SCH2CH2 H 97
.__ ..
6-CI CH3SCH2 H 149
.. __ . ...... _
11 6-CI CH2(OH) H _
12 6-CI CH3CH2CH2 H 75 - 77
.
13 7-Ci CH3 il 142
14 7-CI (CH312CH H
.. . ._ ~_ ~
7-CI CH3SC2H4 H 98
_
16 8-CI CH3 H
17 6,7-CI2 CH3 H
1E~ 7-F CH3 H 230
~ ___ .
19 6-F CH3 H Wax
6-F CH3 C3Hs 182
21 6-F C6HsCH2 ~3Hs
22 7-CF3 CH3 147
23 6-CH3Oc2H4O C2Hs H 107
_ . . .
24 6-CI C2H4OH H 211
. ~, . . .
6-CI CH2-S-Bn __ 170
26 6-CI CH2-S-i.-Pr H 190
_
27 6-CI CH2O-t.-Bu H 12E~
_ ._
28 6-CI C,~tl9 ==-. 115
2 ~
Bn = benzyl
i-Pr = isopropyl
t-Bu = tert.-butyl
Example lll
(3S)-4-N-(Benzyloxycarbonyl)-6-chlors -3-methyl-3,~-di-hydroquinoxalin-2(1 H)-one
The compound of Example IB (1.0 9, 5.1 mmol) was dissolved in 2D ml ~
dichloromethane. 10 ml of 2N aqueous sodium hydrogen carbona~e solution were
added, and 0.9 ml (90%; 5.7 mmol) of benzyl chloroformate was added with ice-
cooling and vigorous stirring. The two-phase system was subsequently stirred for60 hours at room temperature. After 30 hours, another 0.2 ml (1.3 mmol) of benzyl
15 chloroformate was added. When the reaction was complete, the phases wsre
separated, the organic phase was washed once with water and dried (magnesium
sulfate), and the solvent was rernoved in vacuo. The product was purified by silica
gel chromatography with methyl tert.-butyl ether/h~ptane = 1:1 as the eluent This
gave 1.65 g (98%) of a white, foam-like product.
'H NMR (270 MHz, d6-DMSO): ~ = 1.15 (d, J = 7 Hz, 3 H), 4.85 (q, J = 7 Hz,
1 H), 5.20 (d, J = 12 Hz, 1 H), 5.27 (d, J = 12 Hz, 1 H), 6.97 (d, J = 7 Hz, 1 H),
7.19 (dd, J = 8.2 Hz, 1 H), 7.3 - 7.45 (m, ~ H), 7.67 (d, J = 2 Hz, 1 H), 10.81 ppm
(br- s, 1 H).
MS: (M + H)~ = 381
2~
Example IV
(3S)-4-N-(Benzyloxycarbonyl)-6-Chloro-3-methyl-8-nitro-3,4-dihydroquinoxalin-
2(1 H)-one
The compound of Example lll (1.5 9, 4.5 mmol) was nitrat~d in glacial ac~tic acid
(15 ml). A to~al of 5 rnl (124.3 mmol) of fuming nitric acid were added dropwise in
2 ~ 8 ~
the course of 4 hours at O~C to room temperature. The mixture was subsequently
poured into 100 ml of ice-water, and the product, whioh was obtained in the form of
a yellow solid, was filtered off, washed thoroughly wlth water, and dried. Melting
point 85C (subl.).
'H NMR (270 MHz, d6-DMSO): ~ - 1.22 (d, J = 8 Hz, 3 H3, 4.89 (q, J = 8 Hz,
1 H), 5.24 (d, J = 12 Hz, 1 H), 5.31 (d, J = 12 Hz, 1 H), 7.35 - 7.~ (m, 5 H), 7.69
(s, 1 H), 8.Q0 (s, 1 H), 11.11 ppm (br. s, 1 H).
MS: (M + H)' = 376
Example V
(3S)-8-Amino-4-N- (benzyloxycarbonyl)-6-chloro-3-methyl-3,4-dihydroquinoxalin-
2(1 H)-one
The compound of Example IV (1.5 g, 4.0 mmol) was dissolved in 150 ml ofmethanol and hydrogenated at room temperature with Raney nickel catalysis, using1 atm hydrogen. When the calculated amount of hydrogen had been taken up, the
catalyst was r emoved by filtration with suction, and the filtrate was concentrated in
20 vacuo. The product was purified by silica gel chromatography using ethyl
acetate/heptane = 2:1 as eluent. The yield was 0.68 g (49%) of brownish solid ofmelting point 152-154C.
H NMR (270 MHz, d6-DMSO): ~ = 1.11 (d, J = 8 Hz, 3 H), 4.79 (q, J - 8 Hz,
1 H), 5.15 (d, J = 12 Hz, 1 H), ~.24 (d, J = 12 Hz, 1 H), ~.38 ~br. s, 2 H), 6.42 (s,
1 H), 7.3-7.4 (m, 6 H), 19.59 ppm ~br. s, 1 H).
MS: (M + H)~ = 346
2 ~
Example Vl
A) (3S)-6-Chloro-3-methyl-4-N-(3-methyl.2-buten-1-yl)-3,4-dihydroquinoxalin-
2(1 H)-one
The compound of Example IB (1.0 y, 5.0 mrnol) was dissolved in 20 n~l of
acetonitrile and alkylated with 3-methyl-2-buten-1-yl bromide (90%; 0.92 ml,
7.0 mmol) at room temperature in the presence of ~.0 9 (7.0 mrnol) of pulverulent
potassium carbonate. After 7 hours, the reaction had ended. The mixture was
filtered off with suction, the filtrate was concentrated in vacus, and the product was
purified by silica gel chromatography usin~ ethyi acetate/heptane = 1:2 as eluent.
The yield was 0.97 g (72%) of brownish solid of melting point 117-118C (after
crystallization from methyl tert.-butyl ether/heptane).
lH NMR ~270 MHz, d6-DMSO): ~ = 1.02 ~d, J = 8 Hz, 3 I l), 1.74 (s, 6 tl), 3.69 (dd,
J = 14, 8 Hz, 1 H), 3.85 - 3.9 (m, ~ H), 5.19 (m, 1 H), 6.65 - 6.8 (m, 3 H),
10.47 ppm (br. s, 1 H).
MS: (M + H)' = 265
[a]D23 = +168.0~ ~c = 1, MeOH)
B~ (3R)-6-Chloro-3-methyl-4-N-(3-methyl-2-buten-1-yl)-3,4-dihydroquinoxalin-
2(1 H)-one
The compound was prepared by the method described in Example VIA, starting
from the compound of Example IC. Melting point 115-117C (af~er reerystalli7ation
from isopropanol/diethyl ether)
The NMR data agreed with those of the compound deseribed in Exarnple VIA.
[a]D23 = -172 (c = 1, MeC~H)
2 ~
72
C) t3RS)-6-Chloro-3-methyl-4-N-(3-methyl-2-buten-1-yl)-3,4-dihydroquinoxalin-
2(1 H)-one
The compound was prepared by the method described in Example VIA starting
with the compound of Example ID. Melting point 148-14gG (after recrystallization
from isopropanol/diethyl ether)
The NMR data agreed with those of the compound described in Example VIA.
Example Vll
(3S)-6-Chloro-3-methyl-4-N-(2-buten-1 -yl)-3,4-dihydroquinoxalin-2(1 H~-one
The substance was prepared analogously to the compound described in Example
VIA, but with 2-buten-1-yl bromide as the alkylating agent. Melting point 87-88C
(after crystallization from diethyl ether/heptane)
'H NMR (270 MHz, d6-DMSO): ~ = 1.01 (d, J = 8 Hz, 3 H), 1.70 (dd, J = 8, 1 Hz,
3 H), 3.63 (dd, J = 16, 6 llz, 1 H), 3.85 -4.0 (m, 2 H), 5.47 (m, 1 H), 5.75 (m, 1 H),
6.65 - 6.8 (m, 3 H), 10.48 ppm (br. s, 1 H).
MS: (M + H)~ = 251
Example Vlll
4-N- (Isopropenyloxycarbonyl)-3,3,7-trimethyl-3,4-di-hydroquinoxalin-2(1 H)-one
3,3,7-Trimethyl-3,4-dihydroquinoxalin-2(1H)-one (0.4 g, 2.1 mmol~ were dissolved in
10 ml of anhydrous pyridine, and the stirred solution was tre~ted at ro~m
temperature with 0.24 ml (2.2 mmol) of isopropenyl chloroformate. The mixture was
stirred for 6 hours at room temperature and treated with water, thc precipi~ate
which formed was filtered off with suction, washed with water and dried. This gave
0.4 9 (6~%) of eolorless crystals of melting point 185~G.
'H NMR (270 MHz, d,;-DMSO~: d = 1.~ (s, 6 H~1 1.9 (s, 3 H~, 2.25 ~s, 3 I l), 4.7 (m,
2 H), 6.7 - 6.9 (m, 2 H), 7.15 (d, J = 8 Hz, 1 H), 10.6 ppm (br. s, 1 H).
MS: t = 274
2 ~
73
Example IX
(3S)-6-Chloro-4-N- ~4-methoxyphenoxycarbonyl)-3-methyl-3,4-dihydroquinoxalin-
~(1 H)-one
The compound of Example IB (0.5 9, 2.5~ mmol) was dissolved in 10 ml of
anhydrous N,N-dimethylformamide, and 0.41 ml (2.8 mmol) of triethylamine were
added. To the stirred mixtur~ there was first added dropwise 0.4~ ml (2.8 mmol) of
4-methoxyphenyl ohloroformate and, after ~ hours, another 0.21 ml (1.9 mmol).
When ~he reaction was complete (18 hours), the solvent was stripp~d off under
reduced pressure, ~he residue was taken up in ethyl acetate, and the mixture waswashed with water and dried (sodium sulfate). 0.4~ g (54%) of a whit~ solid
remained after concentration. Melting point 187-19ûC (after recrystallization from
isopropanol)
1H NMR (270 MHz, d6-DMSO): ~ = 1.24 (d, J = 8 Hz, 3 H), 3.77 (s, 3 H), 4.94 (q,
J = 8Hz, 1 H),6.97 (dd,J = 8,2Hz, 1 H),7.03 ~d,~l = 8Hz, 1 H),7.2-7.3(m,
3 H), 7.78 (s, 1 H), 10.89 ppm (br. s, 1 H).
MS: (M + H)~ = 347
Example X
(3S)-6-Chloro-4-N-(4-fluorophenoxycarbonyl)-3-methyl-3,4-dihydroquinoxalin-
2(1 H)-one
The compound was prepared analogously to the compound described in Example
VIA, but 4-fluorophenyl chloroformate was used as ar~lating a~ent. Melting point168-170C (after crystallization from isopropanol)
'H NMR ~270 MH~, d6-DMSO): ~ - 1.24 (d, J = 8 Hz, 3 H), 4.94 (q, J = 8 H~,
1H),7.03(d,8Hz,lH),7.2-7.5(m,5H~,7.83(d,J=2Hz,1H),10,90ppm
(br. s, 1 H).
MS: (M + H)~ = 335
2 ~
Example Xl
(3S)-6-Chloro~-N-(4-chlorophenoxycarbonyl)-3-methyl-3,4-dihydroquinoxaiin-
2(1 H)-one
The compound was prepared analogously to ~he compound described in Example
VIA, but 4-chlorophenyl chloroformate was used as acylating agent. Melting point185-188C (af+er crystallization from isopropanol/diethyl ether)
'H NMR (270 MHz, d6-DMSO): ~ = 1.2~ (d, J = 8 Hz, 3 H), 4.94 (q, J ~ 8 Hz,
1 H), 7.04 (d, 8 Hz, 1 H), 7.25 (dd, J = 8, 2 Hz, 1 H), 735 - 7.6 (m, 4 11), 7.80 (s,
1 H), 10.91 ppm (br. s, 1 H).
MS: (M + H)+ = 351
Example Xll
(3S)-4-N - (2-Bromoethyloxycarbonyl)-6-chloro-3-rnethyl-3,4-dihydroquinoxalin-
2~1 H)-one
The compound was prepared analogously to the compound described in Example
VIA, but 2-bromoethyl chloroformate was used for the acylation. Melting point
133-136~C (after crystallization from isopropanol)
2û lH NMR (270 MHz, d6-DMSO): ~ = 1.16 (d, J = 8 Hz, 3 H), 3.7 - 3.8 (m, 2 H), 4.4 -
4.6 (m, 2 H), 4.86 (q, J = 8 Hz), 6.99 (d, 8 Hz, 1 H), 7.21 (dd, 8, 2 Hz, 1 H), 7.74
(d, J = 2 Hz, 1 H), 10.84 ppm (br. s, 1 H).
MS: (M + H)+ = 348
Example Xlll
(3S)-6-Chloro-N-~isopropenyloxycarbonyl)-3-methyl-3,4-dihydroqllinoxalin-2~1 H)-one
The substance was prepared analogously to the compound described in i-~ample
VIA, but isopropenyl chloroformate was used for the acylati~n. Meiting paint
158-159C
2 ~
'H NMR (270 MHz, CDC13): ~ - 1.33 (d, J = 8 Hzl 3 H), 2.02 (s, 3 H), 4.79 (s,
1 H),4.83~s,1 H),5.17~q,J=8Hz,1 H),6.86(d,J=8Hz,1 H),7.12(dd,
J=8,2Hz,1H),7.74(br.s,1H),9.28ppm(br.s,1H).
MS: (M + H)~ = 281
Exarnple XIV
~3S)-6-Chloro-3-methyl-4-N-(vinyloxyearbonyl)~3~4-di- hydroquinoxalin-~(1H)-one
The substance was prepared analogously to the compound described in Example
VIA, but vinyl chloroformate was used ~or the acylation. Melting point 177-179C'H NMR (270 MHz, CDC13): ~ = 1.33 (d, J - 8 Hz, 3 H), 4.96 (dd, J = 14, 2 Hz,
1 H), 5.20 (q, J = 8 Hz, 1 H), 6.83 (d, J = 8 Hz, 1 H), 7.12 (dd, J = 8, 2 Hz, 1 H),
7.2 - 7.3 (m, 2 H), 7.71 (br. s, 1 H), 9.42 ppm (br. s, 1 H).
MS: (M + H)~ = 267
Example X\/ and Example XVI
6-Chloro-3,4-dihydroquinoxalin-2(1H)-one was reacted with 3-methyl-2-buten-1-yl
bromide analogously to the process described in Example VIA. It was possible to
20 isolate two products by silica gel chromatosraphy.
6-Chloro-4-N-(3-methyl-2-buten-1-yl)-3,4-dihydro- quinoxalin-2(1H)-one
Melting point 150-151 C ~after recrystallization frorn ethyl aeetate)
'H NMR (270 MHz, d6-DMSO): ~ = 1.72 (s, 6 H), 3.ô7 (s, 2 H), 3.89 ~d, .1 = 7 Hz,2 H), 5.20 (m, 1 H), 6.7 - 6.8 (m, 3 H), 10.49 ppm (br. s, 1 H).
MS: (M ~ H)~ = 251
6-Chloro-4-N-(3-methyl-2-buten-1-yl)-3-(1 ,1 -dimethyl-2-propen-l-yl)
3,4-dihydroquinoxalin-2(1 H)-one
Melting point 110-112C (after crystalli~ation from heptane)
2 ~ 5
76
'H NMR (270 MHz, d6-DMSO): ~ = 0.94 (s, 3 H), 0.97 (s, 3 H), 1.65 (s, 3 H), 1.66(s, 3 H), 3.77 (dd, J = ~6, 7 H7, 1 H), 4.23 (dd, J = ~6, 7 Hz, 1 H), 4.8 - 4.9 (m,
2 H), 5.02 (m, 1 H), 5.75 (dd, J = 17, 11 Hz, 1 H), 6.6 - 6.7 (m, 3 H), 10.49 ppm
(br s, 1 H).
5 MS: ~M + H)~ = 319
The following compounds of the formula I were synthesize~ from the correspondingunsubstituted quinoxalinones in analogous manner and, K appropriate, derivatizedfurther:
Table 3
R 2
R n~ N
2~6~5
_ . _ __ _ _ _=
O 11~) O N 00 N N N N ~-
I T ~.) O C.)
~: T ~ ~ _ ~ e oN T T
_ _
~: I I I I I I I I I I I :C
_ _ _ _ . _ _
U~ ~ ~ ~ -~, O ~,~ ~ ~ ~.~
Z ~ N C~ ~ L~ ) ~ r- aD a) ~_ ~ r ~_
_--_ _ = _ _ _ _
:
2 ~
r _-5 U C~l
t) ~ o E cl) u~ o ~ ,_ N
. N tD O 0 0 t~5 . ~) ~) cr) C~ O T-
¦~ N N LL O ~ LL I~ u~ O ~ ~ ~ ~_
~ (.) ( ) ~.) O t ) (.) O t~ N t ) t~ ~ t~)
I .
tr I I I I I I I I I I I I I
_' O C) ~ O (.) O ~ O S~) O ~ (~ V
~ CD (D ~) t~ U) ~ ~J (~ ~D CD CD ~D
Z d In tD ~ ~ O) ; N N C~l N C~l ;~ N
2 ~ 5
_ _ _ _ . _
cc I u~ O t ) ~ O O 6 u~ O O ( )
o)
~ t~l N C~l O
cr: I ~ ~ ~ N t~l r~ ~q t l I
_ _ _ . _ _
CC I I I I I I I I I I I I I
tr~ T
G: CD ~D C~ ~D ~ ~ ~D CD ~D ~D ~D ~D tD
. ~ 0~ O> ~ . Cl c7) ~ U ~D ~ 00 ~
Z N C~l N cr) ~ S~ C~ C~ t~) ~ ~ ~) C')
___ _ =_ _. __ _ __ _
s
~ ~ T rl~
~ D ,_ ~ O O o co o
.
tru~u~ ~u~ O O u~u~ O O ,
o
co r~ ~ I I I N
~7 I II I I (~')NI I I
G ~ ~.) O O (..) (~) t ) O O O O t.) t.)
~: :C ~ ~ I~ L
.
~ o o ~ o ~ ~ v o ~ ~ ~) ~ ~
~ CD CD ~D tD tD tD tD tD tD ~ U~- ~0 U~
~ 1~ ~ N~ d- U ) lD I~ t~ ~) O ~ N
Z ~t ~ ~~ ~ ~ ~ ~ ~ ~ ~) Ir) Lr
_ _ __ __ = _
~ `.
2 ~
- - - ~ : ~
~ O N . N ~ N O ~ a) ~ ~ I` I~`)
_ _ _ _
U7 O 1 LU~ O O ~n OU~ O 6 O
~: I I IN N t~)
_ _ _ _
a: I I I I I I I I I I :C :1:
_ _ _ _ _ __ __
c O O ~ ~ ~ O O ~ ~ O ~ ~ ~
CC ~ I` ~` r- t- ~ ~ I~ r- ~ ~ I`_
~ _ _ _ . _ _ _
Z ~ d Ll~ tD 1~ 00 cn O ~ N ~r~ )
I _ _ _ _ _--
2~9~
o _ = _ ___ E ~ E 3
n CD ~ N ~ ~ ., O ~ O ~ N N N
_ _
a: ~ u~ ~ O ,0 O u~ O O ~n O O O
. _ _
~ N
~ _ _ _
c LL IL LL IL ll~ 1~ O," T ~ O ~ t.) V
a: ~ ~D ~C) ~O t- r- ~ I~ ~D (5) Cl:~ ~1 ~
Z ~ ~D t~ ~D 1~ r` N ~ 1` 1`~ 1~ ~ 0
2~6~8~
_ _ _
~ 1~ 1~ N tD CO ~ O ~ _ tC~ ~ ~ 1 J)
B I I ¦ O
! I I I I,~,
L~ I I 1: I I I I I I I I: T
I
I _ C O O O LL C~ ~ V ~ ~ ~~ ~ ~ ~
~ tD ~D tD ~ (D ~D tD ~D t.D CD ~D tD ~D
Z 1~ O 0 N 00 co 0~ 0 CO --0 O a~
~ .
2 ~ 8 ~
In__ N _ _ _ _ =_ __ _
~_ CU N O O ~ OD 'u~ O _ O O ';t
I I I I q II u~ I q I
~ __ _ __
l ~
L ~ . D ~ ~ L~ o D L o ~ _L~
2 ~
- - - = -
~ N O tDa)a:~~Dtt:~ 't N 1~ O O
_ ~N _ _
~ I ~ C .n Z L;N~ ~
~ 8 8 8 8 88 Q 8 o 8 ~ ~
_ _ _ _
~qt"r~~rJ",I:'J ", r) N I`J
~ I II II I I I I II
~: ~ O ~ O V ~ ~ O ~ O
u I I I I I ~ I I I
~ r
_c ~ ~ ~ ~ ~ ~ V ~ t~ ~ ~ ~
G U:) C~:) tD CD CD ~S) ~D CD ~ ~:1 ~D ~
_ _ . _ _ _
. In t~:) I~ :~ o) o ~ N ~) ~ Ir) C~:)
~ O O O O ~ ~ ~_ ~ ~ _- ,_
. __ _ _ --~ _ _
I ~ ~ l a '~
_ _ _ _ _ _
a: O ~ O u~ O u~ O O O O O O
a: I I I I ~ T I
~ ~ o~
Z ~ OD ~ N C~l N N N N t~J N N N
2 ~
87
Key: C5Hg - 3-methyl-2-buten-1-yl
C4H7 = 2-butenyl
CsHl1 = 3-methyl-1-butyl
C6H,1 = 2,2-dimethylcyclopropyl-1-methyl
sC6H11 = 4-methyl-3-penten-2-yl
C3H3 = 2-propen-1-yl
(CH3)2CCHCO = 3,3-dimethylacryl
IPOC = isopropenyloxycarbonyl
ALAC = allylaminocarbonyl
ALOC = allyloxycarbonyl
C4H30 = furanyl
C4H3S = thienyl
CsH4N = pyridyl
Ph = phenyl
,
2 ~
88
Example XVII
6,7-Dimethoxy-3-methyl-3,4-dihydroquinoxalin-2(1 H)-one
4,5-l~imethoxy-1,2-dinitrabenzene (34.2 g, Q.15 mol) was hydrogenated in 500 ml of
5 methanol with Raney nickel catalysis using 1 atm hydrogen. After the calculated
amount of hydrogen had been taken up, the process was stopped, the catalyst
was removed by filtration with suction, and the solvent was stripped off in vacuo.
To remove the water completely, the mixture was taken up hNice in methanol and
reconcentrated. 4,5-Dimethoxy-1,2-phenylenediamine (24.0 g), which remained as abrown oil, was refluxed for 48 hours in 200 ml of ethanol (96%) together with
17.1 ml (0.15 mol) of methyl 2-chloropropionate, with an addition of 21.0 ml
(0.15 mol) of triethylamine. The solution, which was very dark, was concentrated,
the concentrate was taken up in ethyl acetate, the mixture was washed twice withwater and dried (sodium sulfate), and the solvent was stripped off in vacuo.
15 The crude product was crystallized by stirring with diethyl ether (6.2 g, 19%). A
analytically pure sample of melting point 151 C was obtained by silica gel
chromatography using ethyl acetate as the eluent.
H NMR (60 MHz, d6-DMSO): ~ = 1.22 (d, J = 7 Hz, 3 H), 3.63 (s, 3 H), 3.67 (s,
1 H), 3.6 - 3.7 (m, 1 H), 5.62 (br. s, 1 H), 6.40 (s, 1 H), 6.45 (s, 1H), 9.90 ppm
20 (br s, 1 H).
MS: Mt = 222
The following compounds of the formula I were synthesized in analogous manner
and, if appropriate, derivatized further:
.
2 ~ 6
89
Table 4
H
g~
N~H
R
R5
Nr. R1n R3 Rs X M.P. C
. _ _
16,7-(CH30)2 CH3 IPOC O '133
26,7-(CH30)2 CH3 IPOC S
36-C6HsS CH3 C5H9 O 115
.
47-C6HsS CH3 C5Hg O 107
I _
56-C6HsS CH3 H O
I
67-C6HsS CH3 H O
76,7(CH30)2 CH3 H O 151
Key: CsHg = 3-methyl-2-buten-1-yl
IPOC = isopropenyloxycarbonyl
Example XVIII
(3RS)-6-Chloro-4-N-(cyclopropyl)-3-methyl-3,4-dihydroquinoxalin-2(1 H)-one
A) (2RS)-N-(4~Chloro-2-cyclopropylaminophenyl)-(2-brornopropionamide)
4-Chloro-2-cyclopropylaminonitrobenzene (2.10 9, 0.01 mol) was hydrogenated in
100 ml of methanol with Raney nickel catalysis, using 1 atm hydrogen. After the
calculated amount of hydrogen had been taken up, the process was stopped, the
catalyst was removed by filtration with suction, and the solvent was stripped off i
2~6~
acuo. To remove water completely, the mixture was taken up ~vice in methanol
and reconcentrated 4-Chloro-2-cyclopropylaminoaniline (1.80 g), which r~mained in
the form of a brown oil, was dissolved in 50 ml of anhydrous 1,2-dimethoxyethaneand cooled to -60C, with stirring. A solution of 1.1 ml (0.01 mol) of
2-bromopropionyl chloride in 5 ml of anhydrous 1,2-dime~hoxyethane was slowly
added dropwise, and stirring of the reaction mixture was continued ~or 2 hours at
-60 - -70C. The mixture was then allowed to warm ~o approx. -20C and poured
into 150 ml of ice-cold, saturated aqueous sodium hydrogen carbonate soiution.
The mixture was extracted twice usir~g ethyl acetate, and the organic phase was
washed once with water, dried (sodium sul~ale) and concentrated in vacuo. After
crystallization with diethyl ether/pentane, 2.51 9 (79%) of the desired product of
melting point 130C remained.
'H NMR (270 MHz, d6-DMSO): ~ = 0.4 - 0.5 (m, 2 H), 0.7 - 0.8 tm, 2 H), 1.75 (d,
J = 7Hz,3H),2.39(m,1 H),4.72(q,J =7Hz,1 H),5.6(br.s,1 H),6.66(dd,
J = 8, 2 Hz, 1 H), 6.96 ~d, J = 2 Hz, 1 H), 7,21 (d, J = 8 Hz, 1 H), 9.36 ppm (br.
s, 1 H).
MS: (M + H)+ = 319, 317
B) (3RS~-6-Chloro-4-N- (cyclopropyl)-3-methyl-3,4-dihydroquinoxalin-2 ~1 H)-one
The compound of Example XVIIIA (318 mg, 1.0 mmol) was dissolved in 20 rnl of
ethanol (96/o), 0.28 ml (2.0 mmol) of triethylamine were added, and the mixturewas reflux~d for 18 hours. The solvent was removed under reduced pressure, and
the reaction product was purified by silica gei chromatography using ethyl
acetate/heptane = 1 :2 as eluent. The yield was 200 mg ~85%~ of white crystals of
melting point 167C (after crystallization from pentane).
1H NMR (270 MHz, d6-DMSO): ~ = 0.40 (m, 1 H~, 0.63 (m, 1 H), 0.76 (m, 1 tl),
0.98 ~m, 1 H), 1.12 (d, J = 7 Hz, 3 H), 2.47 (m, 1 H~, 3.87 ~q, J = 7 ~z, 1 H), 6.78
(s, 2 H), 7.0 (sl 1 H), 10.46 ppm (br. s, 1 H~.
MS: (M ~ H)~ = 237
2 ~
91
The following compounds of the formula I were synthesized analogously to the
procedure described in Example XVIII using the correspondingly substituted ortho-
nitroanilines and 2-halo carboxylic acid derivatives and, if appropriate, derivatized
further:
Table 5
H
R 1 n~ N\I(~ R 3
R4
R
Nr. Rln R3 R4- - R5 XM.P.C
~ I
1 6-CI CH3 H C6Hs O 191
2 6-CI CH3 CH3 ~3Hs O
_
3 6-CI CH3 CH3 C3Hs S
. _
4 6-CI CH3 CH3 C3Hs
__
6-CI CH3 CH3 C3Hs S
Key: C3Hs = cyclopropyl
C6Hs = phenyl
92 2 ~ 5
Example XIX
7-Chloro-1-N-(cyclopropyl)-3,3-dimethyl-3,4-dihydroquinoxalin-2(1 H)-one
4-Chloro-2-cyclopropylaminonjtrobenzene (2.0 g, 9.4 mmol) was hydrogenated as
described in Example XVIIIA. The resulting 4-chloro-2-cyclopropylaminoaniline
(1.70 g) was taken up in 20 ml of dichloromethane. 1.~ ml (2.01 mmol) of
chloroform, 1.8 ml (2.45 mmol) of acetone and 0.10 g (0.4 mmol) of
benzyltriethylammoniurn chloride were added, and the reaction solution was cooled
to 10C. 4 ml of 50% strength sodium hydroxide solution were slowly added
dropwise with vigorous stirring, during which process the reaction temperature
should not exceed ~0C. After stirring for 5 hours at 10DC, the phases were diluted
and separated. The organic phase was washed once with water, dried (magnesium
sulfate) and evaporated in vacuo. The crude product was purified by silica gel
chromatography using ethyl acetate/heptane = 1:2 as the eluent. the yield was
1.0 g (42%) of white crystals of melting point 132-133C (after recrystallization from
toluene/heptane) .
'H NMR (270 MHz, d6-DMSO): ~ = 0.45 - 0.55 (m, 2 H), 1.05 -1.1 (m, 2 H), 1.19
(s,6H),2.71(m,1H),6.09(br.s,1H),6.71(d,J=8Hz,lH),6.88(dd,J=8,
2 Hz, 1 H), 7.19 ppm (d, J = 2 Hz, 1 H).
MS: (M ~ H)~ = 251
The following compounds of the formula I were synthesized in analogous manner
and, if appropriate, derivatized further:
2 ~
93
Table 6:
R 1 --t~ ~
R 4
1~1 R5
_ _ _
Nr. R'n R3 R4 R5 M.P.C
I _ _
1 6-CI CH3 CH3 CsHs 179
I
L~ 7-CI CH3 CH3 CsHs 171
3 6,7-(CH30)2 CH3 CH3 H
4 6,7-(CH3O)2 CH3 CH3 CsHs _
CH3 CH3 SC6H1l 113
__ ~ C6Hs CH3 H .
7 C~Hs CH3 C5H9 _
8 6-CI CH3 CH3 IPOC 128
9 7-CI C:;H3 CH3 IPOC 169
_ _ , _
7-~H3 CH3 CH3 CsH~ 168
. ,
11 6-CH30 CH3 CH3 H 200
_ . _ _
2~ 12 6-CH3C) CH, CH~, _ 138
13 6/7-COOH CH3 CH3 H > 240
2 ~
94
- ._
Nr. R' R3 R4 Rs M.P. ~C
14 6/7-COOH CH3 CH3 CsH9 180
. .
8-CH3 CH3 CH3 H 140
16 ~-CH3 CH3 CH3 CsHs 160
17 8-CH3 CH3 CH3 IPOC 127
18 6/7-CH3 C2Hs C2Hs H 160
_
19 6-CH3 C2Hs C2Hs CsHs 100
~ _ _ ._
7-CH3 C2Hs C2Hs CsHs 110
21 7-F CH3 CH3 H 120
_
22 7-F CH3 CH3 CsHg 155
23 7-C2tlsO CH3 CH3 H i55
_
24 7-C2HsO CH3 CH3 CsHs 123
6-COOH CH3 CH3 GsHs 245
_
26 7,8-(CH3)2 CH3 CH3 H 196
I _ _ _
27 7,8-(CH3)2 CH3 CH3 CsHs 155
I
28 6,7-(CH3)2 CH3 CH3 H 248
I
29 6,7-(CH3)2 CH3 ~ 3 G5Hg 200
_ _ _ _
6-CI,7- CH3 C:H3 H 211
(2,3-CI2C6H30)
_ .
31 6-CI,7- C:H3 CH3 C5Hg 205
(2~3-cl2c6H3o)
_ , .
32 7-F CH3 CH3 IPOC 175
I _ . _
33 7-C2H,O CH CH3 IPOC 150
2 ~
~5
Nr.Rln R3 R4 Rs M.P. C
346/7-CH3 CH3 CH3 IPOC 152
357,8-(CH3)2 CH3 CH3 IPOC 147
_
366,7-(CH3)2 CH3 CH3 IPOC 161
377-C6H5 CH~ CH~ ~ H 167
¦ 387-C6H50 CH3 CH3 C5Hg 138
397-C6H50 CH3 CH3 IPOC 181
405-CH3 CH3 CH3 H 182
I
¦ 416-CH30, CH3 CH3 H > 240
7-(4-Pyridyl)
426-CI, CH3 CH3 219
7-Piperidino .
l 436/7-CI,7/6- CH3 CH3 H 236
Morpholino
(mixture)
446/7-(N-Methyl- CH3 CH3 H > 240
l piperazin-1 -yl)
I
456/7-CI,7/6- CH3 CH3 H 147
(N-Methyl-
piperazin- 1 -yl)
46 6-CI CH3 CH3 H 152-154
_
L~ 7-CI CH3 CH3 _
¦ 48 6-CICH3 CH3 ALOC 128-129
I _
49 7-CICH3 CH3 ALOC 144
96
Nr. R' R3 - R4 Rs M.P. C
sO 6-CI CHD CH3 COOCH(CH3)2 118
51 7-CI CH3 CH3 COOCH(CH3)2 171
52 7-(4-F-Ph-SO20) CH3 ~H3 H
._ _
537-(4-F-Ph-S02C)) CH3 CH3 IPOC 204
_
546-CI,7-Piperidino CH3 CH3 IPOC 152
6-CI,7- CH3 CH3 IPOC 11 3
Morpholino _
56 6-CI,7-(N- CH3 CH3 IPOC 168
Methyl-
piperazin-1 -yl) ~
~57 6-CI,7-NEt2 CH3 CH3 141
58 6-CI,7-NEt2 CH3 CH, IPOC C)il
10 59 6,7-CI2 CH3 CH3 H 232
6,7-CI2 CH3 CH3 IPOC 171
:
61 7-(N-Methyl- CH3 CH3 H 198
piperazinyl-1 -yl)
62 7-(N-Methyl- CH3 CH3 IPOC 123
piperazinyl-1 -yl)
_ _
63 6-C;H30 CH3Ctl3 IPOC 128
~ __ - ___ . .. - --. _ ., _
15 1~ 7-CI -(CH2)3- IPOC 172
7-CI -(CH2)~,- iPOC 181
66 6-CI -(CH2~3- IPOC 157-158
I
67 6-CI - (CH2)4- I POC 179- 180
2 ~ 3 5
97
_ __
Nr. R' R3 R4 Rs M.P. CC ¦
_ _ I
68 6-Clq CH3 CH3 cOOC2H5 137
I _
69 6-CI CH3 CH3 COOC3H7 125
~ _
Key: c5~3 = 3-methyl-2-buten-1-yl
sC6H'1 = 4-methyl-3-penten-2-yl
IPOC = isopropenyioxycarbonyl
Example XX
3,3-Dimethyl-4-N-(3-methyl-2-buten-1-yl)-3,4-dihydroquinoxalin-2(1 H)-one
The compound was prepared analogously to the compound described in Example
VIA, starting from 3,3-dimethyl-3,4-dihydroquinoxalin-2(1H~-one (J. T. Lai, Synthesis
1982, 71). Melting point 146-147C (after crystallization ~rom methyl tert.-butyl
1 5 ether/heptane)
'H NMR (270 MHz, d6-DMSO): ~ = 1.27 (s, 3 H), 1.68 (s, 3 H), 1.72 (s, 3 I l), 3.88
(d, J = 7 Hz, 1 H), 5.15 (m, 1 H), 6.60 td, J = 7 Hz, 1 H), 6.67 (t, J = 7 H2l 1 H),
6.78 (d, J = 7 Hz, 1 H), 6.87 (t, J = 7 Hz, 1 tl), 1û.33 ppm (br. s, 1 H).
MS: (M + H)' = 245
Example XXI
4-N-(3-Methyl-2-buten-1 -yl)-3,4-dihydroquinoxalin-2(1 H)-one-3-spiro-1 '-cyclohexane
The compouncl was prepared analogously to the compound described in Example
VIA, starting from spiroEcyclohexane-1,3'-(3',4'-dihydroquinoxalin-(1'H)-one)] (J. T.
Lai, Synthesis 1982, 71). Melting point 82-83C ~a~ter crystallization from h~ptane)
H NMR (270 MHz, d6-DMSO): ~ = 1.25~1.75 (rn, 1D H~, 3.75 (d, J = 6 tlz, 2 H),
5.07 (m, 1 H), 6.7 - 7.U (m, 4 H), 10.15 pprn (br. s, 1 H).
MS: (M + H)t = 285
2 ~ 5
98
Example X)(ll
4-N-(3-Methyl-2-buten-1-yl)-3,4-dihydroquinoxaline-2(1 H)-thione-3-spiro-
1 '-cyclohexane
5 The compound of Example XXI (50~ mg, 1.~ mmol) was refluxed for 1.5 hours
under argon together with 370 mg (0.9 mmol3 of ~,4-bis-(4-methoxyphenyl)-
1~3-dithia-2~4-diphosphetane 2,~-disulfide (Lawesson~s reagent) in 10 ml of
anhydrous toluene. The mixture was subsequently ~oncentrated in vacuo, and the
products were isolated by silica gel chrornatography using methyl tert.-butyl
ether/heptane = 10:1 as eluent. The yield was 50 mg (9%) of yellow crystals of
melting point 125C.
'H NMR (270 MHz, d6-DMSO): ~ = 1.1 1.9 ~m, 16 H), 3.64 (d, J = 7 Hz, 2 H),
4.99 (m, 1 H), 6.95 - 7.1 (m, 3 H), 7.18 (d, J = 7 H~, 1 H), 12.2 ppm (br. s, 1 H).
MS: (M + H)+ = 301
3,4-Dihydroquinoxaline-2(1H)-thione-3-spiro-1'-cyclohexane was isolated as a
further product in a yield of 110 mg (26%); yellow crystals of melting point 178C.
~H NMR (270 MHz, cDC13 ~ = 1.25 - 2.2 (m, 10 H), 4.18 (br. s, 1 H), 6.7 - 6.8 (m,
3 H), 6.97 (m, 1 H~, 9.42 ppm (br. s, 1 H).
MS: (M + H)~ = 233.
Example XXIII
(3S)-6-Chloro-4-N- (isopropenyloxycarbonyl)-3-methyl-3,4-dihydroquinoxaline-
2(1 H)-thione
The cornpound of Example Xlll (0.5 ~, 1.78 mmol), dissolved in îO ml of anhydrous
pyridine, was refluxed for 4 hours together with 0.47 g ~2.12 mmol) of phosphorus
pentasulfide. The mixture was concentrated in vacuo, and lhe residue was
chromatographed on silica gel using ethyl acetate/heptane = 1:1 2S eluent. This
gave 0.25 g (47%) of a yellow crystalline solid of rnelting point 148-150C (after
recrystallization from ethyl acetate/heptane).
2 ~
99
H NMR (270 MHz, d6-DMSO): ~ = 1.24 (d, J = 7 Hz, 3 H), 1.96 (s, 3 H), 4.8 - 4.9
(m,2H),5.28(q,J=7Hz,1H),7.22(d,J=8Hz,1H),7.30(dd,J=8,2Hz,
1 H), 7.72 (br. s, 1 H), 12.84 ppm (br. s, 1 H).
MS: (M + H)~ = 297.
The following compounds of the formula I were ~ynthesized in analogous rnanner
~rom the corresponding 3,4-dihydroquinoxalin-2(1H)-ones:
Table 7
H
I
R ~ ~ -- R 3
R 4
R 5
.
Nr. R'n R3 R4 Rs M.P.C
1 CH3 H C5Hg 119
2 6-CI CH3 H CsHg 109- 110
_ .
3 6-CI CH3 HC6H5CH2 92
. _ .. . ,
6-CI H -CH2CH2CS-
6-CI H -CH2CH2CH2CS-
. _ _
6 C6Hs CH3C5H~
_ _
7 6-CI CH3 CH3CsH5 157
_
8 7-CI CH3 CH3C5Hg 160
_ .
7-CI CH3 CH3 H 170
2~6~9~
~oo
_
6-CI CH3 H ALOC 143- 145
11 6-CI CH3 CH3 IPOC 153
_ I
12 7-CI CH3 CH3 IPOC 174 l
_ I
13 6-CI CH3 CH3 175
l 14 6-CI C2Hs H IPOC 176-1.77
6-CI C2Hs H ALOC 159-161 l
I
166,7-(CH3)2 CH3 CH3 CsH9 173
17 6-CI C3H7 H IPOC 154-1~5 l
_ ~ I
18 6-CI C3H7 H ALOC 98-100
_
l 19 6-CI CH3 (2-csH4N)-cH2 175-178
6-CI CH3 H (3-C5H4N)-CH2 77
I _
21 6-CI C H3 CH3 ALOC 153-154
22 6-CI CH3 CH3 COOCH(CH3)2 151
l 23 6-CI CH2SCH3 H IPOC 128
l 24 6-CI H3 CH3 COOC2Hs 163
l 25 6-CI CH3 CH3 COOC3H7 164
l _ .
26 6-CI C2Hs H (2-csH4N)-cH2 162-164
27 6-CI C4Hg H IPOC 132
I _ ~ _
l 28 6-CI CH2SCH3 HCOOCH(Ctl3)2 124
l 29 6-CI CH2SCH3 J (2-csH4N)-cH2 1~9
I .
¦ 30 6-CH30 CH2S~H3 H IPOC _
31 6-CH30 CH2SCH3 HCOC)CH(CH3)2 163
2 ~
101
, r-
32 6-CI ¦ CH2SCH3 H ¦ C~H2C6H4-2-CI __=
Key; CsHg = 3-methyl-2-buten-1-yl
IPOC = isopropenyloxycarbsnyl
5ALOC = allyloxycarbonyl
CsH4N = pyridyl
Example X)(IV
(3RS)-3-Methyl-4-N-(3-methyl-2-buten-1-yl)-2-methylthio-3,4-dihydroquinoxaline
(3RS)-3-Methyl-4-N-(3-methyl-2-buten-1-yl)-3,4-dihydroquinoxaline-2(1 H)-thione
(Table 7, No. 1) (0.49 g, 2.0 mmol) was dissolved in 20 ml of ethanol (96%), andthe solution was treated with 5.1 ml (2.2 rnmol) of a 1% strength sodium ethanolate
1~ solution. After the mixture had been stirred for 15 minutes at room temperature,
û.14 ml (2.2 mmol) of methyl iodide was added dropwise, and the mixture was
stirred for a further 2 hours at room temperature. The reaction solution was
concentrated, and the residue was chromatographed on silica gel. 500 mg (96%) ofa yellow oil were isolated using ethyl acetate/heptane ~ 1:6.
1H NMR d6-DMSO): ~ = 0.96 (d, J = 7 Hz, 3 H), 1.72 (s, 6 H), 2.44 (s, 3 H), 3.71(dd, J = 15, 6 Hz, 1 H), 3.89 (dd, J = 15, 6 Hz, 1 H), 4.00 (q, J = 7 llz, 1 H), 5.20
(m, 1 H), 6.65 - 6.75 (m, 2 H), 7.02 (t, J = B Hz, 1 H), 7.11 ppm (d, J = 8 Hz, 1 H).
MS: (M ~ H)~ - 261
The following compound of the formula i was synthesized in the same manner:
4-lsopropenyloxycarbonyl-2-(isopropenylOXyCarbOnyl)-thiO-3,~,7,8-tetramethyl-
3,4-dihydroquinoxaline.
Melting point: 11~C
2~6~
102
Example XXV
~3Rs)-3-Methyl-4-N-(3-methyl-2-buten-l-yl)-3l4-dihydroquinoxalirl-2(1 H)-one
(3RS)-3-Methyl-3,4-dihydroquinoxalin-2(1~)-on0 (4.86 g, 0.03 mol) dissolved in
50 ml of N,N-dimethylformamide was alkylated with 4.2 ml ~0.033 mol) of 3-methyl2-buten-1-yl bromide (90%) in the presence of 4.60 9 (0.033 mol) of pulverulent
potassium carbonate. The reaction mixture was stirred at room temperature until
reaction of the educt was complete. The solvent was th~n stripped off in vacuo, the
residue was taken up in ethyl acetate and water, the phases were separated, the
aqueous phase was extracted twioe with ethyl acetate, and the combined organic
extracts were washed twice with water. Dryin~ over sodium sulfa~e, concentration in
vacuo and crystatlization from pentane gave 5.80 9 (84%) of white crystalline
product of melting point 92-93C.
lH NMR (270 MHz, d6-DMSO): ~ = 0.99 (d, J = 7 Hz, 3 H), 1.72 (s, 6 H), 3.67 (dd,J = 15, 7 Hz, 1 H), 3.86 (q, J = 7 Hz, 1 H), 3.88 (dd, J = t5, 7 Hz, 1 H), 5.21 (m,
1 H), 6.65 - 6.9 (m, 4 H), 10.31 ppm (br. s, 1 H).
MS: (M + H)+ = 231
Example XXVI
3,3a-Dihydropyrrolo[1,2-a]quinoxaline-1,4(2H,5H)-dione
2-Fluoronitrobenzene (14.1 9, 0.1 mol) and L-glutamic acid (45.0 9, 0.3 mol) were
heated in 100 ml of 2-rnethoxyethanol at 95C, with stirring, and 300 ml of 2N
sodium hydroxide solution were added dropwise. Stirring was then continued for
25 another 3 hours at this temperature. After cooling, the solution was treated with
400 ml of methanol and hydrogenated under atmospheric pressure with Raney
nickel as catalyst.
When the uptake of hydrogen had ended, the catalyst was removed by filtration
with suction, and the solution was concentrated under reduced pressure.
30 The residue was acidified with 250 ml of 2N hydrochloric acid and heatecl in a
steam bath for approx. 30 minutes. The precipitate which resulted in this process
2~6~8~
103
was filtered off with suction, washed with water and alcohol and subsequently
dried, melting point 255C, decomposition.
'H NMR (60 MHz, d6-DMSO): ~ = 1.9 - 2.7 ~m, 4 H), 4.5 (t, J = 8 Hz, 1 H~,
6.8 - 7.3 (m, 3 H), 7.8 - 8.2 (m, 1 H), 10.7 ppm (br. s, 1 H).
MS: (M + H)+ = 202
Example XXVII
7-Phenoxysulfonyl-3,3a-dihydropyrrolo[1 ,2-a~iquinoxaline-1 ,4~2H,5H~-dione
The compound was obtained in analo~ous manner by r0acting phenyl 4-chloro
3-nitrobenzenesulfonate with L-glutamic acid, melting point 140C: (decomp.).
1H NMR (60 MHz, d6-DMSO): ~ = 1.6 - 2.5 (m, 4 H), 4.07 (t, J = 6 Hz, 1 H),
6.7 - 7.6 (m, 8 H), 10.57 ppm (br. s, 1 H).
MS: (M + H)~ = 358
Example XXVIII
3-Carboxymethyl-3,4-dihydroquinoxalin-2(1 H)-one
2-Fluoronitrobenzene (14.1 g, 0.1 mol) and L-aspartic acid (40.0 g, 0.3 mol) were
heated to 95C in 100 ml of 2-rnethoxyethanol, with stirring, and 300 rnl of 2N
sodium hydroxide solution were added dropwise. Stirring was then continued for 1hour at this temperature. After the solution had cooled, it was treated with 500 ml of
methanol and hydrogenated under atmospheric pressure with Raney nickel as
catalyst.
When the uptake of hydrogen had ended, the catalyst was removed by filtration
with suction, and the solution was concentrated under reduced pressure.
The residue was acidified with 500 ml of 2N hydrochloric acid, the mixture was
subsequently concentrated, neutralized with sodium acetate anci extracted with
ethyl acetate. The mixture was dried with sodium suifate, the solvent was stripped
off, and the residue was then obtained which was first oily and crystailized upon
stirring with water, melting point 152-154t::.
2~6~
194
'H NMR (60 MHz, d6-DMSO): ~ = 2.5 - 2.7 (dd partly concealed, 2 H), 4.1 (td,
J = 6, 2 Hz, 1 H), 5.98 (br. s, 1 H), 6.5 - 6.9 (m, 4 H), 10.30 (br. s, 1 H),
12.37 ppm (br. s, 1 H).
MS: M+ = 206
CHN analysis: calculated C 58.2; H 4.8; N 13.6%
found C 58.4; H 4.7; N 13.7%
Example X)(IX
7-Phenoxysulfonyl-3,4-dihydroquinoxalin-2(1H) one
A) Methyl N- [ (2-nitro-4-phenoxysulfonyl)phenyl] glycinate
Phenyl 4-chloro-3-nitrobenzenesulfonate (62.7 g, 0.2 mol) and methyl glyeinate
hydrochloride (100.4 g, 0.8 mol), dissolved in 250 ml of methanol, were treated with
200 ml of triethylamine, and the mixture was refluxed for 15 minutes. After cooling,
the mixture was treated with 1 1 of 2N acetic acid, subjected to filtration with suction
and washed with water. The residue was recrystallized from ethyl acetate and
washed with methanol and diisopropyl ether, melting point 120-123C.
B) 7-Phenoxysulfonyl-3,4-dihydroquinoxalin-2(1 H)-one
Methyl N-[(2-nitro-4-phenoxysulfonyl)phenyl]glycinate (36.6 9, 0.1 mol) was
hydrogenated under atmospheric pressure in a mixture of 250 ml of
N,N-dimethylformamide and 250 ml of methanol, with Raney nickel as catalyst.
When the uptake of hydrogen had ended, the catalyst was removed by filtration
with suction, and the solution was freed from solvent in vacuo. The residue was
dissolved in 40 rnl of 2-methoxyethanol, and the mixture was hea~ed ior one hour in
a steam bath. The resulting precipitate was filtered off with s~ction and washeel with
methanol, melting point 253-254C.
'H NMR (60 MHz, d6-DMSO): g = 4.0 (d, J = 4 Hz, 2 H), 6.6 - 7.6 (m, 9 H),
10.43 ppm (br. s, 1 H).
MS: (M ~ H)+ = 305
2 ~
105
Example XXX
4-(3-Methyl-2-buten-1-yl)-7-phenoxysul~onyl-3,Wihydroquinoxalin-2(1 H)-one
7-Phenoxysulfonyl-3,4-dihydroquinoxalin-2(1H)-one (1.52 g, 5.0 mmol) in 20 ml ofN,N-dimethylacetamjde was stirred for 8 hours at 100~C with 2 ml of 3-methyl-
2-buten-1-yl bromide. After cooling, the mixture was treated with water and
extracted with ethyl acetate. The solution was dried using magnesium sulfate andthen concentrated, and the residue was chromatographed over a silica gel column
using ethyl acetate/heptane = 1:~. The fractions which contained th~ substance
were evaporated on a rotary evaporator, and the product was subsequently stirredwith pentane and filtered off with suction, melting point 132~
H NMR (270 MHz, d6-DMSO): ~ = 1.73 (s, 6 H), 3.90 (s, 2 H), 3.93 (partly
concealed d, J = 6 Hz, 2 H), 5.20 (br. t, J = 6 Hz, 1 H), 6.75 - 7.45 (m, 8 H),
10.66 ppm (s, 1 H).
MS: (M + H)~ = 373
The following compounds of the formula I were synthesized in analogous rnanner
using the corresponding haloaromatic substances and amino acid derivatives and,
if appropriate, derivatized further on nitrogen atom 4:
2~$~
106
Table 8
H
I
R n~ ~R 3
R 4
R 5
. . I
Nr. R1n R3 R4 Rs M.P.C l
_ . I
1 7-C6H5-O-SO2 H CH2OH H 199 ¦
_ I
7-C6H5-O-SO2 H CH2OH CsHs 120
3 7-C6H5-O-SO2 H CH2COOH H 230
decomp. l
. _ I
4 7-C6Hs-o-so2 H CH2COOH CsHs
_ _ _ _
7-C6H5-O-sO2 H CH2CONH2 H 272
decomp.
_
7-C6Hs-O-sO2 H CH2CONH2 C5Hg
7 7-C6H5-O-sO2 H CH2-4-lmi H 216
decomp.
_ _ . , .
8 7-C6H5-O-sO2 H Ctl2-4-lmi C5H,~
_ ._
9 7-C6H5-OO H H H 280
decomp.
_ ..
7-C6H5-CO H H C6H5-CO 277
decomp
_ _ I
11 7-C6H5-C)-sO2 H CH3 _ 148
.. - : .
2 ~
107
_
Nr.R1n R' R4 Rs M.P. C
127-C6H5-O-sO2 H CH3 CsHg Oil
_ _ I
137-C6Hs~S02 H CH3 H 1 9E~
_ _
147-C6Hs~SO2 H CH3 CsHs Oil
157-C6Hs-sO2 H ~H3 IPOC 108
_ _
167-C6Hso-so2 H H H
I _ _ .
177-C~HsSO2 H H COCH3 270
~ _
187-C6H5-OSO2 H CH3 IPOC Resin
Key: CsHg = 3-methyl-2-buten-1-yl
4-lmi = 4-imidazolyl
IPC)C = isopropenyloxycarbonyl
Example XXXI
6-Chloro-7-phenoxysulfonyl-1,2,3,3a-tetrahydropyrrolo[2,1-c]-quinoxalin-4(5H)-one
A) Phenyl 2,4-dichloro-3-nitrobenzenesulfonate
2,6-Dichloronitrobenzene was stirred for 7 hours at 130C with an excess of
20 chlorosulfonic acid. After cooling, the mixture was poured onto ice, the
sulfochloride was filtered off with suction, washeci to neutrality and dried over
sodium hydroxide, melting point 91 C. The resulting sulfochlsride ~29.05 g,
0.1 mol) and phenol (11.5 g, 0.12 mol) were dissol~ed in 150 ml of acetone and
treated with 14 ml of triethylamine at 10C. The mixture was stirred for 1 hour with
25 cooling, stirring was then continued for a further 4 hours at room temperature, the
mixture was then treated with 200 mi of water, the resultin~ precipitate was filtered
off with suction at 10C, washed with water and dried in vacuo at 80 :;, melting
point 102C.
:
:
2 ~
108
B) N-[(3-Chloro-2-nitro-4-phenoxysulfonyl)phenyl]proline
Phenyl 2,4-dichloro-3-nitrobenzenesulfonate (34.8 g, 0.1 mol), 69.0 g (0.6 mol) of
L-proline, 200 ml of 2N sodium hydroxide solution and 200 ml of 2-methoxyethanolwere stirred for 10 minutes at 80C. The clear solution was acidified at 50C using
concentrated hydrochloric acid and poured onto ice. The precipitate was filtered off
with suction, washed with water to neutrality and ~ried at 80C. Melting point
148C (after recrystallization from methanol)
C) 6-Chloro-7-phenoxysulfonyl-1 ,2,3,3a-tetrahydropyrrolo[2, 1 -c]-quinoxalin-
4 (5H)-one
N-[(3-Chloro-2-nitro-4-phenoxysulfonyl)phenyl]proline (38.0 9, 0.075 mol) in 500 ml
of methanol and 25 ml of concentrated ammonia solution was hydrogenated under
atmospheric pressure with Raney nickel as catalyst.
When the uptake of hydrogen had ended, the catalyst was removed by filtration
with suction, the solution was concentrated, the residue together with 2N
hydrochloric acid was heated for approximately 30 minutes in a steam bath, cooled,
subjected to filtration with suction and washed with water to neutrality. Melting point
197C (after recrystallization from glacial acetic acid)
Example X~O(II
8-(4-Methyl-1 -piperazinyl)-3-(2-methylpropyl)-5-phenoxysulfonyl-
3,4-dihydroquinoxalin-2(1 tl)-one
A) Phenyl 2-chloro-4-(4-methyl-1-piperazinyl)-3-nitrobenzenesulfonate
Phenyl 2,4~dichloro-3-nitrobenzenesulfonate (17.4 9, 0.05 mol) and 25 ml of
rnethylpiperazine in 100 ml of isopropanol were refluxed for 10 minutes and
subsequently concentrated. The residue was stirred with 50 mi of 50% methanol,
filtered off with suction, and washed with 50% methanol and finally with water.
Melting point 94-95C (after recrystallization from cyclohexane)
2~3~5~
109
B) N-[(3-(4-Methyl-1-piperazinyl)-2-nitro-~-phenoxysuifonyl)-phenyl]leucine
hydrochloride
Phenyl 2-chloro-4-(4-methyl-1-piperazinyl)-3-nitrobenzenesulfonate t41.1 g, û.1 mol)
and L-leucine (39.3 g, 0.3 mol) were stirred for 8 hours at 95C in a mixture of100 ml of N,N-dimethylformamide, 50 ml of 2-methoxyethanol and 100 ml of 2N
sodium hydroxide sslution. When cold, the reaction mixture was acidified with
concentrated hydrochloric acid. The precipitate was taken up in ethyl acetate, and
the mixture was dried using sodium sulfate and freed from solvent in vacuo. Thisgave an orange oil.
C) 8-(4-Methyl-1-piperæinyl)-3-(2-methylpropyl)-~-phenoxysulfonyl-
3,4-dihydroquinoxalin-2(1H)-one hydrochloride
N-[(3-(4-Methyl-1-piperazinyl)-2-nitro-6-phenoxysulfonyl)-phenyl]leucine
hydrochloride (25.3 g, 0.05 mol) in 250 ml of methanol and 25 ml of glacial acetic
acid was hydrogenated under atmospheric pressure using Raney nickel as catalyst.When the uptake of hydrogen had ended, the ca~alyst was removed by filtration
with suction, the solution was concentrated, and the residue together with 2N ofhydrochloric acid was heated for approximately 10 minutes in a steam bath and
then concentrated in vacuo. The residue was dissolved in water, the mixture was
rendered alkaline using ammonia, and this was taken up in ethyl acetate. The oilwhich remained after concentration was dissolved in 400 ml ~f diisopropyl ether,and the mixture was rendered neutral using ethanolic hydrochloric acid. The
~5 precipitate was filtered off with suction, washed with diisopropyl ether and dried,
melting point 90~C and above (decomp.).
MS: M+ = ~58
The following compounds of the forrnula I were synthesized in analogous manner
using the corresponding haloaromatic substances and amino acid derivatives and,
if appropriate, derivatized ~urther on nitrogen atorn 4:
2 ~ g ~
110
Table 9
l H3
~N H
~0~
¦ Nr. R3 R4 Rs M.P. C
¦ 1 H (CH3)2CHCH2CsHs l
_ ,
~ H GH3 H (HCI)
¦ 3 tl CH3 C5Hg
I _
¦ 4 H H H 126- 127 (base)
I _
H H C5Hg
Key: C5Hg = 3-methyl-2-buten-1-yl
Example XXXlil
(3RS)-4-N-Cyclohexyl-3-rnethyl-3,4-dihydroquinoxalin-2(H)-one
(3RS)-3-Methyl-3,4-dihydroquinox31in-2(1H)-one (0.81 9, 0.005 mol) and 1 ml
(0.1 mol) of cyclohexanone were introduced into 20 ml vf 1,2-dichioroethans.
Trifluoroacetic acid (1.9 ml, 0.025 mol) was addsd dropwise, during which process
a clear solution formed with gentle heating. 2.1 g (0.01 mol) of sodium
triacetoxyborohydride were added, the exothermic reaction was then allowed to
-
:
2~65~5
111
proceed for 30 minutes with stirring, and quenching was then effected by adding
saturated aqueous sodium hydrogen carbonate solution. The phases were
separated, the organic phase was washed with saturated aqueous sodium chloride
solution, dried (magnesium sulfate) and concentrated. The crude product was
chromatographed on silica gel using ethyl acetate/heptane = 1:1. 1.15 g (94%) ofthe d~sired product were obtained, melting point 131-132~C (toluene/heptane).
'H NMR (270 MHz, d6-DMSO): ~ = 0~97 (d, J = 7 Hz, 3 H), 1.0 - 2.0 (m, 10 H),
3 39 (m, 1 H), 3.91 (q, J = 7 Hz, 1 H), 6.68 - 6.94 (rn, 4 H), 10.27 ppm (br. s, 1 H).
MS: (M + H)t = 245.
The following compounds of the formula I were synthesized in analogous manner.
Table lQ
H
R n~
R 3
I R4
R 5
_ _ I
Nr. Rln R3 R4 R5 M.P. C
I _ _
1 CH3 H C2Hs 106 -1 07
I _ _
2 CH3 H CH2C(CH3)3 162
. .
3 CH3 H c-C5~19 120
. _
4 6-CI CH3 H c-~4H7 100
_
6-CI CH3 H CsH,1 94 - 95
~,
.
112 2~59
_
Nr. R'n R3 R4 M.P.C
6 6-CI CH3 H CH2C(CH3)3 158- 160
_
7 6-CI C2Hs H CH2C(CH3)3 158 -1 59
8 6-CI CH3 H CH =CH(:~HO 140 -146
L~ 6-CI CH3 H CH2C--CH3 166-168
6-CI CH3 H 2-Picolyl 198 -199
I _
11 6-CI CH3 H 3-Picolyl 136
I
12 6-CI CH3 H 4-Picolyl 19t - 193
_
13 6-CI CH3 H Furanyl-2-methyl 116- 118
14 6-CI CH3 H CH2C6H4 4-Rr 149 - 150
6-CI CHS H CH2C6H4-4-CN 95 - 96
16 6-CI CH3 H CH2C6H4-4-NO2 117
I
¦ 17 6-CI CH3 H CH2C6H4-3-NO2 125
I
¦ 18 6-CI CH3 H CH2C6Hq-2-NO2 153- 154
I
¦ 19 6-CI CH3 H CH2C6H4-4-CI 122 - 1~3
I . _
L~ 6-CI CH3 H CH2C6H4-3-CI 156 - 157
L~ 6-CI CH3 H CH2C6H4-2-CI 138
¦ 22 6-CI CH3 ll CH2C6H4~-F 147
I _
¦ 23 6-CI CH3 H CH2C6H4-4-C6H5 164-165
I _
~ 6-CI CH3 H C;H2C6H4-4-OC6Hs il
6-CI CH3 H CH2C6H4-4-CH3 60-62
_ _ _
26 6-CI CH3 11 CH2C6H4-4-COC)CH3 139
_ _
27 6-CI CH3 H CH2C6H3-2,~-Cl2 190-191
2 ~ a
113
_ _ _
¦~ ~ R1n R3 R4 Rs M.P. C .
l _ . I
28 6-CI CH3 H CH2C6H3-3,5-Cl2 139-140
I
29 6-CI CH3 H Naphthyl-1 -methyi 164-166
I _
6-CI CH3 H Naphthyl-2-methyl 161-164
_
31 6-CI CH3 H CH2CH2OCH3 78-79
32 6-CI CH3 H Cyclohex-2-enyl Oil
_ _
33 6-CI CH3 H C2H4-C6H5 128
.
34 6-CI CH3 H Thienyl-3-rnethyl 141-142
6-CI CH3 H (5-Methyl~hienyl)- 58-60
2-methyl
36 6-CI CH3 H (3-Methylthienyl)-2- 124
methyl
l _
37 6-CI CH3 H Thienyl-2-methyl 121-123
I
~8 6-CI CH3 H CH2CH = CH-~6H5 _
39 6-CI CH2SCH3 H CH2C6H4-2-CI 128
I _
6-CI CH2SCH3 H CH2C6tl4-2-NO2 134
_
41 6-CI CH2SCH3 H 2-Picolyl Oil
42 6-CI CH2SCH3 H CH2C6H3-2,4-cL2 t43
_ _ _
43 6-CI CH2S-i. Pr H CH2C6H3-2,4-Cl2 Oil
_
44 6-CI CH2S-Bn H Cff2C6H3-2,4-Cl2 Oil
_
6-CI CH2-S-H H CH2C6H3-2,4-Cl2
¦__~ _~ . T-- . .. ...
46 6-CI C2H5 H 2-PiCQiyl 160-162
I _ .
1~_ 6-CI CH3 H (6-CH3)2-Picolyl 158
2 ~ 8 ~
114
Key: CsH11 = 3-methyl-1-butyl
c-C4H7 = cyclobutyl
c-CsH9 = cyclopentyl
Example X)O~IV
(3RS~-3-Methyl-4-N-(3-oxo-1-butyl)-3,4-~ihydroquinoxalin-2(1 H)-one
3-Methyl-3,4-dihydroquinoxalin-2(1H)-one (0.5 y, 3.1 mmol) together with 0.35 ml(4.3 mmol) of methyl vinyl ketone and a catalytic amount of triethylamine were
stirred for 20 hours at room temperature in 20 ml of anhydrous ethanol. Silica gel
chromatography with methyl tert.-butyl ether/heptane = 2:1 gave 620 mg (87%) of
the desired product, melting point 108-109~C (rnethyl tert.-butyl ether/heptane).
t5 1H NMR ~270 MHz, d6-DMSO): ~ = 1.03 (d, J = 7 Hz, 3 H), 2.11 (s, 3H), 2.77 (t,
J=6Hz,2H),3.30(m,1H),3.50(m,1H),3.88(q,J=7Hz,1H),6.68(m,
1 H), 6.78 (m, 1 H), 6.88 (m, 1 H), 10.31 ppm (br. s, 1 H).
MS: (M + H)~ = 233, M~ = 232
Example XXXV
(3S)-6-Chloro-4-N-chlorocarbonyl-3-methyl-3,4-dihydroquinoxalin-2(1 H)-one
The compound of Example IB (2.0 g, 0.01 mol~ in 100 ml of anhydrous toluene was
heated with bis-(trichloromethyl) carbonate (triphosgene) (1.5 g, 0.005 mol) for 1
hour at 80C in the presence of 2 ml (0.û14 mol) of triethylamine. After cooling, the
mixture was washed with water and saturated aqueous sodium chloride solu~ion
and dried (magnesium sulfate), and the solvent was removed under reduced
pressure. The residue (2.5 g) crystalli~ed after stirring with heptane, its purity being
sufficient for preparative purposes. A sample of analytical purity was obtained by
silica gel chromatography using ethyl acetate/heptane = 1:1 as eluent. Melting
point 142-144C.
2 ~
115
'H NMR (270 MHz, d5-DMSO): ~ = 1.25 (d, J = 7 Hz, 3 H), 3.83 (q, J = 7 Hz,
1 H), 6.61 (dd, J = 6, 2 Hz, 1 H~, 6.70 (s, 2H), 10.3 ppm (br. s, 1 H).
MS: (M + H)+ = ~59
5 Example X)O~VI
(3S)-6-Chloro4-N-(2-methoxyethoxycarbonyl)-3-methyl-3,4-dihydroquinoxalin-
2(1 H)-one
1Q To a solution of 0.24 ml (3.0 mmol) of 2-methoxyethanol in 10 ml of anhydrous
1,2-dimethoxyethane there was added 0.16 ~ of a 55% suspension of sodium
hydride in mineral oil, and the reaction mixture was stirred for 30 minutes at room
temperature. 0.50 g (1.9 mmol) of the compound of Example XXXV was
subsequently added, with ice-cooling, and the mixture was allowed to warm to
15 room temperature and stirred for a further 30 rninutes. The mixture was treated with
saturated aqueous sodium chloride solution, extracted several times wi~h ethyl
acetate, the organic phase was washed once with saturated aqueous sodium
chloride solution and dried (magnesium sulfate), and the solvent was removed in
vacuo. After silica gel chromatography (ethyl acetate/heptane = 1:1) and
crystallization from ether/heptane, 0.29 9 (51%) of the desired product was
obtained, melting point 93-94C.
'H NMR (200 MHz, d6-DMSO): ~ = 1.13 (d, J = 7.5 Hz, 3 H), 3.32 (s, 3 H), 3.6 (m,2H), 4.24 (m, 1 H), 4.35 (m, 1 H), 4.81 (q, J = 7.5 Hz, 1 H), 6.98 (d, J = 9 Hz,1 H), 7.2 (dd, J = 9, 3 Hz, 1 H), 7.66 (d, J = 3 Hz, 1 H, 10.81 ppm (br. 2, 1 H).
MS: (M + H)+ = 299
Example XXXVII
(3S)-6-Chloro-3-methyl-4-N-[(phenylthio)carbonyl)l-3,4-dihydroquinoxalin-2(1 H)-one
To a solution of 0.31 ml (3.0 mmol) of thiophenol in 10 ml of 1,2-dimethoxyethane
there was added û.17 g of a 55% suspension of sodium hydride in mineral oil, with
116 20~985
ice-cooling, and the mixture was stirred for 1 hour at room temperature. 0.5 g
(1.9 mmol) of the compound of Example XXXV were introduced, again with ice-
cooling, and stirring was then continued for 2 hours at room temperature. For
working-up, the mixture was treated with saturated aqu~ous sodium chloride
5 solution, extracted twice with ethyl acetate and dried (sodium sulfate), and the
solvent was stripped off. The solid residue was recrystallized frorn
heptane/isopropanol, 0.35 g (35%), melting point 194-195C.
H NMR (200 MHz, d6-DMSO): ~ = 1.10 (d, J = 7 Hz, 3 H), 4.93 (q, J = 7 Wz,
1H),7.08(d,J=9Hz,1H),7.33(dd,J=9,3Hz,1H),7.4-78.6(m,5H),7.78
(d, J = 3 Hz, 1 H), 10.16 ppm (br. s, 1 H).
MS: (M + H)' = 333, (M - C~H5SH + H)' 223
The following compounds of the formula I were synthesized in analogous manner.
15 Table 11
H
I
R n~ J~R 3
R 4
R 5
. _
Nr. R1n R3 ~4 Rs M.P. C
. _
1 6-CI CH3 H COOCH2CH = CHCH3 1 16-1 17
2 6-CI ~H3 H COOCH2= C(CH3)2 87-89
_ . . _
3 6-CI CH3 H COOCH2C----CH 147
I _ _
L~ 6-CI CH3 H COOCH2G ~ CCH3 135
2 ~
117
_. .
R~ n _ R4 Rs M . P. C
S 6-CI CH3 H COSCH2C6Hs 158
6 6-CI CH3 H COSCH2CH = CH2 Oil
_ .
7 6-CI CH3 H COOCH2C(CH3) = CH2 125-127
_
8 6-CI CH3 H COOC(CH3)3
_
9 6-CI CH3 H COO-Cyclohex-2-en-1 -yl
_ .
10 6-CI CH3 H COOCH (CH2OCH (CH3)2)2 Oil
I _
11 6-CI CH3 H COOCH (CH3)2 1 41 -1 42
I
12 6-CI CH3 H COOC2H4N ~CH3)2 Oil
I _. _ _ .
13 6-CI CH3 H COOC2H4SCH3 108-110
I
14 6-CI CH3 H cOSC6H5 194-195
15 6-CI CH3 H COOCH2C6H4-2-NO2 227-231
6-CI CH3 H CoocH2c6H4-3-No2 183-185
17 6-CI CH3 H COO~H2C6H4-4-CI 177-180
I .
18 6-CI CH3 H COOCH2C6H4-2-CI 164
19 6-CI CH3 H COOCH2CH = CHCH2CH3 Oil
_ _ _
20 6-CI CH3 H CO0(3-Picolyl) 160-161
_
21 6-CI CH3 H COO(2-Picolyl) 114-116
22 6-CI CH3 H CoocH2c6H4-4-No2 230-233
_ _ ._ _ _
23 6-GI GH3 H COOCH2CH2C(CH3) = CH2 (:)il
. _ _ _ I
¦ 24 6-CI ~H3 H CO-(4-Nlethylpiperazin-1-yl) _ _
25 6-CI CH3 H CO-N (CH2)~ 218-220
__ I
26 6-CI CH3 H CO-N (CH2)4 ~00-203
I _ _
2~6~9~
118
~ R~n R3 R4 Rs ~ M.P. ~ C
I ...
27 6-CI CH3 H CO-Morpholin-1~yl 193-195
I
28 6-CI CH3 H CO-HNCH2Ph 94-96
I , , ,
29 6-CI CH3 H Cyclopropyl-methyloxy- 119-122
carbonyl