Note: Descriptions are shown in the official language in which they were submitted.
CP-IR-468lC
PROCESS FOR PREPARING A
LINEAR VISCOELASTIC AQUEOUS
LIQUID AUTOMATIC DISHWASHER
DETERGENT COMPOSITION
Background of the Invention
Liquid automatic dishwasher detergent compositions, both
a~ueous and nonaqueous, have recently received much attention,
and the aqueous products have achieved commercial popularity.
The acceptance and popularity of the li~uid formulations
as compared to the more conventional powder products stems
from the convenience and performance of the liquid products.
However, even the best of the currently available liquid
~ormulations still suffer from two major problems, product
phase instability and bottle residue, and to some extent cup
leakage from the dispenser cup of the automatic dishwashing
machine.
Representative of the relevant patent art in this area,
mention is made of Rek, U.S. Patent 4,556,504; Bush, et al.,
U.S. Patent 4,226,736; Ulrich, U.S. Patent 4,431,559;
Sabatelli, U.S. Patent 4,147,650; Paucot, U.S. Patent
4,079,015; Leikhem, U.S. Patent 4,116,849; Milora, U.S. Patent
4,521,332; Jones, U.S. Patent 4,597,889; Heile, U.S. Patent
4,512,908; Laitem, U.S. Patent 4,753,748; Sabatelli, U.S.
Patent 3,579,455; Hynam, U.S~ Patent 3,684,722. Other patents
relating to thickened detergent compositions include U.S.
Patent 3,985,668; U.K. Patent Applications GB 2,116,199A and
GB 240,450A; U.S. Patent 4,511,487; U.S. Patent 4,752,409
(Drapier, et al.); U.S. Patent 4,~01,395 (Drapier, et al.).
The present invention provides a solution to the above
problems~
Brief Description of the Drawings
Figures 1-13 are rheograms, plotting elastic modules G'
and viscous modulus G" as a function of applied strain, for
the compositions of Example 1, Formulations A, C, D, G, ~ H,
I and K, Example 2, A and B, Example 3, L and M and
Comparative Example 1, respectively.
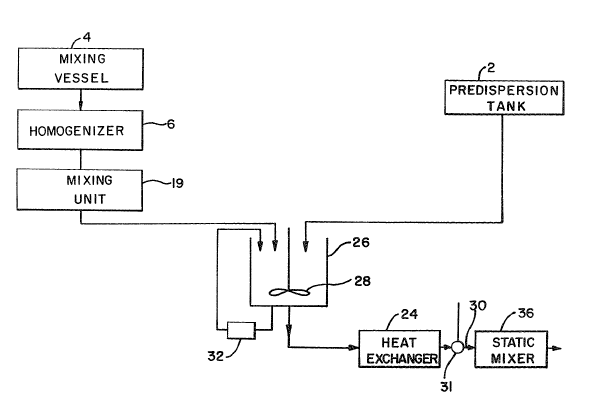
Figure 14 illustrates a schematic diagram of the most
preferred process; Flgure 15 illustrates a from B cutaway view
of a vibrating feeder; Figure 16 illustrates a top view of the
vibrating feeder.
5ummary of the Invention
According to the present invention there is provided a
process for preparing a novel aqueous liquid automatic
dishwasher detergent composition. The composition i9
characterized by its linear viscoelastic behavior,
substantially indefinite stability against phase separation or
settling of dissolved or suspended particles, low levels of
bottle residue, relatively high bulk density, and substantial
absence of unbound or free water. This uni~ue combination of
properties is achieved by virtue of the incorporation into the
aqueous mixture of dishwashing detergent surfactant, alkali
me~al detergent builder salt(s) and chlorine bleach compound,
a small but effective amount of high molecular weight cross-
linked polyacrylic acid type thickening agent, a physical
stabilizing amount of a long chain fatty acid or salt thereof,
and a source of potassium ions to provide a potassium/sodium
~Q~2~
weight ratio in the range of from 1:1 to 45:1, such that
substantially all of the detergent builder salts and other
normally solid detergent additives present in the composition
are present dissolved in the aqueous phase. The compositions
are further characterized by a bulk density o~ at least 1.32
g/cc, such that the density of the polymeric phase and the
density o~ the aqueous (continuous) phase are approximately
the same.
Detailed Descripti.on and Preferred Embodiments
~ process for preparing the compositions of this
invention which are aqueous liquids containing various
cleansing active ingredients, detergent adjuvants, structuring
and thickening agent~ and stabilizing components, although
some ingredients may serve more ~han one of these functions is
disclosed.
The advantageous characteristics of the compositions of
this invention, including physical stability, low bottle
residue, high cleaning performance, e.g. low spotting and
filming, dirt residue removal, and so on, and superior
aesthetics, are believed to be attributed to several
interrelated factors such as low solids, i.e. undissol~ed
particulate content, product density and linear viscoelastic
rheology. These factors are, in turn, dependent on several
critical compositional components of the formulations, namely,
~1) the inclusion of a thickening effective amount of
polymeric thickening agent having high water absorption
capacity, exemplified by high molecular weight cross-linked
polyacrylic acid, (2) inclusion of a physical stabilizing
8 9
amount oE a long chain fatty acid or salt thereof, l3)
potassium ion to sodium ion weight ratio K/Na in the range of
from 1:1 to 45:1, especially from 1:1 to 3:1, and (4) a
product bulk density of at least 1.32 g/cc, such that the bulk
density and liquid phase density are the same.
The polymeric thickening agents contribute to the linear
viscoelastic rheology of the invention compositions. As used
herein, "linear viscoelastic "or" linear viscoelasticity"
means that the elastic (storage) moduli (G') and the viscous
(loss) modull (G") are both substantially independent of
strain, at least in an applied strain range of from 0-50%, and
preferably over an applied strain range of from 0 to 80~.
More specifically, a composition i9 considered to be linear
viscoelastic for purposes of this invention, if over the
strain range of 0-50% the elastic moduli G' has a minimum
value of 100 dynes/sq.cm., preferably at least 250
dynes/sq.cm., and varies less than 500 dynes/sq.cm.,
preferably less than 300 dynes/sq.cm., especially preEerably
less than 100 dynes/sq.cm. Preferably, the minimum value of
G' and maximum variation of G~ applies over the strain range
of 0 to 80~. Typically, the variation in loss moduli G" will
be less than that of G'. As a further characteristic of the
preferred linear viscoelastic compositions the ratio of G"/G'
(tan ~ ) is less than 1, preferably less than 0.8, but more
than 0.05, preferably more than 0.2, at least over the strain
range of 0 to 50%, and preferably over the strain range of 0
to 80%. It should be noted in this regard that ~ strain is
shear strain xlO0.
3 ~
By way of further e~planation, the elastic (storage)
modulus G~ is a rneasure of the energy stored and retrieved
when a strain is applied to the composition while viscous
(loss) modulus G~ is a measure of the amount of energy
dissipated as heat when strain is applied. Therefore, a value
of tan ,^S ,
0.05 < tan ~S < 1
preferably
0.2 c tan r~ ~ 0.8
means that the compositions will retain sufficient energy when
a stress or strain is applied, at least over the extent
expected to be encountered Eor products of this type, for
example, when poured from or shaken in the bottle, or stored
in the dishwasher detergent dispenser cup of an automatic
dishwashing machine, to return to its previous condition when
the stress or strain is removed. The compositions with tan
values in these range3, therefore, will also have a high
cohesive property, namely, when a shear or strain is applied
to a portion of the composition to cause it to flow, the
surrounding portions will follow. As a result of this
cohesiveness of the subject linear viscoelastic compositions,
the compositions will readily flow uniformly and homogeneously
from a bottle when the bottle is tilted, thereby contributing
to the physical (phase) stability of the formulation and the
low bottle residue (low product loss in the bottle) which
characterizes the in~ention compositions. The linear
viscoelastic property also contributes to improved physical
stability against phase separation of any undissolved
2 ~
suspended particles by providin~ a resistance to movement of
the particles due to the strain exerted by a particle on the
surrounding fluid medium.
Also contributing to the physical stability and low
S bottle residue of the invention compositions is the high
potassium to sodium ion ratios in the range of 1:1 to 45:1,
preferably 1:1 to ~:1, especially preferably from 1.05:1 to
3:1, for example 1.1:1, 1.2:1, 1.~:1, 2:1/ or 2.5:1. At these
ratios the solubility of the solid salt components, such as
detergent builder salts, bleach, alkali metal silicates, and
the like, is substantially increased since the presence of the
potassium (K+) ions requires less water of hydration than the
sodium (Na~) ions, such that more water is available to
dissolve these salt compounds. Therefore, all or nearly all
of the normally solid components are present dissolved in the
aqueous phase. Since there is none or only a very low
percentage, i.e. less than 5%, preferably less than 3~ by
weight, of suspended solids present in the formulation there
is no or only reduced tendency for undissolved particles to
se~tle out of the compositions causing, for example, formation
of hard masses of particles, which could result in high bottle
residues (i.e. loss of product). Furthermore, any undissolved
solids tend to be present in extremely small particle sizes,
usually colloidal or sub-colloidal, such as 1 micron or less,
thereby further reducing the tendency for the undissolved
particles to settle.
A still further attribute of the invention compositions
contributing to the overall product stability and low bottle
residue is the high water absorption capacity of the cross-
linked polyacrylic acid-type thickening agent. As a result of
this high water absorption capacity virtually all of the
a~ueous vehicle component is held tightly bound to the polymer
matrix. Therefore, there is no or substantially no free water
present in the invention compositions. This absence of free
water (as well as the cohesiveness of the composition) is
manifested by the observation that when the composition is
poured from a bottle onto a piece of water absorbent filter
paper virtually no water is absorbed onto the filter paper
and, furthermore, the mass of the linear viscoelastic material
poured onto the filter paper will retain its shape and
structure until it is again subjected to a stress or strain.
As a result of the absence of unbound or free water, there is
virtually no phase separation between the aqueous phase and
the polymeric matrix or dissolved solid particles. This
characteristic i9 manifested by the fact that when the subject
compositions are subjected to centrifugation, e.g. at 1000 rpm
for 30 minutes, there is no phase separation and the
composition remains homogeneous.
However, it has also been discovered that linear
viscoelasticity and K/Na ratios in the above-mentioned range
do not, by themselves, assure long term physical stability (as
determined by phase separation). In order to maximize
physical (phase) stability, the density of the composition
should be con~rolled such that the bulk density of the liquid
phase is approximately the same as the bulk density of the
entire composition, including the polymeric thickening agent.
2 ,~ ~
This control and equalization of the densities i9 achieved,
according to the invention, by providing the composition with
a bulk density of at least 1.32 g/cc, preferably at least 1.35
g/cc, up to 1.42 g/cc, preferably up to 1.40 g/cc.
Furthermore, to achieve these rela~ively high bulk densities,
it is important to minimize the amount of air incorporated
into the composition (a density of 1.42 g/cc is essentially
equivalent to zero air content).
It has previously been found in connection with other
types of thickened aqueous liquid, automatic dishwasher
detergent compositions that incorporation of finely divided
air bubbles in amounts up to 8 to 10~ by volume can function
e~fectively to stabilize the composition against phase
separation, but that to prevent agglomeration of or escape of
the air bubbles it was important to incorporate certain
surface active ingredients, especially higher fatty acids and
the salts thereof, ~uch as stearic acid, behenic acid,
palmitic acid, sodium stearate, aluminum stearate, and the
like. These surface active agents apparently functioned by
forming an interfacial film at the bubble surface while also
forming hydrogen bonds or contributing to the electrostatic
attraction with the suspended particles, such that the air
bubbles and attracted particles formed agglomerates of
approximately the same density as the density of the
continuous liquid phase.
Therefore, in a preferred embodiment of the present
invention, stabilization of air bubbles which may become
incorporated into the compositions during normal processing,
such as during ~arious mixing steps, is avoided by pos~-adding
the surface active ingredients, including fatty acid or fatty
acid salt stabilizer, to the remainder of the composition,
under low shear conditions using mixing devices designed to
minimize cavitation and vortex formation.
As will be described in greater detail below the surface
active ingredients present in the composition will include the
main detergent surface active cleaning agent, and will also
preferably include anti-foaming agent and higher fatty acid or
salt thereof as a physical stabilizer.
Exemplary of the cross-linked polyacrylic acid-type
thickening agents are the products sold by B . F. Goodrich under
their Carbopol trademark, especially Carbopol 941, which is
the most ion-insensitive of this class of polymers, and
Carbopol 940 and Carbopol 934. The Carbopol resins, also
known as "Carbomer," are hydrophilic high molecular weight,
cross-linked acrylic acid poly~ers having an a~erage
equivalent weight of 76, and the general structure illustrated
by the following formula:
-- Ç f
H / C ~ J
HO n.
Carbopol 941 has a molecular weight of 1,250,000; Carbopol 940
a molecular weight of and Carbopol 934 a molecular weight of
t~ 9
3,000,000. The Carbopol resins are cross-linked with
polyalkenyl polyether, e.g. 1% of a polyallyl ether of
sucrose ha~ing an average o~ 5. a allyl groups for each
molecule of sucrose. Further detailed information on the
Carbopol resins is available from B.F. Goodrich, see, for
example, the B . F . Goodrich catalog GC-67, CarbopolR Water
Soluble Resins.
While the most favorable results have been achie~ed with
Carbopol 941 polyacrylic resin, other lightly cross-linked
polyacrylic acid-type thickening agents can also be used in
the compositions of this invention. As used herein
"polyacrylic acid-type" refers to water~soluble homopolymers
of acrylic acid or methacrylic acid or water-dispersible or
water-soluble salts, esters or amides thereof, or water-
soluble copolymers of these acids of their salts, esters or
amides with each other or with one or more other ethylenically
unsaturated monomers, such as, ~or example, styrene, maleic
acid, maleic anhydride, 2-hydroxyethylacrylate, acrylonitrile,
vinyl acetate, ethylene, propylene, and the like.
These homopolymers or copolymers are characterized by
their high molecular weight, in the range of from 500,000 to
10,000,000, preferably 500,000 to 5,000,000, especially ~rom
1,000,000 to 4,000,000, and by their water solubility,
generally at least to an extent of up to 5~ by weight, or
more, in water at 2~C.
These thickening agents are used in their lightly cross-
linked form wherein the cross-linking may be accomplished by
means known in the polymer arts, as by irradiation, or,
2,~
preferably, by the incorporation into the monomer mixture to
be polymerized of known chemical cross-linking monomeric
agents, typically polyunsaturated (e.g. diethylenically
unsaturated) monomers, such as, for example, dininylbenzene,
divinylether of diethylene glycol, N,~-methylene-
bisacrylamide, polyalkenylpolyethers (such as described
above), and the like. Typically, amounts of cross-linking
agent to be incorporated in the final polymer may range from
0.01 to 1.5 percent, preferably from 0.05 ~o 1.2 percent, and
especially, preferably from 0.1 to 0.9 percent, by weight of
cross-linking agent to weight of total polymer. Generally,
those skilled in the art will recognize that the degree of
cross-linking should be sufficient to impart some coiling of
the otherwise generally linear polymeric compound while
maintaining the cross-linked polymer at least water
dispersible and highly water-swellable in an ionic aqueous
medium. It is also understood that the water-swelling of the
polymer which provides the desired thickening and viscous
properties generally depends on one or two mechanisms, namely,
conversion of the acid group containing polymers to the
corresponding salts, e.g. sodium, generating negative charges
along the polymer backbone, thereby causing the coiled
molecules to expand and thicken the aqueous solution; or by
formation of hydrogen bonds, for example, between the carboxyl
groups of the polymer and hydroxyl donor. The former
mechanism is especially important in the present invention,
and therefore, the preferred polyacrylic acid-type thickening
agents will contain free carbox~lic acid (C~OH) groups along
the polymer backbone. Also, it will be understood that the
degree of cross-linking should not be so high as to render the
cross-linked polymer completely insoluble or non-dispersible
in water or inhibit or prevent the uncoiling of the polymer
molecules in the presence of the ionic aqueous system.
The amount of the high molecular weight, cross-linked
polyacrylic acid or other high molecular weight, hydrophilic
cross-linked polyacrylic acid-type thickening agent to impart
the desired rheological property of linear viscoelasticity
will generally be in the range of from 0.1 to 2~, preferably
from 0.2 to 1.4~, by weight, based on the weight of the
composition, although the amount will depend on the particular
cross-linking agent, ionic strength of the composition,
hydroxyl donors and the like.
The compositions of this invention must include
sufficient amount o~ potassium ions and sodium ions to provide
a weight ratio of K/Na of at least 1:1, preferably from 1:1 to
45:1, especially from 1:1 to 3:1, more preferably from 1.05:1
to 3:1, such as 1.5:1, or 2:1. When the K/Na ratio i8 less
than 1 there is insufficient solubility of the normally solid
ingredients whereas when the K/Na ratio is more than 45,
especially when it i9 greater than 3, the product becomes too
liquid and phase separation begins to occur. When the K/Na
ratios become much larger than 45, such as in an all or mostly
potassium formulation, the polymer thickener loses its
absorption capacity and begins to salt out of the aqueous
; phase.
12
2 ~ . d ~ ~
The potassium and sodium ions can be made present in the
compositions as the alkali metal cation of the detergent
builder salt(s), or alkali metal silicate or alkali metal
hydroxide components of the compositions. The alkali metal
cation may also be present in the compositions as a component
of anionic detergent, bleach or other ionizable salt compound
additive, e.g. alkali metal carbonate. In determining the
K/~a weight ratio~ all of these sources should be taken into
consideration.
Specific examples of detergent builder salts include the
polyphosphates, such as alkali metal pyrophosphate, alkali
metal tripolyphosphate, alkali metal metaphosphate, and the
like, for example, sodium or potassium tripolyphosphate
(hydrated or anhydrous), tetrasodium or tetrapotassium
pyrophosphate, sodium or potassium hexa-metaphosphate,
trisodium or tripotassium orthophosphate and the like, sodium
or potassium carbonate, sodium or potassium citrate, sodium or
potassium nitrilotriacetate, and the like. The phosphate
builders, where not precluded due to local regulations, are
preferred and mixtures of tetrepotassium pyrophosphate (TKPP)
and sodium tripolyphosphate (NaTPP) (especially the
hexahydrate) are especially preferred. Typical ratios of
NaTPP to TKPP are from 2:1 to 1:8, especially from 1:1.1 to
1:6. The total amount of detergent builder salts is
preferably from 5 to 35~ by weight, more preferably from 15 to
35~, especially from 18 ~o 30~ by weight of the composition.
The linear viscoelastic compositions of this invention
may, and preferably will, contain a small, but stabilizing
2 ~ 8 ~
effective amount of a long chain fatty acid or monovalent or
polyvalent salt thereof. Although the manner by which the
fatty acid or salt contributes to the rheology and stability
of the composition has not been fully elucidated it is
hypothesized that it may function as a hydrogen bonding agent
or cross-linking agent for the polymeric thickener.
The preferred long chain fatty acids are the higher
aliphatic fatty acids having from 8 to 22 carbon atoms, more
preferably from 10 to 20 carbon atoms, and especially
preferably from 12 to 18 carbon atoms, inclusive of the carbon
atom of the carbo~yl group of the fatty acid. The aliphatic
radical may be saturated or unsaturated and may be straight or
branched. Straight chain saturated fatty acids are preferred.
Mixtures of fatty acids may be used, such as those derived
from natural sources, such as tallow fatty acid, coco fatty
acid, soya fatty acid, etc., or from synthetic sources
available from industrial manufacturing processes.
Thus, examples of the fatty acids include, for exam~le,
decanoic acid, dodecanoic acid, palmitic acid, myristic acid,
stearic acid, behenic acid, oleic acid, eicosanoic acid,
tallow fatty acid, coco fatty acid, soya fatty acid, mixtures
of these acids, etc. Stearic acid and mixed fatty acids, e.g.
stearic acid/palmitic acid, are preferred.
When the free acid form of the fatty acid i9 used
directly it will generally associate with the potassium and
sodium ions in the aqueous phase to form the corresponding
alkali metal fatty acid soap. However, the fatty acid salts
may be directly added to the compositiorl as sodium salt or
potassium salt, or as a polyvalent metal salt, although the
alkali metal salts of the fatty acids are preferred fatty acid
salts.
The preferred polyvalent metals are the di- and tri-
valent metals of Groups IIA, IIB and III~, such as magnesium,calcium, aluminum and zinc, although other polyvalent metals,
including those of Groups IIIA, IVA, VA, IB, IVB, VB, VIB,
VIIB and VIII of the Period Table of the Elemen~s can also be
used. Specific examples o~ such other polyvalent metals
include Ti, Zr, V, Nb, Mn, Fe, Co, Ni, Cd, Sn, Sb, Bi, etc.
Generally, the metals may be present in the divalent to
pentavelent state. Preferably, the metal salts are used in
their higher o~idation states. Naturally, for use in
automatic dishwashers, as well as any other applications where
the invention composition will or may come into contact with
articles used for the handling, storage or serving of food
products or which otherwise may come into contact with or be
con~umed by people or animals, the metal salt should be
selected by taking into consideration the toxicity of the
metal. For this purpose, the alkali metal and calcium and
magnesium salts are especially higher preferred as generally
safe food additives.
The amount o~ the fatty acid or fatty acid salt
stabili2er to achieve the desired enhancement of physical
~tability will depend on such factors a~ the nature of the
fatty acid or its salt, the nature and amount of the
thickening agent, detergent active compound, inorganic salts,
$ ~ ~ ~
o~her ingredients, as well as the anticipated storage and
shipping conditions.
Generally, however, amounts of the fatty acid or fatty
acid salt stabilizing agents in the range of from 0.02 to 2~,
preferably 0.04 to 1~, more preferably from 0.06 to 0.8~,
especially preferably from o.oa to 0.4~, provide a long term
stability and absence of phase separation upon standing or
during transport at both low and elevated temperatures as are
required for a commercially acceptable product.
Depending on the amounts, proportions and types of fatty
acid physical stabilizers and polyacrylic acid-type thickening
agents, the addition of the fatty acid or salt not only
increases physical stability but also provides a simultaneous
increase in apparent viscosity. Amounts of fatty acid or salt
to polymeric thickening agent in the range of from 0.08-0.~
weight percent fatty acid salt and from 0.4-1.5 weight percent
polymeric thickening agent are usually sufficient to provide
these simultaneous benefits and, therefore, the use of these
ingredients in these amounts i9 more preferred.
In order to achieve the desired benefit from the fatty
acid or fatty acid salt stabilizer, without stabilization of
e~cess incorporated air bubbles and consequent excessive
lowering of the product bulk denRity, the fatty acid or salt
should be post-added to the formulation, preferably together
with the other surface active ingredients, including detergent
active compound and anti-foaming agent, when present. These
surface active ingredients are preferably added as an emulsion
in water wherein the emulsified oily or fatty materials are
16
28~
finely and homogeneously dispersed throughout the aqueous
phase. To achieve the desired fine emulsification of the
fatty acid or fatty acid salt and other surface acti~e
ingredients, it is usually necessary to heat the emulsion (or
preheat the water) to an elevated temperature near the melting
temperature of the fatty acid or its salt. For example, for
stearic acid having a mel~ing point of 68-69C, a temperature
in the range of between 50C and 70C will be used. For lauric
acid (m.p.=~7C) an e3evated temperature of 3~ to 50VC can ~e
used. Apparently, at these elevated temperatures the fatty
acid or salt and other surface active ingredients can be more
readily and uniformly dispersed (emulsified) in the form of
fine droplets throughout the composition.
In contrast, as will be shown in the examples which
follow, if the fatty acid is simply post-added at ambient
temperature, the composition is not linear viscoelastic as
defined above and the stability of the composition is clearly
inferior.
Foam inhibition is important to increase dishwasher
machine efficiency and minimize destabilizing effects which
might occur due to the presence of excess foam within the
washer during use. Foam may be reduced by suitable selection
of the type and/or amount of detergent acti~e material, the
main foam-producing component. The degree of foam is also
somewhat dependent on the hardness of the wash water in the
machine whereby suitable adjustment of the proportions of the
builder salts, such as NaTPP which has a water softening
effect, may aid in providing a degree of foam inhibition.
17
8 ~
However, it is generally preferred to include a chlorine
bleach stable foam depressant or inhibi.tor. Particularly
ef~ective are the alkyl phosphoric acid esters of the formula
o
HO~ R
OR
and especially the alkyl acid phosphate esters of the formula
HO P OR
dR
in the above formulas, one or both R groups in each type of
ester may represent independently a C,2-C20 alkyl group. The
ethoxylated deri~atives of each type of ester, for example,
the condensation products of one mole of ester with from 1 to
10 moles, preferably 2 to 6 moles, more preferably 3 or 4
moles,
ethylene oxide can also be used. Some examples of the
foregoing are commercially available, such as the products SAP
from Hooker and LPKN-158 from Knapsack. Mixtures of the two
types, or any other chlorine bleach stable types, or mixtures
of mono- and di-esters of the same type, may be employed.
Especially preferred is a mixture of mono- and di-CI6-Cl8 alkyl
acid phosphate esters such as monostearyl/distearyl acid
phosphates 1.2/1, and the 3 to 4 mole ethylene oxide
condensates thereof. When employed, proportions of 0.05 to
1.5 weight percent, preferably 0.1 to 0.5 weight percent, of
foam depressant in the composition is typical, the weight
ratio of detergent active component (d) to foam depressant ~e)
18
generally ranging from 10:1 to 1:1 and preferably 5:1 to 1:1.
Other defoamers which may be used include, for example, the
known silicones, such as available from Dow Chemicals. In
addition, it is an advantageous feature of this invention that
many of the stabiliæing salts, such as the stearate salts, for
example, aluminum stearate, when included, are also effective
as foam killers.
Although any chlorine bleach compound may be employed in
the compositions of this invention, such as dichloro-
isocyanurate, dichloro-dimethyl hydantoi~, or chlorinated TSP,
alkali metal or alkaline earth metal, e.g. potassium, lithium,
magnesium and especially sodium, hypochlorite is preferred.
The composition should contain sufficient amount of chlorine
bleach compound to provide 0.2 to 4.0~ by weight available
chlorine, as determined, for example, by acidification of 100
parts of the composition with excess hydrochloric acid. A
solution containing 0.2 ~o 4.0~ by weight of sodium
hypochlorite contains or provides roughly the same percentage
of available chlorine~ 0.8 to 1.6~ by weight of available
chlorine is especially preferred. For example, sodium
hypochlorite (NaOCl) solution of from 11 to 13~ available
chlorine in amounts of 3 to 20%, preferably 7 to 12~, can be
advantageously used.
Detergent active material useful herein should be stable
in the presence of chlorine bleach, especially hypochlorite
bleach, and for this purpose those of the organic anionic,
amine oxide, phosphine oxide, sulphoxide or betaine water
dispersible surfactant types are preferred, the first
19
$ ~
mentioned anionics being most preferred. Particularly
preferred surfactants herein are the linear or branched alkali
metal mono- and/or di-(C8-CIq) alkyl diphenyl oxide mono- and/or
di-sulphates, commercially available for example as DOWFAX
(registered trademark) 3B-2 and DOWFAX 2A-1. In addition, the
surfactant should be compatible with the other ingredients of
the composition. Other suitable organic anionic, non-soap
surfactants include the primary al~ylsulphates,
al~ylsulphonates, alkylarylsulphonates and sec.-
alkylsulphates. Examples include sodium C~0-C~8 alkylsulphates
such as sodium dodecylsulphate and sodium tallow
alcoholsulphate; sodium C~0-C~8 alkanesulphonates such as sodium
hexadecyl-1-sulphonate and sodium Cl2-C~8
alkylbenzenesulphonates such as sodium
dodecylbenzenesulphonates. The corresponding potassium salts
may also be employed.
As other suitable surfactants or detergents, the amine
oxide sur~actants are typically of the structure R2RINO, in
which each R represents a lower alkyl group, for instance,
methyl, and Rl represents a long chain alkyl group having from
a to 22 carbon atoms, for instance a lauryl, myristyl,
palmityl or cetyl group. Instead of an amine oxide, a
corresponding sur~actant phosphine oxide R2RIPO or sulphoxide
RRISO can be employed. Betaine surfactants are typically of
the structure R2R~N+R"COO-, in which each R represents a lower
alkylene group having from 1 to 5 carbon atoms. Specific
examples of ~hese surfactants include lauryl-dimethylamine
oxide, myristyl-dimethylamine oxide, the corresponding
2 ~
phosphine o~ides and sulphoxides, and the corresponding
betaines, including dodecyldimethylammonium acetate,
tetradecyldiethylammonium pentanoate,
hexadecyldimethylammonium hexanoate and the like. For
biodegradability, the alkyl groups in these surfactants should
be linear, and such compounds are preferred.
Surfactants of the foregoing type, all well known in the
art, are described, for example, in U.S. Patents 3,9~5,668 and
4,271,030. I~ chlorine b7each is not used then any of the
well known low-foaming nonionic surfactants such as
alkoxylated fatty alcohols, e.g. mixed ethylene oxide-
propylene oxide condensates of C8-C22 fatty alcohols can also be
u~ed.
The chlorine bleach stable, water dispersible organic
detergent-active material (surfactant) will normally be
present in the composition in minor amounts, generally 1~ by
weight of the composition, although smaller or larger amounts,
such as up to 5%, such as from 0.1 to 5~, preferably from 0.3
or 0.4 to 2% by weight of the composition, may be used.
Alkali metal (e.g. potassium or sodium) silicate, which
provides alkalinity and protection of hard ~urfaces, such as
fine china glaze and pattern, is generally employed in an
amount ranging from 5 to 20 weight percent, preferably 5 to 15
weight percent, more preferably 8 to 12% in the composition.
The sodium or potassium silicate is generally added in the
form of an aqueous solution, preferably having Na20:SiO2 or
X20:SiO2 ratio of 1:1.3 to 1:2.8, especially preferably 1:2.0
to 1:2.6. At this point, it should be mentioned that many of
2 8 ~
~he other components of this composition, especially alkali
metal hydroxide and bleach, are also often added ln the form
of a prelim.inary prepared aqueous dispersion or solution.
In addition to the detergent active surfactant, Eoam
inhibitor, alkali metal silicate corrosion inhibitor, and
detergent builder salts, which all contribute to the cleaning
performance, it is also known that the effectiveness of the
liquid automatic dishwasher detergent compositions is related
to the alkalinity, and particularly to moderate to high
alkalinity levels. ~ccordingly, the compositions of this
invention will have pH values of at least 9.5, preferably at
least 11 to as high as 14, generally up to 13 or more, and,
when added to the aqueous wash bath at a typical concentration
level of 10 grams per liter, will provide a pH in the wash
bath of at least 9, preferably at least 10, such as 10.5, 11,
11.5 or 12 or more.
The alkalinity will be achieved, in part, by the alkali
metal ions contributed by the alkali metal detergent builder
salts, e.g. sodium tripolyphosphate, tetrapotassium
pyrophosphate, and alkali metal silicate; however, it is
usually necessary to include alkali metal hydroxide, e.g. NaO~
or KOH, to achieve the desired high alkalinity. Amounts of
alkali metal hydroxide in the range (on an active basis) o~
from 0.5 to 8~, preferably from 1 to 6~, more preferably from
1.2 to 4~, by weight of the composition will be sufficient to
achieve the desired pH level and/or to adjust the K/Na weight
ratio.
22
2 ~
Other alkali metal salts, such as alkali metal carbonate
may also be present in the compositions in minor amounts, for
example from O to 4~, preferably O to 2~, by wèight of the
composition.
Other conven~lonal ingredients may be included in these
compositions in small amounts, generally less than 3 weight
percent, such as perfume, hydrotropic agents such as the
sodium benzene, toluene, xylene and cumene ~ulphonates,
preservatives, dyestuffs and pigments and the like, all of
course being stable to chlorine bleach compound and high
alkalinity. Especially preferred for coloring are the
chlorinated phythalocyanines and polysulphides of
aluminosilicate which provide, respectively, pleasing green
and blue tints. TiO2 may be employed for whitening or
neutralizing off-shades.
Although for the reasons previously discussed excessive
air bubbles are not often desirable in the invention
compositions, depending on the amounts of dissol~ed solids and
liquid pha~e densities, incorporation of small amounts of
finely divided air bubbles, generally up to 10~ by volume,
preferably up to 4~ by volume, more pxeferably up to 2~ by
volume, can be incorporated to adjust the bulk density to
approximate liquid phase density. The incorporated air
bubbles should be finely divided, such as up to 100 microns in
diameter, preferably from 20 to 40 microns in diameter, to
assure maximum stability. Although air is the preferred
gaseous medium for adjusting densities to improve phy~ical
23
2 ~ ~
stability of the composition other inert gases can also be
used, such as nitrogen, carbon dioxide, helium, oxygen, etc.
The amount of water contained in these compositions
should, of course, be neither so high as to produce unduly low
viscosity and fluidity, nor so low as to produce unduly high
viscosity and low flowa~ility, linear viscoelastic properties
in either case being diminished or destroyed by increasing tan
~ 1. Such amount is readily determined by routine
experimentation in any particular instance, generally ranging
~rom 30 to 75 weight percent, preferably 35 to 65 weight
percent. The water should also be preferably deionized or
softened.
The manner o~ formulating the invention compositions is
al90 important. As discussed above, the order of mixing the
ingredients as well a~ the manner in which the mixing is
performed will generally have a significant effect on the
properties of the composition, and in particular on product
density (by incorporation and stabilization of more or less
air) and physical stability (e.g. phase separation). Thus,
according to the preferred practice of this invention the
compositions are prepared by first forming a dispersion of the
polyacrylic acid-type thickener in water under moderate to
high shear conditions, neutralizing the dissolved polymer to
cause gelation, and then introducing, while continuing mixing,
the detergent builder salts, alkali metal silicates, chlorine
bleach compound and remaining detergent additives, including
any previously unused alkali metal hydroxide, if any, other
than the surface-active compounds. A11 of the additional
24
8 ~
lngredients can be added simultaneously or sequentially.
Preferably, the ingredients are added sequentially, although
it is not necessary to complete the addition of one ingredient
before beginning to add the next ingredient. Furthermore, one
or more of these ingredients can be divided into portions and
added at different times. These mixing steps should also be
performed under moderate to high shear rates to achieve
complete and uniform mixing. These mixing steps may be
carried out at room temperature, although the polymer
thickener neutralization (gelation) is usually exothermic.
The composition may be allowed to age, if necessary, to cause
dissolved or dispersed air to dissipate out of the
composition.
The remaining surface active ingredients, including the
anti-foaming agent, organic detergent compound, and fatty acid
or fatty acid salt stabilizer is post-added to the previously
formed mixture in the form of an aqueous emulsion (using from
1 to 10~, prefera~ly from 2 to 4~ of the total water added to
the composition other than water added as carrier for other
ingredients or water of hydration~ which is pre-heated to a
temperature in the range of from Tm+5 to Tm-20, preferably
from Tm to Tm-10, where Tm i9 the melting point temperature
of the fatty acid or fatty acid salt. ~or the preferred
stearic acid stabilizer the heating temperature is in the
range of 50 to 70C. Howe~er, if care is taken to avoid
excessive air bubble incorporation during the gelation step or
during the mixing of the detergent builder salts and other
additives, for e~ample, by operating under vacuum, or using
, 8 ~
low shearing conditions, or special mixing operatus, etc., the
order of addition of the surface active ingredients should be
less important.
In accordance with an especially preferred embodiment,
the thickened linear viscoelastic aqueous automatic dishwasher
detergent composition of this invention includes, on a weight
basis:
(a) 10 to 35%, preferably 15 to 30%, alkali metal
polyphosphate detergent builder;
(b) 5 to 15, preferably 8 to 12~, alkali metal silicate;
(c) 1 to 6%, preferably 1.2 to 4%, alkali metal
hydroxide;
(d) 0.1 to 3%, preferably 0.5 to 2%, chlorine bleach
stable, water-dispersible, low-foaming organic detergent
active material, preferably non-soap anionic detergent;
(e) 0.05 to 1.5%, preferably 0.1 to 0.5%, chlorine
bleach stable foam depressant;
(f) chlorine bleach compound in an amount to provide
0.2 to 4%, preferably 0.8 to 1.6~, of available chlorine;
(g) high molecular weight hydrophilic cross-linked
polyacrylic acid thickening agent in an amount to provide a
linear viscoelasticity to the formulation, preferably from 0.4
to 1.5%, more preferably from 0.4 to 1.0%;
(h) a long chain fatty acid or a metal salt of a long
chain fatty acid in an amount effective to increase the
physical stability of the compositions, preferably from 0.08
to 0.4%, more preferably from 0.1 to 0.3%; and
2 ~ ~
(1) balance water, pre~erably ~rom 30 to 75%, more
preferably from 35 to 65%; and wherein in (a) the alkali metal
polyphosphate includes a mixture of from 5 to 3 06, preferably
from 12 to 22% of tetrapotassium pyrophosphate, and from ~ to
20%, preferably from 3 to 18% of sodium tripolyphosphate, and
wherein in the entire composition the ratio, by weight, of
potassium ions to sodium ions is from 1.05/1 to 3/1,
preferably from 1.1/1 to 2.5/1, the compositions having an
amount of air incorporated therein such that the bulk density
of the composition is from 1.32 to 1.~2 g/cc, preferably from
1.35 to 1.40 g/cc.
The compositions will be supplied to the consumer in
suitable dispenser containers preferably formed of molded
plastic, especially polyolefin plastic, and more preferably
polyethylene, for which the invention compositions appear to
have particularly favorable slip characteristics. In addition
to their linear viscoelastic character, the compositions of
this invention may also be characterized as pseudoplastic gels
(non-thixotropic) which are typically near the borderline
between liquid and solid viscoelastic gel, depending, for
example, on the amount of polymeric thickener. The invention
compositions can be readily poured from their containers
without any shaking or squeezing, although squeezable
containers are often convenient and accepted by the consumer
for gel-like products.
The liquid aqueous linear viscoelastic automatic
dishwasher compositions of this invention are readily employed
in known manner for washing dishes, other kitchen utensils and
2~2$~
~he like in an automatic dishwasher, provided with a suitable
detergent dispenser, in an aqueous wash bath containing an
effective amount of the composition, generally sufficient to
fill or partially fill the automatic dispenser cup of the
particular machine being used.
The in~ention also pro~ides a method for cleanin~
dishware in an automatic dishwashing machine with an aqueous
wash bath containing an effective amount of the liquid linear
viscoelastic automatic dishwasher detergent composition as
de~cribed above. The compo~ition can be readily poured from
the polyethylene container with little or no squeezing or
shaking into the dispensing cup of the automatic dishwashing
machine and will be sufficiently viscous and cohe~ive to
remain securely within the dispensing cup until shear forces
are again applied thereto, such as by the water ~pray from the
dishwashing machine.
The invention may be put into practice in various ways
and a number of specific embodiment~ will be described to
illustrate the invention with reference to the accompanying
examples.
All amount~ and proportions referred to herein are by
weight of the compo~ition unless otherwise indicated.
28
2 8 ~
o ,~
a).
o ~ ~
U,~
~o
,~ ~.
U~
.
~, .,~
L` tO
a) O
O , a~ ~ N r l O ~ X
Kl I O N ~ 1 , ~ ~1 0 a~ t`l I I ~i ll~ ,~ (~
,4 ' ~1 ~i N V rl J~ U
~a , Ln ~t' "- (`1 ~ IS) O a~ o o ''~
1~1 N I O t` ~1 , ~')~10 ~` N l ~1 ~1 0 0 (d Ql tQ rl
-~ ' N N V
U l ~ t~l lS) o ~) o O ~
(`3 A A¦ A¦ ~ ~ ~ rl
U~ ~ N ~1 11~ O ~1 ~ O O
0 1 <`~ co I I ~ ~ ~l O ~` N l L-) l rl O O ~ O ~ a~
N ~ A E~ N N ~ ~rl U
a~ Al Al Ur~ a~ ~ a)
0 ~ p ~ ~I Ltl O ~1 ~ ~ O U ~ N
P~ r~l I O N ~ ~ ~ 11 ~ ~1 0 C~ N ~ ~ ~i tll O O ~ rl
a aU N N V . E~U ~
1 0 a~ o o (d
S~ 111 N~1 A ~a O
1 o a~ ~ o o
o I O r~ r~ ~ tn N I ~i ~i O o U ~ ~ U
N ~I V ~ ~1 ~I r~
Ci~ ~ Ul N~ IS) O ~ ~ ~ ol ol td~ ~ U ~ E~
O N I O 1~ ~1 I t71 ~ O ~ N I r-l r1 ~ O O .~ o\ ~D
O I f~ N V uJV (~
Ll I ~ ~ t`~ ~1 U') O ~1 ~7 O O ~) S~
O N I Lf) N rl I t~) rl O t~ N I ~ ~I-rl O O O O
l ~ ~ N ~ O ~1 ~I t~ O O 1o ~ V~ N
O I ~ V
O N 1 117 1~ r-l I ~ Irl O ~ N O ~1 ~1 X JJ ld 11) a~
~1 ~ N N ~ a
a, ~ ~ N ~ L~') O rl ~ ~ O O o i~ h 4~ v
~¢¦ --O N I Ul ~ ~ I r~l ~ O t~ N I ~i ~/ rlO ';t~ N ~ ~
O ~ 1 N V ~4 a~ ~~ a) a~
,~ a~ a~
,~ ~ v 5
Z~ ~ UJ a~ ~ ~ ~ ~ tJ~ rd
a~ h a~ ~ ~ v $
$~a~1 ~v ~d
O ~ U J S~ ~ ~ V p~ ~¢ r~ a
3 ~ ~ a) ~ - . ~ ~ aJ O~o ~_
I a~ v-- a~ ~ N ~v ~ O tJl ~11-- o~ O ~J r-l
I ~v o\-- ~ ~ ~ ~m ,~ ~ -- ,~
a~ ~ a~ ~ o O~o ~ U ~ f~ _ 0\O r~7 ~v--~ ~v v ~ ~ ~ m v ~, a~
I rl 11 N O U) OX ~rl o~ U o\ Ll) ~ rl V !~ rl E~l h ~
,- ~a ~ ~ ~ V s~ ,~ a~ ~ ~ a, ~R
I ~ ~ O _~ ,~ . ~ ~ ,~ u 5~
Fi $~ O Q tr 1~ s v ~ tn o 5: o 3 ~ o 3
~ h O ~ N ~ ~ J ~ d Z a aJ rd O rd o
X t: I a~ o ~ O ~ ~ aJ ~ v
U Z; ~ ~ Z X ~ 4 X ~1 ~ cn c~
Formulations A, B, C, D, E, G, J, and K are prepared ~
~irst forming a uniform dispersion of the Carbopol 941 or 9~0
thickener in 97~ of the water (balance). The Carbopol is
slowly added to deionized water at room temperature using a
mixer equipped with a premier ~lade, with agitation set at a
medium shear rate, as recommended by the manufacturer. The
dispersion is then neutralized by addition, under mi~ing, of
the caustic soda (50~ of NaO~ or KOH) component to form a
thickened product of gel~ e consistency.
To the resulting gelled dispersion the silicate,
tetrapotassium pyrophosphate (TKPP), sodium tripolyphosphate
TP (TPP, Na) and bleach, are added sequentially, in the order
stated, with the mixing continued at medium shear.
Separately, an emulsion of the phosphate anti-foaming
agent (LPKN), stearic acid/palmitic acid mixture and detergent
(Dowfax 3B2) is prepared by adding these ingredient~ to the
remaining 3~ of water (balance) and heating the resulting
mixture to a temperature in the range of 50C to 70C.
This heated emulsion is then added to the previously
p~epared gelled dispersion under low shear conditions, such
that a vortex i9 not formed.
The remaining formulations F, H and I are prepared in
essentially the same manner as described above except that the
heated emulsion of LPKN, stearic acid and Dowfax 3B2 is
directly added to the neutralized Carbopol dispersion prior to
the addition of the remaining ingredients. As a result,
formulations F, H and I, have higher levels of incorporated
air and densities below 1.30 g/cc.
The rheograms for the formulations A, C, D, G and J are
shown in figures 1-5, respectively, and rheograms for
2 ~ j 9
formulations H, I and K are shown in figures 6, 7 and 8,
respectively.
rrhese rheograms are obtained with the System 4 Rheome~er
from Rheometrics equipped with a ~luid Servo with a 100 grams-
centimeter torque transducer and a 50 millimeter parallelplate geometry haviny an 0.8 millimeter gap between plates.
All measurements are made at room temperature 25~1C) in a
humidity chamber after a 5 minute or 10 minute holding period
of the sample in the gap. The measuremen~s are made by
applying a frequency of 10 radians per second.
All of the composition formulations A, B, C, D, G and J
according to the preferred embodiment of the invention which
include Carbopol 941 and stearic acid exhibit linear
viscoelasticity as Reen from the rheograms of figure 1-5.
Formulation E which includes Carbopol 941 but not stearic acid
showed no phase separation at either room temperature or 100F
after 3 weeks, but exhibited 10~ phase separation after 8
weeks at room temperature and after only 6 weeks at 100F.
$ ~
Example 2
This example demonstrates the importance of the order of
addition of the surface active component premix to the
remainder of the composition on product density and stability.
The following formulations are prepared by methods A and
B:
Ingredient
Water, deionized~alance
Carbopol 9~1 0.5
NaOH (50~) 2.4
Na Silicate ~47.5~) 21
TKPP 15
TPP, Na 13
Bleach (1~) 7.5
LPKN 0.16
Stearic Acid 0.1
Dowfax 3B2
Method A:
The Carbopol 941 i9 dispersed, under medium shear rate,
using a premier blade mixer, in deionized water at ambient
temperature. The NaOH i9 added, under mixing, to neutralize
and gel the Carbopol 941 dispersion. To the thickened mixture
the following ingredients are added sequentially while the
stirring is continued: sodium silicate, TKPP, TPP, and
5 bleach..
Separately, an emulsion is prepared by adding the Dowfax
3~2, stearic acid and LPKN to water while mixing at moderate
shear and heating the mixture to 65C to finely disperse the
emulsified surface active ingredients in the water phase.
This emulsion premix is then slowly added to the Carbopol
dispersion while mixing under low shear conditions without
forming a vortex. The results are shown below.
Method B: 20B~
Method A is repeated except that the heated emuls1on
premix is added to the neutrali~ed Carbopol 941 dispersion
before the sodium stearate, TXPP, TPP, and bleach. The
results are also shown below.
Method A Method B
Density l.3a 1.30
Stability (RT-a weeks) 0.00~ 7.00%
Rheogram Fig. 9 Fig. 10
From the rheograms o~ figures 9 and 10 it is seen that
both products are linear viscoelastic although the elastic and
viscous moduli G~ and G~ are higher for Method A than for
Method B.
From the results it is seen that early addition of the
surface active ingredients to the Carbopol gel significantly
increases the degree of aeration and lowers the bulk density
of the final product. Since the bulk density i9 lower than
the density of the continuous liguid phase, the liquid phase
undergoes inverse separation (a clear liquid phase form~ on
the bottom of the composition). This process of in~erse
separation appears to be kinetically controlled and will occur
faster as the density of the product becomes lower.
Example 3
This ex~mple shows the importance of the temperature at
which the premixed ~urfactant emulsion is prepared.
Two fonmulations, L and M, ha~ing the same composition aR
in Example 2 except that the amount of stearic acid was
increased from 0.1% to 0.2% are prepared as shown in Method A
for formulation L and by the following Method C for
formulation M.
Method C ~ 9
The procedure of Method A is repeated in all details
except that emulsion premix of the surface active ingredients
is prepared at room temperature and is not heated before being
post-added to the thickened Carbopol dispersion containing
silicate, builders and bleach. The rheograms for formulations
L and M are shown in figures 11 and 12, respectively. From
these rheograms it is seen that for.~ulation L is linear
viscoelastic in both G' and G" whereas formulation M is non-
linear viscoelastic particularly for elastic modulus G' (G' at
1~ strain-G' at 30% strain > 500 dynes/cm2) and also for G" (G"
at 1~ strain-G" at 30% strain = 300 dynes/cm2).
Formulation L remains stable after storage at RT and 100F
for at least 6 weeks whereas formulation M undergoes phase
lS separation.
Comparative Example 1 ~ 3 9
The following formulation is prepared without any
potassium salts:
Weight
Water ~alance
Carbopol 941 0.2
NaO~ (50%) 2.4
TPP, Na (50%) 21.0
Na Silicate (47.5~) 17.24
Bleach (1~) 7.13
Stearic Acid 0.1
LPKN (5~) 3.2
Dowfax 3B2 0.8
Soda Ash 5.0
Acrysol LMW 45-N 2.0
The procedure used is analogous to Method A of Example 2
with the soda ash and Acrysol LMW 45-N (low molecular weight
polyacrylate polymer) being added before and after,
respectively, the silicate, TPP and bleach, to the thickened
Carbopol g41. dispersion, followed by addition of the heated
surface active emulsion premix. The rheogram is shown ln
Figure 13 and is non-linear with G"/G' (tan ) , 1 over the
range of strain of from 5~ to 80~.
Example 4
Formulations A, B, C, D and K according to this invention
and comparative formulations F and a commercial liquid
automatic dishwasher detergent product as shown in Table 1
above were subjected to a bottle residue test using a standard
polyethylene 28 ounce bottle as used for current commercial
liquid dishwasher detergent bottle.
Six bottles are filled with the respective samples and
the product is dispensed, with a minimum of force, in 80 gram
dosages, with a Z minute rest period between dosages, until
flow stops. At this point, the bottle was vigorously shaken
to try to expel additional product.
The amount of product remaining in the bottle is measured
as a percentage of the total product originally filled in the
15 bottle. The results are shown below.
Bottle Residue
Formulation Residue
A 8
B 10
C 6
D 5
K 7
F* 4
Commercial Product ~20
* The sample separates upon aging.
Example 5
The most preferred process as deplcted on Figures 14-16
was used to prepare the composition of Example 5 for the
manufacture of the viscoelastic gel compositions of the
instant invention comprises the steps of:
(a) forming a predlspersion of at least one surfactant,
a fatty acid or an alkali metal salt of a fatty acid and a
defoamer which comprises the steps of:
(i) adding deionized water at a temperature of
170F to 210F, more preferably 170F to 190F and most
preferably 175F to la5oF~ to a predispersion tank (2);
(ii) adding the surfactant or surfactants with
stirring to the deionized water in the predispersion tank (2),
wherein the concentration of the surfactant i9 30 to 40 wt.
%;
(iii) heating the defoamer to a temperature above
the melting point of the defoamer to transform the defoamer
into a molten defoameri
(iv) adding the molten defoamer with stirring to
the mixture of the deionized water and at least one surfactant
in the predispersion tank (2), wherein the concentration of
the defoamer is 5 to 9 wt. ~;
(~) heating the fatty acid and/or the alkali metal
salt of the fatty acid to a temperature above the melting
point of the fatty acid and/or alkali metal salt of the fatty
acid to transform the fatty acid and/or alkali metal salt of
the fatty acid into a molten fatty acid and/or a molten alkali
metal salt of a fatty acid;
(vi) adding with stirring the molten fatty acid
and/or molten alkali metal salt of the fatty acid to the
mixture of deionized wa~er, at least one surfactant and 3
~efoamer in the predispersicn to form in the predispersion
tank (2) a predispersion solution of the deionized water, at
least one surfactant, defoamer and fatty acid and/or alkali
metal salt of the fatty acid; wherein the concentration of the
fatty acid and/or al~ali metal salt of the fatty acid i9 1. 0
t~ 5.0 wt. ~;
(vii) continuing stirring the predispersion solution
in the predispersion tank (2) for a sufficient period of time
to ensure a uniform predispersion solution, preferably for
to 30 minutes, more preferably 2 to 15 minutes, and most
preferably 3 to 10 minutes;
(b) forming a polymer premix solution which comprises
the steps of:
(i) mixing at least one cross-linked polyacrylic
acid thickening agent such as Carbopol 941, Carbopol 940,
Carbopol 614 and/or Carbopol 624 with deionized water in a
mixing vessel (4) at a tempera~ure of 50F to 80F, most
preferably at 50F to 75F; and
(ii) transferring the mixture of the polyacrylic
acid thickening agent and the deionized water from the mixing
ve~sel (4) into a premix tan~ agitator (6) or in line
homogenizer (6) to further mix and dearate the premix solution
to the solution has obtained a Brookfield viscosity at room
temperature using a #6 spindle at 50 rpms of 10,000 cps to
60,000 cps, more preferably 15,000 cps to 50,000 cps wherein
the unneutralized premix solution has less than 2.0 volume ~
of entrained air bubbles, more preferably less than 1.5 volume
~ and most preferably less than 1.0 volume ~.
An especially preferred method of Eorming the
unneutralized premix solution of the polyacrylic acid
38
.
thickening agent and the deionized water is to employ a funnel
shaped vibrating eeder (7) as depic~ed in Figures 2 and 3
tha~ has a bo~tom opening (8) at the bottom of the feeder (7)
and a ring (9) wlth a bore (not shown) continuous there
through and a plurality of water inlet apertures (10), wherein
the ring (9) is joined to a water inlet source (13) and the
ring (9) is affixed to the upper inner surface (12) of the
feeder (7) at a point just below the upper rim (15) of the
feeder (7) whi.ch has an open top (19). A continuous stream
(11) of water comes from aperature (10) o~ the ring (9) and
cascades down the inner surface (12) of the feeder (7) towards
the bottom opening (8) of the feeder (7). Alternati~e to the
ring (9~ with aperature (10) other water delivery mearls are
contemplated such as a spray assembly positioned over the open
top the feeder (7). The solid polyacrylic acid thickening
agent (23) i9 dropped from above the feeder (7) into the
feeder (7) and the thickening agent (23) contacts the stream
(11) of water on the inner surface (12) of the feeder (7) and
the thickening agent i9 wet by the ~tream of water and forms a
mixture of the thickeniny agent and the water, wherein the
mixture is continuously discharged through the bottom opening
(8) of the feeder (7) through a cylindrical ~haped member (3)
having a bore (5) therethrough, wherein the cylindrical shaped
member (3) is joined at one end to the bottom of the feeder
(7) and at the other end to a Dilumett homogeneous mixer (16),
into an in line Dilumett homogenous mixer (16) sold by Arde-
Barinco or alternatively a Dispac-Reactor which is a 3 stage
rotor/static homogenizer sold by IKA Co. of Germany or any
other suitable in line homogenous mixers and the unneutralized
premix solution i9 pumped to premix mixing tank, wherein the
resultant Brookfield viscosity at room temperature at a #6
spindle at 50 rpms is 10,000 cps to 60,000 cps, more
preferably 15,000 cps to 50,000 cps, wh~rein the
unneutralized premix solution has less than 20 volume ~ of
entrained air bubbles, more pre~erably less than 1.5 volume
and most preferably less than 1.0 ~olume ~.
(c) neutralizing the polyacrylic acid thickening agent
ln the unneutralized premix solution which comprises the step
of adding to the unneutralized premix solution a sufficient
amount of an alkali metal silicate to substantially neutralize
the polyacrylic acid thickening agent in a neutralizing mixing
unit (19) to form a neutralized premix solution. The
preferred method of neutralizing consists of mixing the premix
solution of the polyacrylic acid thickening agent and
deionized water in a neutralization mixing unit (19), wherein
the concentration of the polyacry~ic acid thickening agent in
the premix solution is 0.25 to 10 wt. ~, more preferably
1.0 to 9.0 wt. %, and most preferably 2.0 to 8.0 wt. ~,
with an aqueous olution of the alkali metal silicate, wherein
the concentration of the alkali metal silicate in the aqueous
solution i9 40 to 70 wt. ~, and an in line static mixer is
the neutralization mixing unit (19). The resultant
neutralized premix ~olution of the neutralized polyacrylic
acid thickening agent and deionized water has a ~rookfield
vi~cosity at room temperature at a #2 spindle at 50 rpms of
1,000 Cp9 to 20,000 cps, more preferably 1,500 cps to
15,000 cps and most preferably 2,000 Cp9 to 10,000 cps and
the pH of the neutralized premix solution is at least 10,
more preferably at least 10.5 and most preferably at least
11.0;
2r~
(d) Forming the viscoelastic gel composition in a main
.ixing vessel (26) having a stirrer unit (28) whlch comprises
the steps of:
(i) Adding deionized water at a temperature of
45F to 80F, more preferably 50F to 75F, to the main
mixing vessel (26);
(ii) optionally, adding with stirring a colorant to
the deionlzed water in the main mixing vessel (26);
(iii) adding the neutralized premix solution with
stirring to the main mixing vessel (26);
(iv) adding an aqueou~ solution of an alkali metal
hydroxide such as sodium hydroxide, wherein the concentration
of the alkali metal hydroxide in the a~ueous solution is 20
to 60 wt. ~, with stirring to the mixture of deionized water
and neutralized premix solution in the main mixing vessel
~26);
(v) adding an aqueous solution of potassium
~ripolyphosphate, wherein the concentration of the potassium
tripolyphosphate in the aqueous solution is 50 to 70 wt. ~,
wi.th stirring to the mixture o~ deionized water, neutralized
premix solution and alkali metal hydroxide in the main mixing
vessel (26) wherein it is understood that potassium
polypyropho~phate can be readily employed in place of
potassium tripolyphosphate;
(vi) adding an hydrous sodium tripolyphosphate with
3tirring to the mixture of deionized water, neutralized premix
solution, alkali metal hydroxide and potassium
tripolyphosphate in the main mixing vessel (26); and
(vii) adding the predispersion solution with mixing
to the mixture o~ the deionized water, neutralized premix
solution, alkali metal hydroxide, potassium tripolyphosphate,
2 ~
sodium tripolyphosphate to form a solution (A) of the
eionized water, neutralized polyacrylic acid thickening
agent, alkali metal hydroxi.de, sodium tripolyphosphate,
potassium tripolyphosphate, alkali metal silicate, at least
one surfactant, defoamer and fatty acid and/or alkali metal
salt of the fatty acid, wherein if any fatty acid was
employed, the fatty acid at this point in the process has been
neutralized in situ to the alkali metal salt of the fatty
acid;
(e) transferring solution (A) through a heat exchanger
system (32) to increase the temperature of solution (A) to
140F to 200F, more preferably 145F to 165F, and recycling
said solution (A) into the main mixing vessel (26);
(f) adding the heated solution (A) in the main mixing
vessel (26) with stirring an aqueous solution of an alkali
metal hypochlorite such as NaOCl, wherein the aqueous solution
of NaOCl contains 5 to 50 wt. ~ of NaOCl, more preferably
7.0 to 25 wt. ~, to form solution (B) which comprises
solution (A) together with the alkali metal hypochlorite;
(g) cool.ing the solution (B) through an in line cooling
heat exchanger (24) to a temperature of 70F to 90F to form
the viscoelastic gel composition which has a density of 1.28
to 1.42 grams/liter, more preferably 1.32 to 1.42
grams/liter and most preferably 1.35 grams/liter and has les~
than 2 volume % of entrained air bubbles, more preferably
less than 1 volume ~, and most preferably less than 0.5
volume ~, wherein the viscoelastic gel composition has a
Brookfield viscosity at room temperature using a #4 spindle at
20 rpms of 1,000 to 10,000 cps, more preferably 2,000 to
8,000 cp~, as measured just after it is made and a Brookfield
viscosity after one week at room temperature at a #4 spindle
42
at 20 rpm of 4,000 cps to 12,000 cps and more preferably
3,000 CpS to 10,000 cps;
(h) optionally, adding per~ume with mixing in line by
injection through an injection part (31) into the transfer
line 30 carrying the viscoelastic gel composition; and
(i) mixing for 1 to 10 minutes in an in line static
mixer (36) the mixture of the viscoelastic gel composition and
the perfume to form a scented viscoelastic gel composition.
The formulation of Example s which was prepared
using the vibrating ~eeder (7) and the Delumett homogenous
mixer (16) as set forth in step (b)(ii) is in weight ~;
W_~ght
Dowfax 3B2 0.8
LPKN 158 0.158
Stearic Acid ~ 0.06
NaO~ (33%)
KTPP (60~) 33.92
NaTPP (3~ H20) 5.26
Sodium Silicate (47.5~) 20.83
Carbopol 614 1.0
NaOC1 (13~) 8.995
Colorant 2 O. 003
Perfume 3 0.05
In the production of the above formula the
temperature of the deionized water in step (a)(i) was 1~0F;
the concentration of the Dowfax 3B2 in step (a)(i) was 36.78
wt. %, the concentration of the LPKN in ~tep (a)(iii) was
7.356 w~. % and the concentration of stearic acid in step
(a)(v) was 2.759 wt. %; stirring in step (a)(vi) was 5
minutes; the temperature of the deionized water in step (b)(i)
was room temperature, and the Brookfield viscosity of the
premix solution in step (g)(ii) after the in line homogenous
I stearic acid - 50~ C~8 acid + 50% Cl6 acid.
2 colorant - C1 Direct Yellow 28/C1/9555 sold by Sando~
Chemical.
3 perfume - Highlights III perfume sold Bush Bach Aken.
43
- mixer was 25,000 cps at room temperature at a #6 spindle at
jO rpms and had less than 1.0 volume ~ of entrained air
bubbles; the concentration of the Carbopol 614 in the premix
solution was 4.8 wt. ~; the Brookfield viscosity at room
temperature at 50 rpms at #2 spindle was 5,880 cps; the
deionlzed water which was added to main mixing vessel in step
(d) was room temperature; the temperature of the heated
solution (A) in step (e) was 180F; and the temperature of the
cooled solution ~ in step (g) was 80F; mixing of the perfume
in step (i) was 5 minutes.
The formulation was analyzed as follows:
Brookfield viscosity
at R.T. at ~4 spindle
at 20 rpms - unaged sample 4200 cps
15 Brookfield viscosity
at R.T. at #4 spindle
at 20 rpms
1 week aged sample 7850 cp~
Density 1.38 grams/liter
2~ P2Os 12.2 wt. ~
Appearance translucent
Solids 41.01 wt.
Available chlorine 1.15 wt. %
Amount of unbound 4 ~0 .25 wt.
25 water solids wt. %
pH (1~ solutiGn) 11.5
-
4 200 grams of product was placed in a funnel containing
filter paper and allowed to filter for 24 hours. The filtrate
(water) i9 collected in a beaker and weighed. The ~ of
unbound water equals weigh~ of the filtrate divided by 2. In
both of these samples no water was collected thereby setting
forth that there i9 less than 0.25 wt. % of unbound water in
the sample. A sample of Example 1 of U.S. Patent 4,836,946
was tested and it showed a 2~ wt. % o~ unbound water.
44