Note: Descriptions are shown in the official language in which they were submitted.
2~6~7~
PROCESS AND DEVICE FOR DECOMPOSITION OF SPENT
ION EXCHANGE RESINS
The invention relates to a method for the decomposition of
solid harmful substances, for example spent radioactive ion
exchange resins, which are continuously delivered together
with a reaction aid to a reactor chamber maintained under
normal pressure, and decomposed by means of hydrogen peroxide,
which is continuously delivered to the reactor chamber in
metered amounts.
Such a method is known from US-A 4,624,7~2, in accordance with
which the reactions react ill an aqueous medium in which the
decomposition products gradually accumulate. As a reaction
aid, a catalyst, for e~ample an iron salt, is continuously
supplied to the reaction zone, such that it provides a
catalytical effect at a temperature below the boiling point of
the liquid. The reaction speed of the catalytic process is
comparatively slow; and it is only possible to increase it by
using increase~ pressure which, however, should be avoided for
reasons of safety. Because it is necessary to supply fresh
catalyst material continuously in order to maintain the
reaction, the increasing amounts of mineral salts remain as
mineral waste, which must be disposed of. The process must be
performed in batches, because the reaction chamber must be
emptied every time it is filled with decomposition products.
It is known to utilize ion exchange resins for processing
radioactive materials. Following their use, these resins
represent a lar~e portion of low- to medium-contaminated
atomic was~e, depending on the content of radioactive
materials. Most of the commercially employed ion exchange
materials are synthetic resins. In view of the growing demand
for capacity for special waste and because of its limited
availability, a pre-treatment of the spent ion exchange resins
to concentrate their volume and weight is necessary and
Pconomical prior to further storage processing for safe
disposal of the waste. The known pre-treatmant processes,
whether dry or we~, result in completely different radioactive
,
.
20~7~1
materials in respect to their chemical ~nd physical
properties, which are commonly advantageous for their further
safe processing.
As is known, the dry processing of spent ion exchange resins
is performed either by incineration or pyrolysis. In spite of
considerable advances in these methods, technical safety
problems are still present during dry incineration of
radioactive waste, as mentioned in:
Valkiainen, M., et al., Nucl. Technol., 1982, S8, 248-255;
Johnson, T.C., et al., Trans. Am. Nuc. Soc. 1979, 32, 19-28.
The disadvantages of the incineration process in particular
are:
- a complicated waste-gas treatment system is required due
to large volumes of radioactively contaminated high
temperature;
- flying ashes are easily dispersible materials and must be
carefully treated;
- the handling of wastes and residue~s can impart harmful
doses of radioactivity to the operation personnel;0 - the costs of construction, maintenance and operation of
radioactively contaminated incineration systems are
relatively high.
Among the wet processes, acid digestion is the most
extensively studied method to reduce the volume of radioactive
combustible waste, according to:
U.S. Patent 4,313,845
U.K. Patent 2 050 682 B
Although this process seems to be attractive compared with
incineration, it still has some disadvantages:0 - The process operates under drastic conditions, namel~
hi~hl~ concentrated acids and temperatures between 230C
and 270C;
- a large amount of energy is consumed in order to maintain
the required reaction temperature;
.
' ' ' .' , , '
.
2 ~ 7 ~ ;~
- a complicated waste-gas treatment system is necessary due
to the evolution of corrosive oxides of both s~llfur and
nitrogen;
- recycling of the used concentrated nitric and sulfuric
acids is required.
Furthermore, it is known from K. Lohs, D. Martinez,
Entgiftungsmitel-Entgiftungsmethoden, Vieweg, Braunschweig,
1978, pp. g4-108, to decompose toxic organic solid waste
chemicals by means of hydrogen peroxide, in which iron sulfate
is used as the catalyst. The reaction temperature in this
case lies below 100C in order to pravent decomposition of the
peroxides. The reaction is performed in batches in the liquid
medium, so that ~here is a large volume of waste.
It is the object of the invention to improve the reaction
speed of the previously mentioned method of decomposition for
the purpose of reducing the volume of organic harmful
materials, in particular of spent, radioactively contaminated
ion exchange resin, under safe operatillg condition~, and to
provide a device for its simple, safe and reliable execution
under easily controlled temperature and pressure conditions.
The ob;ect is attained in that starter energy, generated by
means of a heating wire energized at the start of the reaction
or a catalyst material s~pplied only at the start of the
reaction and which exothermally decomposes the hydrogen
pero~ide, is used as a reaction aid, and in that the residual
liquid generated during the decomposition of the harmful
materials is continuously filtered at the bottom of the
reactor chamber and conducted into a terminal reæervoir, and
that the metering of the hydrogen peroxide is controlled in
3~ such a way that the decomposition of the harmful matarials
takes place in a temperature range between 105C and 140C.
Advantageous embodiments of the method and of the device are
recited in the dependent claims.
' ` ' .:
~ '' ' `
;:
.
2~5~7~1
The reaction temperature is preferably maintained at a level
between 105C and 135C, depending on the desired
decomposition speed, the size of the installation, the foam
level and the preset volume o~ supply of reaction components
and the metering of the residual solution.
The advantage of the invention consists in particular in that
the cost of operation, the cost of construction and the cost
of maintenance of such equipment are low and the gas
discharged can ~e prccessed quite simply in a closed emission
processing apparatus.
It is particularly advantageous that no external energy input
is required and under certain operating conditions free energy
for external use can be made available.
In the process it is advantageous that, when the oxidizing
agent is used sufficiently sparingly, only decomposition and
not complete oxidation of the ion exchange resin can be
achieved.
It is advantageously possible, by this process and apparatus,
to achieve reliable and certain decomposition of other types
of combustible non-radioactive waste an~ other radioactive,
particularly alpha-contaminatedl waste without harm to the
environment and thereby to achieve energy consexvation.
In order to be able to carry out the process in a simple and
secure manner, an apparatus for the chemical decomposition of
radioactively contaminated ion exchange resins is described,
which permits a fully automatic control without the need for
continual intervention in the radioactive control region.
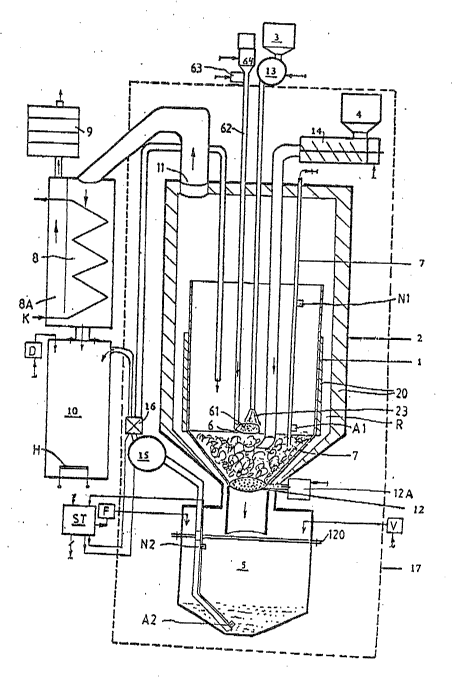
Fig. 1 shows schematically a decomposition apparatus, with
a vertical cross-section of the reactor.0 Fig. 2 shows a detailed cross-section having a replaceable
filter.
20~674:L
Figure l shows schematically the functionally essential
combinations of parts and a reactor; their dimensions and
proportions are typical but variable, provided the function is
not adversely affected.
The system consists of a shaft funnel reactor vessel (l),
which is surrounded by an outer protective jacket (2). A
hydrogen peroxide tank (3) is positioned outside the
controlled radioactive security zone (17), and the ion exchage
resin container (~), provided with a metering device (14) and
a return pump (15) for the spent fluid, is positioned in this
security zone (17).
The reactor (1) and the protective jacket (2) are connected at
their bases via a releasable connector (120) to a terminal
reservoir (5) which receives both fluid residues during the
operation and also solid residue after a certain period of
operation. The bottom exit (12) of the reactox (1) is
advantageously in the form of a filter layer made of sintered
glass, which allows only the fluid resiclue to pass through
until, when opened, it allows the solid residue to pass into
the terminal reservoir (5).
Various metering devices are provided in order to provide a
continuous charging of the reactor (1) from the tank (3) by
means of a first pump (13), from the container (4) by means of
a controllable ~eed device (14) and from the terminal
2S reservoir (5) by means of a second pump (15) in a controlled
and regulated manner.
In order to start the reaction, a catalytic material is
introduced. This is preferably iron sulfate. The catalyst
(61) is advantageously introduced into the reactor (1) via a
long handled spatula (6) right under the exit (23~ of the
oxidant, which is advantageously in the form of a nozzle. ~he
spatula (6) is movable to various heights on the spatula shaft
(62) by an adjustable drive (63); the reaction speed can thus
:~ ,
: .
2~7~1
be influenced. The spatula shaft (63) is tubular, terminating
at its lower end in the spoon-like concave spatula (6), and
carrying a funnel arrangement or a metering device (64) for
the introduction of the catalytic material at its upper end.
Both the r~actor vessel (1) and the protective jacket (2) are
surrounded with a thermally isolating layer (20) so that, due
to available energy from the decomposition reaction, heating
of the feed material to the required reaction temperature
takes place. Process control is achieved by one or more
temperature probes (7~ whose signal or signals are fed to
control e~uipment (ST), which so controls the metering
equipment (13, 14, 15), the catalyst-feed equipment (64) and
the catalyst positioning equipment (63) that a suitable
predetermined temperature range for the decomposition reaction
is quickly reached and then maintained.
The waste gas is fed via outlet (11) in the jacket (2)
to a cooled condensor (8) and thereafter through an HEPA-
filter (9). The condensate produced in the condensor (8) is
collected in a receiver (10) and from ~here discharged.
The decomposition process is started by means of solid iron
sulfate producing an exothermic decomposition reaction with
preferably 60% hydrogen peroxide. A physical energy input is
therefore not necessary, which simplifies the apparatus. The
reactive hydrogen peroxide attacks the introduced resin, and
the resul~ing decomposition of the resin proceeds
exothermically, so that more heat is liberated into the
reaction medium. This combined heat in the reaction medium
further decomposes introduced hydrogen peroxide solution,which
thereby attacks more ion exchange resin. This reaction
proceeds further according to the feed of both components,
which is ultimately controlled by the measurements made in the
reactor vessels. The reaction produc~s are solid residues,
liquid residue solutions and waste gases such as steam.
'
:. -
2~7~
The solid residues, which collect at the bottom of the shaft
reactor, contain most of the radioa~tive materials. If
present, cesium, which is highly soluble, is contained mainly
in the residue solution.
The residue solution, which is collected in the terminal
reservoir (5), is preferably returned to the reactor (1) for
the purpose of evaporation utilizing the heat of reaction, so
that its volume is reduced.
The handling of the residual solution depends on its quantity
and degree of activity, which in turn depends on the type and
combination of waste to be decomposed and on the type and
quantity of radioactive substances therein, such as in the
following ways:
- If the resiclual solution has a relatively low
radioactivity, then it is fed, irrespective of its
amount, to condensate container (10) outside the
controlled security zone (17) via a first route through
adjustable two-way valve (16).
- If the residual solution contains a high specifi~
radioactivity and is produced in a large quantity, it is
chemically treated in the terminal reservoir (5) in order
to precipitate the radioactive material and then returned
via a second route through the two-way valve (16) to the
reactor (1), where the precipitate is filtered out.5 - I~ the residual solution has a high spe~ific activity,
but only a small quantity is produced, it is mixed with
the solid residue and then solidified in the terminal
reservoir (5) by additives and then removed.
For the control of the above procedure, temperature, level and
activity sensors (7, Nl, N2, A1 and A2) are provided in the
xeactor (l) and in the terminal reservoir (5), the signals
from these sensors being fed to the control e~uipment (ST).
The latter controls the two-way valve (16) and tha feed of
precipitant (F) and the additives (V), which produce the
2~7~
solidification, to the terminal reservoir (5), on an ongoing
basis according to preset values. Continuing operation
following the catalytic start is maintained by continuous
metering of resin and controlled additions of oxidant. Upon
the formation of foam, the feed of reactants are additionally
regulated when the foam reaches a predetermined level in the
reactor. Even an occasional excessive foaming is not critical
because the foam can overflow through the annular gap (R)
between the reactor (1) and and the protective jacket (2) into
the terminal reservoir (5). Moreover, it is possible to add a
known antifoaming agent~
Further, the filter layer (1~) is movable by the control
device (ST) via a controlled drive (12A), so that evacuation
of the solid residue into the terminal reservoir (5) can be
brought about by a sideways sliding the filter layer, after
the reaction chamber (1) has been filled to a predetermined
level and after a succeeding completion of the decomposition
reaction and, if necessary, after a precipitation reaction and
the completion of the following filtering of the precipitated
material.
An ~xample of the precipitating agent (]?) is, when radioactive
cesium is contained in the radioactive residue solution, a
concentrated solution of tri-potassium fe~rohexacyanide,
whereby the cesium is bound and can be filtered and
2S concentrated as solid residue in the terminal reservoir.
An example of solidifying additives (V) for the residue is
water-compatible polyester, which is added to the terminal
reservoir (5) for binding purposes.
The waste gas and waste steam are fed to the condensor (8) and
then through an HEPA-filter. This section of the process
depends principally on the decomposition of organic ion
exchange rPsins. Advantageously, it is not necessary to carry
out complete oxidation in the radiation controlled zone. If
,
~$74~l
the concentration of the hydrocarbon in the condensate in the
condensate container (10) is relatively high, it can there, or
even later if necessary, be oxidiz~d further, e.g. if
necessary with hydrogen peroxide. For such a secondary
reaction, thè addition of hydrogen peroxide is provided at a
controlled dosage (D) and an additional heater (H) may ~e
built into the condensor (8). This is purposely insulated.
The resulting decomposition products of the additional
oxidation, namely carbon dioxide and steam, leave the
container (10) through the vent (8A) of the cooler (8) and
further through the filter (9). The main reactor in the
controlled zone is then not overloaded.
A part of the apparatus is shown in Fig. 2 provided with an
alternative filter arrangement. In this case, a replacement
15 of the filter layer (12) during the operation is possible, in
case precipitated fine residues clog the filter (12) and a
sliding operation as shown in Fig. l is not sufficient to
clean the filter. For the filter replacement, a horizontal
transport track (21) is provided which leads directly over the
filter through the protective jacket (2) and the wall of the
reactor (1). A filter carrier (22), closed from above,
containing a replacement filter (12') is provided on the
trans~ort track and is insertable into the reactor (l). The
used filter (12) is positioned in a holder (28) closable to
the outside. It lets itself ~e guided to fall into the
terminal reservoir (5) and is disposed of with the solid
residue. After closing the holder (28), the replacement filter
(12 t ) is guided freely out of a holder (24) in filter carrier
(22), so that it is positioned in the holder (28) and taken up
there. Then the filter carrier (22) is drawn back to its
laterally outermost position, where engaging edges (25, 26) of
the reactor chamber walls and the protective jacket (2) hold
back the reactor contents. Electromagnets and a motor drive~
or pneumatic or h~draulic motors, are provided for the
35 guidance of the holders ~28, 24) and the carrier (22).
: . ~
;: ~ .. .. .
20~l~7~
The embodiments shown above are preferred, very simple and
easily controllable constructions which can be modified by
persons skilled in the art. In particular, instead of the
catalyst feed apparatus for starting the reaction, another
source of energy introduction can be used, e.g. in the form of
an electrical heating apparatus, or a heating wire.
Furthermore, a filter exchange may be carried out from below
in the region of the terminal reservoir instead of from the
side, which obviates the need for the sluice valve and the
transport apparatus. In this case, several filters pivotable
at the edge of the exit of the reactor chamber may be
provided, which are controllably pivoted and removed from
their holdars. Upon such an exchange of filters, however, the
solid contents of the reactor chamber are deposited into the
terminal reservoir. From there, the fluid can be pumped back
into the reactor chamber, if this is necessary.
For the purpose of providing a solidifying agent it is
possible to arrange a tubular device in the terminal
reservoir, so that an homogeneous solid product can be
produced.
The above invention provides a simple and comprehensive
syst~m for the decomposition of organic ion exchange resins
and has various advantages, as follows:
- The apparatus is very simple and compact since it
essentially consists of a reactor vessel and a protective
~acket, and requires low construction and maintenance
costs~
- The operational costs are very low since the single
active chemical which is consumed is commercially
available hydrogen peroxide and its use is minimized.
- No continuing external source of heat is required since
the reaction energy is sufficient due to insulation.
- The handling of the waste gas and the equipment therefor
are extremely simple since the production of highly
.
,
' ' ' ' '
7 ~ ~
11
aggresive s~llfur and nitrogen oxide gases is avoided,
which was a disadvantage of the prior acid digestion
procedure.
- The process is extremely advantageous since it is carried
out at normal temperature and pressure and requires no
external heat and concentrated acids. This results in
guaranteed degrees of safety and reliability through
known preventive measures.
- Noticeably large reductions of volumes and weights of
waste to be handled are achieved, which minimizes the
cost of suhsequent handling and storage of quantities of
wastes.
- The process lends itself to the treatment of other kinds
of wastes, e.g. combustible, non-radioactive material,
and other nuclear, particularly highly toxic long-lived
alpha-waste produced in the various steps of the fuel
cycle.
The process can be adapted and used as an enerc3y source
if corresponding technical improvements are made.
20 - Radioactive materials are not blown away on account of
the low operational temperature and therefore they are
not propagated, which in contrast t:hereto is a
disadvantage of the prior art incineration process.
- The concentration of most of the radioactive nuclides in
a small fraction of inorganic resiclue which ~athers at
the base of the reactor facilitateæ the subse~u nt
solidification, if the residue is delivered directly into
the terminal storage container, where it is mixed with
binding matarial.
30 - The process is easily controlled by the regulation of the
hydrogen peroxide feed~
- The apparatus is so simple and compact that it can be
constructed at the site of the waste production, thereby
eliminating signi~icant handling, storage and transport
costs ~or the waste material.
- ' ~ "' . :
. ,
- :~