Note: Descriptions are shown in the official language in which they were submitted.
2 ~ _ ~ r ~ ,.~ 9
wosl/o66ol PCT/US90/06363
--1-- .
3IODEGRADABLE POLYMERIC
~ .
BACK ROUND OF THE INVENTION
1. Field of the Invention
. _ _ _
This invention relates to biodegradable polymeric
compositions.and to articles fabricated therefrom. More
particularly, this invention relates to such composit ions
' which exhibit improved biodegradabili~y when exposed to
environmental effects such as sunligh~, heat, water,
oxygen, pollutants, microorganisms, insects, animals and
mechanical forces such as wind and rain.
2. Prior Art - '
. 15Many discardable packaging items such as bags and
: containers are destined, a~er a relatively short~
functional life to arrive as a significant component of
urban garbage. .Because-of the increased use of.plastics
in the fabrication of these discardable packing materials,
i~ has been proposed to make throwaway materials from
biodegradable plastics to ameliorate waste disposal
problems. "''
owever,:the low-cost~high volumne packagingici .
'' materials such'as;polyethylene,:polypeopylene, polys~yrene
25~'and poly(ethylene terephthalate):are~not natura'lly
' biodegradable~ ~Severàl methods have been proposed to
' enhance'the'biodegradabi:lity of such polymeric materials
and/vr to develop other useful buodegradable polymeric
materials. For example, U.S. Patent No. 4,016,111
;30 discloses~that;compo'sitions.'~'Qf~e~hylene acrylic acid
copolymer and a starchy material are biodegeadable. U~'S.
a't'ent'iNo.'-4,'337,181~~describes~a:.biodegradable composition
''''~contaLning 'up'`to''abo'ut:60%'"gelatinized:starchTand:.various
le'v'els ~f'êt'hyle'nè"~'a'c`ryl^ic'acid:copolymer-.cand~-opt.ionally
polye~hy^lenei.~''3U.'S¢'~Patent:'No~ 4,016,'117 describes a '`.
biodegradable composition which comprises-.a:synthetic^~"
resin, a b'iodegr'a'dable granular filler~such-as natural
. . - . ::
.,. ' .~.
` :-
w~gl/~66ia~ 3 -2- PCT/US9Q/06363
starch, and a substance which is autooxidizable to yield a
peroxide which attacks the carbon to carbon linkages of
the resin. PCT Appln. WO 8B/09354 describes a degradable
polymer composition which is a blend of a normally stable
chemically saturated polymer such as polyethylene, a less
stable chemically unsaturated polymer or copolymer such as
a styrene/butadiene block copolymer, or natural rubber, an
anti-oxidant active over a limited period and a latent
pro-oxidant such as an organic salt of a transition metal,
e.g. cobalt naphthenate, which may optionally.include
filler particles of a directly biologically sensitive
material such as a natural starch, a derivative of natural
starch, a natural protein, a natural cellulose or a sugar.
Previous efforts to make non-biodegradable polymers
biodegradable by blending them with biodegradable fillers
and other additives have not been successful. Existing
biodegradable plastics are deficient.in properties
required in most packaging applications and are more
expensive than commonly used packaging plastics.
~urthermore, such materials often fail to degrade in a
reasonable period of time to the point where they lose
their structural integrity and fall apart. This loss in
physical~iproperties,~l,embrittlement.and disintegration are
.required in-order~to lessen the volume of such articles in
landfills, or;to allow.release of the conten~s of the
25 .plastic package (such as in the case of yard waste? or .
..cause the fragmentation and,disappearance.of .litter and so
;,3..:~ SUMMA Y.OF THE.INVENTION ~
~,J, ! j ~ia~t ~r317~ .. Theipres53ent~inventioni;isldirected to b~odegradable I .
;s~polymer.. composition ~which~obvijates one~or more of;.the ~ I
n~def~cts~of.conventiona.}.biodegr~3idableires n~ The ::
Ij composition~of.ijthis inyent;ion:.comprisesi~and intimate
:.: .mixture.of .~ I s~ r ' I ~t~ d.5
a) ~one:or more~polymers::rand~ *~ * ,.
I
i ~''
., ............. . . . -
, . . . . . . .
W~ gl/06601 Z~ ~r ~9 PCTIUS90/06363
;;`` ~3~
~ b) an effective amosunt nf one or more particulate
fillers which comprise one or more degrada~ion enhancing
materials which enhance the biodegradation of the
polymers, which materials are associated with one or more
biodegradable and degradable saEening materials which
inhibit the activity of said degradation enhancing
materials during said association whereby on
' biodegradation or degradation of said safening material -'
the activity of said~enhancing materials is completely or
partially restosred. '
- Another aspec5 of this invention relates to an
article of manufacture fabricated totally or in part from
the biodegradable composition of this invention.
Sevéral beneficial effects are provided by the
invention. For example, low cioncentrations of the
particulate filler are required in'order to ps~ovide an
accep~able level of disintegration of the composition
within'a reasonable period of time. The degradatiosn
enhancisng'material is released when the composition is
exposed to environmental (biodegradation) conditions, and
20--is not time dépendent'. Thus,':there is no danger or
substantially reduced'danger of'premature degradation and
associated shelf'l'ife problems. ~A~wide ~ariety`of
degradation enhancing materials and safening materials
having a wide ~ariety-of properties can'be used,'which
facilitates'the use of the invention`with a wide variety
' ~ 'of"polymers''and'environmental conditions. Some usèful
degradation'~enhasncing materials not only cause fàilure of
't'hérpolymer''due-to'ltheir own?'àctivity, bùS~ also'promote
fùrthe'r'''bio~egradation of thé safen'ing material an'd/or
other biodegradabl'e` additives tha`tlmay;ibe'in the'~
('' 'I''com'position'~'c'au'i'ng'a cascading 'effèce'.'"~
~ .!O.l i~l'l i :-ts;; ';~ RIEF~'DEscRIpT ~ ~'5''' -~-
!~ 35 i-~'s~ Thi's''invent'Lo'n1'can''be~'be~er3~undërs~'oo'd':fr'om'w'a '
' '?'consideration'~oe'ith`e ispe'c'ificat'ion'in'conjunction'with the
~ 'drawingi~ in which: '
`~ ' ' ~:
,` . , .
;` :
WO9~/06601 ~ PCT/~S90/06363
Figure l is a graph indicating the degree to which
high density polyethylene is protected by beta-
cyclodextrin.
Figure 2 is a graph indicating the conductance of the
~ormation and degradation of a beta-cyclodextrin sodium
dodecyl sulfate complex.
Figure 3 is a graph indicating the conductance of the
~ormation of a beta-cyclodextrin cetyl pyrdinium chloride
complex and of a starch/cetyl pyridinium chloride complex.
Figure 4 is a graph indicating the conductance of the
formation and degradation of a corn starchisodium dodecyl
sulfate complex.
, DESCRIPTION OF THE PREFERRED EM~ODIMENTS
The composition of this invention comprises two
:~essential ingredient. One essential ingredient is a
polymeric resin. ,The type of polymeric resin used may ~.'
vary widely.. Illustrative of useful resins are aromatic,
, aliphatic and cycloaliphatic polyamides such as
2~ poly(m-xylylene adipamide), poly~p-xylylene sebacamide),
~,., poly,2,2,2-trimethyl-hexamethylene terephthalamide), poly
(piperazine sebacamide).,,.poly.~metaphenylene ~
-isophthalamide) ~Nomex), poly ! p-phenylene ..; ,,-
terephthalamide),~,(Kevlar): the copo,lyamide of.~30
. ,hexamethylen.e.,diammon,ium .L~,ophthalate.and ?% -
:.. hexamethylene,,diammonium,adipate, the~copolyamide .o~ up to
30~jbis-(,amidocyclo-hexyl)m~ethylene~terephthalic~acid
n~ a.ndicaProlact~am~polyhexame~thylene adipami,de ~nylon 66),
poly~butyrolactam) ~nylon 4), ~ poly~ ~9i-amLnonoanoic, acid)
tnylon~9),,.poly~enantho,lactam);(nylon~-7), ~
; poly~caprylla,c,tam)`~nylon 8,), polycaprolactam~!nylon 6),
poly ~p-phenylene terephthalamide), polyhexamethylene
- sebacamide,(nylon 6jlO),~polyaminoundecanamide ~nylon ll),
polydodecono-lactam (nylon 12), polyhexamethylene
35 ~is,pp,hthalam~ide,~cpolyhexamethylene,,lt~ereph~thalamide, ~-'' ' "
n~ .poIycaproamide,; poly~nonamethylene azelamide~j(,nylon 9,9),
, ::
- - , . ., ... ~ . .
: ~ ., ~ . - : '''
W091/06601 ~ ~ 9 PCT/US90tO6363
poly(decamethylene aæelamide) (nylon 10,9),
poly(decamethylene sebacami de ~ ( nylon 10,10),
poly[bis-(4-aminocyclothexyl) methane 1,10- `-
decanedicarboxamide] (Qiana) (trans), or combination
thereof; and aliphatic, cycloaliphatic and aroma~ic
polyesters such as poly(l,4-cyclohexlidene dimethyl
eneterephathalate) cis and trans, poly~ethylene-l,
5-naphthalate), poly~ethylene-2,6-naphthalate), poly(l,
4-cyclohexane dimethylene terephthalate) (~rans),
poly(decamethylene terephthalate), poly(ethylene
terephthalate), polytethylene isophthalate), poly(ethylene
oxybenozoate), poly(para-hydroxy benzoate),
poly(dimethylpeopiolactone), poly(decamethylene adipate),
poly(ethylene succinate), poly(ethylene aæelate),
poly(decamethylene sebacate), poly(~ dimethyl- :.
'propiolactone), and the like.
Also illustrative of useful polymeric resins are
polymers copolymers formed by polymerization of ,~
-unsaturated monomers of the formula: :
~ Rl R2-C = CH2
whereLn~
j ...Ri and R2 are the same'-or different'a'nd'are ''
hydrogen,hydroxy,'ha'logen, ~alkylcarbonyl, carboxy, ~
alkoxycarbonyl, heterocycle or alkyl or ary"l ei'ther -
.unsubstituted or:substituted with one or more substLtuents
;7i selected from the group;consisting of alkox'y, cyano,-
hydroxyj.alkyl::and aryl,.-'lllustrative''of 'such`polymèrs of
~ L'B-unsaturated~monomers.-are'polymers including .
; ~po}ystyrene-, polyethylen'e,`plyprop'ylene'~:~poly't'l-o'ctadencë),
.~. cpolyl~obutylene,~poly(l-pë'ntene), poly(2-méthylstyre'ne),
polyt4-methylstyrene), poly(l-hexene), po'lytl-'pentene),
.po~y(4-me~hoxystrene),epoly(5-methyl''l-hexene~
npoly~4-methylpen~ene)~-/Jpoly`~l''butenë), polyvinyl ;~ F
2chloridè,~polybu.tylene, polyàcrylonitrile,`~poly'(me't~'yl
pentene~ poly(vinyl3alcohol)',~"poly(vinylacëtat'e')"'~
~ polyt'vlnyl`~utyral), poly~vinyl"chl'oridê), poly(vinylidene
'' ~ ' : - - ':"
, : .
.
. ... , , ..... i
. . .
- . ~ .
, . . :, . : .. , ,, . .. ~. :,, . : .
2 ~ 9
WOgl/066~1 PCT/US90/06363
... . . f~
-6-
chloride), vinyl chloride-vinyl acetate chloride
copolymer, poly(vinylidene fluoride), poly~methyl
acrylate, poly(methyl methacrylate),
poly~methacrylo-nitrile), poly(acrylamide), poly(vinyl
fluoride), poly(vinyl formal), poly(3-methyl-
l-butene), poly(l-pentene), poly(4-methyl-1-butene),
poly(l-pentene), poly~4-methyl-1-pentence, poly(l-hexane),
poly(5-methyl-1-hexene)~ poly(l-octadence), poly~vinyl-
cyclopentane), poly(vinylcyclothexane), poly(a~vinyl-
naphthalene), poly(vinyl methyl ether), polytvinyl-
ethylether), poly(vinyl propylether~, poly(vinyl
carbazole), poly(vinyl pyrolidone), poly(2-chlorostyrene),
poly(4-chlorostyrene), poly(vinyl formate), poly(vinyl
butyl ether), poly(vinyl octyl ether), poly(vinyl methyl
ketone), poly(methylisopropenyl ketone),
poly(4-phenylstyrene) and the }ike.
Preferred resins for use in the composition of this
invention are resins which are commonly used in the
fabrication of packaging materials such as polyethylene,
polyethylene terephthalate, polystyrene, polyurethane,
polyvinyl chloride, polypropylene, polycarbonate and
blends of such materials. The above list of preferred is
merely intended to be representative of useful and
preferred resins,.and other resins which are.used as
packaging materials.may!also be used. ~In the.most
referred embodiments of this invention, the.resins of
choice.are polyethylene (high density, low:density and
llnear~low density), polyethylene terephthalate,:polyvinyl
ehloride, polyurethane;~and.blends of-such:polymers.t.
The second essential ingredient.of the.composition of
this,invent.ionji.s~.~an-effective amount:of a particulate '
fil?er.~;"The.filler is~a~material whichiis.designed~to be
inactive during~th~e3use of the composition and~whLch~
enhances~the degradat.ion~off~thelpolymer on.!exposure~of the
composition~of thi~-~,invention~to a suitable environment,as
for~examp}e~a{ga~rbage~dump or landfLll.~The~filler.lis ~ :
comprised...oj~a,degradation-enhancing~material~which is.
,, ", . . . . .. . .
~ effectivejto enhance3the.jdegradation,of~.the;polymeric
1 . . . :
:
.
. , .
, ~ . ... : . . . .
. ~; , , . , : .
W091/06601 ' PCT/U~90/~6363
7-
material and a biodegradable safening agent which inhibits
the activity of the enhancing material. In operation, the
safening material associates with the degradation
enhancing agent, thereby completely or partially
inhibiting its activity during the association. On
exposure of the composition to a suitable environment
containing agent(s) effective to biodegrade the
biodegradable safening material, the activity of the
enhancing material is competely or partially restored,
which results in an enhancement of degradation of the
10 pOlymer-
As used herein, ~association~ is any chemical,physical or like interaction between the biodegradable
safeni~g material and the degradation enhancing material
which completely or partially inhibits the biodegradation
-enhancing characteristics of the degradation enhancing
material, and which allows a complete or partial
restoration of such characteristic's on the biodegradation
of the biodegradable safening material. The nature of the
association between the biodegradable safening material
and the degradation enhancing materiàl may vary widely and
'essentially depends on the properties of these two
ma'terials. The'only requirement is`that this'association
'~'''inhibits the activity of the enhancing'material during the
association', and that this activity'is totally or ~ ~'
part'ially restored on biodegradation'of'the sa~ening
material.': 'Cert`ain represe'ntative associat'ion's~'include
: ionic and covalent bonding as for example in the case of
th~e'ion'i~cibon'ding of a''~met''al~'with cellulose'asrin'sodium
cellulose-,~the ioni`c'bonding'of-metal ~saLts' ànd io'n`ic
30`"~'speciè's`su'c'h'l~as''metallsalt's!of fatty'acids'~as or ëxample
s'odlum s~earate and various'covàlent fatty 'àcid l '1
der'i'vativ~e~s suc`~'as''''f'att'y''acid dèr'iva't'ives'`of-~protè`ins or
~"; ^}/'cà'r~ohy'dra'te's'.'^~Other fo''èms-of'ass'oc'iàtionCinclùdè non '
co'v''à'le''t*l'a'ssodiationfs'~''sucEi3~'à's'inte'rc'al''àtion',i~'in'ci'ursion~lC~
35'~ i;complexation~,'ic'h'ëiat~loh~`an'd'non-specific~à'dsorption'as for' 'i
examplë the'"'i'nclu~lon~!'of''a'hydro'phob'ic surfactant or
''`stress'cracking agen~iwithin the''cavity o;aicyclodextrin
, .
.
WO91/06601 PCT/US90tO6363
-8- ~:
molecule. Still other forms of association includes
incapsulation where the enhancing material is physically
encapsulated and surrounded by the safening material.
Suitable safening agents may vary widely. Any
material which is degradable and which is capable of
inhibiting the biodegradation enhancing activity of the
enhancing material can be used. As used herein, a
material is "degradableR where it degrades as a eesult of
exposure to the environmental effects of sunlight, heat,
water, oxygen, pollutants, microorganisms, insects and/oc
animals Usually such materials are naturally occurring
and are usually "biodegradableq. As used herein,
~biodegradableU materials are those which are degraded by
microorganisms or by enzymes and the like produced by such
microorganisms. Illustrative of suitable safening
materials are starches and starch derivatives such as rice
and maize starch, dextrin, cyclodextrin, amylose,
. amylopectin, defatted or solvent extracted starch, and the
like. Other useful safening materials include sugars and
derivatives thereof, such as sucrose, dexteose, maltose,
mannose, galactose, lactose, fructose, glucose, glyamic
acid, gluconic acid,.maltobionic acid, lactobionic acid,
. lactosazone,.glucosazone, and the,like. Still other
useul sa~ening materials.are cellulos~ and derivatives
thereof such as esters...of cellulose as for example,
.triacetate cellulose,..acetate cellulose, acetate- .
.. .. . .. . . .. . . . . . . .. .. . . . . . .
.. butyrate cellulose,~nitrate cellulose.and sul~ate
; .. ... cellulose, ethers of.cellulose.as for example,.ethyl ether
. ~ceLlulose,~hydroxymethyl ether.cellulose!~hydroxypropyl
. .. ether cellulose, carboxymethyl.ether.celLulose,.~,
; 30 ~-~ ^ .......................... . ...... . .
;,;.l.n~;ethylhydroxy ether..cellulose! and..cyanoethylether ether
cellulo~e,~ether-esters o~`cellulose as for?cexample,
apetoxyethyll~ether~cellulose~lproplonoxypropyl~celluloser
.and? benzoyloxypropyljce}lulose.and~urethanel~cellulose as
,foriexample~J~phenyl.;urethane ce}lulose.~Other.~useful
..,~3,5 :`~ safening;.~agents include;peoteins;suchJas~,zein!~soy~.protein
..or proteLn,hydrolysat.es,icasein, collagen,~elastin,.
. ialbumins and the like and! llgnins. Useiul biodegradable
.
. . - . ~. . . .:: . . . . . , -
2 ~ $ ~.?~ 9
WO~1/06601 ~ .`.P~/US90/06363
safening materials also include fats and fatty acids such
as mono-, di- and tri-glycerides derived from animal or
plant material and the common derivatives of these fats
such as fats obtained from peanut oil, corn oil, coconut
oil, cottonseed oil, palm oil and tallow, and fatty acids
such as oleic acid, stearic acid, lauric acid, myristic
acid and palmitic acid; biodegradable anti-oxidants such
as tocophenols, rosemary trosemari-quinone) and mustard
seed extracts, ascorbic acid and compounds closely related
to vitamin C such as ascorbic acid-2-phosphates and
ascorbic acid-6-fatty acid esters propionic acid; and
biodegradable polymers such as poly(glycolide),
poly(tetramethylene carbonate), poly(lactide),
poly(glycolide co-lactide), poly(caprolactone),
poly(tartaric acid), poly(ethylene-co-ketone acetal),
poly(hexamethylene azelate), poly(decamethylene '
succinate), poly(decamethylene azelate)! poly(ethylene
succinate) 9 poly(hexamethy}ene sebacat@), poly(ethylene
azelate), poly~r3-methoxy-4-hydroxy styrene), polytamino ''
triazole), poly(hydroxy butyrate), poly(hydroxyvalerate),
~ 'poly(hydroxy butiyrate-co-hydroxy valerate),
poly(dihydropyranj, poly(spiro ortho carbonate) and
poly~l-phe'nylalan'ine/ethylene glycoljl,6-diisocyanato
'~ ihexane);' ''~
' ' ;Preferred biodegradable safening materials are-starch
-5 ~and-starch derivatives'such as~cyclodëxtrins,.fats, fatty
, ., , .. . . , . ,.. - , . ~-, ;, . . . . .
~ - 'acids'~and biodegradable polymers such as poly(carbonates),
J ` and'homopblymers and copolyrmers derived from the .................... '''
polymer-ization-of hydroxy alkanoic acids and their
iderivat'ives'su'ch'as polylZ~l-hydroxy butyrate),
3 ZZ~ r~ r j, ~ ,~ "
poly~lactide), polyglycolic acid and copolymers thereof`~
: and particularly preferred biodegradable safening .. ,
mater1als'are starchès and starch derivatives and
biodegradable polymers derived from the polymerization of
hydroXyalkanoic acids and their derivatives ~. Most.i ~
9 Z;~ ,t ~ i r ,~ ~Z ~t C Z l ' ~ `5 ~
preferred b~odegradablé sa~ening agents~are cyclodextrin'sZ,
~A poly~beta-'hy'droxybutyrate), poly(lactides)
poly(glycolide) and block copolymers containing
WO91/06601 2 ~i~J..~19 PCT/US90/06363 ~
1 0 ~
3-hydroxybutyrate glycolide and/or lactide recurring
monomeric units.
~ seful degradation enhancing materials include any
material which in an unassociated form is capable of
directly or indirectly enhancing the degradation of a
polymeric material to some extent and which is capable o~
association with a biodegradable safening material which
inhibits the activity of the enhancing material, such
activity being restored on biodegradation o the
biodegradable safening material. Useful enhancing
materials may vary widely. Illustrative of useful
materials are stress cracking agents as for example,
surfactants. Useful surfactants include, anionic,
cationic, zwitterionic and nonionic surfactants.
Useful anionic sur~actants include alkali metal,
ammonium and amine soaps and alkali metal salts of
alkyl-aryl sulfonic acids, sodium dialkyl sulfosuccinate,
sulfated or sulfonated oils such as glycocholic acid
sodium'salt~ glycodeoxycholic acid sodium salt, sodium
dioxychalate, cholic acid sodium salt, l-deconesulfonic
acid sodium salt, caprylic acid sodium salt, sodium
dodecyl sulfate, taurocholic acid sodium salt,
t'au'rodeoxycholic acid sodium salt, sodium decyl sulfate,
sodium octyl sulfate, sodium hexyl carboxylate,.sodium
';-hep'tyl carboxylate,~'so'dium octyl carboxylate,.sodium nonyl
5- ^carboxylate, sodium decyl caeboxylate and sod~um dodècyl
~-'carboxylate, disodium 'dodc'yl phosphate, disodium 4-alkyl
3-sulfonatosuccinates and sodium dodecyl benzènesulfonate.
;Us'efu'l~c'ationic su'rfa"cta'nts i`nciude salts.oe long
chain primary,'isecondar'y 'and tertiary amines such as ~:
.'~'o'le'ylaminë acetate, cetylam'ine acètatè, didodecyiamine
lactate',7the acetate of'aminoethyl-amino ethyl.stearamide, .
dLlau'royl triethylene~'tetramine diacetate, and .-...-~i.
aminoet'hyi-2~hépt''adëcenyl'~niidazolLné' acetate' ~
quatërnary~s'alts''such as'cétylpyrïdinium bromidë,~ ~ ;'c
35! L^1hex'odecyl ethyi morpholinium ch1Orlde, didodecyl ammonium
chloride, cetyipyridinium chloride, dodecyltrimethyl- :
- ammonium bromide, he'xadecyl trime~hylammonium bromide,
'
'' .
., ..... . .. , , .. . . . .. . .. : ..
W~91/0660] ''.".; PCT/US90/06363
~`., - - 1 1 -
tetradecyl trimethylammonium bromide, dodecyl ammonium
chloride, cetyl trimethyl ammonium bromide, benzalkonium
chloride, decomethonium bromide, methylben~ethonium
chloride, 4-picoline dodecyl sulfate, sodium
perfluorooctanoate, sodium hexyl sulfosuccinate, sodium
octyl sulfosuccinate, sodium cyclohexyl acetate, sodium
cyclohexyl propionate, sodium cyclohexyl butanoate and
sodium cyclohexyl sulfamate.
Useful zwitterionic surfactants include N-alkyl-N,N-
dimethyl-3-ammonio-1-propane sulfonates such as N-decyl-
N,N-dimethyl-3-ammonio-1-propane~ N-dodecyl~N,N-dimethyl-3-
ammonio-l propane, N-hexadecyl-N,N-dimethyl-3-ammonio-1-
propane, N-octyl-N,N-dimethyl-3-ammonio-1-propane, and
N-dodecyl-N,N-dimethyl 3-ammonio-1-propane, D,L-alpha-
phosphatidyl choline and dipalmatioyl.
Useful nonionic surfactants include n-alkyl-D- '.
glucopyranosides and n-alkyl-D-maltosides such as
decyl-D-glueopyranoside, dodecyl-D-glucopyranoside,
heptyl-D-glucopyranoside, octyl-D-glucopyranoside,
20 nonyl-D-glucopyranoside, decyl-D-maltoside, dodecyl-D- . .
; maltoside, heptyl-D-maltoside, octyl-D-maltoside, and
nonyl-D-maltoside, condensation products of higher Eatty
: alcohols with alkylene,oxides, suchtas-the'reaction
product o~ oley} alcohol with 10 ethylene oxide unit's;
condenstation products of.alkylphenols with alkylene
~ oxides, suchjas the reaction products of isooctylphenol,
: j o~c.~tylphenol and nonylphenol.with from abut 12-to aboùt 30
.~ ,e~hylene.oxide,units; çondensation.pro'ducts of higher`'
~fa$ty acjid.a~ides;i~with.5.or.;more alky'lene oxide units such
- ~ as ethylene oxLde units;:~polye~hyl glycol esters of long
chain fatty~acids,lsuch as.~tetraethyleAe glycol
monopalmitate, hexaethyleneglycol monolaurate,`'i''' ';`
~nonaethyleneglycol dioleate,~tridecaéthyle'neglycol
mojnoa.rachidata,-triosaethylene"glycol~-mono~ehënate,;~''i . .
5 ~1tjricosae~5h,yleneglycoldLbehanat!e,ipolyh'ydric~àicohoï;'
par~ial~hi~her~fatty.~àcid-.est.eri~"suc'h'5as~'sorbitan'i' `'9
trisearat~e,;ethylene~ox.ide~condensat'ion prodù'cti~3Of~
polyhydric .alcohol.parital hi~her ~atty.esters, and their
1:
' :
. .
.
~', .
WO91/06601 ~ PCT/US90/06363
-l2~
inner anhydrides (mannitol-anhydride, called Mannitan, and
sorbi~ol-anhydride, called Soebitan), such as glycerol
monopalmitate reacted with l0 molecules ofethylene oxide,
pentaerythritol monooleate reacted with 12 molecules of
ethylene oxide, sorbitan monostearate reacted with io to
15 molecules of ethylene oxide: long chain polyglycols in
which one hydroxyl group is esterified with a higher fatty
acid and the other hdroxy group is etherified with a low:
molecular alcohol, such as methoxypolyethylene glycol S50
monostearate (550 meaning the average molecular weight of
the polyglycol ether).
A combination o~ two or more of these surfactants may
be used. For example, a cationic surfactant may be
blended with a nonionic surfactant, or an anionic
surfactant with a nonionic surfactant.
Other useful degradation enhancing material include
olefins especially mono, di and tri unsaturated fatty
acids, triglycerides and derivatives such as lipoproteins
and lipopolysacharides agents capable of a change in
physical properties..upon exposuee to environmental
conditions such as moisture,.for example, bentonite `clay;
oxidizing agents such as.halogens and heterosubstitut'ed
anions,.especially~tri iodide-(I3); strong acidsj strong
bases and certain salts such as'trifluoroacitic'acid,
dichloroacetic acid,:hydrochloric''acid," lithium bromide,.
25 r Z~Lnc clorid,e, sodium cyanide,'phospho'ric acid, 'nitric
.~. aci~d, sulfuric.acid,-~sodium'.hydroxide, lithium hydroxide,
. apd.sodium~alkoxide;.and transition ~,etal salts'(cobalt,
copp;er,~nickel,~zinc, manganese,~ cadmiùm,ii'iron) alone oc
in combinat~ion,.with organic compounds!such as fatty acids
~e.g, steara~e or naphthenate, and the~i'ike)jasisàlt
''complexes..J;~-$~,ji..i,:;i'; ~""' "'i''' ''~`'''
~Still~,c,ther useeul;degradation enhancina, ~,ateriàis , '~
:include~sc-lvepts and,other-materials'~which'ë'nh'àn'ce the'
degr~adation...,of ,jspecif ic ,po lymers ;~as':!fo~ ;éxampl!e ~, ' hèxarle/~
35 heptane~ ,$icQbutyl.~acetate,- 5-me't'~yl"'2'-'hiexatnc,ne,'J~ :
2-pentanone,,J,,3,-pentanone,^.ctoluenè,Scycl'o`hexanë,jJànd~,'
.t.etrahydro~uran which~are~'detrimèntal~to~p'o'ly~bu'tadienes);
~' .
W~91/0660~ PCT1US90/06363
t : -13-
xylene, p-xylene, tetralin, decalin, tetrachlocoethylene,
n-butyl acetate, diphenyl ether, butyl sterate, squalene,
glycol dipalmitate, tripalmitin which are detrimental to
polyethylene, pol~(propylene) and poly(tetrafluoro-
ethylene~; N,N-dimethylformamide, N,N-dimethylacetamide,
Y-bUtyrolactone, nitric acid, hydroxyacetonitrile,
dimethylformamide, ethylene carbonate, propylene
carbonate, malonitrile, su~cinonitrile, sulfuric acid,
which are detrimental to polyacrylonitrile; cyclohexanone,
cyclopentanone, and tetrahydrofuran which are detrimental
l~ to poly(vinyl chloride); acetonitrile, heptanone-4,
isoamyl acetate, n-butyl chloride, heptanone-3, .
n-propanol, acetone, benzene, n-butyl acetate, n-butyl
chloride, and chloroform which are detrimental to
poly(methylmethacrylate) benzene, toluene, butanone, and
cyclohexanone which are detrimental to poly(styrene);
phenol, o-chlorophenol, aniline, cyclopentanone, ethylene
carbonate~.cyclohexanone, benzyl alcohol, acetic
anhydride, and dimethyl formamide which are detrimental to
. poly~oxymethylene?; o-chlorophenol, phenol,
2~ trifluoroacetic acid, and dichloeoacetic acid which are
detrimental to poly(ethylene terephthalate, nylon 6 and
nylon 66;and.mineral acids such as HCl,..HBrj-H2SO4j..
and H3PO4, trifluoroacetic acid, inorganic salts such
as Ca(SCN~2,jLiSCN,iNaSCN, LiI, NaIj KI, and
K2~gI4l), strong bases such as LLOH, and~NaO~
tetraalkyl-bases,~metal complex solutions..such as
(lcu!~NH3~4](~oH)2/~dimethyl~sul~oxlde~J-and .i ^ :.
dimethylfocma~ide which,are1detrimental.-;to:.cellulosic
.~,m~aterials. r,~;i J i~
."3J~ .Preferred;.degradation ~nhancing materials are stre~ss
cracking.~agents~which are.materials which cause the;1
.. ...
..,.. r,.polymer~com~osition-to~crack,i,and;:particularly.pceferred
` ... ,i~degradation~.. enhancing materials:are surfactants... More
~preferred~surfactant~s.1are~capable;of!inot only-degrading
35~ theplasSic.~but~a1jso~exbibit~a benefici:al;effect..byit;
enhancing~he biodegrada~ion of any::biodegradable~ e.~
.. component i~corporated into-the plastic... Most.preferred .
.:
.
, .
WOsl/06~0~ 2~ 9 -14- PCT/USsO/06363
for use in the practice of this invention as degr~dat ion
enhancing materials are nonionic surfactants, or mixtures
of nonio~ic surfactants and other types of suefactants.
Particularly preferred for use in the practice of this
invention are nonionic surfactants such surfactants are
capable of not only degrading the polymees but also
exhibit a beneficial effect by enhancing the
biodegradation of any biodegradable component in the
polymer. Preferred nonionic surfactants for use in the
practice of this invention are alkylarylpolyethers, such
as the condensation products o~ alkylphenols, such as
octylphenol, nonylphenol and isooctyphenol, and alkylene
oxides, such as ethylene oxide fatty acid alkanol amides;
poly-alkoxylated alcohols, such as polyethoxylated
tridecanol, idotridecyl alcohol adduct with ethylene
oxide and fatty alcohol polyethers.
The particularly biodegradation safening materials
and degradation enhancing:material selected in any-
particular situation will depend on a number of factors
including the capability of the biodegradation safening
20 . material for inhibiting the activity of the degradation
enhancing material, the level and nature of the activity
of the.;degradation enhancing material, t~e poly~0ric
: material.stability.of the complex,-especially as i~'
pertains-to processing'condi~ions for:the polymeric' .
mateial and the effect.that the degradation enhancing
material.has upon the biodegradation of-incor.porated
components of.theipolymer, the propoied use'of~thë'' ' '
'~ composition~and::article:ifabricate'd therefrom,'thè~'cost of
~he composition and aeticle fabricated ~herefrom, the ~.............. .
.30 .;;?usable~lifetime of~theicomposition'and~àrticle'fabricated
.therefrom, the tLme'after:'exposure t!o'biodegradat'ion
environental):'conditions which degra`dati'on''is"~desired to
3-itake~place,~s'~For. example,~'when' the'degradation~'enhancing
,?~:imaterial~is a-isurfactantj~the~biodegràdation-safening-; .
; 35 material::~is.. preferably-a~'carbohydrat'e-s'u'ch as!st~arch or a
: starch.derivative.~includLng 'amylos~j' lineae-?~
polysaccharides and cyclic~polysaccarides''such'as'' '
~' -
.~: . .. . . ,.. .... ..... .. , .. - . .,, . , . , .. . - , , - . . . . . .. . . . .
.' ' ' ~ . ' . , ' . ', . . .
WO91~6601 ~$~ 9 ` ~ ~ ~ PCT/US9~/06363
-15-
cyclodexteins which function to form an inclusion co~plex
and encapsulate the surfactant and protect the polymeric
material from the deteriorating effects of the
surfactal~-. Biodegradation of the carbohydrate releases
the surfactnat which can enhance the degradation of the
polymeric material.
Similarly when the biodegradation enhancing material
is potassium triiodide, the biodegradable safening
. material is preferably a polysaccharide such as a starch
which is capable of complexing the salt which functions to
pre~ent or retard the formation of the halogen, I2 which
adversely affects the polymeric material. Here again, on
biodegradation of the starch, the I2 is released to
enhance the degradation of the polymeric material.
The co~position of the invention includes an
'Aeffective amount~ of the particulate filler. As used
herein, an ~effective amount~ is an amount which when
activated is sufficient to enhance the biodegradation of
the polymeric-material to any extent. This amount may
...~. vary widely and depends on a number of factors such as the
20 .amount and activity of the degradation enhancing material
:. in the fiLler and the-like. In~general, the amount of '
,' E,iller,employ,ed~is-at.,~least, about,-,O,.~OO,l,i,we.ight,percent -~.,based on the total weight.,,of the composition.-.-In the
preferred embodiments of,.the,.invention, .the amount of the ,,
5 ,~filler is from~.about~Ø05 to.about 40 weight percent based
.~,on the total weight of the composition,.~,and in ~he ,
~, particularly-~-preferred embodiments of.the invention.is
i...Er.om~about o.ol~to~about~i2oi~AweightFpercent on the
: aforementioned.basis.!!,Amongst.these.,particularly
rpr.efer.red -embodiments; most~preferred.-are ,those ,~
embodiments~.in.,.which~the amount~of the~filler, is"rom
abou,t; l to~abo.ut,ilO~weightrpercent~bas~d..o,n-lthe..total
n~.weight of,the-~c,o,,mposition~ v,~t .,~ t~Ci.' ..~ .tt~
~dL; ~he filler,ctis-yi~particulate form to~.al.lowi;dispersion
; of the ~iller in the polymeric material.1c,~In~thej~preferred
'~ t~C~ t~:embodiments;.~-Pf :thel~inv,en~ion,~the~part.iclezsize is equal
~ to ori~less than.about;lOOO.~um.. ;~he lowee limit,in~
:
WO91/0660l ~ a~ ~ 9 PCTtUS90/06363
particle size is not critical, and in the preferred
embodiments of the invention, the size of the particles is
as small as possible which facilitates the dispersion of
the ~iller in the polymeric ~aterial. In the more
preEerred embodiments of the invention, the particle size
is from about 0.1 to about 500 ~m, and in the most
pceferred embodiments of the invention, particle size is
from about l to about 300 ~m. Amongst the most preferred
embodiments of the invention, tho~e in which the particle
size is from about 2 to about 200 ~m are the embodiments
of choice.
In addition to the above-described essential
components, the composition of this invention can include
various optional components which are additives commonly
employed with polymers. Optional components include
fillers, nucleating agents, plasticizers, impact
modifiers, chain extenders, pigments, colorants, mold
release agents, antioxidants, ultra violet light
stabilizees, lubricants, antistatic agents, fire
retardants, and the like. These optional components are
well known to those of skill in the art, and accordingly,
will not be described herein in detail.
!'` ;~i ~':,Th'e''compos'ition'may fu'rther-comprise additional'
'particulate biodegradable~fillers which ~urther enhance
"-' t'he'~r'ate of biodegradation of the composition.
5 -'Illustrative:of'useful and pr'efer'red~fille'rs 'are those
materials described as useful for-biodegradable safening
';mater'ials. Preferred fillers are~starches. '
r~ The amount of b'iodegr'a'dable~fille'r m'ay vary widely
and'~:amounts ~normally used lin the art~maylbe used. -
Howeverj- in the~prefe`rred 'embodimets of~the invention, the
'amo'unt o~'such biodegradable filler is~not more;than about
!3o~wleight~percent~ basedton-the~total weight:of the~5
composition and more preferably not-more~than about~20
we~fghtt~percent~an~ most'~pre'ferably~not-more tha~n about l0
~35`'J'weight~'percent'.;~ le ''~~
i The~-composition~'bf,this inven~ion~can berprepa~ed by
blending or mixing the essential ingredients,~ and other
.
~ g
W~ 91/~6601 ' ' .~ PCT/VS90/06363
-17-
optional components, as uniformly as possible employing
any conventional blending means. Appropriate blending
means, such as melt extrusion, batch melting and the like,
are well known in the art and will not be described herein
in greater detail. In one useful procedure, the blent~ing
procedure can be carried out at elevated temperatures
above the melting point of the polymer and the nucleating.
agent either preformed, or as individual components of the
agent separately oe as a combination of the components in
a suitable form as for example, granules, pellets and
preEerably powders is added to the melt with vigorous
stirring. Alternatively, all or a portion of ~he various
.
components of the filler can-be masterbatched.or
preblended with the polymer in the melt and this premixed ...
or masterbatch added.to.the polymer in the melt in amounts .'.
- ~suEficient to provide the desired amount''of the filler in
the polymer product. "'Stirring is continued until a
.homogeneous composition is1for~ed. 'Blending temperatures ~ :'
and blending pressures, and the order of addition of the
vaeious components are not critical and may be varied as
20 ~ desired provided that a substantially homogeneous :
. composition results. The blending procedure can be .'
car.ried:out.at~ elevated~temperatures,:in:whi'chLcàse the ''
.polymer component is melted:and the-~filler and~othér
optional ingredients a're admixed therewith by vigorou~ly . .:.
.ii..stir.ring the melt.~Similarly, th'e`"'various solL'd ' ;
components -can be -granulatéd;iland the granulat'-'d'' ~
. c.omponents~mixed dcy~in;a suitable-blender,`' or''for ~ :
example,l(.alBanbury.mixer~, ~''s uni'forml'y''a`s`"pos'sibie, then
: m~lted'in an~extruder~randr'extruded w'ithlc'o'oli'ng.~
ni~.The~.composit'ions ac'cor'ding'toi:t'he~.inv'ent'i'orn''~are'~ ;'
.thermoplastic.~biodegradable~im'at~èrial's''from~which'molded .
articles.^of manù'factu're~can~be produce'd~''by te:conventional
JO.,shapin~g',.pro~esses~~ suchl~a's~.'me~ spi'nn'lng',~'casting, ~
l`nject.ion~molding ~and~-`extru'd'ing'.'~ Thè compositi'ons~ of' ;
' 35 thls inven'tion!~are'~'especi,~ y'~useful'for"'fa~r"ic'at~io~n of
'~ ;extruded films,?ae~;for'~'ex'amplië~ f~ m~s `for use~rin''food
packaging ?rSuch fi-lms.can be .fabr'icated using conventional
'
,
wosl/0660l 2~ ~ ~ 18- pcT/vsso/o6363
film extrusion techniques. Such films foemed from the
composition o~ this invention are biodegradable such as
being buried or composted with other garbage, the film
degraded by evironmental effects such as sunlight, heat,
water, oxygen, pollutants, microorganisms, and the like.
The following examples are presented to more fully
illustrate the invention and are not to be construed as
limitations thereon.
EXAMPLE I
A) Preparation of the ~-Cyclodextrin tCD) Com~lex
The molecular complex of CD with Igepal was
prepared by combining the individual soluble components
and recovrerng the precipitated complex. In 100 ml of
15 water 15 grams of-CD was dissolved by heating. A 5 ml ,,
solution of 32~ Igepal ~Igepal C0630), a non-ionic
surfactant obtained from GAF Corporation was warmed to
reduce its viscosity and then added dropwise into the hot
CD solution over a period of about 15 minutes wi~h
constant stirring. The solution was next alowed to cool
slowly to room temperature and then maintained at 4 C foe
approximately,l15 houes.; The resul~ant pre,cipitated~;
complex~was recovered by filteation and then air dried.
, .. . . . .
~. ,,To ei~timate the ratio of CD to Igepal in the complex
a known,weigh~ of complex was,subjected to extensive acid
hydeolysis so as to fully degrade the cyclodextrin to its ' ,
component glucose units,,which could then be measueed
Using a,glucoi3,e analyzer (Beckman Instrumen~s). The
amount of cyclodextrin!in ,thej,complex,,can;thus be `;,;
determined,~7 glucoise molecules per l ~-cyclodextrin
. ". ~ ... . ... ~ . .
molecule) with th,e,,~remainder,~o the;,weight ofithei~complex
due,~,~t~,o,;,the Igepal~ ,In,on,e,example,~lSO mg of the,complex
was hy~d~rolyze~d~by;~re~qf~luxLng~for~l.5 hours ,at~i:100 C!in?~100
ml,o~~rl M~Cl~ The-gl,ucose"-pr,oduced ~was,measured and
accounted,,~for,~115.,3~mg of ~the;<samplelwtih -38 mg .~
re~presenting the cont,ribution of~,the Igepali Using the
,mo,lecu,lar,weights, of .the cyclodextri'n and~Igepal-the'ratio
'..
,g~/06601 ~ Y PCI/U~;90/06363
of cyclodextrin to Igepal in the complex was determined to
be approx ~ately 1.6 to 1.
B) Testing of the Complex. To test the ability of
the complex to function as a biologically released
destructive agent a method was developed to measure the
destructive effect of the release of the stress cracking
agent, Igepal. The assay method developed for this
purpose uses small pieces of polyethylene which are
st~essed by gentle bending in the middle of a 1 cm by 3 cm
sample. The ends of the pieces are held together to
maintain the gentle stress and thus provide a point o~
failure for the plastic and reduce the time for the assay
and the samples are immersed in the test solution. Normal
crazing and stress c~acking will occur withou~ the
introducton of an external stress, bu~ in a longer time
fram. This assay method was first used to determine the
, appropriate concentration of Igepal to be used for for
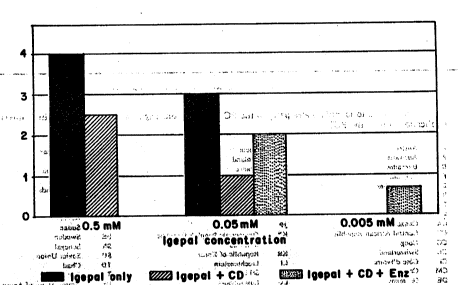
stress cracking. The results are set ~orth in Figure 1.
As shown in Figure l, a concentration of greater than 0.05
,"20,,, ,mM was found to-completely, or nearly comple~ely, break
; the plastic samples in the stress cracking assay within 24
hours at 60C. The p`rotective effect of the cyclodextrin
encapsulant was demonstrated in a similar~experiment in
which cyclodextrin was added to the'aforementioned testing '
mixture (0.05 mM Igepal). The Igepal,,no,,longer,,,broke thè
plastic samples and stress cracking was either ,low or not
measurable. To demonstra~e the biodegradability of such a
i~ r ~
complex and ~he results of the rèlease of the Igepal, the
same test ~as:run, except that the enzyme alpha' amyl'ase
' ,30 ,,.which,.is,.known.to~degrade cyclodextrins was added to'the
~ tm~ x~t~ure.~ he~result-3Llo~flthLstè~xperiment is also depicted
"I 3,in,F,lgure,l ~andj~shows~hat,a;~normal mi~crobial,enzyme,,is
capablerof relea~ing ~he-,a~detrimental~agent-~(Igepal)
from ~thejc,omp,l,~x ,l~wlth~CD)f~and,~hat,3lthe~fr~ee~agentj,can
",elst,~r~Y,i,ltj,h,,e Plag,~lC~ oJ,~ ? J . '1 :''.i 'l'i'~ '!.'` ~,` !
. . ' ~
,, -. ` , :,, ~ ' . : . :
- , ~ : ' . . , . . : , , . : .:
- ' - . : ' . , : '.
W~9l/06601 PCT/US90/06363
-20-
C) ~ ~ ~ =~ '
polyethylene (HDPE). ~Cyclodextrin and the complex o CD
with Igepal were incorporated into HDPE to produce a
plastic product of ten mil thickness containing the
approptiate additives at a level o~ lO~i by weight. The
physical properties of the final products (and a control
plastic containing no additive) are shown in the following
Table 1.
TABLE 1
leA~
Sample Wt~ @ yield @ break
-ID filler psi psi
(1) Control
0 40~0 1741
. (2j I~epal Complex in beta-Cyclodextrin
- 10 :~. 3803 1636
(3) beta-C~lodextrin-
3703 3309 .
..
TABLE 1 ~cont'd)
.. . Elonyation . - Tensile .~ .
Q yield @ break . .Impact .~
ft-lb/in
.3 ~493.0 ~ 84.6`
`(2)'4 ~ 52-9 ,, 37.9 ,
.~~.(3)~ .8. ~18.g .~ 1.2
To'determine'if thé ^complex'in the plàstic was sùsceptible
sto~iodegradation, the1samplës were placed in a;n enzyme
soiution'containLnc'~àlpha`j'àmylase and glucoamylasë which . :
~make~possible ~t~e~.degra~dàtionJof the cyclodëxtrLn 'and its;F. ~ ;
conversion to glucose. Glucose is readiiy measùrëd using
an instrument isuch as a glucose analyzer lBechman
.
':~ ' . . ~, ,. , . . ' , ~ , . .. . .
' ' ' . '. . ' ' ' ' ' .' . '' . '.' ". " . ' '.~ ' ' . " ' ' . '.. .' ' ' .
W091/06601 ~ "1,~L~'9 PCT/US90/06363
-2l-
Instruments). ~he results of this experiment are shown in
the following Table 2.
' TABLE 2
~ degradation of cyclodextrin control:
Time (h) HDPE + complex HDPE ~ cyclodextrin no en2yme
only
O O` O O ..
10 2.8 .40 ~ .92
l9 .73 .94 0
201 Z.3 0
.
The production of glucose during the time period of the
test shows that the cyclodéxtrin within the plastic can be
degraded by microbial enzymes, As shown in the tests
described above, the deg~radation of the cyclodextrin
~.. .. . . . . .
portion of the cyclodextrin/Igepal complex will liberate
the Igepàl. The Igepal will in!turn cause stress cracking
of the plastic, With the samples chosen for the testing
o~ the incorporation of the complex into the plastic ~10%
i loading) it was not possible"to show the result of stress
cracking in our standard test~within a short period of
25 time. In part, this is due to t~e difference in the low ~ "
~''''tëmpèra~ùres uséd to a-ccelërat'e 'a'nd''t~est'~'the'''st'ress
J: cracking, Furthermore, in order to demonstrate 1 - '
~ '~ `' cycLodextrin degradation (glucose production) the 10%,
:, ~f i ',S`',~j'J ~(~tf~f'i! '~2r; ~ i ;,a~f5~ t.' .~
loading was appropriate, however, this relatively high
1~ ' '? l C'S~ r~ 'f~ f~ .3 i?~
~' 30 level Oe complex addition caused a siufficient change in ~;
the'physical properties so as to affect the response of "
the plastLc to stress cracking,failure.
~ f i~ J '~ ?f~ f ~ Uir~ 'r,f~ t~i'~ ?~ Ir.~ '.~
~ ~ "r,`f i ?,5~ t~ ' r"~
' ' '' ' ~'
WO9~/0660l Z ~ PCT/US90/06363,,~
-22-
EXAMPLE II
Complexation and Degradation of a ~-Cyclodextrin With
Sodium Dodecyl Sulfate (SDS) and of ~-Cyclodextrin and
Corn Starch Wlth Cetyl PYridinium Chloride (CPC). Using
the procedure of Example I, the complexation of
cyclodextrin with destructive agents other than Igepal was
demonstrated using positively and negatively charged
surfactants. In both cases the formation and degradation
of the complex were monitored by changes in the
conductance of the solution. As the low molecular weight,
charged surfactant was immobilized by the formation of a
complex with cyclodextrini and conductivity decreased.
Upon addition of an appropriate enzyme to degrade the
cyclodextrin, the conductivity again rose indicating the
release of the destructive agent in solution. An example
of this effect is shown in Figures 2 and 3 in which the
formation and degradation ~by the addition of the enzyme
alpha-amylase) of a beta-cyclodextrin complex with SDS and
and the formation of a beta-cyclodextrin complex and a
corn starch complex with CPS are measured by conductivity
changes.
.. ,...,.. .: , ,
EXAMPLE I I I
.. . :. . . .... . . . .
, ! ' ~ Using '
the procedure of Example'I, the compléxa~ion and
, degradation of corn''starch 5Pearl Starchj was demonstrated
with sodium dodecyl suIfate. As shown"by monitoring
conductivity'changés ~Figure 4) starch can complex a
''strëi's,~-cracking'surfàctan~'~SDS), and release it upon
enzymat ic degradat ion. Since starch has been widely used ,,
as an addit ive to piastics, it foLlows that thè
starch/surfactant complex could b~ added then the
suractant released upon exposure to conditions which
35 pcomote biodegradation. c~'.
~,
,' ' ,
. . , . ~ . , , ~ . .: ,
; . . ~ , ; .
,- .'. ... - ,' ` . ,:, .: '. .. '' '