Note: Descriptions are shown in the official language in which they were submitted.
i 2070252
A POLYMERIC ARTICLE,
SUCH AS A MEDICAL CATHETER,
AND METHOD FOR MAKING THE SAME
INVENTORS:
Charles E. Larsen, Leonard Pinchuk and Thomas D. Weldon
BACKGROUND OF THE INVENTION
The present invention relates to articles of softened
polymeric material and/or articles of polymeric material which
have portions of differing softness, pliability or
flexibility, and to a method for making such articles. More
particularly, this invention relates to medical catheters,
such as thin-walled angiographic catheters, guiding catheters,
angioplasty catheters, urinary tract catheters,
gastroenterology catheters, and the like, and to a method for
making such catheters wherein the catheter includes an
elongated body or shaft portion and a softer or more pliable
or flexible distal end portion which is integral to its
adjacent shaft portion. Even more specifically, this
invention relates to a three zone catheter that includes 1)
a relatively stiffer body portion, 2) a softer, or more
pliable or flexible distal end portion which may terminate in
3) an even softer or more pliable or flexible tip end portion.
Catheters are widely used in the medical field for a
variety of applications, including both diagnostic and
therapeutic procedures. Depending on the particular medical
application, it is often desirable for different parts of the
catheter to exhibit different physical characteristics. For
example, radiological catheters are widely used in
angiographic applications where it is necessary to administer
a fluid at a location within the cardiovascular system of a
patient. Because these catheters are inserted into and passed
through the blood vessels of the vascular system, they
necessarily must have a very small outside diameter. On the
other hand, because the radiologist usually desires to
administer large boluses of radiopaque dyes or the like at
high flow rates to obtain the sharpest x-ray image, the
c
2 270252
catheter lumen should have the largest possible inside
diameter (i.e., the catheter should have the thinnest possible
wall). However, competing considerations which limit the
thinness of the catheter wall are the need for high strength
to withstand the high pressures of liquid injection, which may
exceed 1000 psi, and the need for a high degree of tensile
strength and stiffness in order to allow the catheter to be
pushed, guided and manipulated through the vascular system of
the patient without kinking or buckling. Accordingly, in
addition to being thin-walled, catheters such as angiographic
catheters should have a shaft or body portion with good
mechanical stability, burst strength and kink resistance.
Yet, it is often desirable for the same catheter to have
a more pliable distal end portion. For example, many
specialty catheters such as diagnostic catheters and urinary
stent catheters are shaped at a distal end portion into
specific configurations to reach difficult vessels and/or to
locate a portion of the catheter within the various vessels,
sinuses and cavities. The distal end portion of a so-called
Judkins catheter, as an example, is bent into a bell-like
shape to help insert the tip into the coronary arteries. The
distal end portion of a coronary diagnostic catheter is
typically formed into a pig-tail or loop to allow lodgement
of the catheter tip in the heart ventricles. Similarly, a
urinary stent may utilize a double pig-tail, i.e., one on each
end to allow lodgement of the catheter tips in the kidney
calyxes as well as the urinary bladder. Because many of these
catheters are inserted into the patient along and over guide
wires, it is desirable for this bent or formed portion to be
more flexible or pliable than the body portion of the catheter
to enable sliding of the catheter over the guidewire without
significantly distorting the guidewire.
Further, because catheters typically must be able to
reach distant vessels within the body without damaging,
tearing or causing trauma to the various tissue, the tip end
portion of the catheter is preferably even softer and less
traumatic than the body and adjacent portion of the distal end
of the catheter. Unless the context indicates otherwise, for
purposes of this description "distal end" means generally the
portion or area at the end of the catheter which is located
t
2U7~2~2
- farthest from the physician or other medical personnel using
the catheter. The term "mid-distal end" is used herein with
reference to a part of the distal end portion which does not
extend to the very tip end of the catheter. "Tip end" is used
herein to refer to the most distal end portion of the
catheter. For example, an angiographic catheter of the type
which must be passed through blood vessels and/or into the
ostium of a coronary artery should not cause trauma to the
lining of the vessel or to other tissue, such as a heart
leaflet valve. Accordingly, in order to reduce the risk of
trauma, it is desirable that such a catheter, while having a
body and/or mid-distal end portions which meet the above
criteria, also have an even softer or more flexible tip or tip
end portion.
The prior art has attempted in several different ways to
address the need for a catheter which has different portions
exhibiting different characteristics. As described, for
example in U.S. Patent No. 4,551,292, a catheter with a more
rigid body and a softer tip may be provided by separately
molding the body portion and the distal or tip portion of
different materials and joining them by either heat sealing,
sonic sealing, solvent bonding or other fusing techniques.
One drawback with such a "two-piece" yr "three-piece"
catheter, however, is the possibility that the distal or tip
end may separate from the body of the catheter during a
medical procedure. Fused multi-piece catheters have an abrupt
transition from the harder portion end to the fused-on softer
portion. This abrupt transition focuses stresses on the fuse
joint which renders it more susceptible to kinking and further
risks separation. Regardless of the medical procedure,
inadvertent separation of the distal or tip end from the
catheter body or shaft during the procedure is to be avoided.
With vascular catheters such as angiographic catheters which
are used in cardiovascular procedures and are often used in
close proximity to the heart, separation can create risk of
serious harm to the patient. Far example, a separated
catheter may reduce blood flow to the heart or actually lodge
within the heart and require immediate surgery to remove. In
the best of circumstances, a separated end is a great
4 2070252
inconvenience for the surgeon and, at worst, is
life-threatening and possibly fatal to the patient.
Unfortunately, the potential for end separation is
increased in thin wall catheters, such as angiographic
catheters or guiding catheters, where the reduced wall
thickness may not provide a sufficient amount of
cross-sectional area to allow for the secure attachment of the
distal or tip end portion of the catheter to the catheter body
or shaft. For similar reasons, thin wall catheters of
two-piece or three-piece construction require more precise,
laborious and time-consuming assembly techniques and test
procedures to better insure the security of the bond between
the end and shaft and to provide smooth, continuous and
confluent inner and outer walls of the catheter to prevent
snagging of the catheter within a guiding catheter or of a
guidewire within a catheter lumen. Finally, fused-multi-piece
construction makes assembly of multilumen catheters extremely
difficult.
Accordingly, the prior art also discloses several efforts
to provide a catheter having a softer end portion but wherein
the end portion is of a one-piece or fuseless construction
with the body portion of the catheter. For example, U.S.
Patent No. 4,753,765 describes a catheter having a two-layered
tubular body portion with a rigid inner sheath and a more
flexible outer sheath. The end portion is an integral
extension of the flexible outer sheath that is co-extruded
over the rigid inner sheath and extends beyond the distal end
of the inner sheath.
The precision co-extrusion process described in U.S.
Patent No. 4,753,765, however, has many drawbacks. One
drawback is that the extrusion is difficult to control,
particularly for thin wall catheters, as it utilizes an
underlying material having a significantly higher melting
point than the top extruded material. This dissimilarity of
materials may also adversely affect bonding. Another drawback
is that thin tubes require a wire mandrel support for the
tubing which can be quite costly. Also, the process described
in U.S. Patent No. 4,753,765 is not conducive to mass
production because the underlying sheath is discontinuous, and
this may also significantly increase the expense of
..
~~~~CJ
manufacturing these catheters. Another drawback with this
type of catheter is the abrupt transition between the hard and
soft sheaths. Finally, the requirement for multiple layers,
in particular for a three zone catheter (having a body or
5 shaft portion of certain hardness and a softer mid-distal end
portion and an even softer tip end portion) , may increase the
overall outside diameter of the catheter and/or reduce the
diameter of the lumen, both of which are to be avoided.
The prior art also discloses a catheter body and tip of
relatively soft material, with braiding provided along the
body portion to provide the desired stiffness and burst
resistance. Braiding a thin wall catheter, however, is a
difficult and expensive process which results in greater
waste, higher than desirable wall thicknesses, and increased
costs. Moreover, braiding a three zone catheter is even more
difficult and costly to perform.
Another prior art patent, U.S. Patent 4,960,410,
describes a two piece construction having a helix cut through
the wall of the distal tip of the catheter to provide
flexibility and pushability. The portion is then sheathed
with a thin softer tube to complete the catheter wall. One
drawback with this catheter and method for making such a
catheter is that cutting the helix, sheathing and bonding the
sheath to the inner catheter is both laborious and costly.
In addition, although this construction provides a pliable or
flexible distal zone, it does not provide a softer end or tip
portion that is atraumatic to the blood vessel lining.
Another technique for forming a fuseless catheter of
differing characteristics is described in U.S. Patent No.
4,963,306. This patent discloses a novel method of making a
fuseless, thin-walled catheter without co-extruding multiple
layers of plastic, braiding or bonding a separate tip. In
general, the method includes subjecting the body or shaft
portion of an extruded polymeric tube of the catheter to solid
state polymerization, while shielding or excluding the distal
portion from the solid state polymerization process. The
effect of the solid state polymerization process is to make
the body or shaft portion of the catheter harder and more
rigid. The distal portion, however, in which solid state
polymerization has been retarded, remains softer and more
2U7U~~i
pliable. Accordingly, although solid state polymerization
effectively hardens a polymer and can be used to harden the
body portion of a catheter relative to the distal portion, it
cannot be used to soften an existing polymer or catheter tube,
for example, for making the very tip end of an existing
catheter tube even softer and atraumatic.
Still another prior art patent, Japanese Patent No.
1,545,771, describes an integral soft-tip catheter made by
dipping the leading edge of the catheter into a solution of
a plasticizes dissolved in a solvent that is a good solvent
for the plasticizes but a "poor" solvent for the polymer
comprising the catheter. A "poor" solvent for the polymer is
identified as a solvent that will not cause the polymer to
swell or otherwise adversely effect the polymer comprising the
catheter.
Unfortunately, the method described in Japanese Patent
No. 1,545,771 requires a substantial amount of time
(approximately four hours for thin-walled catheters) to even
minimally plasticize the intended flexible portion. Further,
this method limits the plasticizes that can enter the polymer
to relatively small mobile molecules of the plasticizes. When
the catheter is used, these smaller molecules can also more
easily migrate out of the catheter and increase the possible
risk of adverse tissue reactions. The higher molecular
weight, low mobility plasticizers such as
hexylbenzenesulfonamide are not readily imbibed or absorbed
in sufficiently substantial amounts into the polymer system
with these solvents.
Finally, the process described in Japanese Patent No.
1,545,771, utilizing a "poor" solvent, does not permit the
desired amount of plasticizes, typically approximately 25~ by
weight, to be absorbed into the polymer for a particularly
soft tip. Thus, while the amount of plasticizes absorbed may
be sufficient to somewhat reduce the bending moment or
increase the flexibility of the distal portion of a catheter,
it is insufficient to provide a distal tip end of a catheter
which is particularly soft so as to further minimize the risk
of trauma to the lining of blood vessels and/or tissue.
CA 02070252 2003-11-26
76664-70
7
SU1~1ARY OF THE INVENTION
In general, the present invention is directed to
an article of polymeric material which is softened, in whole
or in part, after formation and, more particularly, to an
article of polymeric material having portions of differing
softness, pliability or flexibility, and to a method for
making such an article by contacting the article or a
portion of the article with a swelling agent to swell the
material and with a plasticizer to allow for the migration
or absorption of the plasticizer into the polymer.
The article may be contacted by the swelling agent
and plasticizer in different ways, but is preferably
contacted by immersion, such as by dipping, of the article
or desired portion of the article into the swelling agent
and plasticizer. Although the article may be contacted with
the swelling agent and plasticizer sequentially, it is
further preferred to immerse the article or the desired
portion of the article in a solution which contains both the
swelling agent and the plasticizer.
In contrast to the prior art, this method provides
for substantial absorption of plasticizer into the polymer,
including higher molecular weight and lower mobility
plasticizers such as N-hexlbenzenesulfonamide, resulting in
a particularly soft and pliable article or portion, with
lower risk of subsequent migration of the plasticizer from
the polymer.
According to one aspect of the present invention,
there is provided a method for making a polymeric article
comprising: providing an article of a polymeric material;
contacting said article with a swelling agent; and
contacting said article with a plasticizer.
CA 02070252 2003-11-26
76664-70
7a
According to another aspect of the present
invention, there is provided a method for making a catheter
having at least a body portion and an end portion, said end
portion being more pliable or flexible than said body
portion, the method comprising: forming an elongated, tube
of polymeric material, said tube having a lumen and having a
body portion and an end portion; contacting said end portion
with a swelling agent; and contacting said end portion with
a plasticizer.
According to still another aspect of the present
invention, there is provided a catheter comprising an
elongated tube of polymeric material, said polymeric tube
having at least a generally elongated body portion and an
end portion, said end portion being softer or more pliable
than said body portion by reason of said end portion having
been contacted with a swelling agent and a plasticizer.
In accordance with further aspects of the present
invention, the swelling agent is an agent which will cause
the polymeric material to expand without significant
deleterious affect to the polymer. Preferably, the swelling
agent is a solvent for the polymer and the plasticizer, used
at a temperature sufficiently low and for a contact time
period sufficiently short so as to not dissolve the polymer.
Further, the solvent is preferably compatible with the
plasticizer to provide a combined solution that allows the
contacting step to be carried out simultaneously by dipping
into the solution. The use of a solution of plasticizer and
swelling agent acts synergistically to further increase the
i :oval
2U7~0252
absorption of plasticizes into the polymer as compared, for
example, to a sequential contacting process.
In accordance with further details of the present
invention, the solution of plasticizes and solvent comprises
about 10% - 98% solvent and 2% - 90% plasticizes and, more
preferably, about 50% - 90% solvent and about l0% - 50%
plasticizes. For a polyamide or polyurethane polymeric
material, the preferred plasticizes is N-
butylbenzenesulfonamide or N-hexylbenzenesulfonamide and,most
preferably, the former. For polyamide material the preferred
swelling agent is formic acid, and for polyurethane material,
the preferred swelling agent is methylene chloride.
After the step of contacting, the residual swelling agent
on or in the polymeric material is neutralized. This may be
achieved by air drying or by contacting the article with
neutralizing agent, such as by dipping the article into an
alkaline liquid, e.g., a solution of ammonia. After
neutralizing, thermoforming, grinding or other steps may also
be used to form the article into the desired size and shape.
As noted above, the present invention has particular
application in the manufacture of medical catheters having
portions of differing softness or flexibility and, in
particular, the manufacture of three zone medical catheters
of the type having a shaft or body portion of certain
hardness, a softer mid-distal portion and an even softer tip
end portion. The method includes providing an elongated tube
of a polymeric material having a lumen within, and contacting
an end portion with a swelling agent to swell the polymer and
a plasticizes to allow migration of the plasticizes into the
polymer.
As described above, the end portion of the catheter may
be contacted with the swelling agent and/or plasticizes in
various different ways without departing from the present
invention. However, the end portion of the catheter is
preferably contacted by dipping it into a solution of the
swelling agent and the plasticizes.
The swelling agent, plasticizes and solution are
generally as summarized above. For the manufacture of medical
catheters the end portion is preferably contacted with the
r..
2U702~2
- solution for about 1-30 minutes, with such time being variable
depending on, inter alia, the thickness of the catheter, the
quantity and strength of solvent and plasticizer in the
solution, and the temperature of the solution. Fcr making a
catheter having more than one portion of differing softness,
an end portion of the catheter, e.g., the distal end portion,
may be dipped into the solution for a selected period of time
and then partially withdrawn, leaving the remainder, e.g., the
tip end portion, in the solution for a longer period of time,
resulting in portions of differing softness.
1~ 207022_
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view, partially broken away, of
an assembled angiographic catheter according to the present
invention:
Fig. 2 is a longitudinal, cross-sectional view along line
2-2 of Fig. 1;
Fig. 3 are elevation views of different steps in carrying
out the method of the present invention as employed in the
manufacture of a catheter.
Fig. 4 are elevation views of different steps of carrying
out the method of making a catheter in accordance with another
aspect of the present invention:
Fig. 5 shows further steps which may be employed in the
method of the present invention, as utilized in the
manufacture of a catheter:
Fig. 6 shows another step, the forming of a pigtail shape
on the distal end of the catheter, which may be employed in
the method of the present invention.
v
11 . 207022
DETAILED DESCRIPTION OF THE DRAWINGS
The present invention is directed to an article of
polymeric material and to a method of making a polymeric
article, such as catheter, wherein the entire article or a
portion of the article can be made softer and more flexible
or pliable. Although the following detailed description of
the preferred embodiment concerns a fuseless three-zone
thin-walled angiographic catheter and the method of making
such a catheter, it should be understood that the present
invention is not limited to catheters in general or to
three-zone angiographic or fuseless catheters in particular.
The present invention may be used for other polymeric
articles, such as catheter balloons or other articles, as well
as for catheters in general, including, guiding catheters,
angioplasty catheters, urinary tract catheters,
gastroenterology catheters, and the like having or requiring
two or more portions of different softness or flexibility.
Turning to the attached drawings, the depicted catheter
and the method for making it will be described generally
before turning to more detailed aspects of the catheter and
method. Fig. 1 depicts a three-zone angiographic catheter 10
which includes an elongated, fuseless polymeric tube generally
at 12 having a body or shaft portion 14, a generally distal
end portion 15, a mid-distal end portion 16, and an atraumatic
tip end portion 18 at the most distal portion of the tube.
A hub member 20 is secured to the proximal end of tube 12.
As seen in Fig. 2, a longitudinal lumen 22 extends
through the elongated fuseless polymeric tube 12 from a
generally coaxial bore 24 in the hub member 20 through the
body portion 14, the mid-distal end portion 16, and tip end
portion 18, terminating at end orifice 26 within the tip end
portion.
Although depicted with a single lumen, the present
invention is also applicable to catheters having a plurality
. of lumen, e.g. , a fluid flow or instrument insertion lumen and
an inflation lumen for inflating a balloon associated with the
catheter. The tip end portion 18 may be mechanically tapered,
ground or manicured such that the wall thickness at distal tip
end portion 18 may be less than the wall thickness in
c: ,.:.-
12 2U7025~
mid-distal end portion 16 or body portion 14. Similarly, the
inner lumen diameter at distal orifice 26 may be thermoformed
to a diameter less than that of lumen diameter 22 at mid-
distal end portion 16 and body portion 14, as is frequently
encountered in catheter designs.
The elongated fuseless polymeric tube 12, including the
body portion 14, the mid-distal end portion 16 and the tip end
portion 18, may be constructed of a unitary sheath or cylinder
which has been extruded in a single pass out of a precision
extrusion device, although other techniques, such as injection
molding, may also be used to form the tube. As shown in Figs.
1 and 2, tip end portion 18 is preferably of one-piece,
integral, fuseless construction with the mid-distal end
portion 16 and catheter body 14. Alternatively, the
mid-distal end portion 16 and tip end portion 18 may be of
one-piece construction and fused to catheter body 14 by the
use of heat, solvent, sonic or radio, frequency energy or other
suitable bonding method.
Mid-distal end portion 16, and tip end portion 18, may
have any of a variety of configurations, depending upon the
medical procedure to be performed, without departing from the
present invention. For example, the distal end portion 15 may
exhibit a selected degree of curvature depending on the
particular procedure in which the catheter is to be used. An
example of this is shown in Fig. 6, which depicts a so-called
"pig tail" distal section often used in catheters which must
pass through a valve of a human heart. Further, as noted
above, the tip end portion 18 may exhibit a gradual tapering
of the outside diameter which decreases toward the distal
orifice 26 or the inside diameter at distal lumen 22 may be
smaller than the lumen diameter at mid distal end portion 16.
With reference to Fig. 3, a one piece, fuseless catheter,
according to the present invention is manufactured by first
extruding a tube of a polymeric material onto an air mandrel,
a wire mandrel or other means, typically by utilizing a
conventional wire coating extrusion apparatus in order to form
a continuous cylindrical extrusion. The extruded tube is cut
to the desired length for the elongated fuseless polymeric
tube or catheter, and the mandrel (if a solid one is used) is
CA 02070252 2002-12-11
76664-70
13
removed. The elongated tube 12 is then placed on a stiff
wire support mandrel or the like.
The portion of the tube that is t:o form the body
portion 14 of the elongated fuseless polymeric tube 12 or
catheter is preferably exposed to a high temperature zone as
part of a solid state polymerization procee~s described in
detail in U.S. Patent No. 4,963,306. The distal end portion
15, including mid-distal end portion 16 and tip end portion
18 of the tube may be exposed to the solid state
polymerization, but, as described in the above-identified
patent, preferably is protected or shielded from the solid
state polymerization process, thus rendering the mid-distal
portion 16 and tip portion 18 more flexible: than body
portion 14.
Although the process described in U.S. Patent No.
4,963,306 results in a catheter having a more pliable mid-
distal end portion 16 and tip end portion .18, in accordance
with the present invention, the tip end portion 18 of the
polymeric tube 12 is made even softer and more pliable than
the mid-distal portion 16 and considerably softer than
catheter body 14 as a result of the addition of plasticizer
to the tip portion 18 following extrusion (or other
formation) of the tube 12.
In general, the plasticizer is added to the
polymeric material of the catheter by contacting the tip end
portion 18 with a solution including a swe:Lling agent
selected for the particular polymeric mate~_ial used in
making the catheter and a plasticizer also compatible with
the polymeric material and, preferably, compatible with said
swelling agent. The swelling agent tempor<~rily causes the
polymeric material of the tube to swell or expand, allowing
CA 02070252 2002-12-11
76664-70
13a
the plasticizes to enter or migrate into the expanded
polymeric material. The catheter may be contacted with
swelling agent and plasticizes by spraying, wiping,
immersing, dipping or other means, although immersion by
dipping is the preferred method for contacting the swelling
agent and plasticizes. Although the swelling agent referred
to herein is preferably a liquid, it is also within the
scope of the present invention to use other types of
swelling agents which may now exist or later become known
which are not liquid. However, one further advantage of a
14
liquid solvent which is compatible with the plasticizes is
that when used in solution with the plasticizes, the swelling
agent also may act as a carrier to further help the
plasticizes enter the polymeric material. The introduction
of a plasticizes into the tip portion 18 of the tube 12 gives
the tip portion 18 a degree of pliability, softness or
flexibility greater than the body portions 14 and mid-distal
section 16 which may or may not contain a plasticizes.
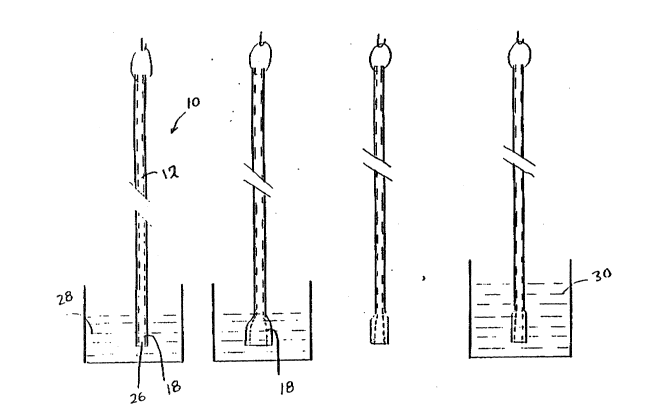
As shown in Figures 3a-3d, the tip end portion 18 of
catheter 10 (with distal orifice 26), is preferably immersed
in bath 28 as shown in Fig. 3a, contacting the tip end portion
18 of the tube 12 with a solution which includes a swelling
agent, preferably a solvent, and a plasticizes. In response
to the swelling agent, tip end portion 18 preferably swells
to a diameter 10% to 300% in excess of its original diameter
as is shown in Fig. 3b. As tip end portion 18 swells by
reason of the action of the swelling agent, plasticizes is
simultaneously carried into the polymeric catheter. The
catheter tip end portion 18 is immersed in the plasticizing
solution for a period of time sufficient to soften tip end
portion 18 to the desired degree. For example, for catheters
having a wall thickness less than .020 inches (.0508 cm),
adequate immersion times range from 1 to 30 minutes. The
catheter is then removed from the bath and the swelling agent
is evaporated-off by exposure to ambient air for a period of
days, evacuation at room temperature and/or exposure of the
catheter tip to hot air, as shown in Fig. 3c. Prior to being
evaporated-off, the tip may be neutralized in bath 30 as shown
in Fig. 3d, with an appropriate neutralizing agent. After
evaporation and/or neutralization of the swelling agent, the
catheter diameter shrinks. Depending on the amount of
plasticizes that has entered or been absorbed by the polymeric
material, however, the diameter of the catheter portion may
not completely return to its original dimensions.
Figures 4a-4c shows an alternative method of making the
catheter in accordance with the present invention. In Fig.
4a, tip end portion 18 of catheter 10 is immersed in a bath
32 comprising only the swelling agent. Tip end portion 18 is
immersed in the swelling agent for a selected period time
allowing the tip portion l8 to swell to the desired degree.
f ,:.
15 207022
Catheter 10 is then removed from the swelling agent bath and,
as shown in Fig. 4b, tip end portion 18 is then immersed in
a bath 34 comprising the plasticizer. The tip portion 18
remains immersed in the plasticizer bath for a period of time
so as to sufficiently soften tip end portion 18 to the desired
degree. After removal from the plasticizer, catheter 10 may
be dried in the manner described above.
Once the plasticized catheter has been allowed to dry,
the tip end portion may, if necessary, be conformed to its
original or required outer dimension by surface grinding the
swollen section on a centerless grinder or the like.
Similarly, the catheter can be conformed to its original
dimensions, or to any other dimension, or geometry, if so
desired, by thermoforming means. One preferred technique of
thermoforming is shown in Figs. 5a, b and c. As shown there,
w heat shrink tube, such as Teflon, or preferably a Freon TF
swollen silicone rubber tube 36, whose preswollen inner
diameter is that of the desired final outer diameter of the
catheter, is placed over the swollen tip end portion 18 as
shown in Fig. 5a using a mandrel 38 in the lumen of the
catheter 10. The catheter is then heated in the vicinity of
the swollen area by placing tip portion 18 in hot block 40 at
an appropriate temperature to evaporate-off the swelling agent
in the silicone rubber, and thus return it to its original
dimensions or to shrink the heat shrink band (Fig. 5b). The
shrinking portion of the tube or band compresses the tip end
to its desired dimension, which it retains by action of the
heat. Figure 5c shows the completed catheter after removal
of the silicone'rubber forming tube. It should be understood
that this thermoforming step can also be performed in
conjunction with removing the swelling agent from the
catheter.
Turning now to a more detailed description of the present
invention in the preferred embodiment, the elongated fuseless
polymeric tube is preferably made of extrudable or otherwise
formable polymers such as polyamides and polyurethanes. The
preferred polyamide materials include Nylon 6, Nylon 6/6,
Nylon 6/9, Nylon 6/10, Nylon 9, Nylon 11, Nylon 12 and other
available Nylons, such as Novolon (Nylon 11) material from-
Novoste Corporation, Aguadilla, Puerto Rico. Also included
16 270252
with the polyamide are blends or copolymers of polyamide with
polyether or polyesters, with radiopaque fillers or the like,
to provide thermoplastic elastomers of polyamides.
Where the polymeric tube is made of polyurethane, the
preferred polyurethanes include those synthesized from the
reaction products of macroglycols, macroglycols that are amine
terminated, diisocyanates and chain extenders. Examples of
suitable macroglycols include polyetherdiols, such as
polytetramethylene glycol, polypropylene glycols, etc.,
polyesterdiols, polycarbonatediols, aliphaticdiols,
etherlessdiols and the like. Suitable diisocyanates include
1,4-methylenebisphenyl diisocyanate (MDI), toluene
diisocyanate, 1,4-methylene biscyclohexane diisocyanate(HMDI)
or hydrogenated methylenebisphenyl diisocyanate, isophorone
diisocyanate, linear 1,6-hexamethylene diisocyanate, and the
like. Suitable chain extenders include aliphatic diols and
diamines such 1,4-butanediol, 1,6-hexanediol,ethylenediamine,
methylene dianiline, 1,4-cyclohexanediamine and the like and
combinations of the above. Although the above reactants form
linear, thermoforming polyurethanes or polyureas or
combinations thereof, the invention also can be applied to
thermosetting polyurethanes and polyamides made by
incorporating reactants with hydroxyl or isocyanate
functionality greater than 2.
As described above, the polymeric tube 12 is precision
extruded or otherwise formed of the desired dimensions,
length, inside and outside diameters and wall thickness for
the particular desired applications. By way of illustration
only, a typical angiographic catheter of the type described
herein will be made of a tube so as to form a catheter having
a French size of from about No. 2 to about No. 9. For such
a catheter, the wall of the tube should be as thin as possible
(and the inside diameter as large as possible) to permit high
fluid flow rates or passage of therapeutic devices while
maintaining the necessary strength and stiffness in the body
portion of the tube. Such a catheter may have a wall
thickness from about 0.007 to about 0.015 inches (0.017 cm to
0.0381 cm) , although a range from 0.002 to 0.020 inches (0.005
to 0.0508 cm) may also be employed.
~ ~ ~'~'~ ~ 2
Following formation of the polymeric tube 12, and the
solid state polymerization described in U.S. Patent No.
4,963,306, the portion of the tube which will comprise the tip
end portion 18 or a part of said tip end portion of the
catheter is contacted with a swelling agent/plasticizer
solution. As noted above, the solution preferably comprises
a swelling agent and a plasticizes. The swelling agent is
selected to cause the polymeric material of the tube 12 to
expand or swell, thereby allowing the plasticizes to migrate,
imbibe, absorb, diffuse or seep into the polymeric material.
Also, the swelling agent can act as a carrier, where the
combination of swelling agent and plasticizes swells the
polymer to a greater extent than just the swelling agent
alone.
The swelling agent selected should be a swelling agent
suitable for the particular polymeric material, either
polyamide or polyurethane, and for the plasticizes used. In
particular, the swelling agent should have a strength or
concentration sufficient to cause the polymeric material to
expand, but without significantly weakening or destroying the
polymeric material. The swelling agent is preferably a
material which, when exposed to the polymer at a selected
temperature (usually a temperature lower than that at which
- the polymeric material will dissolve) causes swelling of the
polymer rather than dissolution or etching of the polymer.
The swelling agent should also be capable of being removed
from the polymer after the plasticizes is in place with
subsequent shrinkage of the polymer and entrapment of
plasticizes. And finally, the swelling agent is preferably
capable of swelling the polymer at least about 10% to a
maximum of about 300% to enable fast and thus economical
uptake of plasticizes into the polymer.
Examples of swelling agents for the preferred polyamide
material include formic acid, acetic acid, cresols, phenols,
chromic acid and the like, applied at a temperature lower than
that at which dissolution or significant damage of the polymer
may occur. For example, formic acid has been found to work
well with the polyamide material described above to expand
the
material sufficiently without significantly weakening the
polyamide. Especially good results for polyamide have been
18
~01025~
obtained using a solvent concentration of 88% formic acid
(available from Fisher Scientific), although more dilute or
more concentrated formic acid may also be used depending upon
the thickness of the polymeric tube and the solution
temperature. A preferred temperature for swelling Nylon 11
and 12 is formic acid at about 45°C. At this temperature,
nylon 11 and 12 reversibly swell approximately 10% - 30%
larger than its original size. Nylon 11 and 12 tend to
dissolve in formic acid at temperatures greater than 100'C.
Examples of swelling agents for polyurethanes are
methylene chloride, 1,1,1-thrichloroethane, methylethyl-ketone
and the like. As With the polyamides, these solvents may
dissolve polyurethane at higher temperatures. However, when
the temperature is maintained below the temperature where
dissolution may occur, the polyurethane will swell but will
not dissolve. A preferred swelling agent for polyurethane is
methylene chloride at room temperature. Catheter samples
comprised of polyurethanes will swell from 10% to 300% when
exposed to this swelling agent for a sufficient amount of
time.
With respect to the plasticizes, the preferred
plasticizes is one that is capable of being combined with the
selected swelling agent (in solution) and must also be
compatible with the polymeric material of the tube. Also
desirable is that the plasticizes be of sufficient molecular
weight and chemical composition such as to resist leaching
out, or evaporating out of the polymeric material when used
in its intended physiological environment.
Such plasticizers for polyamides include aromatic and
aliphatic sulfonamides, such as N-butylbenzene sulfonamide,
N-hexylbenzenesulfonamide, N-decylbenzenesulfonamide,
N-cyclohexylbenzylsulfonamide, toluene derivatives of the
above such as p-toluenesulfonamide,
N-ethyl-p-toluenesulfonamide, N-butyl-p-toluenesolfonamide,
N-hexyl-p-toluenesulfonamide, N-decyl-p-toluenesulfonamide,
N-cyciohexyl-p-toluenesulfonamide; N-alkyl aliphatic-
sulfonamides; orthophosphates, such as monooctyl diphenyl
phosphate, cresyl phenyl phosphate and xylenyl diphenyl
phosphate; esters of aliphatic carboxylic acids, such as
19 zt~~n~
dioctyl azelate and dioctyl sebacate; lactams; lactones;
cyclic ketones; adducts of oxycarboxylic acids with alkylene
oxides; amylcyclohexanol carbonate and tetrahydrofurfuryl
carbonates: chlorinated aromatic hydrocarbons and ethers
thereof; adducts of polyamides with ethylene oxide:
condensates of urethanes and fvrmaldehydes; condensates of
isocyanates and polyoxy compounds; and amorphous polyamides.
Such plasticizers for polyurethane include any of its
reactants, for example, polyether glycols, polyester diols,
such as dibutyldiglycol adipate, caprolactam, castor oil,
polycarbonate oligomers, monooctyl diphenyl phosphate,
aliphatic diols, aliphatic amines; aromatic and aliphatic
sulfonamides such as N-butylbenzene sulfonamide,
N-hexylbenzenesulfonamide, N-decylbenzenesulfonamide,
N-cyclohexylbenzylsulfonamide, toluene derivatives of the
above such as p-toluene-sulfonamide,
N-ethyl-p-toluenesulfonamide, N-butyl-p-toluenesolfonamide,
N-hexyl-p-toluenesulfonamide, N-decyl-p-toluenesulfonamide,
N-cyclohexyl-p-toluenesulfonamide; N-alkyl
aliphaticsulfonamides and the like.
In general, where the plasticizes is combined with the
swelling agent in a solution, the solution may comprise
variable amounts of swelling agent and plasticizes, including
between 10% - 98% swelling agent and 2% - 90% of the
plasticizes, depending, in part, on the strength of the
swelling agent, the processing time permitted and the
thickness of the polymeric tube 12. Preferably, between 50%
- 90% of the swelling agent and 10% - 50% of the plasticizes
may be employed. It can be expected that less swelling agent
will be required when a more concentrated form is used.
Conversely, when a weaker (less concentrated) swelling agent
is used, the solution may comprise more of the swelling agent
component and a longer immersion or dwell period may be
required.
For manufacture of the preferred polyamide thin-wall
catheter, the preferred plasticizers for quick imbibition or
adsorption into the polyamide material are
N-butylbenzenesulfonamide and N-hexyl-benzenesulfonamide.
These plasticizers are soluble in formic acid, the preferred
swelling agent for Nylon 11 and 12. When the preferred
I - .
2~ 2070252
solvent and plasticizes is used, the solution preferably
comprises about 2/3 part of 88% formic acid to 1/3 part of
sulfonamide plasticizes. Swelling solutions comprised of
these mixtures of N-butylbenzenesulfonamide and formic acid
swell Nylon 11 and 12 to diameters 60% larger than formic acid
alone, as N-butylbenzenesulfonamide and
N-hexylbenzenesulfonamide behave synergistically to achieve
these high swelling percentages.
For thin-walled polyurethane catheters, these catheters
are softened by immersing the ca~heter segment into a solution
of 50/50 methylene chloride and N-butylbenzene sulfonamide (or
N-hexylbenzenesulfonamide) at room temperature for
approximately 10 minutes. The catheter section is swollen
approximately 50% larger with this softening solution.
To even further speed the entry of plasticizes into the
polymer, the solution is preferably maintained at a
temperature at or above normal room temperature but not so
high as to significantly cause the polymer to dissolve or the
swelling agent to significantly evaporate-off. For the
preferred polyamide polymer, solvent and plasticizes, a bath
temperature of about 45°C is preferred, although this may be
varied, depending on the tube thickness and solvent strength.
For making a thin-walled angiographic catheter, the tip of the
tube or catheter is immersed in the preferred solution for
approximately 3 to 12 minutes. For the preferred polyurethane
catheter, swelling agent and plasticizes, the preferred
temperature is room temperature and the preferred time is
approximately 10 minutes.
As noted above, time and temperature requirements may be
adjusted depending on the wall thickness of the polymeric tube
and the concentration of solvent. For example, polymeric
tubes having a wall thickness greater than the preferred range
of 0.004-0.015 inches (.0101 to .0381 cm) for angiographic
catheters may require a longer dwell time and/or an increased
temperature. Similarly, if the solution is at a lower
temperature, such as room temperature, a longer immersion time
would be required to effect the addition of plasticizes to the
polymeric material of the tip portion. The tip may be
contacted with solution in several different ways but is
preferably contacted by directly immersing or dipping the tip
21 2~~~L~~
portion into a solution bath. Other techniques which may also
work include spraying or wiping. Typically, only the most
distal portion of the tip, such as the last one-half ( 1/2 )
inch (approximately 1.27 cm), is contacted with the solution,
although the entire tip end portion or mid-distal end portion
could also be contacted without departing from the present
invention.
After removal of the tube or catheter tip from the
solvent/plasticizer solution, the catheter or tube, if
subjected to an acidic solution, such as formic acid and
N-butylbenzenesulfonamide, is preferably subjected to a
neutralization step to neutralize any residual formic acid
swelling agent. Air neutralization or evaporation may be
employed. Preferably, however, the catheter or tube is
neutralized by contacting the tip with an alkaline solution
having a buffered or non-buffered pH of 7 or greater. In the
preferred embodiment, the tip portion is immersed or dipped
into a solution of ammonia having a pH of approximately 10 and
allowed to dwell in the neutralizing solution for several
minutes.
As a final step, the polymeric tube may be dried in open
air for a period of time to allow any remaining solvents to
evaporate. It has been found that a drying period of
approximately two (2) days at room temperature is sufficient.
The drying time can be accelerated, however, by heating the
catheter. Temperatures of 60°C for thirty minutes are
sufficient to effectively flash off remaining swelling agent.
Following drying, tip end portion 18 may be ground to
remove any remaining swelling and/or to form a tapered or
manicured configuration. Similarly, as described above, the
swollen catheter tip may be thermoformed with shrink tubing
(with a mandrel in its lumen) to provide a desired wall
thickness and inner and outer diameter. A desired curvature
may also be imparted to the mid-distal and/or tip end portion,
depending upon the procedure for which the catheter is to be
employed. In accordance with well-known thermosetting
technique, the catheter tip is formed into the desired shape
(as by inserting the tip over a mandrel or wire form of a
desired shape) and by dipping the tip into hot water for a
i
22 2070252
short period of time to set the shape of the tip. Such a
technique is also disclosed in U.S. Patent No. 4,963,306.
Fig. 6 shows another embodiment of the catheter of the
present invention. This catheter includes an elongated
fuseless polymeric tube 12 which also has a body portion 14
and a tip portion 18 which includes a "pig tail" curvature.
In this embodiment, essentially the entire distal portion
is contacted with the solvent/plasticizer solution
10 described above, preferably by dipping for approximately two
(2) minutes. The mid-distal portion is withdrawn from the
solution and approximately two (2) centimeters of the most
distal tip end portion 18 are then contacted (allowed to
remain in the solvent/plasticizer for an additional five to
15 ten minutes. Usually this process is performed prior to
formation of the pig tail curvature. This.results in a distal
end portion itself having parts with different amounts of
plasticizes, the tip end portion 18 having the greatest amount
of plasticizes and being the most pliable or flexible.
Neutralizing and drying steps discussed above may also be
performed.
To obtain the so-called "pig tail" curl, the polymeric
tube is placed on an inner mandrel 42 having the pig tail
configuration shown in Fig. 6. The curled mandrel 42 with
attached tube 12 are immersed in hot water or other solution,
including hot air, which acts to relieve internal stresses
set the shape of the tip to conform to that of the mandrel,
in a manner well known in the art.
Without implying any limitation to the present invention,
the following examples are offered for illustrative purposes
only:
Example 1 (Example of making the plasticizes):
N-Hexylbenzenesulfonamide was made in the following
manner: Pyridine (7.91 g) was mixed with 1-hexylamine (10.12
g) and cooled in an ice bath. Benzenesulfonyl-chloride (10.12
g) was then slowly dripped into this cooled solution with
continuous stirring of the exotherming reaction. This
solution was dissolved in 100 ml methylene chloride, and
23 2U1U252
washed with equal amounts of distilled water ~n a separatory
flask. The upper eluent was discarded and the wash repeated
three times with 1 molar hydrochloric acid, once with 2%
sodium bicarbonate and again with saturated sodium chloride
solution. After proper separation, the lower product
containing solution was transferred to a beaker and heated to
evaporate-off the methylene chloride leaving the product,
N-hexylbenzenesulfonamide which was stored in a bottle until
used.
Example 2 (example of making the plasticizer):
N-Decylbenzenesulfonamide was prepared in the same manner
as Example 1, however, 15.73 g of 1-decylamine was used
instead of 1-hexylamine.
Example 3:
Nylon 11 catheter tubing is extruded in a conventional
manner with a French 7 outer diameter (0.222 cm) and a 0.007
inch ( . 017 cm) wall. A 100 cm length of this tubing is placed
over a wire mandrel and the distal 5 cm is immersed in flowing
cold water and the remaining portion of the catheter is
exposed to hot air (140°C) for 30 minutes. The catheter is
removed from the oven and water and the distal 0.4 cm of the
resultant two zone catheter is then dipped in a warm solution
(40-45°C) of 33% N-butylbenzenesulfonamide (Plastohall BSA,
from C.P. Hall, Co.) in 88% pure formic acid (Fisher
Scientific) for 8 minutes at which point the catheter tip
swells approximately 10%-30%. The resultant three-hardness
zone catheter is then removed from this solution and dipped
into a solution of 1% ammonium hydroxide for 5 minutes to
neutralize residual formic acid. The catheter is then dried
at 50°C for one hour. (It could also be dried at room
temperature for about two days.) The outer diameter of the
catheter is then ground to provide a slight taper toward the
tip.
24 2070252
Example 4:
A Shore 75D hardness polyurethane catheter tube is
extruded in a conventional manner with a French 7 outer
diameter (0.222 cm) and a 0.00? inch (.017 cm) wall. A 100
cm length of this tubing is placed over a wire mandrel and the
distal 0.4 cm tip is dipped in a room temperature solution of
50% N-hexylbenzenesulfonamide (from example 1) in methylene
chloride (Fisher Scientific) for 12 minutes at which point the
catheter tip swells approximately 50%. The resultant
two-hardness zone catheter is then removed from this solution
and a 2 cm section of preswollen, in Freon TF, silicone
rubber tubing (0.222 cm, ID. 0.300 cm wall), is placed over
the swollen polyurethane area and the distal 1 cm portion
heated in a shallow hole in a temperature controlled hot plate
(100°C) for 5 minutes. Heating caused the swollen catheter
tip to flow back to its original French 7 diameter with 0.007
inch (.017 cm) wall. The tip hardness was measured as Shore
70A.
Example 5:
The most distal 8 cm of the inner member component and
the most distal 2 cm of the outer member component and the
nylon balloon component of a Nylon 12 angioplasty multi-lumen
balloon catheter (wherein a guidewire passageway is defined
within the inner member and a balloon inflation passageway is
defined between the inner and outer members) were dipped into
a solution of 33% N-butylbenzenesulfonamide in formic acid for
10 minutes, removed, neutralized and dried, as in Example 3
above. The softened inner and outer member sections were
stretched and clamped over an inner mandrel and heated to
110°C for 10 minutes to thermoform the softened sections back
to its original diameters. The inner and outer members and
balloon were then assembled into an angioplasty multi-lumen
balloon catheter wherein the distal section was soft and
flexible and the nylon balloon section and balloon legs or
ends were soft and easy to pull down for insertion into a
small diameter guiding catheter.
25
Example 6:
The most distal 10 cm of a polyurethane catheter, similar
to the one described in Example 4, as exposed to a solution
of 50% castor oil in methylene chloride for one hour. The
most distal 0.4 cm was then immersed in a solution of 50%
N-decylbenzenesulfonamide, from Example 2, for an additional
minutes. The resultant catheter was dried in an oven at
50'C for one hour, rinsed in methylene chloride for 5 seconds
10 to remove surface plasticizer and the tip centerless ground
to its original diameter. The resultant three-zone catheter
had a Shore 75D proximal end, a Shore 55D mid-distal end and
a Shore 80A most distal atraumatic tip.
Example 7:
The distal 0.4 cm tip of a 10 cm long section of Shore
Hardness 90A polyurethane tubing was immersed in a bath
containing 50% N-hexylbenzenesulfonamide in methylethyl ketone
for 10 minutes. The catheter was removed and the methylethyl
ketone was evaporated-off in room air overnight. The 10 cm
length, with the softened 0.4 cm tip, which measured Shore 60A
in durometer, was then fused to a Shore 55D polyurethane tube
to provide a three zone catheter with an atraumatic tip.
The foregoing description and examples are for purposes
of illustration and not limitation, and the scope of the
present invention is defined by the appended claims. Although
the present invention has been described most specifically in
connection with making a catheter having a distal end portion
of different degrees of flexibility, the present invention
also has other applications such as application in the
manufacture of balloons for balloon angioplasty catheters.
Balloon angioplasty catheters are typically inserted through
the lumen of a guiding catheter located within the vascular
system of a patient and extending to the proximity of the
blockage site. It is normally desirable to use the smallest
possible diameter guiding catheter.
However, one difficulty sometimes experienced is that
upon deflation, the balloon does not deflate to a lay-flat
position along the catheter. As a result, the balloon will,
i
26 ~ 2U70~5?
on occasion, make it difficult to withdraw the angioplasty
catheter through the guiding catheter. When this occurs, both
guiding and angioplasty catheters must be withdrawn, even
though it is often medically preferable to allow the guiding
catheter to remain in place for subsequent procedures and to
reduce any risk associated with vascular collapse. Treatment
of the balloon with the method described above (i.e.,
contacting it with a swelling agent and a plasticizer) to
soften the balloon is believed to provide enhanced properties
which will result in a more complete collapse of the balloon
upon deflation, and allow withdrawal of the angioplasty
catheter through the smallest possible guiding catheter.
This is but one example of further application of the
present invention in addition to those described in more
detail above. For these reasons, the present invention is not
limited to the specific embodiments described in this
specification, but is defined according to the appended
claims.