Note: Descriptions are shown in the official language in which they were submitted.
; 2,~7~-~9~
WO91/08718 ~ ; ' ' PCT/US90/07233
-1-
HOLLOW VISCUS PROSTHESIS AND METHOD OF IMPLANTATION
BACKGROUND OF THE INVENTION
Field: This invention relates to products and
techniques used in the repair or reconstruction of the
walls of organs or other body parts which have been
breached by accidental trauma or resected by surgical
necessity. More particularly, this invention relates to
lo implantable prostheses capable of being incorporated into
the surrounding tissue for restoration of portions of
walls of organs or replacement of entire organ walls when
such organs or body parts are not susceptible~to
reconstruction by traditional techniques, such as grafting
15 or end-to-end anastomosis.
State of the Art: There are numerous diseases
or conditions for which replacement of a hollow viscus or
partial reconstruction of an organ wall is indicated. The
20 more common of these diseases or conditions are non-re-
sponding stricture of the esophagus; esophageal carcinoma,
the incidence of which is reported as 1% of all malignant
lesions and 4% of gastrointestinal malignancy; non-re-
! sponding neurogenic bladder with large volume urinary
25 retention, neurogenic bladder being present to some degreein probably 83% of diabetic patients; urinary tract ob-
struction which may involve ureter or urethra obstruction
as the result of trauma, congenital defects or neoplasia;
and neoplasia of the urinary bladder which comprises 2.5%
30 of all cancer deaths.
There are many other diseases or conditions for
which replacement or reconstruction of an organ or tissue
area would be invaluable, but the technology for doing so
has been lacking. Examples of these are various diseases,
35 such as cancer or idiopathic mucosal ulcerative colitis,
which frequently result in much pain, discomfort, and
inconvenience due to the new stomal opening and collecting
equipment which must be worn. There are also circum-
stances under which, for exam~le, the trachea must be re-
40 sected, and the removal of more than 8 centimeters of
,`
~, . .
.: : , , . : : . .................................. : .
: ., . : . : , . . ... :: .. .
' " . ' J
WO91/08718 ~ 7 0 2 9 ~ PCT/US90/07233
trachea precludes an end-to-end anastomotic repair. At
present, there is no consistent tracheal substitute.
The aforementioned diseases and conditions have
often been treated by surgical substitution of another
5 hollow viscus for the diseased one, or by substitution of
tissue from other organs, as in graft reconstructions.
For example, the esophagus may be replaced by a sub-
stituted portion of the colon, or more commonly by esopha-
gogastrectomy. The lower urinary tract may be treated by
10 partial to total cystectomy with ureteroileostomy. Add-
itionally, the use of a reservoir ileostomy and mucosal
proctectomy with ileoanal anastomosis has been utilized
following coelectomy, although more recent procedures
using ~echanical stomal occluding devices have been ad-
15 vanced.
All of these procedures require prolongedsurgical manipulation, have a fair number of post-opera-
tive complications, and require, in some cases, an ex-
ternal collecting device. In addition, there are some
20 obvious aesthetic effects which are unsatisfactory in some
of these procedures.
In addition to the use of donor tissues or organ
replacements to treat these conditions, polymers and
metals, alone or in combination, have been used for esoph-
25 ageal or lower urinary tract substitution. Many materialshave been suggested for use as prostheses for tracheal,
esophageal, and urinary tract replacements. For example,
E. F. Bergman, in "The Experimental Replacement of Por-
tions of the Esophagus by a Plastic Tube," 135 Annals of
30 Surqery, March 1952, pp. 337-43, suggested the use of
methacrylate or polyethylene tubing as a replacement
following esophageal surgery. See also, Battersby, et
al., "Esophageal Replacement with Plastic Tubes," A.M.A.
;~ Archives of Surqery, 1954, pp. 400-09 (disclosing lam-
35 inated layers of polyethylene and Nylon); Barnes, et al.,
"Replacement of Portion of Canine Esophagus with Composite
Prosthesis and Greater Omentum," 64 Jr. of
Thoracic and Cardiovascular Suraerv, December, 1972, pp.
t : " " .
., .. . ~ . -
.,~
;, .. .
: ' '~ . ' ' . -
-
.
., ' .
. ~
~ v~ y l~
WO91/08718 PCTIUS90/07233
~ .
--3--
892-96 (disclosing use of polyurethane); Fukushima, et
al., "Seven-year Follow-up Study After the Replacement of
the Esophagus with an Artificial Esophagus in the Dog," 93
Suraerv, January, 1983, pp. 70-77 (disclosing a silicone
5 rubber tube with outer Dacron mesh). E. Michelson, et al.
review and discuss the many devices for tracheal replace-
ment which have been tested in "Experiments in Tracheal
Reconstruction," 41 Jr. Thoracic and Cardiovascular Sur-
qerv, June, 1961, pp. 748-58. And Gore-Tex~ has been used
10 with ureteral replacement with limited succçss. S. Var-
ady, et al., "Ureteral Replacement With a New Synthetic
Material: Gore-Tex," 128 Jr. of UroloqY, July, 1982, pp.
; 171-75.
Several significant problems have been observed
15 when synthetic prosthetic devices are used for replacement
or reconstruction, including leakage of the anastomosis
between the device and the surrounding tissue, infection,
; dehiscence of the anastomotic suture line with subsequent
extrusion of the prosthesis into the lumen or outside the
20 body, occurrence of fistulous tracts, and migration of the
prosthesis~ These problems may arise as a result of the
type of prosthesis being used and the technique of im-
plantation. For example, leakage of the suture line is
generally the result of improper or inadequate suturing
25 and the particular method adopted for interfacing the
prosthetic tube with mùcosa. Infection may result from
leakage at the anastomotic site, or from failu~re of
aseptic technique during implantation of the prosthesis.
Migration and suture line dehiscence, however,
30 are the result of the unique properties of epithelium.
Epithelium will continue to grow along a substrate until
it contacts other epithelium, and epithelium is innately
unable to permanently heal to anything but mucosa. With a
non-biodegradable prosthesis, mucosa begins to migrate
~' 3S inside the prosthesis, but the mucosa soon outgrows its
blood supply since vessels cannot penetrate certain poly-
mers, e.g., SilasticR, resulting in a slough of mucosa
back to the suture line. Subsequently, dehiscence of the
, . . .
,",.
`,' ' ' ' ' " ' ' '' ~ ' ' '
WO91/08718 2 0 7 0 2 9 ~ PCT/US90/07233
.
suture line occurs. The mucosa then grows outside the
~ prosthesis, receiving its blood supply from the connective
! tissue surrounding the prosthesis. This results in ex- -
trusion of the prosthesis into the lumen, or the formation
5 of a fistulous tract.
- Some of the problems encountered with
implantation of synthetic substrate materials have been
; overcome by the application of collagen to the synthetic
substrate. Collagen, a fibrous protein which is the major
10 extracellular structural protein of connective tissue and
r, ' bone, forms a structural continuum in binding cells to-
gether to form tissue. The findings of Y. Shimizu, et al.
in "Studies on Copolymers of Collagen and a Synthetic
Polymer (First Report)," 5 Biomat.. Med. Dev. Art and
15 Orq., 1977, pp. 49-66, "Studies on Composites of Collagen
and a Synthetic Polvmer (Second Report)," 6 Biomat.. Med.
Dev.. Art. and ora., 1978, pp. 375-391, and "Study on
Composite of Collagen and Synthetic Polymer," Proceedinas
; of the Second Meetina of ISAO, April 1979, suggested that
20 a copolymer of collagen and a synthetic material is highly
effective for use as a prosthetic biomaterial because of
the formation and growth of natural connective tissue
encouraged by the collagen. A collagenous prosthetic
; implant with a non-absorbable fabric reinforcement was
.. .
25 disclosed in U.S. Patent No. 3,272,204 to Artandi, et al.,
and collagen-coated silicone rubber useable in surgical
treatments was disclosed in U.S. Patent No. 3,955,012 to
Okamura, et al.
; In prior synthetic substrate-collagen
30 copolymers, the collagen has been shown to encourage
epithelial growth across the substrate. The collagen is
, eventually absorbed by the body, leaving a layer of
`~ epithelial tissue positioned on top of the synthetic
substrate. Although epithelialization across the sub-
35 strate does occur in these devices, significant problems
have still been encountered. For example, the growing
epithelial layer lacks vascularization and growth event-
ually is restricted. It has also been found that
.j; .
. .
', . '' ' ~ ~ ~ - ,
.. : .
'' :~ ' ' ' ' ' :
.. . : .
: -
W O 91/08718 ,,2j,~,~ PC~r/US90/07233
~' :
--5--
epithelialization may not occur completely across the sub-
strate. Further, growth cannot be controlled and excess
connective tissue growth may result in stenosis of the
tract. Ineffective methods of collagen application have
5 also led to implants which leak.
The problems associated with implantation of
synthetic prosthetic devices, as enumerated above, may be
overcome by providing a prosthesis which will encourage
primary interfacing between the prosthesis and the body's
10 connective tissue which will promote epithelial growth
within the lumen of the prosthesis or organ while promot-
ing vascularization and anchoring of connective tissue
through the pores of the synthetic material.
SUMMARY OF T~E INVENTION
The instant invention presents a unique material
for implantation into the tissue or viscera of a body
20 which addresses the difficulties encountered with prior
synthetic prosthetic forms. That is, through controlled
application of collagen to a synthetic substrate, the
invention encourages regulated growth of connective tissue
for ultimate incorporation of the device into the sur-
; 25 rounding body tissue.
The invention includes a porous syntheticpolymeric substrate to which collagen has been applied so
that the pores of the substrate become occluded with the
~ collagen, thereby rendering the substrate impermeable to
; 30 liquid and bacterial invasion. This impermeability is
particularly important when the invention is used in
urinary tract, respiratory tract, or gastrointestinal
tract for reconstruction or replacement.
The synthetic substrate may generally be formed
35 of any tissue-compatible and relatively inert synthetic
~,`! polymer, such as polyethylene, polyurethane, poly-
propylene, silicone rubber, or teflon. The pores formed
therethrough may have uniform or irregular shape and size.
Irregularity of porpsity, however, enhances the strength
40 of epithelial bonding to the substrate and also controls
~;
r
WO91/08718 2 0 7 0 2 9 ~ PCT/US90/07233
~'
-6-
the amount of granulation tissue (initial connective
tissue) forming therethrough. A porous synthesized metal-
lic material, such as tantalum, may also be used in the
invention. "Synthetic", as used herein, refers to any
5 material, whether metallic, polymeric or of natural ori-
gin, which is not otherwise naturally occurring in the
;; desired form and which must be specially fabricated for
the purposes of this invention.
Controlled growth of connective tissue into the
10 pores of the substrate is important in assuring optimal
incorporation of the invention into the body. That is,
the rate at which the collagen is absorbed by the body,
and the resulting formation of the connective tissue
therefor, affects the rate of epithelialization across the
15 substrate and affects the ultimate rate of incorporation
of the device. For example, it has been observed in pre-
vious collagen coated implants that if insufficient con-
nective tissue ingrowth occurs through the substrate to
,~ the lumen, epithelial growth from the suture line toward
20 the center of the implant stops. This is due to
outgrowth of the blood supply in the connective tissue
resulting in sloughing of the epithelium back to the
suture line. If the epithelium does not grow to cover the
; ingrowing connective tissues, the connective tissue
i 25 continues to grow and produces stenosis of the lumen of
the viscus. Controlled application of collagen to the
substrate ameliorates insufficient ingrowth or excessive
ingrowth of connective tissue into the substrate, and
regulates optimal growth of connective tissue.
Enhancement and control of connective tissue
; ingrowth into the substrate, and subsequent epithelializa-
tion, may be furthered by the addition of fibroblasts to
the collagen as a coating on the substrate. Fibroblasts
?' are fibrillar cells found in abundance in connective
`~~ 35 tissue, and are the source of collagen in connective
tissue. When added to the collagen, fibroblasts interact
with the collagen to the substrate, and produce their own
collagen, thus promoting the ingrowth of connective tissue
, ~ .
.. . ...... .
` " ~ '
;
:
:-, .
.~ .
.
WO91/08718 2 ~ PCT/US90/07233
~ .
--7--
on the substrate to effect complete incorporation of the
implant into the surrounding tissue.
The collagen coating may be cross-linked by any
of several means, including irradiation, ultraviolet
5 light, glutaraldehyde, or polyglycerol polyglycidylether
(PPE). Cross-linking causes increased density and
strength of the collagen and causes the collages to expand
slightly within the substrate pores to increase the
anchoring of collagen to the substrate. Although cross-
l0 linking increases the density and strength of the collagencoating to the substrate pores, cross-linking may not be
required or even desirable in some applications.
In addition to the porous synthetic polymer used
as a substrate, other synthetic materials may be
15 associated with the substrate which lend elasticity to the
implant or prosthesis as needed. That is, in some
applications a certain amount of elasticity is desirable.
An example would be prosthetic implants for the urinary
bladder where expansion and contraction of the implant is
20 required during filling and evacuation. Typical elastic
materials which might be used include silicone rubber
~ (SilasticR) or pOlyurethanes
; Optimum efficacy of the implant may be achieved
through association of mucosal epithelium from the sur-
25 rounding viscus or tissue with the intraluminal surface of
the implant. The epithelial lining of many organs is im-
portant to the function of that organ. For example, the
, mucosal lining of the trachea contains cilia which move
j debris out of the lungs. A graft of mucosal epithelium
30 from the trachea or nasal sinus may be attached to the
surface of the implant or prosthesis which will be
directed intraluminally. With vascularization through the
synthetic substrate and epithelialization, the
intraluminal graft may eventually assume its function.
! 35 The incorporable material of the present
invention may be adaptable to a variety of medical or
; surgical applications. For example, the incorporable
material may be produced in the form of a patch for use in
,. . : . . . ~,, . , :
,,: , . .:: : ~ .
WO91/08718 2 ff7 0 2 3 ~ PCT/US90/07233
repairing or reconstructing holes in a viscùs produced by
accidental trauma or necessary surgical resection, or for
expanding a stenotic lumen. As an example of need for
this application, nine percent of infants placed on
5 ventilators develop subglottic stenosis. This often pro-
gresses to the point where the glottis is totally
occluded. At present, there is no prosthesis available to
expand the stenosed glottis and a costal cartilage must be
utilized. This procedure usually requires a prolonged
10 period of tracheostomy and a seton in the glottis. The
use of a patch that will epithelize rapidly and is totally
incorporated may decrease the morbidity in the more severe
cases of subglottic stenosis and repair in these patients.
Alternatively, the incorporable material may be
15 formed into a tube for implantation into a trachea, eso-
phagus, or intestine where end-to-end anastomosis is made
impossible. Most esophageal resections are performed for
cancer of the esophagus or cancer at the cardioesophageal
junction. Esophageal resections are also required for
20 some benign lesions and chemical burns. The use of
radiotherapy or chemotherapy, either individually or in
combination, has not eliminated the continued need for
surgical resection of the esophagus for the management of
carcinoma of the esophagus. The non-surgical palliative
25 use of the intraluminal esophageal tube to maintain a
lumen for the passage of saliva and food has proved to be
generally unsatisfactory and inferior to surgical
resection as a palliation of esophageal cancer. The
intraluminal tube is sometimes used as a last resort, or
30 not used at all.
Both palliative esophageal resection and wide
~`j extended resection of carcinoma of the esophagus, with and
without the use of chemotherapy and irradiation, provide
' most patients with a very limited survival time of less
35 than two years from time of diagnosis. Despite the
prognosis of limited survival time, many patients are
still subjected to a major surgical procedure which may
include surgical invasion of both the thorax and abdomen
. .~
:: . .. . . . . .. .
:
,,
.
,
WO91/08718 2UP~ J~ ~ PCT/US90/07233
/~..`:?.
(~,5,;
_ 9 _
for production of a graft. At present, the maximum amount
of trachea that can be successfully resected is six to
eight centimeters due to tension. A Nevile prosthesis (a
medical grade silicone copolymer tube with polyester-
5 covered Dacron sewing rings at either end) is sometimesused, but this does not permit intraluminal connective
tissue ingrowth or epithelial ingrowth, and it often
extrudes or forms fistulas. These major surgical
procedures, in mostly elderly or debilitated patients, may
lO have a high rate of morbidity. The use of an artificial
segment of esophagus would allow the surgical procedure to
be limited to the thorax and possibly the neck, without
the necessity of an abdominal procedure. The elimination
of the abdominal procedure would allow most patients
15 immediate restoration of nutrition postoperatively through
the gastrointestinal tract. It would also eliminate the
problems of post-laparotomy ileus and provide a shorter
postoperative hospitalization for these patients who have
prognostically a very limited survival time.
! 20 In an alternative embodiment, the invention may
be used as a prosthetic replacement for an entire organ or
organ system. For example, urinary bladder replacement is
indicated following cystectomy for bladder cancer.
Reported cases of bladder cancer range from 37,000 to
25 40,000 cases per year, about 25~ of which eventually
require cystectomy. Additionally, neurogenic disorders
and pelvic exenteration (selective evisceration) for
cervical or rectal cancer or contracted urinary bladder
often require urinary tract diversion or cystectomy.
30 Current methods for post-cystectomy urinary diversion
utilize intestinal conduits, usually from ileum or colon.
These procedures have a high incidence of complications as
well as requiring the use of external appliances, making
the patients urologically handicapped.
Many different methods of repair have been
proposed and tested over the last few decades, but most
methods have failed or have proven insufficient due to
infection, inflammation, rejection, extrusion,
, .
. . : :.
.. . . , . ... : .
WO91/08718 2 0 ~ D ~ 9 ~ PCT/US90/07233
--10--
obstruction, leakage, or fistula formation. The only
methods which have met with even a modicum of success are
those in which a portion of the bladder was left intact,
thereby providing a source for generation of transitional-
5 cell epithelium.
The present invention, by being capable of
incorporation into the surrounding tissues, overcomes the
consequences associated with previous prosthetic devices,
and provides a functional device in such applications.
BRIEF DESCRIPTION OF THE D~AWINGS
FIG. l is a perspective view in partial cut-away
illustrating a porous synthetic substrate;2
FIG. 2 is a cross-section view of a substrate
illustrating the open-celled arrangement of pores;
FIG. 3 is a cross-section view of a substrate in
contact with collagen;
FIG. 4 is a cross-section view of an implanted
prosthetic device illustrating ingrowth of connective
tissue and a layer of epithelium on the intraluminal and
extraluminal surface;
FIG. 5 is a cross-section view of a prosthetic
25 device illustrating host graft epithelium on a
intraluminal surface;
FIG. 6 is a perspective view of an embodiment of
i the invention comprising a prosthetic patch;
FIG. 7 is a perspective view of an embodiment o~
30 the invention comprising a hollow tube; and
FIG. 8 is a perspective view in partial cut-away
of an embodiment comprising a prosthetic bladder.
DETAILED DESCRIPTION OF THE ILLUSTRATED EMBODIMENTS
The invention generally includes-a porous sub-
strate material which is contacted with either collagen or
a mixture of collagen and fibroblasts. A layer of
40 autogenous graft or tissue-cultured mucosal epithelium may
be applied to one surface of the material, the intra-
. .
. ' ' ' :
:' . ' . : ..
,
.
..
WO91/08718 2 ~j 7 ~ PCT/US90/07233
--11--
luminal surface, to promote growth of host mucosal epithe-
lium. The prosthetic material of the invention may take
any form, including an implantable patch, prosthetic tube, -
or prosthetic organ. The basic material and prosthetic
5 forms are discussed more fully below.
The 8ubstrate
The substrate of the material may be any porous
synthetic material which is tissue-compatible, including
metallic materials and organic polymers. Organic polymers
i which are inert in the body are preferable, such as poly-
; ethylene or polyurethane. In a preferred embodiment, the
15 substrate is a polyethylene material which has formed
therethrough a substantial number of irregularly shaped,
open-celled pores. An example of such material is Medpor
manufactured by Porex ~edical Co. (Fairburn, Ga.). As
illustrated in FIG. 1, the pores 22 in synthetic material
20 20 of this type are pores which are irregular in shape and
size and do not necessarily define a straight path. The
diameter of the pores or the pore opening width, as gener-
ally measured by the diameter of a sufficiently small
~' object being able to pass therethrough, can vary from a
;l 25 fraction of a micron to a few hundred microns. For the
purposes of this invention, a pore size of between about
100 microns and 360 microns is preferred.
The thickness of the substrate affects the rate
and amount of connective tissue ingrowth. That is, the
30 thicker the substrate, the greater the depth of the pores
through which the çonnective tissue must grow. Therefore,
the thickness of the substrate may vary from between about
; 0.25 millimeters (mm) to about 1.50 centimeters (cm).
When a thin sheet of this porous synthetic
35 polymer is viewed in cross-section, as illustrated in FIG.
2, it can be seen that the pores 24 follow a tortuous and
irregular path. A substantial number of the pores are
open-celled 25, meaning that there is an opening to the
pore at both surfaces of the substrate. ~here may,
40 however, be a number of pores which are close-celled 26;
'~
.. ~ : - . : - . ,
., . : . . . . . .
W O 91/08718 2 0 7 0 2 9 i PC~r/US90/07233
-12-
that is, having an opening only to one surface only. The
irregular porosity in the substrate provides a stronger
adherence of collagen or collagen and fibroblasts to the
substrate. Additionally, the ingrowth of connective
5 tissue through the irregularly shaped pores provides
greater strength and adherence of the tissue to the
substrate leading to assured incorporation of the
prosthesis into the living tissue.
The Colla~en
The substrate is contacted with collagen in any
manner which allows adherence of the collagen to the sub-
15 strate and which causes the collagen to fill the pores
sufficiently to produce a liquid-impermeable seal. As
illustrated in FIG. 3, the collagen 28 substantially fills
the pores of the substrate 30 and defines a thin layer of
collagen 32 on one surface of the substrate.
The prosthetic material of the present invention
allows controlled ingrowth of epithelium by controlling
the ingrowth of vascularized connective tissue. This is
done by varying the thickness and/or density (i.e the
concentration of collagen in solution) of the collagen
^~ 25 applied to the substrate, and by varying the thickness of
the substrate. Thus, where the collagen or collagen-
fibroblast coating on the substrate is thinner, more rapid
connective tissue ingrowth occurs. Conversely, when the
collagen coating is thicker and/or more dense, less
connective tissue ingrowth occurs. The thickness of the
collagen may be from about 0. 025 mm to about 1.00 mm.
The more rapid the connective tissue ingrowth,
the more rapid the epithelial growth. Therefore, more
;~ connective tissue ingrowth and epithelial growth at the
point of suturing may be encouraged by a thinner and less
dense layer of collagen or a thinner substrate at that
point. Conversely, stenosis of the lumen produced by
excess connective tissue growth in the inner portion may
be avoided by a thicker and more dense layer of collagen
40 or a thicker substrate. This will allow the
. . .
:' '
. . , , ., ~ ..
'~
.:
23~29 j~ `
W O 91/08718 ~ PC~r/US90/07233
~ ' ~.
-13-
epithelization to c~ntinue. The rate of absorption of
collagen, and subsequent ingrowth of connective tissue,
may also be controlled ~y varying the depth to which
collagen or collagen and fibroblasts are applied in the
5 pores of the substrate. Collagen absorption may also be
controlled by cross-linking of collagen, as described
below.
FIG. 4 illustrates how collagen 36 is replaced
by connective tissue 38 which grows into pores 40 of the
10 substrate 42. The vascularization 44 of the connective
tissue retains the viability of the tissue so that
epithelium 46 may grow.
Any type of non-antigenic collagen may be used
to coat the substrate. Preferably, atelocollagen (Koken
15 Tokyo, Japan) is used. Atelocollagen is an enzyme-
solubilized collagen lacking in the strongest antigenic
determinants and, as a result, has little antigenic
,i activity to the body. Atelocollagen is biodegradable,
shows significantly little inflammatory response, and is
20 particularly suitable for sealing the pores of the
substrate due to its viscosity.
Atelocollagen purchased from Xoken Co., Ltd., (5
-18, Shimoochiai 3-chome, Shinjuku-Ku, Tokyo, 161 Japan)
is kept in its dry form in a refrigerator at approximately
25 40 F. The dry atelocollagen is solubilized in 0.01M
CH3COOH (glacial acetic acid) in a suitable size (usually
20 ml) glass vial with a screw cap.
As previously noted the rate of collagen absorp-
;~ tion may be regulated by varying the density of collagen
30 which is applied to the substrate. Density, as used in
j this disclosure, refers to the concentration of collagen
molecules in solution. The concentration of collagen may
vary from one to five percent.
By way of example, a 5% solution of collagen is
35 prepared by adding one gram of solubilized collagen tonineteen milliliters (ml) of 0.10M CH3COOH. The solution
is maintained for about 24 hours to completely dissolve
the collagen. The solution is stirred two to three times
~,
: .
i................................ .
: , . .
, ~ . .
. ~ , , .
.. : .. , .. ~ , :
. . ~ ' ' ' ' '
;i , , . ~
.
~ i , ,
~'' ' ; - ' ' '`:- .' '
W O 91/08718` ~ 0 7 0 2 g ~ PC~r/US90/07233
-14-
during this period. The collagen is then stored in the
refrigerator. Once the collagen is dissolved, the solu-
tion may be used for up to four months after it is
prepared if no signs of contamination are present. The
5 concentration of collagen in the solution is determined
according to the desired use of the device. Thus, if fast
dissolution of the collagen is necessary, a lower
percentage of collagen is used. If slower connective
tissue ingrowth is required, a higher collagen percentage
10 is used.
One method of contacting atelocollagen with the
substrate may be obtained by the method set forth in the
following example:
Exam~le A:
A piece of Medpor (Porex, Inc., Fairburn, Ga.)
having a pore size of approximately 100 to 300 micrometers
20 was cut with scissors to an approximate patch size of two
centimeters (cm) by four cm. The patch was weighed,
measuring 14 gm. An equal amount of prepared 5%
solubilized collagen, 14 gm., was weighed out. The
collagen was spread over the Medpor with a spa~ula to coat
25 the upper surface of the Medpor. The coated Medpor was
immediately immersed in 100 ml of 5% NaCl solution for one
hour. The collagen became cloudy and began to produce
random, non-parallel fibrils of collagen. Alternatively,
the coated piece of Medpor may be immersed in 100 ml of
30 phosphate buffered saline (PBS) immediately after coating
the piece of Medpor. This procedure produces native or
parallel placed fibrils of collagen.
Adherence of Collaaen to the 8ubstrate
The contacting of collagen to a porous substrate
` of irregular porosity increases the anchoring of the col-
lagen to the substrate due to the collagen seeping into
40 the labyrinthine cavities of the pores. As a result of
this mechanical anchoring, additional bonding between the
,~
.. . , , '~ '
- : :
.
-
~ WO91/08718 2 0 7 0 2 9 ~ PCT/US90/07233
... :
-15-
collagen and substrate is not necessary. Further, when
atelocollagen is mixed with fibroblasts in culture medium,
as set forth in Example C, below, the fibroblast culture
medium causes the atelocollagen, a normally gelatinous
5 substance, to become more liquid in consistency. The
? contacting of atelocollagen and fibroblasts to the
substrate, in that liquified state, increases the ability
of the atelocollagen to seep into the irregular pores of
the substrate so that further bonding is not required.
Cro~-Lin~ina of Collaqen
Two and one-half percent to five percent
15 atelocollagen is normally very soluble and will not
maintain itself in a fluid environment. In order to
decrease the solubility of atelocollagen and have it
persist longer before being absorbed by the body, it may
be cross-linked. Cross-linking increases the density of
20 the collagen by inducing linking between the collagen
polymer chains. The greater the degree of cross-linking
the more dense and less biodegradable is the collagen.
; Thus, it can be appreciated that the rate of absorption of
collagen is dependent upon the concentration of collagen
in the coating, the amount of cross-linking between these
collagen chains, and the degree of porosity or void volume
of the pores in the substrate.
Cross-linking is a technique well known in the
art and can be accomplished by many methods, including
30 irradiation, ultraviolet light, or chemicals such as
glutaraldehyde.
Methods for inducing cross-linking by use of
glutaraldehyde are discussed in D. T. Cheung and M. E.
Nimni, "Mechanism of Crosslinking of Proteins and
35 Glutaraldehyde I: Reaction with Model Compounds," 10
Connect. Tiss. Res. 187-99 (1982) and D. T. Cheung and M.
E. Nimni, "Mechanism of Crosslinking of Proteins by
Glutaraldehyde II: Reaction with Monomeric and Polymeric
Collagen," 10 Connect. Tiss. Res., 201-16 (1982), the
40 contents of which are incorporated herein by reference.
.. . . . .
"1~ ' ' ' ' , ' '
.
,
~ ; .', . . ~ ' : " -
; ~ ,
. . . .. .
2~ 7~29~
W O 91/08718 '.'~ PC~r/US90/07233
-16-
Methods for inducing crosslinking by use of irradiation
are discussed in R. J. Davidson and D. R. Cooper, "The
Effect of Ultraviolet Irradiation on Acid-Soluble
Collagen," 105 Biochem. J., 96S-69 (1967), D. R. cooper
5 and R. J. Davidson, "The Effect of Ultraviolet Irradiation
on Soluble Collagen," 97 Biochem J., 139-47 (1965), and T.
Miyata, T. Sonde, A. L. Rubin, and K. H. Stenzel, "Effects
of Ultraviolet Irradiation on Native and Telopeptide-poor
Collagen," 229 Biochim Biophys. Acta, 672-680 (1971), the
10 contents of each of which is incorporated herein by
reference.
An example of induced cross-linking follows:
, .j
15 ExamPle B:
A substrate measuring 2 cm x 4 cm was coated
with collagen (as described in Example A, above), and
precipitated by immersing the coated substrate patch in 5%
20 NaCl solution. One hundred milliliters of glutaraldehyde
crosslinking solution were prepared by adding together 50
;~ ml of MeOH and 50 ml of H2O. The solution was maintained
in a 150 ml Erlenmeyer flask on a magnetic stir plate.
The pH of the solution was adjusted to 11.5 by adding 0.5
25 ml of 1 N NaOH. Forty milliliters of 25% glutaraldehyde
were added to the solution, and the pH was adjusted to 12
by the addition of 0.1 ml of lN NaOH. The solution was
stirred slowly for one hour on the plate.
Immediately following precipitation in the
30 collagen with NaCl, the patch was immediately placed into
glutaraldehyde solution in a capped vial. The patch was
completely submerged in the solution.
The vial was placed in a shaking water bath at
25-26C. for two hours to produce a high degree of cross-
35 linking. (Lesser time is required if faster dissolutionof the collagen is required.)
After two hours had lapsed, the patch was
removed from the glutaraldehyde solution and placed in 100
'~ ml. of phosphate buffered solution containing sodium azide
., .
:,"
-
,, .
.,. '
'`. ' ' , ~ : : ' . ,
~WO91/08718 2 07D2 9 ~ ` PcT/us9O/07233
(NaN3). (Phosphate buffered solution is prepared by
adding 0.272 gm/l potassium phosphate (KH2P04), 1.136 gm11
sodium phosphate (Na2HPO4), and 8.474 gm/l sodium chloride
(NaCl) to 250 ml distilled water, then adding a quantity
5 of distilled water sufficient to make 1000 ml of solution.
To that is added 1 gm/l of sodium azide (NaN3).)
i The patch in PBS and sodium azide was kept in a
shaking water bath for 5 to 7 days at 25-26C. The solu-
; tion was changed twice a day for the first 3 days, and at
10 least once a day thereafter. This procedure o~f changing
solutions was performed to remove any unreacted
glutaraldehyde which might have been left in the collagen.
At the end of this period of time, the patch was
kept in saline solution with sodium azide and kept in the
15 refrigerator until 24 hours prior to surgical implant.
Approximately twenty-four hours before implant,
the patch was removed from the sodium azide-saline bath.
It was then placed in a sterile container with sterile
saline. The saline was changed, using sterile techniques,
; 20 five to six times in the first 12 hours. The final change
was into sterile saline containing 1 gm/1000 ml cèfadyl
solution. The patch was stored in the refrigerator during
this time.
At this point, devices prepared by the foregoing
25 procedure are considered ready for implantation, and are
to be handled using sterile techniques.
:
The Fibroblasts
The substrate may be contacted with either
, atelocollagen alone, as described above, or an admixture
of atelocollagen and fibroblasts may be applied to the
substrate. Fibroblasts are present in all types of
,~ 35 fibrillar tissues and membranes, such as areolar, dense
fibrous, and elastic tissue, and are the source of
~ collagen fibrils. Fibroblasts which have been tissue-
S cultured are added to atelocollagen and are then contacted
~ with the porous substrate. The fibroblasts produce their
,,
,, ~,' ' ''. ' ' ' ' .,' .. ' ' ' . ' ' " ' ' .~ ' ' ' ' ;'`'' '
4'~'' , ........... ' i ~ ' '
~`, "., . '` - . ' ' ' ' ' ' , ' ' , '-
i''-" '. ' ; ' ~, ' ' ''
' ', ' , ' " , , ~
WO9l/087l8 ~ 207029~ PCT/US90/07233
-18-
own collagen and cause the atelocollagen to shrink and
become more dense. Because this shrinking and increased
density causes the collagen-fibroblast coating to adhere
more strongly to the pores of the substrate, cross-linking
5 is not required. Additionally, cross-linking may damage
or kill the living fibroblasts. The living cellular
content formed by the fibroblasts within the substrate en-
hances the ingrowth of connective tissue. This living
membrane within the substrate should also transfer
10 nutrients across the substrate more rapidly than cross-
linked atelocollagen. The transfer of nutrients is
important to the growth of connective tissue and therefore
ensures better survival of free mucosal or tissue-cultured
mucosal grafts.
One method of contacting a porous substrate with
a collagen and fibroblast mixture may be illustrated by
the following example.
,
20 ExamDle C:
Fibroblast Culturina
Canine buccal mucosa is harvested, washed with 1
25 liter (l) phosphate buffered solution (PBS), and cut with
a knife into small explants of approximately 2 cm by 2 cm.
These explants are placed on the upper seam of a 25 cubic
cm flask and are fed 9 ml of Dulbecco's modified Eagle
medium (DMEM) with 10% Fetal Bovine serum (FBS).
Dulbecco's modified Eagle medium is a tissue
culture medium purchased from Gibco Labs, Grand Island,
N.Y. The medium is prepared by mixing together 1000 ml of
distilled, deionized water, 10 grams (g) DMEM powder
(#430-1600, Gibco Catalog), 3.7 g sodium bicarbonate, 0.3
35 g l-glutamine, 4.76 g N-2 hydroxyethylpiperazine-N'-2-
~ ethanesulfonic acid (HEPES), 2.5 mg/10.5 ml Amphotericin-
: B, and 10 ml Penicillin-Streptomycin. The pH of the
mixture is raised to 7.3 with lM NaOH. The mixture is
filtered using sterile techniques. Then 100 ml of fetal
; 40 bovine serum (FBS), purchased from Hyclone Labs, Logan,
., .
.
- . . . ~ . - . . - -
~WO91/08718 2 0 7 a ~ 9 ~ PCT/USgo/07233
~ ,' ,?
-19-
Utah, is added. Fetal bovine serum is blood serum which
is extracted from fetal calf blood, and it provides a
nutrient medium for cell growth.
These flasks are incubated at 37O centigrade (
5 at a slight angle so that the medium comes only to the
seam of the flask. Fibroblasts begin to grow out of the
explants and to migrate down into the flask. After three
; weeks, when the fibroblast cells are growing well, the
cells are transferred to culture dishes and allowed to
l0 proliferate in DME culture medium for seven days.
Fibroblast Coatinq
J1 lSPatches of MedporR porous synthetic substrate
measuring 2 cm by 3 cm are coated with l ml of l.25%
atelocollagen applied by pipette. The coated patches are
allowed to stand for 16 to 24 hours at 37C to seal the
pores of the substrate. Thereafter, l ml of l.25%
20 atelocollagen containing approximately 4 x lo6 fibroblasts
, i8 pipetted on each patch. The coated patches are then
placed in 5 ml DME with 10% FBS. As the fibroblasts
proliferate, they begin collagen production of their own.
After two weeks, the patches are tested for leakage. This
25 is done by placing the patch on a ring abQve the medium
dish. One-half milliliter of DMEM is pipetted onto the
patch, and the patch is observed for five minutes to
detect any filtering of DMEM through the patch. If any
leakage is observed, another l ml of l.25% collagen and
30 fibroblasts is added to each patch as before. Testing for
leakage is done every two weeks until the patches are
sealed.
:
; 35 Exam~le D:
Autogenous Graft Epithelium
Many types of cells of epidermal and endodermal
40 origin, when placed on a membrane-like extracellular
matrix, such as collagen, are enhanced by the culture
environment such that cell proliferation,
~, '
WO91/08718 2 0 7029~ PCT/US9~/~7~33
-20-
cytodifferentiation, and gene expression are promoted.
Such growth and differentiated function has been
demonstrated using human hepatocarcinoma and Ewing's sar-
coma, bovine vascular endothelium from aorta, pulmonary
5 artery, umbilical vein, vena cava, bovine capillary
endothelium from adrenal cortex, brain cortex, corpus
luteum, and human upper respiratory epithelium from nasal
polyps. Such cells were previously resistant to
successful attempts at culture. However, when placed in
lO serum-supplemented culture on the collagen, they attach
firmly, migrate, and proliferate to form a flattened, non-
overlapping, closely apposed epithelial layer. Functional
characteristics, such as mucous secretion, ciliary
movement, expression of tissue specific antigens, and
15 other similar characteristics are demonstrated. Generally
such cultures resemble the in vivo counterparts in each of
the ways thus far measured.
It is clear that the use of autogenous grafts of
epithelial tissue, such as buccal mucosal epithelium, will
20 enable the prosthetic device to become incorporated moré
readily into the host tissue by allowing the prosthesis to
become physiologically functional within a shorter period
of time. As used herein, "autogenous" refers to donor
tissue taken from the host viscus or surrounding tissue.
25 The tissue may be taken from the same individual upon whom
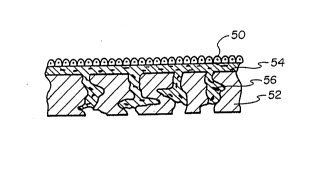
the surgical procedure is being performed. FIG. 5
illustrates the placement of graft epithelium 50 on a
substrate 52 which has been coated with collagen 54 and
fibroblasts 56.
Autogenous graft epithelium may be applied to
the collagen- or collagentfibroblast-covered substrate in
either a direct manner, or through ln vitro culturing.
These two procedures are illustrated by Example E and
Example F, respectively.
~ 35
;~ Example D
A collagen-coated substrate, readied for
` 40 implantation, is placed within a surgical bowl. The sub-
...... ... .. . .. ... . . ................. . .. . . .
. . -.
O91/08718 ~ PCT/US90/07233
-21-
strate is then pre-clotted with blood taken from the
patient. A strip of buccal or respiratory mucosa l/2 cm
wide is harvested from the patient. The strip is sutured
onto the lumen surface of the substrate with absorbable
5 suture. The lumen surface may be coated with a synthetic
biodegradable material such as fibrin glue to protect the
graft. The substrate is sutured into place in the area to
be repaired. During the implant procedure, the device is
kept moist with Dulbecco's tissue media.
Exam~le F:
Patches of Medpor (Porex, Inc., Fairburn, Ga.)
15-are soaked in saline with 0.1% cefadyl for approximately
48 hours to remove any residual ethanol or sodium azide in
which they are stored. They are then pre-equilibrated in
Dulbecco's modified Eagle tissue culture medium for
approximately 24 hours.
~ 20 Surgically excised canine buccal or urothelial
; tissue is washed in phosphate buffer saline (PBS) and the
top portion of the dermis and the epithelium are removed
using a dermatome (slicer) set to cut at 500 um. The
lower dermis is discarded. The epithelium-dermis is then
25 cut into small explants approximately l to 2 mm2. These
explants are placed on the collagen-coated side of the
patch or that which will become the inter-luminal surface
l (approximately 5 explants per cm2). The patches are then
placed in 60 x 15 mm tissue culture dishes and are fed 5
30 ml of tissue culture medium [Keratinocyte Growth Medium
(KGM), Clonetics Corp., San Diego, CA]. For urothelium,
KGM is mixed with Dulbecco's modified Eagle medium (DMEM)
j~ in a ratio of 90 KGM:10% DME containing 10% FBS. The
; patches are cultured at 37 for 14 days. At that time,
35 the patches are epithelized sufficiently to permit
implantation.
~; Once the prosthesis has been implanted, the col-
lagen or collagen-fibroblast layer prevents bacteria or
contamination from pe~etrating into the substrate while
.
; , . .. . . : .
: . . . : . - ,, ~ :
, -
- : ~
W~91/08718 2 0 7 0 ~ 9 ~ PCT/~590/07233 ~
-22-
allowing connective tissue (granulation tissue) to grow
into the substrate from the surrounding tissue. As
illustrated in FIG. 4, by the time the collagen,
collagen/fibroblast layer has been biodegraded by the
invading connective tissue, the pores 40 are filled with a
bacteriocidal layer of granulation tissue 38 (immature
connective tissue). This granulation tissue provides the
blood supply necessary for the epithelium to survive and
for the epithelium to ingrow from the suture site.
lO Collagen fibroblasts also allow the osmotic transport of
nutrients from the surrounding tissue to keep the
epithelium viable and growing.
Prosthetic Forms
The basic material of the prosthesis may be
formed into any number of devices for use in
reconstruction of areas of damage, or for replacement of
20 entire viscera. In one embodiment, the material may be
formed into a patch, or single layer of collagen-covered
substrate, as illustrated in FIG. 6. This particular
embodiment may be used to reconstruct areas of damage in
tissues or viscera where end-to-end anastomosis of the
25 tissue or viscera is impossible. The patch may be made in
any shape or size as dictated by the area to De
reconstructed. FIG. 6 illustrates, by way of example, a
rounded piece of porous substrate 60 which has been
. .
~ coated, in part, by a film of collagen 62, and is thus
; 30 ready for implantation.
In an alternative embodiment, a tube 64, as
illustrated by FIG. 7, can be formed from the porous
synthetic substrate and contacted with collagen 66 or
collagen and fibroblasts. An additional layer of
35 autogenous graft epithelium may be added. The tube thus
formed can be used for reconstructing the trachea, esopha-
gus, or varying lengths of the intestinal tract where end-
to-end anastomosis is made impossible by resection of too
much tissue.
, `
,., ... : . :. .
':: . : ' ' ': - ' -
091/08718 ~ ~ D;2~ PCT/US90/07233
-23-
In another alternative embodiment, the basic
material may be formed into a prosthetic organ, such as a
urinary bladder, for replacement of the natural organ.
FIG. 8 illustrates a urinary bladder 68, shown in partial
5 cut-away, formed from porous synthetic substrate 70. In
this embodiment, it may be desirable to attach an
autogenous layer of, for example, bladder mucosal tissue
76 to the intraluminal surface, as shown in FIG. 8. The
ureters 72 and urethra 74 of the patient have been sutured
lO to the prosthesis.
Examples illustrating procedural techniques for
implantation of the various embodiments of the device, and
results of incorporation of the device are given below.
EXAMPLE I:
Trachea or alottis patch implants
The trachea or glottis of a patient is
longitudinally incised. A patch is selected which is the
same longitudinal length as the defect. The width of the
, defect is determined by the size of the area to be
patched. Patches measuring 2 by 3 cm having coatings of
25 5% collagen, 2 l/2% collagen, 2 l/2% collagen/fibroblast,
and 2 l/2% collagen fibroblasts with buccal mucosa tissue
culture have been implanted. The mucosa is dissected from
the cartilage creating a 2 mm flange of tissue.
The mucosal flange is sutured to the luminal
~ 30 aspect of the patch with a continuous preplaced absorbable
c suture. After the mucosal suture is tied, the cartilage
is placed in the gtoove or under the flange and fixed with
interrupted non-absorbable sutures and pledgets.
There was no evidence of air loss or infection.
j 35 The initial patches of S% collagen dislodged and did not
show ingrowth. Patches of 2 l/2% collagen demonstrated
ingrowth through the edges of the patch but no epithelial
growth from the edges. Patches of 2 l/2% collagen/fibro-
blast demonstrated tissue growth in the center of the
40 patch. Patches of 2 l/2% collagen fibroblast buccal
.,, ~ .
.~
. .
,. ' , ,, ,, ~ . .
, : . . :~ , : .
, ,:, . ': , - , . . .
, ~ . , . . ~ . -
,, - ~ :
,
:, ,, ~ . '. ~ ~ . :, :
WO91/08718 2 0 ~ 0 2 9 ~ ~ ~ PCT/US90/07233
-24-
mucosa demonstrated tissue growth in the center of the
patch. Patches of 2 l/2% collagen fibroblast buccal
mucosa demonstrated tissue ingrowth with some evidence of
tissue cultured mucosal survival. The groove shaped patch
5 demonstrated that mechanical fixation can occur without
connective tissue ingrowth.
Tracheal tube implants in dogs up to 7 cm long
with s% collagen coated tubes demonstrated complete
connective tissue ingrowth through the substrate and an
l0 intraluminal connective tissue layer. Stenosis of the
center of some substrates occurred because of excess
connective tissue growth.
Epithelial ingrowth from the sutures line
occurred up to 2 cm from each end. one tube, with a free
15 buccal mucosal graft demonstrated complete epithelization
of a 7 cm long tube.
EXAMPLE II:
, Five percent collagen coated tubes up to 5 cm
lo~g were implanted in an isolated bowel segment in dogs.
The bowel segment was isolated. The mucosa-submucosa was
dissected from the sero-muscular layer. The mucosa was
25 sutured inside the lumen of the implant with pre-placed
continuous absorbable suture. The sero-muscular layer was
sutured on the outside of the implant with continuous
suture of non-absorbable material. One end of the
isolated bowel was closed; the other end was brought to
30 the outside as a colostomy. The implant was wrapped with
' omentum and the omentum was sutured in place about the
tube. There was no leak or infection. All tubes demon-
strated a luminal layer of connective tissue by 4 weeks
post-operatively. At sixty days, there was evidence, in
`, 35 some tubes, of mucosal migration from the suture line
toward the center of the device for a distance of .6 mm
from each end. (Normal bowel will only show .8 mm of
epithelial growth.) There was production of mucus and
fluid and high bacterial count in the isolated bowel.
, . .
. .
.: . ' , . ' . . ' : .: .: .' . ' ,
; 02~
091/08718 PCT/US90/07233
-25-
EXAMPLE III:
Patches measuring 2 by 4 cm in size with a
coating of 5% collagen, 2 l/2% collagen, 2 l/2%
5 collagen/fibroblasts with autologous uroepithelium or
buccal epithelium tissue cultured onto the collagen were
implanted in the urinary bladder of dogs. The urinary
bladder was exposed. A deffect the same size as the patch
was created in the bladder. The mucosa-submucosa was
lO dissected from the sero-muscular layer creating a 2 mm
flange. The mucosal layer was sutured to the~cultured
~lumen) surface of the patch with a continuous suture of
absorbable material. ~he sero-muscular layer was sutured
to the outside of the patch with absorbable continuous
15 suture. The patch was covered with omentum and sutured in
place. There was no evidence of infection or urine
leakage. Most patches demonstrated connective tissue
ingrowth around the edges. The 2 l/2% collageh/fibro-
blast/cultured patch demonstrated connective tissue
20 ingrowth in the center of the patch and some evidence of
tissue cultured cell survival.
~,)
.~ .
,~ . .
:, .
. .
",
, ,,
'~
, .
., .
.
. . , : .
. . .
: . . . :.,.. . . : . . ,: - ., .- : : .