Note: Descriptions are shown in the official language in which they were submitted.
2~386
WO 91/06523 PCT/EP90/01763
Description
Proces~ for the preparation of fluorQbenzenes and fluoro-
toluenes
The present invention relates to a process for the
preparation of substituted or unsubstituted fluoro-
benzenes and/or substituted or unsubstituted fluoro-
toluenes from the corresponding fluorobenzaldehydes. The
substituted or unsubstituted fluorobenzenes and fluoro-
toluenes are important intermediates, for example in the
preparation of pharmaceutical products.
Hitherto, fluorobenzenes have been prepared from the
corresponding substituted or unsubstituted anilines by
diazotization and subsequent replacement of the diazo
group by fluorine. Thus the synthesis of fluorobenzene by
diazotization of aniline hydrochloride, conversion of the
resulting benzenediazonium chloride into the tetra-
fluoroborate and subsequent heating has long been known
(G. Balz and G. Schiemann, Ber. 60 [1927] 1186, 1188;
D.T. Flood, Org. Synth. Coll. Vol. II tl943~ 295).
1,3~Difluorobenzene could be obtained analogously by
heating benzene-1,3-bisdiazonium tetrafluoroborate in a
yield of 31~, relative to the m-phenylenediamine as the
starting compound (G. Schiemann and R. Pillarsky, Ber. 62
[1929] 3035-3043, especially 3039). The diazotization of
3-fluoroaniline in anhydrous hydrogen fluoride in the
presence of either ammonium fluoride or tertiary amines
or dimethyl ~ulfoxide likewi e led to 1,3-difluoro-
benzene. The yields were reported as 46 to 73%
(US-A 4,075,252 and US-A 4,096,196).
Similarly low yield~ (27 to 40%) were obtained by G. 9alz
and R. Pillarsky (loc. cit.) for the prepara~ion of
1,4-difluor~benzene from p-phenylenediamine.
The thermal decomposition of the substituted benzene-
diazonium hexafluorophosphates in place of the tetra-
20703~
wo 91/06523 - 2 - PCT/EP90/01763
1uoroborates resulted in many cases in higher, but still
not economically satisfactory, yields of the correspond-
ing fluorobenzenes (K.G. Rutherford et al., J. Org. Chem.
26 [1961] 5149-5152).
Fluorinated toluenes are obtained analogouSly from the
corresponding C-methyl-substituted anilines.
The decarbonylation of aromatic aldehydes on catalysts
containing a metal of the platinum group was described by
J. Smolik and M. Kraus (Collect. Czech. Chem. Commun. 37
[1972] 3042-3051).
It has also been proposed (DE Application No. 38 24 141.2)
to prepare fluorobenzenes by catalytic decarbonylation on
zeolite catalysts from the corresponding fluorobenzal-
dehydes. The preparation of fluorotoluenes from fluoro-
benzaldehyde~ is only possible by this process by start-
ing from fluoromethylbenzaldehydes.
By means of the invention it is now possible to prepare
fluorobenzenes or fluorotoluenes as desired from the
fluorobenzaldehydes.
The present inven~ion relates to a process by which
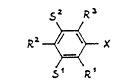
benzaldehydes of the formula (II) (see Patent Claim 1),
in which R1, R2 and R3 are, independently of each other,
hydrogen, $1uorine, chlorine and/or Cl-C3-alkyl, at least
one of these radicals being fluorine, and S1 and s2 are,
independently of each other, hydrogen and~or radicals
which reduce the electron density at the aromatic
nucleus, such as chlorine, but preferably hydrogen, are
reacted at 150 to 600C on a catalyst, activated by
treatment with hydrogen, comprising at least one trans-
ition metal of subgroups VII to VIII of the PeriodicTable of Meyer/Mendeleev, to give compounds of the
formula (I) (see Patent Claim 1), in which X is H or a
methyl group and Rl, R2, R3, Sl and s2 have the meaning
given above. If the reaction i~ carried out without a
207038~
WO 91/06523 - 3 - PCT/EPgO/01763
carrier gas or with a carrier gas which is inext, under
the reaction conditions, to the compounds participating
in the reaction, decarbonylation to the corresponding
fluorobenzenes principally ensues, whereas, using hydro-
S gen as the carrier gas, hydrogenation to the correQpond-
ing fluorotoluenes principally takes place. The prepara-
tion of fluorotoluenes from fluorobenzaldehydes requires
the presence of hydrogen.
The catalysts suitable for the process according to the
invention contain one or more elements of subgroups VII
to VIII, i.e. manganese, rhenium, iron, cobalt, nickel,
ruthenium, rhodium, palladium, osmium, iridium or plat-
inum, preferably platinum and/or cobalt and/or platinum
and rhenium. They can be applied to the usual supports,
such as Al2O3, silica gel or activated charcoal. The
catalysts employed can be in principle also all conven-
tional zeolites which have been doped by Lmpregnation,
ion exchange or other conventional methods with an
element of subgroups VII to ~III. The catalysts used
according to the invention are employed advantageously in
the form of moldings or extrudates. Fluidized-bed
catalysts can also be used.
The process according to the invention is preferably
carried out at temperatures from 180 to 440C. I~ can be
carried out both in the liquid phase and, preferably, in
the gas phase. In the gas-phase reaction, a carrier gas
such as nitrogen or C02 or, especially when the prepara-
tion of fluorotoluenes is envisaged, hydrogen can also be
admixed.
The total pressure i~ generally between 0.1 and 100 bar,
preferably between 1 and 20 bar. Particularly preferably,
however, the pressure employed is atmospheric or the
backpressure resulting from the transport of the gaseous
starting materials through the for example fixed
catalyst.
2070386
WO 91/06523 - 4 - PCT/EP90/01763
The weight hourly space velocity (unit weight of the
starting material per hour and per unit weight of the
catalyst - dimension: h~l ~ = WHSV = Weight aourly Space
Velocity) is preferably between 0.1 and 10 h-1.
The gas mixture leaving the reaction zone can be worked
up by conventional separation methods, for example by
cooling using fractional condensation. Preferably,
however, the desired reaction products are obtained in
pure form by distillation.
In the following examples, U denotes conversion rate,
SDFB the selecti~ity for 1,3-difluorobenzene and SDFT the
selectivity for 2,4-difluorotoluene.
Examples
1 to 5~ A tube reactor having a diameter of 30 mm and a
length of 600 mm was charged with 34 g (S0 ml) of a
commercial catalyst containing approxLmately 0.5% by
- weight of platinum and approximately 0.3% by weight of
rhenium on aluminum oxide as support. Hydrogen ~25 l/h)
and 2,4-difluorobenzaldehyde (14 ml/h) were passed over
the heated catalyst bed. The reaction products were
cooled and the condensed fractions were analyzed by gas
chromatography. The following results were obtained:
Example T/C U/% SDFB SDFT
l 195 99 8 92
2 212 99 13 87
3 220 98 25 76
4 370 99 83 17
S 450 99 86 14
6) In the apparatus described in ~xample l, 50 ml of the
catalyst mentioned there were treated for 3 hours at
350C with hydrogen and subsequently the trial from
~ID70386
WO 91/06523 - S - PCT/EP90/01763
Example 1 was repeated using an identical quantity of
nitrogen instead of hydrogen at 440C. 1,3-Difluoro-
benzene was formed virtually exclusively during this
procedure at a conversion rate of 50% (SDFB = 99~)
7 to 9) If, in place of the catalyst used in Examples 1
to 6, a catalyst containing approxLmately 0.5% by weight
of palladium on SiO2 as a support was used and the
procedures of Examples 1 to 5 were otherwi~e followed,
the following results were obtained:
Example T/C U/~ SD~ SDFT
7 200 55 76 24
8 240 99 93 6
9 280 99 98 2
10) A tube reactor having a diameter of 20 mm and a
length of 600 mm was charged with a catalyst, which had
been prepared ~y extruding 50~ by weight of finely-
powdered cobalt and 50% by weight of ~-Al203 to form
extrudates having a diameter of 2 mm, and the catalyst
was treated for 3 hours at 300C with hydroqen. Using
nitrogen (lS l/h) as the carriex gas, at 400C and a WHSV
of O.S h-l, 1,3-difluorobenzene was obtained with a 6elec-
tivity of 99~ in addition to traces o~ 2,4-difluoro-
toluene, at a conversion rate of the 2,4-difluorobenz-
aldehyde of 45~.
11 to 14) Repetition of the trial described in Example 10
using hydrogen (15 l/h) as the carrier ga~ instead of
nitrogen produced the following values:
3 O EXamP1e T/ C U/ 96 SDFB SDFT
11 290 20 9 88
12 316 2~ 10 88
13 350 25 18 80
14 4~0 28 23 68
WO 91/06523 - 6 - PCT/~ 907/~
15 to 17) The tube reactor described in Example 10 was
charged with 20 ml of an extruded rhodium/zeolite cata-
lyst containing 1% by weight of rhodium. This catalyst
had been prepared from zeolite H-ZSM 5 (SiO2/Al2O3 = 90)
by ion exchange with RhC13-xH20 (38% Rh), subsequent
drying at 110C, calcining at 350C and subsequent
treating for three hours with hydrogen at 350C. 2,4-Di-
fluorobenzaldehyde was then passed through the reactor at
10 ml per hour in a hydrogen stream of 15 1. The follow-
ing results were obtained:
Example TtC U/~ SD~SDFT
-
360 90 83 17
16 420 94 94 6
17 440 94 55 5
18 to 20) If, in place of the catalyst used in Examples
15 to 17, a catalyst containing 2.5% by weight of rhodium
on ~-Alz03 was used, and the procedures of Examples 15 to
17 were otherwise followed, the following results were
obtained:
Example T/C U/% SDF~ SDF~
18 190 99 95 5
19 322 99 96 4
350 99 97 3
21) If, in place of the catalyst used in Examples 15 to
17, a catalyst containing 1~ by weight of platinum on a
charcoal support was used, and the procedures of Examples
15 to 17 were otherwise followed, 1,3-difluorobenzene
was selectively obtained in the reaction of 2,4-difluoro-
benzaldehyde in a flow of nitrogen at 370C.
22 to 25) The tube reactor described in Example 10 was
charged with 20 ml of a platinum/zeolite catalyst. This
catalyst had been prepared from 100 g of zeolite H-ZSM 5
(SiO2/Al2O3 = 90) by impregnating with a solution of
2070386
WO 91/06523 - 7 - PCT/EP90/01763
1.65 ~ of tetraammine platinum(II) chloride in 300 ml of
water, subsequent drying at 110C, calcining for four
hours at 350C and subsequent treating for three hours
with hydrogen at 350C. 2,4-Difluorobenzaldehyde was then
S passed through the reactor at 10 ml per hour in a
hydrogen stream of 15 l. The following results were
obtained:
Example T/C V/% SDF~ SDFT
.
22 200 88 18 82
23 250 92 60 40
24 350 96 97 3
450 98 99
26) A tube reactor having a diameter of 50 mm and a
lS length of 600 mm was charged with 20 ml of a granulated
catalyst containing 5% by weight of rhodium on activated
charcoal as support. 2,4-Difluorobenzaldehyde was passed
through the reactor at 15 ml per hour in a nitrogen
stream of 15 1 at 165C. 1,3-Difluorobenzene was obtained
in a selectivity of 99% at a conversion rate of 40~.
27 to 29) In the tube reactor described in Example 1,
20 ml of the platinumtrhenium catalyst mentioned there
were arranged. 2-Chloro-6-fluorobenzaldehyde was then
passed through the reactor at 10 ml per hour in a hydro-
25 gen stream of 15 l. The followin~ results were obtained:
Example T/C U/% S~ S~T
-
27 200 98 9 83
28 300 99 20 66
29 400 95 34 61
S~ = selectivity for 1-chloro-3-fluorobenzene
S~T = selectivity for 2-chloro-6-fluorotoluene
30) If, in a trial set-up as described in Example 1, a
catalyst was used which contained approximately 5% by
2070386
WO 91/06523 - 8 - PCT/EP90/01763
weight of nickel on an al~minum oxide support, at a
temperature of 470C, a WHSV of 0.78 h~1 and a hydrogen
carrier gas stream of 15 l/h, 1,3-difluorobenzene and
2,4-difluorotoluene were obtained in a weight ratio of
S about 13:1 at almost complete conversion, but with
markedly decreased selectivity.