Note: Descriptions are shown in the official language in which they were submitted.
W~ ~1/06036 ! ~Pcr/us90/~X~
-1- 2070389
1 COATED PA~TICLES A~ID METHODS OF COATING PARTICL~S
Backqround of the Invention
This invention generally relates to methods of
coating particles; and, more specifically, to methods that
are well suited for coating nanoparticles and to the
particles formed by those methods.
Nanoparticles are particles having a size on the
10 order of magnitude of 10 7m. While such particles are known,
per se, heretofore, these particles have not been extensively
used. For several reasons, though, it is believed that
nanoparticles will have very important commercial
applications in the future. For example, in some cases, such
15 as in photographic emulsions, nanoparticles coated with a
given material may be suitable replacements for, and cost
less than, larger, solid particles made from that given
material. In addition, nanoparticles can be constructed so
as to exhibit an enhanced plasmon resonance effect, which is
20 the enhancement of electromagnetic fields in and around those
particles, and these particles may be used in numerous
specific methods and devices to enhance photo or optical
processes ~hat occur in those me hods and devices. In other
applications, nanoparticles may e very useful as nucleation
25 centers, which may be used to form larger particles having
specific constructions.
SUMMARY OF THE INVENTION
An object of this invention is to provide new,
30 coated particles, and new methods for coating particles.
Another object of this invention is to coat
WO9l/060~6 2 0 7 0 ~ 9 Pc-r/~s90/~n
1 nanoparticles with one or more layers of various types of
materials such as metals, polymers and halides.
A further object of the present invention is to
provide a nanoparticle, and a method that may be used to form
a nanoparticle, having a dielectric core coated with metal
halide, such as silver halide.
A further object of this invention is to provide a
method that may be used to coat dielectric nanopart~cles with
a layer of a metal.
Another object of the present invention is to
provide a nanoparticle, and a method that may be uséd to form
a nanoparticle, having a dielectric core covered with one
shell of metal and another shell of a metal halide, e.g.,
silver halide.
These and other objects are attained with various -
methods for coating particles and the particles formed by
those methods. A f irst embodiment of this invention is a
method for forming metal halide, e.g., silver halide, coated
dielectric particles, comprising the steps of providing a
source of metal ions and a source of halide ions in a liquid
carrier having dispersed therein charged colloidal dielectric
particles, and reacting the halide ions with the metal ions
in the presence of the dielectric particles to form coatings
of metal halide over individual dielectric particles. A
second embodiment of this invention is a method of forming
metal coated dielectric nanoparticles, comprising the steps
of providing a source of metal ions, a source of an alcohol
and a source of a ketone in an anaerobic liquid carrier
having dispersed therein charged dielectric particles, and
exposing the liquid carrier to light to cause the metal ions
W~ ~l/06036 " PC~lUS90/~X~
- 2~703~
1 to be reduced and form metal coatings over individual
dielectric particles.
In accordance with a third embodiment of this
invention, metal coated dielectric nanoparticles are formed
by a method comprising the steps of forming metal halide
coated nanoparticles, and exposing the coated nanoparticles
to light and a reducing agent to change the metal halide to
metal to form metal coatings over individual nanoparticles.
In a fourth method according to the present invention, metal
coated dielectric nanoparticles may also be formed by a
method comprising the steps of providing a source of metal
ions, a source of halide ions and an election hole scavenger
in a liquid carrier having dispersed therein negatively
charged colloidal dielectric particles, reacting the metal
ions with the halide ions in the presence of the dielectric
particles and the election hole scavenger to form metal
halide coatings over individual nanoparticles, and exposing
the liquid carrier to light and reducing agent to change the
metal halide coatings to metal coatings.
A fifth embodiment of this invention is a method of
forming metal coated dielectric nanoparticles, comprising the
steps of providing a liquid carrier having dispersed therein
dielectric nanoparticles having metal halide on the surfaces
thereof, changing at least a portion of the metal halide on
individual nanoparticles to metal, adding metal ions and a
sulfate reducing agent to the liquid carrier, and forming
metal coatings on the nanoparticles from those metal ions,
wherein the metal on the nanoparticles acts as a catalyst and
accelerates the formation of the metal coatings.
With all of the above-described methods, it is not
necessary that any formed coating of a particle completely
W091/06036 2 0 7 0 3 89 P~r/~ M~
~.
1 cover the core or an underlying coating of the partlcle.
Complete coverage of the core or of another coating may be
preferred for certain applications, though, and can also be
obtained using the methods of this invention.
A first nanoparticle according to this inventions
comprises a dielectric core, and a metal halide coating over
this dielectric core. A second nanoparticle according to the
present invention comprises a dielectric core, a metal
coating disposed immediately over that core, and a layer of
metal halide disposed immediately over that metal layer. A
third nanoparticle according to the present invention
comprises a dielectric core, a layer of metal halide disposed
immediately over that core, and another layer of metal
disposed immediately over the metal halide coating. A still
another nanoparticle according to the present invention
comprises a dielectric core, a layer of one metal disposed
over that core, and a layer of another metal disposed over
that first metal layer.
Any of the nanoparticles formed in accordance with
the present invention may be provided with an outer coating
of a polymer material to prevent the particle from reacting
chemically with any medium or environment in which the
particle is used. Also, it may be desirable to provide these
nanoparticles formed according to the present invention with
interior coats of a polymer material to prevent other coats
of the particle from chemically reacting with each other, or
to prevent the core of the particle from chemically reacting
with a coating of the particle. In addition, in any
nanoparticle according to this invention, it is not necessary
that any given coating of the particle completely cover the
core or another coating of the particle. Such complete
W~` '`I /06036 PCr/l,'590/06~)09
.2070389
1 coverage of the core or of another coating may be preferred,
however, for certain nanoparticles or for certain uses.
Further benefits and advantages of the invention
will become apparent from a consideration of the following
detailed description given with reference to the accompanying
drawings, which specify and show preferred embodiments of the
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1 through 9, which are not drawn to scale,
show various coated nanoparticles of the present invention.
Figures 10 through 18 outline several procesges of
this invention that may be used to form the particles shown
in Figures 1 through 9.
Figure 19 is a transmission electron micrograph of
silver-coated silver bromide nanoparticles.
Figure 20 is a transmission electron micrograph of
silver coated silver bromide nanoparticle treated with
ammonia.
Figure 21 shows various optical extinction spectra
of silver coated silver bromide nanoparticles either
untreated or treated with ammonia. The spectra of (a) to (d)
are spectra of various illuminated solutions of Ag, Br, and
EDTA. In going from (a) to (d), the illumination time
increases. The spectrum in(e) is a typical spectrum observed
after the addition of ammonia to any of the above solutions.
Figure 22 shows computed extinction efficiencies
for silver-coated silver bromide particles in water. The
diameter of the core particle is 20 nm and the thickness of
the silver coats are indicated in nm. The spectrum marked
solid is that of a homogeneous 20 nm diameter silver sphere.
WO 91/06036 PCI/U.~90/~6009
- 2070389 f
l Figure 23 is an optical extinction spectrum of a
measured silver coated silver bromide nanoparticle and two
computed e~tinction spectra. The measured spectrum lies
between the two computed spectra. In the upper curve all the
5 silver in the coat is assumed to come from the solution. In
the lower curve all of the silver is assumed to come from the
reduction of AgBr at the particle surface.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Figures 1 through 9 show various particles all
formed in accordance with the present invention; and
generally, each of these particles comprises a dielectric
core and one or more coatings over that core. The cores of
all of these particles have sizes on the order of magnitude
15 of a 0.S to 200 nanometers, and thus are referred to as
nano-cores or nanoparticles. With some of these particles,
such as those shown in Figures 1 through 3, and 7 and 8, one
coating of each particle is comprised of silver halidei and
with some of these particles such as those shown in Figures 2
20 through 6, 8 and 9 at least one coating of each particle is
comprised of a metal.
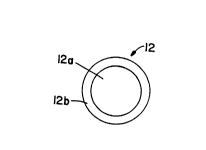
Particle 12 of Figure 1 consists of core 12a and
coating or shell 12b, the core consists essentially of a
dielectric material such as silica, and the shell consists
25 essentially of silver halide. Further, with this particle,
shell 12b is disposed immediately over and substantially
completely covers core 12a. This particle does not itself
include any metal and thus does not exhibit the plasmon
resonànce effect. However, the silver halide in the particle
3O may be changed to metal silver, either to form a layer of
metal silver on the particle or to help form a layer of
w~1/06036 PCI/US~/~
--- 20703~9
another met~l thereon, and to thereby form a particle that
does exhibi the plasmon resonance ef~ect.
In particle 14 of Figure 2, a metal coating such as
silver, copper, aluminum, gold or palladium is disposed
5 between the dielectric core and the silver halide coating to
increase the sensitivity of the silver halide to light. This
increased sensitivity is caused by the plasmon resonance
effect produced by the metal coating. More specifically,
particle 14 consists of dielectric core 14a, metal coating
lO 14b disposed immediately over and covering that core, and a
layer of silver halide 14c disposed immediately over and
covering layer 14b.
Particle 16 of Figure 3 is similar to particle 14
of Figure 2 in that both of these particles include a
dielectric core, one coating of silver halide and a second
coating of a metal. The order of these coatings in particle
16, though, is the reverse of the order of these coatings in
particle 14. More specifically, particle 16 consists of
dielectric core 16a, a layer of silver halide 16b disposed
immediately over and covering core 16a, and a layer of a
metal 16c disposed immediately over and covering the silver
halide coating 16b.
Particle 20 of Figure 4 comprises a dielectric core
20a, a layer of one metal 20b disposed over core 20a and a
layer of another metal 20c disposed over metal layer 20b.
Preferably, coating 20b completely covers core 20a, and
coating 20c completely covers coating 20b. Particle 22 of
Figure 5 is one specie of the general type of particle shown
in Figure 4. More specifically, particle 22 comprises a
dielectric core 22a, a layer of silver 22b disposed over core
22a, and a layer of another metal 22c disposed over the
WO 91/06036 P~r/U~
12ao7D389 ~~'~
1 silver yer 22b with the specific particle configuration
shown in Figure S coating 22b completely covers core 22a, and
coating 22c completely covers coating 22b.
For certain applications, it may be preferred to
provide the nanoparticles of the present invention with an
outer coating of a polymer material, for example, to prevent
the particle from reacting chemically with any medium or
environment in which the particle is used. This principle is
generally illustrated in Figures 6 and 7. Figure 6 shows
particle 24 comprising core 24a, first coating 24b and second
coating 24c. Core 24a consists essentially of a dielectric
material, coating 24b consists essentially of a metal and is
disposed immediately over core 24a, and coating 24c consists
essentially of a polymer material and is disposed immediately
over shell 24b. Coating 24b may completely cover core 24a,
and coating 24c may completely cover coating 24b. Figure 7
shows particle 26 comprising core 26a, first coating 26b and
second coating 26c. Core 26a consists essentially of a
dielectric material, coating 26b consists essentially of
silver halide and is disposed immediately over core 26a, and
shell 26c consists essentially of a polymer material and is
disposed immediately over and completely covers shell 26b.
Coating 26b may completely cover core 26a, and coating 26c
may completely cover coating 26a.
It may also be desirable to provide nanoparticles
of this invention with interior coatings or shells of a
polymer material, either to prevent other coatings or shells
of the particles from chemically reacting with each other, or
to prevent the core of the particle from chemically reacting
with a coating or shell of the particle. This principle is
W' ')1 /06036 PCr/US90/06009
-~- 2070389
l generally illustrated in Figures 8 and 9, which disclose
particles 30 and 32 respectively.
Particle 30 is similar to particle 16 in that both
of these particles include an inside dielectric core, a first
5 metal coating and a second, silver halide coating.
Particles 30 and 16 differ in that the former particle
includes a third, polym~ ic coating disposed between the
metal coating and the silver halide coating. More
specifically, particle 26 includes core 30a and coatings 30b,
c and d. Core 30a consists essentially of a dielectric
material, and coating 30b consists essentially of a metal and
is disposed immediately over and core 30a. Coating 30c
consists of a polymeric material and is disposed immediately
over shell 30b, and shell 30d consists essentially of silver
halide and is disposed immediately over shell 30c. With the
specific arrangement illustrated in Figure 8, coating 30b
substantially completely covers core 30a, coating 30c
substantially completely covers coating 30b, and coating 30d
substantially completely covers coating 30c.
Particle 32 is similar to particle 20, and both of
these particles include a dielectric core and two metal
coatings or shells. Particle 32 includes a further polymeric
coating located between the two metal coatings to chemically
insulate these two metal coatings from each other. To
elaborate, particle 32 comprises core 32a and coatings 32b, c
and d. Core 32a consists essentially of a dielectric
material, and coating 32b consists essentially of a metal and
is disposed immediately over core 32a. Coating 32c consists
essentially of a polymeric material and is disposed
immediately over coating 32b; and coating 32d consists
essentially of a metal, which may or may not be the same as
WO ~1 /06036 PC~/l,'S90/116009
~7 0389 -''J-
1 the meta of coating 32b, and is disposed immediately over
polymeric coating 32c. Each of coatings 32b, c and d may
form a respective one, at least substantially complete shell.
With such an arrangement, coating 32b substantially
5 completely covers core 32a, coating 32c substantially
completely covers coating 32b, and coating 32d substantially
completely covers coating 32c.
Figures 1-9 are only representative of nano-
particles formed according to the present invention, and in
10 particular, only illustrate the general relationship between
the cores and the coatings or shells of the particles. In
any nanoparticle of this invention, the particle and the rore
thereof may have any suitable shapes, and specifically, the
particles and the cores may have shapes other than spherical.
For instance, the particles and the cores may be cylindrical
or ellipsoidal, have a thread-like shape, or be crystalline
shaped. The actual crystal form of the core may be any
suitable form; and, for example, these cores may be:
Tetragonal crystal forms,
Orthorhombic crystal forms,
Monoclinic crystal forms,
Triclinic crystal forms,
Isometric crystal forms,
Hexagonal crystal forms.
Also the shapes of the nanoparticles may change as
they are made.
Further, any suitable dielectric material may ~e
used in these particles; and, in particular, the dielectric
material may be linear or non-linear.
As used herein, the term "dielectric" material
refers to a material which is a non-conductor or a
w~ ~l/06036 Pcr/u~s90/n~n
207~)38g
semi-conductor. The conductivity of this material may range
from as low as 0, but preferably 10 40, to as high as 106
mhos. In a preferred em~odiment, the conductivity ranges
from 10 40 to 105 mhos. In a most preferred embodiment the
5 conductivity ranges from 10 30 to 104 mhos. Examples of
dielectric core include glass, silica, cadmium sulfide,
gallium arsenide, polydiacetylene, lead sulf ide, titanium
dioxide, polymethylacrylate (PMMA), silver bromide, carbon
fi~ers, copper sulf ide, silver sulf ide and the like.
In addition, as the term is used herein, "metal"
includes any material having a negative dielectric constant,
and so can include super conductors, conducting polymers,
materials with an anomolous dispersion of carrier electrons,
and heavily doped semi conductors where free carrier electron
15 motion dominates the dielectric function.
The types of metals that can be coated onto the
dielectric core in accordance with the methodology of the
present invention include the transition metals, the
lanthanides and the Group IIIA metals. The especially
20 preferred transition metals include the Group VIII and IB
metals, and Group IIIA metals, especially copper, silver,
gold, iron, nickel, palladium, platinum, cobalt, rhodium
iridium, ruthenium, aluminum and the like. Especially
preferred metals include copper, silver, gold, nickel,
25 palladium, platinum and aluminum.
In accordance with the present invention, the
metal-halide coated nonoparticles can be prepared by
providing a source of metal ions and a source c r halide ions
in a liquid carrier having dispersed therein charged
30 colloidal dielectric particles and reacting the halide ions
with the metal ions in the presence of the dielectric
WO91/06036 pcr/~9o/~K~
207038~
l particles to form coatings of metal halide over individual
dielectric particles.
For example, using this procedure, silver halide
coated dielectric particles, such as particle 12 of Figure 1
5 can be prepared. More specifically, this procedure,
generally, comprises the steps of providing a source of
silver ions and a source of halide ions in a liquid carrier
having dispersed therein charged colloidal dielectric
particles, and reacting the halide ions with the silver ions
in the presence of the dielectric particles to form coatings
of silver halide over individual dielectric particles.
Figure 10 outlines one preferred method for
carrying out this process but this procedure is exemplary and
is equally applicable to the other metal halides coating
dielectric particles. This method generally comprises the
steps of providing an aqueous solution including negatively
charged colloidal dielectric nanoparticles, positively
charged silver ions, and a halide, and reacting the halide
with the silver ions to bond, or grow, coatings of silver
halide on, and preferably completely covering, individual
dielectric particles. Preferably, the concentrations of
dielectric particles, silver ions and halide in the solution,
and the length of time over which the coatings are allowed to
grow on the dielectric particles, are selected so that
coatings of a uniform preselected thickness are grown on
those particles. The specific order in which the dielectric
particles, the silver ions and the halide are added to the
aqueous solution is not critical; and, for example, the
dielectric particles may be dispersed in the solution, then
the silver ions may be added, and then the halide may be
added.
w~ ~l/06036 Pcr/uss~ n
; 2Q7~389
1 With a preferred process, after the dielectric
particles are added to the solution, the pH of that solut~on
is adjusted to and thereafter maintained at a level ~lightly
above 2, and even more preferably, between about 3 d 5.
5 With this procedure, the dielectric particles do no_ have to
be negatively charged when they are added to the solution,
and, instead, the acidity of the aqueous solution causes the
dielectric particles to become negatively charged once the
particles are in the solution. Further, with the preferred
process, the initial concentration of the silver ions in the
solution is relatively low, less than 10 4M; the initial
concentration of the halide in the solution is slightly
greater than, such as about 10% greater than, the
concentration of the silver ions in the solution; and also,
the solution is co~stantly stirred while the halide is being
added to it.
The silver ions may be added to the solution in any
suitable form, and for instance, these ions may be added in
the form of a silver salt that is soluble in aqueous
solution, e.g., silver nitrate. Likewise, the halide that is
added to the solution may be any suitable halide, such as an
alkali halide, e.g., sodium bromide, potassium bromide,
potassium chloride, sodium chloride and the like. In
addition, any suitable dielectric may be used in the
above-discussed process, and the dielectric may be linear or
non-linear and may have any suitable shape and size. For
example, the dielectric particles may be spherically shaped
silica particles. When, first, the d;electric particles are
these silica particles, second, the silver ions are added to
the solution in the form of silver nitrate, and third, the
halide is sodium bromide, then the silver from the silver
--1 L_ PCr/US9O/060~
l nitrate reacts with the bromide from the sodium bromide to
form silver bromide, which bonds to and forms layers over the
silica particles.
A metal co~ting on a dielectric particle, such as
coating 14b of particle 14, or coating 24b of particle 24,
may be made by a process, generally, comprising the steps of
providing a source of metal ions, a source of a secondary
alcohol, preferably a lower secondary alkanol containing 3-7
carbon atoms and a source of a ketone preferable containing
3-7 carbon atoms in an anaerobic liquid carrier having
dispersed therein charged dielectric particles, and exposing
the liquid carrier to light, prefe~ably ultraviolet light to
cause the metal ions to attach to the dielectric particles
and form metal coatings over individual dielectric particles.
As used herein, the term lower alkyl, when used
alone or in combination, contains 1-7 carbon atoms. These
alkyl groups may be straight chained or branched and include
such groups as methyl, ethyl, proply, isopropyl, butyl,
sec-butyl, isobutyl, t-butyl, pentyl, amyl, hexyl and the
like
As used herein, a secondary alkanol refers to a
lower alkyl alcohol in which the hydroxy group is attached to
a secondary carbon. Such groups include isopropanol,
sec-butanol, and the like.
The preferred ketone is acetone.
Figure 11 outlines one preferred method for
carrying out this process. This method generally comprises
the steps of providing an aqueous solution including
negatively charged colloidal dielectric particles, metal
ions, isopropanol and acetone; removing oxygen from the
solution; and exposing the solution to ultraviolet light to
cause the metal ions to attach to the
W~'`1/06036 PCr/li'S90/~6009
1 2070389
1 dielectric particles and form metal coatlngs completely
covering in~lividual dielectric particles. Preferably, the
concentrations of the dielectric particles, the metal ions,
the isopropanol and the acetone, and the length of time the
5 solution is exposed to the ultraviolet light are selected so
that coatings of a uniform preselected thickness are formed
on the dielectric particles.
In the above-discussed procedure, without wishing
to be bound, it is believed that the acetone absorbs energy
from the ultraviolet light and then reacts with isopropanol
to form isopropyl radicals. These radicals are powerful
reducing agents and cause metal ions that have become
attached to the dielectric particles to form metal molecules.
The particular order in which the dielectric particles, the
metal ions, the isopropanol and the acetone are added to the
aqueous solution is not critical; and, for instance, the
isopropanol and acetone may be added to the solution, the
dielectric particles may then be dispersed in the solution,
and then the metals may be added.
In the above procedure, it is preferred that the
light sourze used contain ultraviolet light. It is preferred
that the light source contain wavelengths from 150-550 nm.
The preferred wavelengths range from 200-400 nm.
Furthermore, it is preferred that the intensity of
light used range from 50 watts to 1.5 kilowatts, with the
preferred intensity ranging from 250-1000 watts. Especially
preferred intensity range from 350-550 watts, with an
intengity of about 450 watts being the most preferred.
In a preferred method, as with the method outlined
in Pigure 10, after the dielectric particles are added to the
solution, the pH of the solution is adjusted to and
WO 9l/n6036 PCr/US90/~''
2070389 -~f'-
1 thereafter maintained at a level slightly above 2, and even
more preferably, between about 3 and 5. In thls way, the
dielectric particles do not have to be negatively charged
when they are added to the solution and the acidity of the
5 aqueous solution causes the dielectric particles to become
negatively charged. In addition, the initial concentration
of the metal ions in the solution is relatively low, such as
2 x 10 4M, and the initial concentrations of the ketone,
e.g., acetone, and secondary alcohol, e.g., isopropanol ln
the solution are about equal to each other and much greater
than, such as about 400 times greater than, the initial
concentration of the metal ions in the solut~on. In
ad~ition, preferably the solution is stirred while exposed to
the ultraviolet light.
Numerous specific types of metal coatings may be
made using a procedure as described above, and for example,
the process may be used to form silver coated dielectric
particles, gold coated particles or palladium coated
particles. In addition, the metal ions may be provided in
the solution in any suitable manner; and, for example, these
ions may be provided by adding a water soluble metal salt
such as silver nitrate, to the solution.
Moreover, any suitable dielectric may be used in
the above-discussed process, and the dielectric may be linear
of non-linear and may have any suitable shape and size. For
instance, the dielectric particles may be spherically shaped
silica particles. When such dielectric particles are used,
and the metal ions are added to the solution in the form of
silver nitrate, then the ultraviolet light, in combination
with the acetone and the isopropanol, causes the silver ions
w~ ~1/06036 PCT/US~/~K~
~7 2070389
1 to bond to and form metal silver coatings over the silica
particles.
The following example illustrates this process for
forming a metal silver coating over the silica particles.
3o
wo sl/n603~ Pcr/us~o/~ ~
~ 2070389
EXAMPr,E 1
An aqueous solution is prepared by mixing the
following solutions in a 50 ml beaker:
5 (1) 0.5 ml of 0.0~ M AgN03
(2) 0.5 ml of O.S0 M Sio2 of low porosity particles. The
particle diameter was chosen to be between 5 to 20
nanometers, although other sizes can be readily substituted,
(3) 1.5 ml of pure isopropanol,
lO (4) l.S ml of pure acetone.
All chemicals used are of reagent grade quality, unless
otherwise specified. The above mixture are diluted with 16
ml of distilled water, and the pH adjusted to be between 4 to
5 by dropwise addition of a 0.01 M nitric acid solution. In
15 this pH range, the silica particles are negatively charged,
causing the positively charged silver ions to be bound to the
surface. After thorough mixing by stirring for one minute
using a magnetic stirrer, the sample is transferred to a W
photolysis vessel, equipped with a quartz window and
20 provision for careful deoxygenation by bubbling nitrogen gas
for one hour. It is important that no oxygen be present in
the solution. The sample is irradiated by a 450 Watt Hg-Xe
lamp for one hour, with gentle stirring continued by means of
a magnetic stirrer. The solution color, and consequently the
25 thickness of the coat, can be controlled by adjusting the
period of illumination by W light. This forms the basis for
the preparation of the silver coated silica particles in the
present example.
3o
W~91/~6036 P~/U~9~ K~
1~- 2070389
1 Metal, e.g., silver coated dielectric particles may
also be made by a process employing photoreduction of metal
(e.g,, silver) halide, and one such exemplary process is
outlined in Figure 12. In this process, silver halide coated
5 dielectric particles are made, for example, by the process
discussed above in connection with Figure 10, and then the
coated particles are exposed to light to change the silver
halide coatings over the individual particles to metal silver
coatings.
Preferably, though, a more integrated process is
used to form metal coated dielectric particles. This
process, generally, comprises the steps of providing a source
of metal ions, a source of halide ions and a source of an
electron hole scavenger in a liquid carrier having dispersed
therein negatively charged colloidal dielectric particles,
reacting the metal ions with the halide ions in the presence
of the dielectric particles and the electron hole scavenger
to form metal halide coatings over individual dielectric
particles, and exposing the liquid carrier to light to change
the sliver halide coatings to metallic silver coatings.
Figure 13 outlines a preferred method to implement this
process using silver ions. In accordance with this method,
dielectric particles are dispersed in a solution including
silver ions, a halide and an electron hole scavenger, and the
silver ions react with the halide to form silver halide
coatings over, and which may completely cover, individual
dielectric particles. The solution is then exposed to
ultraviolet light, and this light changes the silver halide
coatings to silver coatings. Preferably, the concentrations
of the dielectric particles, the silver ions, the halide and
the electron hole scavenger in the solution, and the length
2 0 7 0 3 8 9 ~ s~o/~
1 of time the solution is exposed to the ultraviolet l~ght are
selected s~ that coatings of a uniform preselected thickness
are formed on the dielectric particles.
Preferably, with this process, the initial
5 concentration of metal (e.~., silver) ions in the solution i~
greater than the initial concentration of the halide in the
solution; and for instance, the former concentration may be
about 5 time the latter concentration. The metal ions may be
in the solution in any suitable form, and for instance, if
10 silver is the metal ion, these ions may be added to the
solution in the form of a metal salt that is soluble in
aqueous solutions, e.g., silver nitrate. Similarly, the
halide that is added to the solution may be any suitable
halide, such as alkali halide, e.g., sodium bromide,
potassium bromide, sodium chloride, potassium chloride and
the like. Further, any suitable dielectric may be used in
this process, and the dielectric may be linear or non-linear
and have any suitable shape and size. For example, the
dielectric particles may be spherically shaped silica
particles. When (i) the dielectric particles are the silica
particles, (ii) the silver ions are added to the solution in
the form of silver ni~rate, and (iii) the halide is sodium
bromide, then the silver from the silver nitrate reacts with
the bromide from the sodium bromide to form silver bromide
coatings on the dielectric particles; and the ultraviolet
light, in the presence of EDTA, then reduces the silver
bromide coatings to metallic silver.
3o
W~1/06036 PCT/US~/~X~
-21- 207D~89
1 EXA~PLE 2
The following example illustrates this process for
forming silver coated dielectric particles. Metallic silver
on SiO2 particles can be obtained by photoreduction of silver
halides, which are typically prepared in the presence of
excess Ag ions. A hole (h ) scavenger, EDTA, is added to
the solution. One ml of a 0.002 M NaBr solution is added to
19 ml of a solution which is prepared in a 50 ml beaker by
mixing the following:
lO ~1) 1 ml of 0.01 M AgNO3,
(2) 0.5 ml of 0.50 M of low porosity SiO2 particles. The
particle diameter was 12 nanometers, although other sizes can
be readily substituted,
(3) 1 ml of 0.02 M EDTA,
15 (4) 16 ml of distilled water.
After thorough mixing, the solution is transferred to a 1 cm
W quartz cuvette and exposed to a 375 Watt tungsten halogen
light source. Under these conditions, very little light is
actually absorbed since the colloidal AgBr has a very low
20 absorbance above 350 nm. A possible mechanism for the
reduction process is given by: -
AgBr -~ AgBr(e + h )
AgBr + e -----~ Ag + Br
EDTA + h -----> product
Br + Ag (excess) -----> AgBr
The duration of illumination, which is in the order of
minutes, determines the color of the silver coated silica
particles. This color is a result of the thickness of the
silver layer, and can range from yellow to a purplish gray.
3o
W09l/06036 PCrt~S90/~MX9--
2 0 7 0 3 8 9 -?~-
1 Once the silver coated silica spheres are prepared,
they are purified by dialysis and then placed in a sodium
dodecyl sulfate micellar solution, or a micro emulsion.
3o
Wl' 9 1 /06036 P(~/USg~)/0600~
~- 207038~
1 A variation of the process described above may be
employed to form metal coatings other than silver ~n
nanoparticles, and this variation utilizes the fact that
metallic silver on the dielectric particles will act as a
5 catalyst to help grow metal coatings on those particles from
other metal ions in the solution. In accordance with this
variation, which is outlined in Figure 14, a liquid carrier,
or a solution, is provided including dielectric particles
having silver halide formed on those particles, at least a
lO portion of the silver halide on individual nanoparticles i8
changed to metallic silver, and ions of a metal are added to
the solution to form coatings of that metal completely
covering individual dielectric particles, with the metall~c
silver on those particles acting as a catalyst to accelerate
15 the formation of the metal coatings. These metal ions may be
added to the solution in any suitable manner, and for
instance, conventional photographic developing solutions may
be added to the solution to reduce the metal ions.
Only minute amounts of metallic silver are needed
on the dielectric particles to help grow the metal coatings
thereon; and hence, in the above-described process, it is
only necessary to form minute amounts of silver halide on the
dielectric particles. Alternatively, complete coatings of
silver halide may be formed on the dielectric particles, with
only minute amounts of the silver halide on individual
particles being changed to metallic silver on individual
particles, and then these minute amounts of metallic silver
may be used to help form metal coatings completely covering
the silver halide that remain on the dielectric particles.
The resulting product comprises a dielectric core, a first
coating of silver halide that substantially completely covers
wogl/06036 ~r/us90/~n
2070389 ,,~
1 the electric core, and a second coating of a metal that
completely covers the layer of silver halide.
The following example illustrates the coating of
silver on an dielectric core of silver bromide. The silver
5 bromide nanoparticles exposed briefly to intense W light in
the presence of EDTA have optical extinct~on spectra similar
to those computed for distribution of silver coated silver
bromide nanoparticles. By intense, it is meant that the
intensity of the light ranges from S0 watts to 1.5 kilowatts,
10 with the preferred range being 250-SS0 watts, and the most
preferred having a range of 350-SS0 watts.
As clearly shown by the following discussion, with
shorter exposure time, the plasmon resonance maximum is
shifted to lower wavelengths, a result consistent with theory
15 50 long as the coat thickness increases with exposure to
light. The resonance maximum of the distributions of coated
particles can be controllably shifted to 600 to 700 nm.
3o
W~l/06036 Pcr/usso/~
-2,- 2~70389
1 EXAMPLE 3
Silver bromide colloids were prepared by rap~dly
mixing equal volumes of AgN03 and NaBr solutions. A growth
stabilizer (SDS) and an electron donor (EDTA) were added
immediately after precipitation. Typically the final
concentrations were 1 x 10 4 M Br , 4 x 10 4 M Ag , 5 x 10 4
M SDS, and S x 10 4 M EDTA. The concentrat~on of SDS was far
below the critical micellization concentration (~o 2 M).
Freshly prepared solutions were exposed to light from a 450
lO Watt Hg-Xe lamp for a few seconds. With the shortest
exposures the spectra appeared blue. With longer exposures
the solutions appeared orange. When ammonia, which dissolves
AgBr by forming complexes with Ag~, was added to any of the
illuminated solutions the color changed to a yellow color
15 characteristic of small metallic silver colloids.
The particle size distributions were characterized
with transmission electron microscopy (JEOL 1200EX). A
typical micrograph is shown in Fig 19. A size distribution
consistent with the limited micrograph data is the log normal
distribution.
N(r) = NOexp(-((ln(r) - ln(rm))/ln(s))2),
with rm equal to 1 nm or less and s in the renge of 4 to 4.5
nm. The size distributionc ~s determined by TEM did not
appear to change markedly wlth exposure to light.
After the addition of ammonia to any of the
illuminated samples only small particles having diameters 5
nm or less were observed in the TEM (Fig 20). The most
likely interpretation is that only part of the AgBr was
reduced to Ag during the illumination and that the larger
particles are AgBr/Ag composites.
wo sl/06~36 Pcr/~s~o/06~
207 0389 ~
l Example optical extenction spectra measured shortly
after exposure are shown in Figs. 21a) to d). The exposure
time and/or EDTA concentration, and hence the reduction of
Ag , increases in qoing from a) to d). The peak extlnctton
5 shifts to shorter wavelengths as the illumination time ts
increased. This result is consistent with theory so long as
the coat thickness increases with exposure. A spectrum of
the ammonia treated solution, shown in Fig 21e), is typical
of homogeneous silver nanoparticles. The general shapes of
lO the above spectra are readily reproducible. At comparable
illumination times, in the absence of Br , the appearance of
color in a given sample is negligible.
Theoretical optical extinction spectra of
individual silver coated spheres are shown in Fig. 22. The
15 peak of the theoretical extinction shifts from red to blue as
the ratio of coat thickness to core radius increases. This
data is consistent with the measured spectra where the
absorption maxima shifts toward the blue as the time of
exposure increases, since the coat thickness should increase
with exposure time. The computed spectra are very sensitive
to the coat thickness. The measured spectra are much more
broad than the spectra shown in Fig. 22 because of the
distributions of core diameters and coat thicknesses.
The magnitudes of the extinction spectra are also
characteristic of silver coated particles. For example, ~t a
wavelength of 700 nm the extinction cross section per unit
volume of silver is lOO's of times larger in a silver coated
nanoparticle havinq the appropriate ratio fo core ra~ius to
coat thickness that it is in a solid silver sphere. The fact
that the theoretical extinction is so large can be used to
help verify that the particles are coated with silver.
wnsl/06036
-~7-
1 However, since there is a broad distribution os sizes care
must be taken in ma~ing the comparision.
Here we started with the size distribution of core
particles described by the above eguation, then used trial
5 and error to determine the distributions of coat thicknesses
required to match the measured spectra, and then found that
the magnitudes of the spectra were within the range of values
expected from the initial concentrations of Ag and Br .
The assumptions made in computing the spectra are
10 as follows:
1. The reduced silver is in the form of a smooth
coat on the surface of a spherical AqBr
particle. The extinction efficiencies were
computed using the separation of variables
solution for concentric spheres based on
algorithms.
2. The size distribution of the core particles is
described by the log-normal distribution of the
above eguation. The values of No were
determined by setting the total volume of all
the particles prior to illumination in the
distribution egual to the volume of AgBr. The
initial total volume of AgBr was determined by
solving the ionic equilibria eguations
including the Ag+-EDTA complex.
3. The size distribution of the coat thickness is
a Gaussian, typically with a standard deviation
of 2 to 8 nm.
4. The silver coat may be formed either from the
reduction of the silver halide of the initial
particle, or from the reduction of Ag from
W091t06036 PCT~US90/~X9 -
2~7~389 -2~-
1 solution. Computations have been done for each
of the two limiting cases.
5. The total extinction is computed by numerically
integrating over distributions of core radii
and coat thicknesses.
be( ~ Nn(rc)Ng(t)Q(rc~t~mc~mt~l)~ r2drcdt
where Nn is the size distribution of the cores,
Ng is the size disbribution of the coats, Q i8
the extinction efficiency, mc is the refractive
index of the core, and mt is the refractive
index of the coat. Typically the integrations
over cores were from r=2 to r=18.
6. The refractive index of the silver was computer
from the data of Hagemann et al. in J. OP. Soc.
Am., 65, 742-744 (1975) and ~erker, in J. OD.
Soc. Am. B., 1327-1329 (1985) either by itself,
or combined with a Drude model in which the
increased electron scattering at the surfaces
of the very thin coat was taken into account.
The refractive index data of Johnson and
Christy in Phv. Rev. B, 6, 4370-4379 ~lg72) was
also used for some computations not shown.
Linear interpolation was used to obtain the
values of refractive index at points not in the
data.
7. The refractive index of AgBr was obtained by
combining the data from White, J. ~E~. Soc. Am,
62, 212 (1972) and James, "Theory of the
Photographic Process,!' McMillan (1977) p 216.
Fig. 23 shows a measured spectrum and two computed
spectra. In the topmost curve the Ag in the coat is assumed
w~)sl/u6o36 PCT/US~/~Xn
-2-~- 20 7038g
l to come only from the solution, i.~., the AgBr cores are not
reduced in size as the coat grows. In the bottom curve the
Ag in the coat is assumed to come only from the reduction o
AgBr at the surface of the particle and so the core shrinks
5 as the coat grows. Since the measured curve lies between the
two computed spectra, the magnitudes of the plasmon enhanced
extinction is in the range of values computed.
The main parameters that can be adjusted in fitting
the distributions to the spectra are: 1) the thickness and
lO standard deviation of the coats and the limits of the
numerical integration for the coats. 2) the size
distribution and the limits of integration for the cores. 3)
the date for the refractive index of silver. 4) the
fraction of the reduced silver that came from solution. The
15 computed spectra are very sensitive to the distributions of
cores and coats chosen and 'co the limits of integration,
which also define the size c stributions. The computed
spectra depend on the refractive index of silver used.
However, by varying the size distributions, similar spectra
can be obtained with the different models for silver. The
effect of the different assumptions about the source of the
Ag for the coat can be seen in Fig. 23. In a preliminary
experiment without excess silver a spectrum similar to that
shown in Fig. 21d) was generated.
Without wishing to be bound, it is believed that
the silver coat is formed by the coalescing of many small
silver particles. The coat may also contain some AgBr or
voids, but it is homogeneous enough to have a refractive
index similar to that of bulk islver. The bonds between the
particles may be relatively weak because the coat breaks into
Wosl/n6036 PCT/US90/~K~ -
2070389
1 many small particles when the solution is treated with
ammonia.
It might have been thought that the spectra could
be accounted for by nonsperical silver particles. The fact
5 that ammonia, which dissolves AgBr but not Ag, reduces the
spectrum to that of small solid silver particles, and the
fact that the particles in the TEM do not have large
eccentricites, argue against this hypotheses. Also, the
particle shapes do not seem to be related to the colors of
10 the solutions.
In summary, the predicted tunability of the
surface-plasmon resonace frequency and enhanced extinction at
longer wavelengths was experimentaly confirmed with Ag-AgBr
colloidal composites. The particles scatter as if the Ag is
15 smoothly coated on the AgBr.
Silver coated dielectric particles may also be
formed by a process utilizing chemical reduction of silver
ions by hydroquinone at elevated temperatures. The following
example, generally outlined in Figure 15, illustrates this
20 process.
3o
W~91/06036 PCT/US~/~X~
31 2 07 03 8g
~XAMPLE 4
100 ml of a s ~ica solution (particle diameter 7
nm) which had been purified by overnight dialysis was
transferred to a 250 ml beaker, and the pH adjusted to 4,0 by
5 dropwise addition of 0.01 M nitric acid. THis was heated to
90C, and 0.01 M AgNO3 solution added dropwise under gentle
stirring to achieve the final concentration shown in the
table below. After about 2 minutes, sufficient quantity of
0.01 M hydroquinone was added in a similar manner. The
10 reduction to metallic silver takes place gradually over a
time period of about five minutes, accompanied by a color
change from pale yellow to dark brown. The rate of silver
deposition by this method can be controlled by varying the
temperature between 85 and 95C. A transparent solution is
15 obtained in every case, and is allowed to cool and then
purified by dialysis.
The following table summarizes the experimental
conditions, including final concentra- ~ns, which were used
in four different sets:
I II III IV
Sio2 1% 1% 1% 1%
AgN~3 S.OxlO 4 l.OxlO 3 l.SxlO 3 2.0xlO 3
Hydro- S.OxlO 5 l.OxlO 4 1.5xlO 4 2.0xlO 4
quinone
The amount of silver deposited increases from I to
IV, and is evident from the color of the solution (light
yellow to dark brown). Electron microscopy also provided
evidential support. The optical absorption spectra show the
presence of a single peak maximum at about 400 nm.
WO91/06036 PC~/US~/~X~ -
2~389 _3~_
1 ELECTRON MICROSCOPIC RESULTS:
Solution 1 consists of particles which are smaller
and better defined, appear darker, and were in the size range
of 10 to 30 nm. In solution II, III and IV, the particle
5 size range was found to be between 40 to 100 nm, the
particles were similarly dark, but contained elongated as
well as spherical shapes. The final size distribution may be
due in part to the non uniform size of the silica core
particles, found to be between 7 to 11 nm by electron
lO microscopy.
With all of the processes described above, after
the coated particles are prepared, they may be removed from
the solution in which they were prepared by dialysis, and
then placed in a sodium dodecyl sulfate micellar solution or
15 a micro emulsion. Additional coatings of either silver
halide, a metal or a polymer, may then be added until the
desired final configuration is reached. Polymer coating of
any of these particles may be readily achieved in a solution
by the well known emulsion polymerization method, in which a
suitable amount of monomer and initiator have been added.
For instance, the following process, outlined in
Figure 16, shows how a polymer coating may be made on a
silver coated particle.
The following aqueous stock solutions were
Prepared
~I) 0.1 M ~H2 PO4'
(II) 0.1 M NaOH,
(III) 2% solution of sodium salt of styrene sulfonic acid,
NaSS (comonomer),
(IC) 3% solution of K252O8.
W~9l/06036
Pcr/usso/0600s
~ ~07038~
l All solutions were prepared in doubly distllled
wate , and all chemicals were reagent grade.
131.6 ml of a 1% solution of the silver coated
silica particles were transferred to a three necked flask. 8
5 ml of solution IV, followed by 6.4 ml of solution II, were
added with constant stirring using a magnetic stirrer. The
flask was equipped with a condenser, and a platinum
thermometer, which, in combination with a thermoregulator and
a heating mantle, allowed regulation of the temperature of
lO the flask to 65 ~ 1C. At this temperature, nitrogen gas was
bubbled through the mixture continuously, and 30 ml of
styrene added. After 15 minutes, 10 ml of solution III were
added, and after another 20 minutes 4 ml of the initiator,
solution IV, were added. Depending upon the thickness of the
15 polymer film desired, the reaction can be terminated by
addition of 25 ml of a 1% solution of hydroquinone, and
cooling the reaction mixture to room temperature. The
particles are filtered, washed several times with doubly
distilled water, resuspended in water, and further purified
by dialysis.
3o
w09l/06036 Pcr/usso/~x~
2e7~389
1 EXAMPLE 5
~ oating of carbon fibres with copper was carried
out by photochemical reduction of Cu using highly reductive
short lived l-hydroxy-l-methylethyl radicals. These radicals
5 were produced in situ by illuminating a mixuture of 1 M
acetone and 1 M propanol-2 with a W source of Hg-Xe lamp
operated at 450 watt. The reaction can be presented by
(CH3)2CO --~ (CH3)2CO
(CH3)2CO + (CH3)2CHOH ~ 2(CH3)2COH
2(CH3)2COH + Cu+2 > 2(CH3)2CO + Cu + 2H
nCu ~Cu
Two different solutions of Cu (1 x 10 2 M and 1 x
10 3 M) were used to achieve two different coating
thicknesses. Both solutions contained 1 M acetone, 1 M
propanol-2, and carbon fibers. The illumination time was two
20 hours.
These coated fibres, washed with distilled water
and observed under an optical microscope, show a very fine
and smooth coating and visibly exhibit a metallic lustre of
copper. The amount of copper on these fibres was detected
25 using atomic absorption spectroscopy after removing the coat
with 1 M nitric acid. The presence of copper on these fibres
was also confirmed using Energy Dispersive Spectroscopy
(EDS), which shows a peak for copper. The thickness of the
coat can be controlled by the copper concentration in
3 solution and the duration of illumination. It can be readily
varied in the range of tens of nanometers to microns.
WO 91/06036 PCrtlj.S~0/lKOO~J
- 2Q7~389
l The processes discussed abo~e may be used in
various combinations to form particles of a desired
configuration. For example, Figure 17 generally outlines a
procedure to make particle 20 of Figure 4. First, metal
coating 20b is formed over dielectric core 20a, for example
using the method illustrated in Figure 13; and then silver
halide coating 20c is made over metal layer 20b, for instance
by generally following the method shown in Figure 10.
Similarly, Figure 18 generally illustrates a procedure to
make particle 22 of Figure 5. In this procedure, first,
metal coating 22b is formed over dielectric core 22a, for
example by the process described above in connection with
Figure 11, then polymer coating 22c is applied over coating
22b, and then silver halide layer 22d is formed over coating
22c, for example by generally following the procedure
discussed above in connection with Figure 10.
Methodology of the present invention are not
limited to coating metals on a nanoparticle. Methods
described herein can also be used to plate metals onto a
substrate. This is especially useful if the substrate is a
catalyst support system. By using methodology described
herein, the catalyst support system can be coated with
metals, e.g., Group VIII metals and provide a surface on
which catalysis can occur. Catalyst support systems include
zeolites, polystyrene, metal oxide, (e.g., titanium oxide)
polymer support and the like.
The methods of the present invention can be used to
coa~ other surfaces. For example, methodology of the present
invention can be used to coat dielectric material, such as
silica, glass, cadmium sulfide, gallium arsenide,
polydiacetylene, lead sulfide, PNMA, silver bromide, carbon
fibers, copper sulfide, silver sulfide, glass fibers, and the
like.
WO9l/06036 ~cr/~ x~ -
2~70389 ~ ~
1 The methods of the present invention can also be
used to plate metals onto other metals, especially Group VIII
metals.
The metals that can be plated onto the substrate
5 include the lanthanide, the Group VIII metals, the Group IA
metals and the Group IIIA metals. The preferred metals are
silver, copper, gold, iron, nickel, palladium, platinum,
cobalt, rhodium, iridium, ruthenium and aluminum.
One of the preferred methods for coating metals
10 onto the surface of a substrate entails providing a source of
metal ions, secondary lower alkanol and a lower alkyl ~etone
distributed in an anerobic liquid carrier containing said
substrate and exposing the liquid carrier to light to cause
the metal ions to form metal and to plate onto the surface of
15 the substrate. This procedure is used in the same manner as
that described herein for coating a dielectric particle with
the secondary alcohol, alkyl ketone, source of metal ions and
light.
The other procedure for coating metals ~e.g.
20 silver) onto surfaces is the photoreduction method described
herein. A substrate is coated with metal ~e.g. silver)
halide by reacting a source of metal ~e.g. silver) ions with
a source of halide ions on the surface of a substrate in an
anerobic carrier containing an electron scavenger and
25 optionally a growth stabilizer. The substrate surface is
then illuminated with light, whereby the metal (e.g. silver)
halide coatings is converted to metal (e.g. silver) coating.
This procedure is thus used in the same manner as described
herein for coating dielectric particles.
3o
W~9l/06036 PCT/~S90/~X~
_.7_ 2 Q 7 0389
1 Although the text hereinabove refers to halides,
the halides may be replaced with organic anions, thereby
forming other metal complexes. A property of these organic
anions is that they must be capable of forming stable
5 complexes. These organic ions include such anions as
acetate, formate, citrate, EDTA, malonate, and polypeptides
prepared from the natural amino acids, such as poly GLU, poly
ASP, and the like.
While it is apparent that the invention herein
10 disclosed is well calculated to fulfill the objects
previously stated, it will be appreciated that numerous
modifications and embodiments may be devised by those skilled
in the art, and it is intended that the appended claims cover
all such modifications and embodiments as fall within the
15 true spirit and scope of the present invention.
3o