Note: Descriptions are shown in the official language in which they were submitted.
W~ 91/06035 2~ 73 ~ ,9 PCT/US90/06008
hIGHT-SENSITI~E ~ECORDING MEDIA
BACKGROUND OF THE INVENTION
This invention generall~ relates to light sensitive
recording media such as a light-sensitive silver-halide
emulsion.
Light sensitive xecording media are used in many
different applications; and, f or exc~ple, light ~ensitive
silver halide emulsions are often used as photographic
10 emulsions. The requirements for a commercially successful
photographic silver halide emulsion are quite severe~ Among
other needs, such emulsions must be highly sensitive, have
fine grain, sharpness and abundant latitude, and have
sufficiently high optical density and sufficiently low fog
density. In addition, the emulsions must be highly
processable, easy to develop and to wash, but at the same
time, the ~mulsions must be able to hold or fix an image and
be highly resistant to various ohemical agents. Further, it
; is iMportant that the photographic and processing properties
of the emulsions be stable over lengthy pieriods of time prior
to use, that the quali y of the emulsions b~ highly
dependable and repxoducible, and that the cost of produci~g
. the emulsions be low. .
Numerous specific light sensitive emulsions are
known that, to one degree or another, satisfy these
requirements; and sever~l known emulsions contain particles
r
of silver halide dispersed as a colloid or i~ gel, with
i either the construction or properties o~ the particles b~ing
designed to improve or enhance the ~uality of the emulsions.
For instance, U.S. Patent 4,484,877 discloses a
, light sensitive silver halide emulsion whicb comprises silver
,,
,
~ 35
.,
.f
wogl/06035 2 ~7 ~ 9 rcT/us9J/o6
halide grains composed of a core and a shell. The core
i consists essentially of silver halide containlng silver
iodide, and the shell covers the core and consists
essentially of silver bromide, silver chloride or ~silver
chlorobromide. The shells have thicknesses from 0.01 to 0.1
micrometers.
U.S. Patent 4,72~,602 discloses a light sensitive
silver iodobromide emulsion containing silver iodobromide
grains composed of a core and a shell. The core
substantially comprises silver iodobromide containing at
10 least about 5 mol percent of silver iodide; and the shell
substantially comprises iodobromide having a lower silver
iodide content than ~he silver iodobromide content of the
core, or the shell substantially comprises silver bromide.
i The relative standard deviation of the silver iodi~e content
15 of the individual grains of the emulsion is lower than about
20 percent.
U.S. Patent 4,639,410 discloses a silver halide
color photographic light sensitive material including a
core-shell type silver halide emulsion. The shells in the
emulsion consist substantially of silver bromide, but they
may contain silver iodide, silver chloride or silver
iodochloride; and the core of each sphere is preferably
silver iodobromide, although it may contain a silver halide
other than silver iodobromide, such as silver chloride.
All o~ these emulsions, as well as most or even all
other conventional silv~r halide photographic emulsions,
contain relatively large amounts of silver and color films
contain relatively large amounts of dyes and/or precursors of
dyes. Because of the high cost of silver, it is very
desirable to provide a silver halide photographic emulsion
.
.~ .
~'
, .
W~91/06035 Pcr/~s~o/
containing less silver and less dyes and dye precursors than
1 thes~ conventional emulsions.
Light sensitive recording media are also used as
optical disks or plates to record data. In such
applications/ a first light beam, referred to as a write
5 beam, is passed over the recording medium in a giv~n pattern
to alter the morphology of the recording medium over a path
or selected areas, ~hich thereby represents stored data.
After this change in morphology, the portions of the
recording medium that were exposed to the write beam are very
10 much less able to absorb light than are the portions of the
; recording m~ um that were not exposed to the write beam.
An~ther light beam, referred to as a read beam, of
; an intensity low enough so that it does not change the
morphology of the recording medium, can then be passed over
15 that medium. The read beam is reflected or transmitted when
it strikes a portion of the recording medium previously
exposed to the write beam, while the read beam is absorbed
when it strikes a portion of the recordiny medium not
previously exposed to the write beam. In this way, the read
20 beam can be used to determine, or read, the data stored in
the recording medium.
Heretofore, optical recording media were not made
in a manner that takes advantage of the plasmon resonance
effect, which is an effect that increases the intensity of
certain electromagnetic fields. In accordance with the
present invention, it has been determined that optical
r~cording media c~n be made that effectively employ the
plasmon resonance effect to significantly improve the
sensitivity of the recording media to light and other
electroma~netic radiation in the optical spectrum.
. .
., .
~.
~ 35
.,
,
A
~,; . .: .' '. . , . ~ ' '
WO 91/0603~ ~'7~ h ~9 PCT/US90/06008
Th~ plasmon resonance effect is shown by certain
1 small particles and this effect includes the enhancement of
electromagnetic fields at certain frequencies inside and near
the particle, and the enhancement of the scatteringj
absorption and extinction of certain frequencies of light.
5 The extinction is defined as the sum of the absorption and
scattering. The extent to which a particle exhibits the
plasmon resonance effect depends on a number of factors,
including the size and shape of the particle, the material or
;~ materials from which the particle is made, and, in a particle
10 made of a plurality of materials, the order, number, shape
and dimensions of the materials from which the particle is
made. For example, the plasmon resonance effect may be
enhanced in particles that have sizes on the order of
; magnitude of tens of nanometers, and thus are commonly
15 referred to as nanoparticles. Because of the above
considerations, nanoparticles that consist of a number of
materials are of special interest because they can be made to
exhibit an enhanced plasmon resonance effect in a selected
electromagnetic frequency range. One consequence of the
frequenc~ dependence of the plasmon resonance effect, and
hence the frequency dependence of absorption and scattering,
-~ is that the particles are colored and consequently can be
; useful in color photography, color printing, color copying,
etc. Any fre~uency at which a particle exhibits the plasmon
resonance effect is xeferred to as a resonance frequency or a
plasmon resonance frequency of the particle.
It has been recognized for many years that the
plasmon resonance effect in small metal particles can be
responsible for absorption and scattering phenomena of
electroma~netic radiation.
.~ .
., ~
. , .
,
... .
~, :
"
,
~"
WO;~I/0~035 5 PC~/US90/060
c~
Recently Ker~er et al. in Phy. Rev. B, 26,
l 4052-4062 (1~82), have recognized that by designing composite
nano particles comprised of metal and dielectxic layers, the
~ plasmon resonance can be greatly enhan.ced. This now makes it
: possible to use the effect in many processes from nonlinear
optics to photochemical catalysisO
Consider, for example, a spherically shaped nano
particle suspended in a medium, and cc~nsisting of a 5pherical
core made of a dielectric material surrounded by a shell made
of a metal. When electromagnetic radiation of a wavelength
much longer than the size of the particle ls incident on that
particle, the radiation scattering coefficient, a~, of the
particle, including the effect thereon of the plasmon
resonance effect, is given by the equation:
al~ 2/3 i~(e?-el) ~el-2e~) + q3(26~+ ~ ~l-e )
I (e2~2e3) ~el~2e21 - q ~2e2-2e3) lel-e2)J
where, q= a/b
. a-2~ b/~
: i is the imaginary number, ~ J
a is the radius of the core of the particle,
:~ b is the radius of the particle,
is the wavelength of the incident electromagnetic
radiation,
E 1 is the dielectric constant of the core,
E2 is the dielectric constant of the shell, and
~3 is the dielectric constant of the surrounding
medi~n.
:.
.~ . .
,,
:
WO9l/06~35 ~ PCT/US9(~/06~08
In the computations for this patent application,
1 all the coefficients of any significant mag~itude were
included. As mentioned above, optical recording media have
not previously been made so as to utilize fully the plasmon
resonance effect. Pursuant to the present invention, by
5 providing an optical recording medium with selected nano
particles, the plasmon resonance effect can be effectively
employed to enhance dramatically the photoprocesses that
occur in the media.
In the present invention, particles are designed
lO and made which use various features of the plasmon resona}lce
effect to lmprove and enhance photographic emulsions and the
photographic process. First, particles which enhance the
fields are used to increase the intensity in the silver
halide layer of the particles and hence to enhance the
15 sensitivity of the silver halide to a given intensity of light
incident upon the emulsion. Second, coated particles, which
enhance the absorption and scattering of light with a much
smaller amount of silver than required with solid silver
particles, are used to decrease the amount of silver needed
in photographic emulsions, prints and films made with silver.
` Third, coated particles, which enhance the
~requency-dependent absorption and scattering, and hence are
colored, are used to decrease the amount of dyes needed in
photographic prints and films.
~` SUMMARY OF THE INVENTI 0~7
.. _
An object of this invention is to provide enhanced
optical responses ln light sensitive media.
i
..
; 35
-,:
.,
;
, .
WOgl/06035 ~7~ PCr/US~0/06008
~` 2~7~
Another object o this invention is to reduce the
1 amount of silver needed in a light sensitive silver halidè
emulsion.
Another object of this invention is to reduce the
amount of dyes and dye precursors needed in a light sensitive
5 emulsion.
Another object of the present invention is to use
silver halide coated dielectric particles in a light
sensitive recording medium.
A Eurther object of this i,nvention is to use
' 10 particles comprising silver halide coated dielectric cores in
a light sensitive silver halide emulsion, where those
particles also include a metal shell to enhance the
sensitivity of the silver halide to light.
Still another object of the present invention is to
15 utilize the plasmon resonance effect to enhance the
sensitivity of an optical recording medium.
Another object of this invention is to provide an
optical recording medium with a multitude of nanoparticles,
each of which is made to exhibit the plasmon resonance
20 effect, to enhance the photo processes that occur in the
~ recording medium.
'IA These and other objectives are attained with light
,j
'I sensitive recording media constructed according to the
'- present invention. With a first embodiment, the medium is a
,~, 25 light sensitive silver halide photographic emulsion,
~, comprising a colloid or gel, and a multitude of silver halide
' particles dispersed in that colloid. Each of these particles
- includes at least a core surrounded by a shell, one of the
core and shell includes silver halide, and the other of the
core and shell includes a dielectric. Preferably, one of the
:"'
.
'
; 35
.
.
.
- , ., ~ . . . ~ , , "
WO9l/06035 -8 PCr/US90/0600X
2~
core and shell consists essentially of silver halide, the
l other of the core and shell consists essentially af the
dielectric material, and this dielectric material is
substantially free of silver.
With a second embodiment, the recording medium is a
5 light sensitive optical recording disk or plate, comprising a
solid, light reflecting or light transmitting substrate, a
colloid applied onto the substrate, and a multitude of
particles dispersed in that colloid. Each of these particles
includes a core surrounded by a shell, and at least one of
lO the core and shell consists essentially of a metal.
Pre~erably, the other of the core and shell consists
essentially of a dielectric; and more specifically, the core
of each of these preferred particles consists essentially of
a dielectric material, and the shell of each of these
15 particles consists of a metal.
Further benefits and advantages o~ the invention
will become apparent from a consideration of the following
detailed description given with reference to the accompanying
drawings, which specify and show preferred embodiments o~ the
invention.
BRIEF DESCRIPTION OF ~HE DRAWINGS
. .
Figure 1 diagrammatically illustrates a
photographic emulsion accordin~ to the present invention.
Figures 2-5 and 5A~ which are not drawn to scale,
show particles that may be used in the emulsion of Figure 1.
Figures 6-11 show the light absorption efficiency
of solid silver spheres and silver coated silica spheres at
wavelengths of 355r~, 382nm, 414nm, 497nm, 621nm and 828r~
respectively.
.~ .
` 35
-
W~91/06035 PCT~US90/06008
Figures lla-llf show similar wavelength dependent
1 spectra observed in the scattering and extinction spectra.
Figures 12 and 13 show optical ~ecording media also
according to the present invention.
Figures 14-27, which are no-t drawn to scale, show
various particles that may be used in the optical recording
media of Figures 12 and 13.
Figures 28-36 outline several processes that may be
used to ~orm the particles shown in Figures 2-5 and 14-27.
Figure 37 is a transmission electron micrograph of
silver-coated silver bromide nanoparticles.
Figure 38 is a transmission electron micrograph of
silver coated silver bromide nanoparticle treated with
ammonia.
Figure 39 shows various optical extinction spectra
- 15 of silver coated silver bromide nanoparticles. (a) to ld) are
; spectra of illuminated solutions of Ag, Br, and EDTA with
specific concentrations. In going from a to d the
illumination time increases. (e) typical spectrum observed
` after the addition of ammonia to any of the above solutions
either untreated or treated with ammonia, which were measured
~''t shortly after light exposure at different illumination times.
Figure 40 shows computed extinction efficiencies
for silver coated silver bromide nanoparticles. The diameter
of the core particle is 20 nm and the thickness o the silver
coats are indicated in nm. The spectrum marked solid is that
of a homogeneous 20 nm diameter silver sphere.
Figure 41 is an optical extinction spectra of a
measured silver coated silver bromide nanoparticle and two
computed spectra. The measured spectrum lies between the two- 30 computed spectra. In the upper curve all of the silver is
assumed to come ~rom the reduction of AgBr at the particle
; surfacP.
''
.,
~ 35
.,,'~ .
;
WO91/06035 Z~3fc~ 9 ~ Pcr/us90/0~008
... . ~,
DETAILED DESCRIPTION OF T}~E PREFERRED EM~3ODIME:NTS
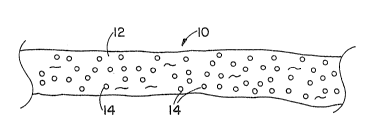
Figure 1 illustrates a first light sensitive
recording medium 10 according to the present invention. This
medium is a light sensitive silver halide photographic
emulsion, comprising a colloid or gel 12, and a multitude of
silver halide particles 14 dispersed in that colloid.
Figures 2-5 show four types of particles, referenced at 16,
20 and 22, and 24, respectively, that may be used in emulsion
10. Each of these particles includles at least a core
surrounded by a shell; and in each of these particles, one of
the core and shell includes silver halide, and the other of
the core and shell includes a dielectric material.
Preferably, the one of the core and shell consists
essentially of silver halide, the other of the core and shell
consists essentiially of a dielectric material, and moreover,
this dielectric material is substantially free of silver.
For example, particle 16 consists o~ core 16a and sh~ll 16b,
the core consists essentially of a dielectric material.
~` The term "dielectric" material or core as used
herein, refers to a material which is a non-conductor or a
semi conductor. The conductivity of the material may range
from 0, ~ut preferably as low as 10 40 to 106 mhos. In a
prefierred embodiment, the conductivity ranges from 10 40 to
` 105 mhos. In a most preferred embodiment, the conductivity
-~ 25 ranges from 10 30 to 104 mhos. Examples of dielectric
materiaI includes glass, silica, cadmium sulfide, gallium
arsenide, polydiacetylene, lead sulfide, titanium dioxide,
polymethylacrylate (PMMA), silver bromide, carbon fibers,
copper sulfide, silver sulfide, and the like.
3
~,.
` 35
,~
~ . .
~!
". ': . ' . . ' , . . ~ . ' .: , ' ' ,' ,, . :'
: ' .
W~ 91/06035 ~ PC~t~JS~0/06~08
s
The shell consists essentially of silver halide. s
1 Further, with this particle, shell 16b is disposed
immediately over and subs~antially completely covers core
16a.
In particle 20, a metal coating such as silver,
5 copper, aluminum, gold or palladiurn is disposed between the
dielectric core and the silver halide shell to increase the
sensitivity of the silver halide to light~ This increased
sensitivity is caused by the plasmon resonance effect
produced by the metal coating. Moxe specifically, particle
20 consists of dielectric core 20a, metal coating 20b
disposed immediately over and covering that core, and a layer
of silver halide 20c disposed i~nediately over and covering
layex 20b.
If it is desired to use a layer of metal between
15 the dielectric core and the silver halide shell, it may be
preferred, as is done in particle 22, to separate or space
the metal from the silver halide to prevent interference in
the de~elopment of the latent image. In particular, particle
22 consists of dielectric core 22a, a layer of silver 22b
! 20 disposed immediately over and covering core 22a, a layer of
9 dielectric material 22c such as a polymer, disposed
immediately over and substantially covering the silver layer,
~3 and shell 22d formed of silver halide disposed immediately
over and substantially completely covering layer 22c.
It is not necessary to the present invention in its
`; broadest sense that the dielectric material and the silver
; halide of the particles used in emulsion 10 form the core and
~shell of the particle, respectively, and Figure S shows a
fourth particle 24 that may be used in emulsion 10 and which
comprises core 24a comprised of silver halide and shell 24b
` ~ .
~ 35
'',
~i
, -
.,.,.~: : , . ~ .
W~91/0~035 -12- Pc~/usso/o6o~i~
2~ Y~ ~
comprised of a dielectric material. with the particle 24
1 shown in Figure 5, it is also desira~le for some emulsions to
provide the particle with a layer of metal (not shown) to
enhance the sensitivity of the silver halide of the particle;
and if this is done, to further provide the particle with a
still further coating of a dielectIic material [also not
shown) between that metal layer ancl the silver halide core of
the particle to prevent interference in the development of
the latent image.
Coated nanoparticles can be designed and made which
use features of the plasmon resonance effect to improve
photographic emulsions. Plasmon resonances of nanoparticles
can be used to improve both the recording (writing) of an
image and the regeneration (xeading) of the image. In the
next two paragraphs the ways in which plasmon resonant
particles can be used in photography are explained briefly.
A more detailed discussion follows.
When light from a scene illuminates a typical
silver halide photographic emulsion it generates very small
silver particles in the silver halida grains. The pattern of
small silver particles form a latent image. It is often
desirable to increase the sensitivity of an emulsion to
light, i.e, to make an emulsion in which a latent image can
be formed with less light. By replacing the solid silver
halide particles in an emulsion with coated nanoparticles
where there is a silver layer underneath the silver halide
layer, the sensitivity o~ the silver halide to light can be
enhanced. A layered particle having the appropriate
thicknesses of dielectric~ silver, polymer, and silver halide
can have a plasmon resonant enhancement o~ the light in the
silver halide layer. The enhanced optical intensity induces
, . .
`'" ' .
. 1 .
. A;
_, ~
~ .
'.
WO91/06035 -13- PCT/US90/06008
2 ~ ~9
the ~ormation of more latent image centers in a plasmon
l resonant particle than in solid silver halide particles
illuminated with the same light intensity. Hence, the
plasmon resonance effect can be used to increase the
sensitivity of a given amount of s.ilver halide to light, and
can improve the recording of an image.
After a typical silver halide emulsion is exposed,
the small clusters of silver in the latent image act as
nucleation centers for the reduction of the rest of the
silver in the grain and/or silver :Erom solution. In black
and white photography, the absorption and scattering by the
reduced silver is responsible for the image observed when
illuminated with light. In color photography, the reduction
of the silver is coupled to the generation of dyes. When
illuminated with white light, the dyes in the amulsion absorb
certain wavelengths and consequently the film tran$mits
and/or reflects the complementary wavelengths. The solid
silver particles generated after development in black and
white photographs can be replaced with silver coated
nanoparticles that use less silver to absorb and scatter more 20 light. The same volume of silver can be much more absorbing
when it is coated at the right thickness onto the surface of
a dielectric nanoparticle. so it is possible to reduce the
amount of silver required in the photograph. Also, the dyes
`~ generated during development in a color photograph can be
replaced with plasmon resonant silve~ coated nanoparticles
that, when illuminated with white light 9 reflect and transmit
colored light. By replacing expensive dyes with coated
nanoparticles, the cost of film can be reduced. So, plasmon
resonant coated nanoparticles can be used tv reduce the cost
of both color and black and white photogr~phs.
:..
,
-~ 35
`'
, . .
:', : ,;-.,., .:; '. .. ;; '
WO91/06035 1~, PCT/US~0/06008
2~a~
Particles must be designed to enhance those aspects
1 of plasmon resonance required for each particular purp~se.
The next five paragraphs state in more detail the aspects of
plasmon resonance used in the coated nanoparticles, how these
aspects are used in light recording devices, and the
advantages of using particles having such resonances.
Improvements in recording the image are discussed in (1).
Improvements in reading or regenerating the image are
discussed in (2)-~4). Improved paxticles to ,bsorb stray
light are discussed in (5)O Emulsions combining some of the
particles are discussed in ~6).
(1) Coated nanoparticles having a plasmon resonant
enhancement of the light in the silver halide layer of an
emulsion can be used to increase the sensitivity of a given
amount of silver halide to a given incident intensity of
light. Such coated particles can be of use in recording an
image in either color or black and white photography" One
type of appropriate nanoparticle has a dielectric core, a
first coat of silver, a second coat of a polymer, and a third
coat of a silver halide. The thicknesses of the core and
f. 20 coats must be chosen to enhance the light intensity at the
appropriate wavelength range in the silver halide layer. An
advantage of using these particles is an increased
sensitivity of the emulsion and a decreased requirement for
silver.
(2) Coated particles having a plasmon resonant
enhancement of the absorption of light can be used as the
absorbing regions in black and white films. Prior to
;i development, the coat of a particle is a layer 4f silver
halide. During development, the silver halide is reduced to
a coat of silver that has the correct thickness for a plasmon
.
~,
~, 35
: .
.,
.
:
,, ., .. .. . . :.. , , . . .,. .. , . :, :
W ~ 1/06035 2 ~ 9 PCT/Ui~90/0600
r~sonance. Such nanoparticles require a relatively small
l amount of silver to scatter and absor~ light as efficiently
as do solid silver particles. These particles can be used to
d~crease the amount of silver needed in photogra~hic
emulsions, prints and films. For black and white
photography, a group of particles having plasmon resonances
at differ~nt frequencies should be combined in the emulsion.
An advantage of using such particles is that less silver is
required. Also, since a smaller particle can have a
significant absorption and a thinner layer of particles i5
required, there can be some increase in the resolution of the
film.
(3) Coated particles, which enhance the
frequency-dependent scattering and absorption (i.e. the
extinction), and hence transmit light that is colored, can be
used to decrease the amount o~ dyes needed in photographic
~ilms and slides. For applications where the light is
transmitted through the emulsion, colored light can be
generated using either scattering or absorption or any
combination. The particles in a color photographic film or
slide that go into the layer whose transmitted light is to
appear red (for example), are particles that, when developed,
absorb and scatter blue and yellow light, while transmitting
the xed. The particles, before development, have a layer o~
silver halide that has a thickness such that, when developed,
the resulting thickness of the silver coat and the thickness
o~ the-other layers result in a particle that absorbs and
scatters as desired. The advantage of using such particles -
is that they will replace dyes which are expensive.
(4) Coated nanoparticles having a plasmon resonant
light scattering spectrum can be used to decrease the amount
~'
. .
,,
~ 35
,, .
~,
'
:,
: ~...... -.,i, ': . . '.. ' ,. ; . . .
W~91/06035 -16- PCT/US90/~6008
of dyes needed ln color prints since the light reflected rom
1 these particles is colored. These particles should have a
relatively large plasmon enhancement of the frequency
dependent scattering and a relative~].y smaller enhancement of
the frequency dependent absorption. Relatively large
5 nanoparticles, typically having diameters in the range of 60
to 200 nanometers, should be used since their ratio of
scattering to absorption is larger. The particles, before
development, have a layer of silver halide that has a
thickness such that, when developed, the resulting thickness
lO of the silver coat and the thicknesses of the other layers
result in a particle that scatters as desired. The particles
in a photographic print placed in the layer of the emulsion
that is to appear red ~for example), are particles that, when
developed scatter red light. Such particles can also be used
- 15 for color copiers and colored inks.
(5) Coated nanoparticles having a plasmon resonant
enhancement of the absorption of light can be used to
decrease the amount of silver needed in the absorbing layers
of photographic emulsions, prints and films. These particles
20 should have a more enhanced absorption than scattering and
hence are particularly small, in the range 5 to 60
' nanometers. Such particles can be used to replace the
Cary~Lea sols now used to absorb stray light in both black
and white and in color films. They can also be used as the
absorbing regions in black and white films. An advantage of
3 using such particles is that less silver is re~uired.
(6) Emulsions in which combinations of the above
particles and e~ects, and particles in which the above
. advantages and effects are combined are also useful. An
example of a particle in which (13 and (2) is employed is a
.
., .
, . , , . , ." , . .
-17-
WO9l/0603~ PCI/US~0/06008
~7 ~
particle having a dielectric core, a silver coat, a polymer
1 coat and then a silver halide coat. The plasmon resonance
enhances the fields in the silver halide layer. When thP
silver halide is developed to silver, the plasmon.resonances
in the scatterin~ and absorption b~ the particle provide the
advantages descri~ed in (2) above. The particles described
in (5) can also be used in the same emulsion.
To achieve the advantage~; described in ~2), (3) and
: (4) above, it is preferable that the silver halide coat is
reduced to a relatively smooth silver coat. With reference
to Figure 5A, one way to force the reduced silver to form a
: smooth coat on ~.he dielectric is to precoat the original
particles 16 with a polymer 16C that is porous enough to
allow ions to pass. For example, the coat 16C can prevent
Ag+ ions from migrating to sites on the surface next to the
polymer. The polymer 16C encourages the Ag~ to go to the .
positions where growth is desired. Such a polymer coat can
be made in a manner similar to that specified herein.
Also, independently of the photographic process,
this is another way to make silver coated particles.
Figures 2-S are only representative of
nanoparticles that may be used in the practice of the pre~ent
invention, and in particular, only illustrate the general
-; relationship between the cores and the shells of the shown
, particles. In any nanoparticle used in this invention~ the
particle and the core thereof may have any suitable shapes,
and specifically, the particles and the cores may have shapes
other than spherical. For instance, the parti.cles and the
cores may be cylindrical or ellipsoidal, have a thread-like
shape, or be crystalline shaped. The actual crystal form of
3o
, .
.
.. . .
, : ~ . ~; , .... . ..
WO91~06035 ZC~ 3~3 PCr/usgD/0600g ~ ~
. .
the core may be any suitable form; and, for example, these
1 cores may be:
Tetragonal crystal forms,
Orthorhombic crystal forms,
Monoclinic crystal forms,
Triclinic crystal Eorms,
Isometric crystal forms,
- Hexagonal crystal forms.
Furthermore, the emulsion 10 may include a mixture
of particles of different sizes and shapes, and the emulsion
may include silver halide particles of the type convention-
ally used in photographic emulsions. Still further, any
suitable dielectric material may be used in the particles
employed in this invention; andr in particular, the
dielectric material may be linear or non-linear. In
: 15 addition, as the term is used herein, "metal" includes any
:~ material having a negative dielectric constant, and so can
include superconductors, conducting polymers r and materials
;~ with an anomalous dispersion of carrier electrons, and
; heavily doped semiconductors where free carrier motion
dominates the dielectric function.
The development of emulsion 10 is based r in part,
on the fact that at various wavelengths of light, silver
. coated dielectric spheres may be made that absorb and scatter
`; light at a much higher efficiency than do solid silver
spheres of the same size, and this fact is demonstrated in
Figures 6-11.
Figures 6-11 show the light absorption efficiency
of solid silver spheres and silvex coated silica spheres at
. various wavelengths of light; and in particular, at
wavelengths of 3S5nm, 382nm, 414nm, 497nm, 621nm and 828nm
,''
,; .
.
: 3~
:' .
,
.
., .
..
Wog1/0603~ 19- P~T/US90/0600
,~ ~9
respectively. The efficiencies were computed using a
1 procedure described by Toon and Ackerm~n, Applied Optics,
1931. The light absorption efficiency~ Q, of the particles
is plotted along the vertical axis of each Figure; and a size
parameter, x, equal to2~r/~ where r is the radius of the
particle and ~ is the wavelength of interest for the
respective Figure, is plotted alon~ the horizontal axis of
each Figure. Curves 32a, 34a, 36a, 40a, 42a, and 44a of
Figures 6-11 indicate the light absorption ef~iciencies of
solid silver spheres. Curves 32b-d, 34b-f, 36b-e, 40b-g,
42b-f and 44b-e indicate the light a~sorption efficiencies of
silver coated silica spheres; and in particular, each of
these curves represent a constant value (shown in
parenthesis) for the ratio of the diameter of the internal
silica core to the diameter of the whole coated sphere.
Thus, for instance, the values determined from
curve 32b of Figure 6 are for silver coated silica spheres
comprising an internal silica core having a diameter that is
~` half the diameter of the whole coated sphere, and the
absorption values determined from curve 36d of Figure 8 are
for silver coated silica sphere~ comprising an internal
`1 silica core having a diameter that is 0.6 times the di~meter
of the whole coated sphere.
For example, Figure 6 shows that at a wavelength of
355nm, for a coated sphere having an outside diameter of
about 113nm ~x=l.0~ and including an internal silica core
having a diameter half that of the whole coated sphere, the
absorption efficiency of that coated sphere is about 0.93.
~; Similarly, Figur~ 9 shows that at a wavelength of 497nm, for
;~ a coated sphere having an outside diameter of ahout 79nm
- 3O ~x=0.5) and including an internal s.ilica core having a
W091/06035
Pcr/usso/06oos
;2Z~Zi7
dia~eter that ls 0.7 times the diameter of the outside
l diam.eter of the w~Ie Z~o~ted ~ re~ t~e ahsarpZt~Z~n
efficiZ~ncy of that coated sphere is about 5.2.
Flgures 10 and 11 show th,at, for spheres having
outside diZ~meters between 1 and 100 nanometers and for light
; 5 in the wavelength range of G21 to ~28 nanometers, spherical
particles comprised of silica cores coated with an
appropriate thickness of silver absorb many times more light
than do solid silver spheres of the sam.e diameter. For
example, Figure 10 shows that at a wavelength of 621nm,
first, the absorption eEficienoy of a solid silver sphere
having an outside diameter of about 198 nanometers (x= 1.0)
is about 0.3, and second, a coated sphere having the same
outside diameter and an internal sili.ca core having a
diameter 0.9 times the diameter of the whole coated sphere,
has an absorption efficiency of about 2.0, which'is more than
six times the absorption efficiency of the solid silver
sphere of the same size~ FiZ~ure 11 shows that at a
wavelength of 828nm, first, the absorpticZn efficiency of a
solid silver sphere having an outside diameter of about 132
20 nanometers (x= 0.5) is about O.2, and second, a coa~ed sphere
having the same outside diameter and an internal silica core
having a diameter 0.9 times the diameter of the whole coated
sphere, has an absorption efficiency of akout 5.8, which is
', about twenty-nine times the aksorption efficiency of the
solid silver sphere of the same size.
FiZ~ures 6 and 7 show that, in the near ultraviolet
wavelenyths (at wavelengths of 355 and 382 nanometers), the
solid silver spheres absorb more light than do the silver
.' coated silica cores, at least with spheres between 1 and 100
. 30 nanometers in diameter. However, with scZme oE the larger
'~, 35
. Z
. .
". .. .
W~ 91/06035 ~ 9 -21- PCr/US~0/06~08
spheres, some of the silver coated particles absorb as much
1 or more light in the ultraviolet wav~lengths than do the
solid silver spheres. Also, with some of the larger sizes of
the spheres, the silver coated particles and the~solid silver
spheres have similar absorption efficiencies. The absorption
cross section of a sphere is the absorption e~ficiency of
-~ that sphere multiplied by ~r2, where r is the radius of the
; sphere.
Similar wavelength dependent spectra are also
- observed in the scattering and extin~tion spectra. With
smaller sized spheres, say 10 nm, the absorption is greater
than the scattering and with larger sized coated spheres, say
; 200 nm, the scattering can be greater than the absorption
when there is an appropriate thickness of core to coat.
Figures lla and llb show~ dependent spectra, and (2) ~c~ 15 when the particles are small. FigurP llc shows Q5~ Q~ when
diameter~ lOOnm. Figures lld and lle show Cabs/Vol for
coated sphere ~than for the solid sphere especially when
500 nm.
Because the light absorption profile of each coated
particle is a function of the diameter of the intexnal silica
core, the thickness of the coating on that core, and the
wavelength of the light incident on the particle, a desired
light absorption profile can be obtained for a film emulsion
~-~ by combining appropriate amounts of differe.~ sizes o~ coated
particles, which in certain instances might be in combination
with uncoated particles. To avoid rep0ating the phrase
"absorption, scattering or extinction profile" too many
times, in the followiny description of the synthesis of a
profile we refer to it only as an absorption profile although
.,
:-
~ 35
,,
,
WO9l/06035 ~D~3 -22-- PC~/iS~0/06008 ~
the method woxks just as well for scattering or extinction
l spectra.
One way to synthesize an absorption profile is to
take a multitude of coated particles that absorb~light over a
wavelength range of interest, to separate that multitude of
particles into groups according to the sizes or coating
thicknesses of the particles, and then to form a mixture of
particles from these groups, with the amount or proportion of
particles taken from each group weighted so that the
resulting mixture has the desired absorption profile.
For instance, assume that a particular absorption
profile, D(~), is desired over the wavelenyth range of
; visible light, and that the initial multitude of coated
~ particles absorbs light over this wavelength range, with
; these particles being dispexsed in water and with the
dielectric cores of the coated particles having uniform
diameters of approximately 3Onm. The coated particles can be
grouped according to their coating thicknesses; and, for
` example, the particles can be separated into 14 groups having
coating thicknesses of 1.5nm, 1.75nm, 2.0nm, 2.25nm~ 2.5nm,
3.0nm, 3.5nm, 4.0nm, 4.5nm, 5.0nm, 6.0nm, 7.0~m, 9.0nm, and
12.Onm respectively. The light absorption of each group of
- partieles can be expressed as a function of the wavelength of
light incident on the particles; and, more generally, the
absorption of th~ ith group of particles can be expressed as
Ai(~), where ~ is the wavelength of the light incident on the
particles.
The actual absorption profile, T(l), of a mixture
~- containing N groups of particles ca~ ~e expressed as follows:
~; N
T (~ ai Ai (~)
~ ial
.
i~
~ 35
.,,
'`
`, !
.~
~.... ....... ... , .
~r 0~035 ~9 - ~ 3~ PCT/US90/06008
where ai is the proportion of the ith yLuup ~f part~cles i~--
l the mi~t~x~ ~a a~hiev~ th~ desil~ ah~OlptiO~ profile, the
ai (that is al, a2...aN~ are chosen so that T(~ is equal to
or closely approximates D(~). Any suitable procedure may be
used to determine or to estimate the ai. According to one
very well known technisue, the ai can be estimated to
minimize the sum, or the integral, C, of the squares o~ the
difference between D(~ and T(~ . According to this
technique,
C = S (1)(,{) - T(,~ ) )2dl
The set of ai that minimizes this difference can be
found or suita~ly estimated by many known methods such as by
a gradient search procedure (for example, as disclosed in
"Practical Methods of Optimizat.ion," by Fletcher (1981)).
Numerous computer programs are also known and may be used to
estimate or determine the set of ai that minimizes the
di~feren~e between D(l) and T(l).
The mixture of coated particles needed to achieve
the desired ~bsorption profile is then made by combining the
. N groups of particles, each weighted by the associated ai.
Another way to obtain a film emulsion with a
desired light absorption profile is to form the film emulsion
from a series of layers, with the layers containing different
particle sizes or typesO
Figures 12 and 13 illustrate two embodim~nts of a
second type of light se~sitive recording medium~ generally
reference at 50a and SOb respectively, and also according to
the present invention. This medi~m is a light sensitive
optical recording disk or plate, comprisiny a solid, light
reflectiny or light transmitting substrate 52 and a multitude
`
'
.
.
WO91/06035 ~ 9 ~74~ Pcr/us~o/
of particles 54 caxried by that substrate. The particles 54
l may he carried directly by substrate 52, as shown in Figure
12; or, as illustrated in Figure 13, the particles 54 may be
dispersed in a colloid or gel 56 that is applied~onto the
substrate. Rach of the particles 54 includes a core
surrounded by a shell, and at least one of the core and shell
consists essentially of ~ metal. Preferably, the other of
the core and shell consists essentially of a dielectric
material; and more specifically, prefera~ly the core of each
o~ these particles consists essentially of a dielectric
material, and the shell of each particle consists of a metal.
To record data in medium 50a or 50b, a light beam,
referred to as a write beam, of suf~icient intensity to
change the morphology of the coated particles 54, such as by
melting the silver coating or the dielectric core, is passed
over the recording medium in a given pattern to change the
morphology of the particles over a path or selected areas, to
thereby represent stored data. Because of this ahange in
morpholosy, the portions of the medium that were exposed to
the write beam are very much less able to absorb light than
are the portions of the medium that were not exposed to the
, write beam.
-~ Another light beam, referred to as a read ~eam, of
an intensity low enough so that it does not change the
~- morpholosy of the particles 54, c~n then be passed over the
recording medium. The read beam is reflected or transmitted
when it stri~es a portion of the recording medium previously
exposed to the write beam, while the read beam is absorbed
when it strikes a portion of the recording medium nat
previously exposed to the write beam. In this way, the r~ad
~j
.
,
. ,
. ~
:,
.,~ i . . ... .. .. .
-, : - .,: , : .
W~91/06035 ~C~ 9 PCT/US90/ObO08
beam can he used to determine, or read, the data st~red in
1 the recording medium.
As will be appreciated by those of ordinary skill
in the art, in any nanoparticle use~d in this invention, the
particle and the core thereof may have any suitable shapes,
and specifically, the particles and the cores rnay be
cylindrical or ellipsoidal, have a thread-like shape, or be
crystalline shapedO Also, the write! beam may be used to
change these particles in various specific ways to produce
the desired results. Moreover, with the particles 54 that
include dielectric material, any suitable dielectric material
may be used, and in particular, that dielectric may be a
linear or a non-linear material. In addition, as the term is
used herein, "metal" includes any material having a negative
~ dielectric constant, and so can include superconductors,
- 15 conducting polymers, materials with an a~omalous dispersion
; of carrier electrons, and heavily doped semiconductors where
free motion dominates the dielectric function.
The following considerations are helpful in forming
and selecting suitable or preferred particles for use in the
recording media illustrated in Figures 12 and 13.
i) at light wavelengths at which the plasmon
resonance effect shown by certain particles is significant
~referred to as resonance frequ~.~ies~, the electromagnetic
-` fields around the particles are enhanced,
ii) the electromagnetic fields, or the heat caused
by the absorptian of those fields, in and around certain
particles can cause those particles to change so that they
- absorb or scatter less l ~ht,
iii) the plasmon resonance effect exhibited by
small metal-dielectric layered particles is dependent on the
,'
:
.
: ~ : ~. : ........ . . .... .
, . . . . :
~ 26-
WO9l/~S03s ~3 PCT/US90/06008
,, ,,.
size and dielectric constants of those layers, and p~rticles
1 can be made so that only a comparatively small change in the
physical structures of the particl~s results in significant
changes in the way in which, or the extent to which, the
particles absorb or scatter light, and
iv) magnetic materials become non magnetic when
heated to or above the Curie points of the materials.
Based on the above facts, particles 54 for
recording media 50a and 50b can be made so that, on the one
hand, a write beam can be used to change significantly the
ability of the particles to absorb or scatter light, but on
the other hand, the ability of the particles to absorh or
scatter liyht will not change significantly in the absence of
the write beam.
For example, with a first general class of
particles, the particle may comprise a core and first and
- second layers of materials over that core, with each of these
layers comprised of a material different than the material of
the other layer, and the write beam may cause these two
materials to react with each other to form a third material
; 20 having a dielectric constant di~ferent than the dielectric
~: constants of the original materials of the particles. Figure
14 shows a particle 60 comprising core 60a, first layer 60b
:~ and second layer 60c, and one of these layers 60b and 60c is
a dielectric, such as an oxide, and the other of these layers
60b and 60c is a metal, such as silver. The energy of the
write beam may cause layers 60b and 60c to react with each
other to form a dielectric, silver oxide. The altered
particle is shown at 62 in Figure 15, and this particle
consists of core 62a and the formed dielectric layer 62b.
:~ 3 Because the formed particle 62 does not have a layer of
.
:
,~,
, . .
`', . ' .
,~
.: , ,
. ` , ~' :
W~ I/06035 ~ s~9 -27- PCT/USsO/~6~8
metal, that particle does not exhibit the plasmon resonance
1 e~fect; and, con --quently, the light -bsorptio~ and
reflection charac~eristics of particlc 62 are significantly
different than the light absorption and reflection
characteristics, respectively, of particle 60. '.
As another example, Fi~ure 16 shows a particle 64
comprised of a core 64a and first and second layers 64b and
64c, and each of these latter two layers is comprised of a
respective one type of dielectric material. The write beam
may cause these dielectric materia].s to react with each other
to ~orm a third dielectric material having a dielectric
constant different than the dielectric constants of the
materials used to form layers 64b and 64c. This altered
particle is shown at 66 in Figure 17, and the particle
consists of core 66a and the formed dielectric material 66b.
With a second general class of particles,
represented by particle 70 of Figure 18, the particle
includes a core 70a and one or more outside layers 70b and
70c, with one of these layers being comprised of a monomer.
The write beam causes the monomer layer to polymerize,
changing that layer to a solid th~ has a dielectric constant
: different than the dielectric constant of the monomer. The
formed particle is shown at 72 in Fi~ure 19; and the particle
co~ rises core 72a, ~irst layer 72b and polymex layer 72c.
To make particle 70 itself, it may be preferred to form or
apply the layer of monomer 70c onto the core 70a of the
! particle at low temperatures.
With a third general class of particles, the write
beam is used to change the shape of the particle and ~hereby
change its light absorption or reflection characteristics.
For example, Figure 20 shows a non-spherical particle 74
'~
. .,
3~ :
. ~,
.,
,. . .
.. ,.:
~ ~ 7 ~ 3 PCr/VS90/06008
comprised of a core 74a and first and second shells 74b and
1 74c. The energy from the write be~r that is abs~rbed by th~
particle 74 causes shell 74c to melt:, and then the surface
tension between the remaining shell 74b and the medium in
which the particle is suspended, causes the shell and the
coxe to become spherical. The formed particle is shown at 76
in Figure 21, and the particle comprises core 76a and shell
76b. The plasmon resonance effect exhi~ited by particle 76 is
different than the plasmon resonance effect exhibited by
particle 74, and thus the light absorption and light
; lO reflection characteristics of the former particle are
`~ different than those of the latter particle.
With a fourth general class of particles, the write
beam is used to melt the outside layer of the particle, and
this melted material then mixes with and changes the
dielectric constant of thé medium surrounding the particle or
of the substrate on which the particle is carried~ For
instance, Figure 22 shows particle 80 comprising core 80a and
shells 80b and 80c, dispersed in a medium 82. In use~ the
write beam melts shell 80c, and the material from the shell
mixes with medium 82. The result of this process is
illustrated in Figure 23, which shows particle 84, comprising
core 84a and shell 84b, dispersed in a medium 86. The
plasmon resonance ef~ect exhibited by particle 84 in medium
86 is dif~erent than the plasmon resonance effect shown by
particle 80 in medium 82. As a result, the light absorption
and reflection characteristics of particle 84 in medium 86
~- are different, respectively, than the light a~sorption and
re~lection characteristics of particle 80 in medium 82.
With a fifth class of particles, the write beam is
used to melt the outside layer of the particle and to change
. .
.,
~,
: . . .. . .. .. ... . .
: . . ,, . . ... ; . :
W~ ~l/06035 Zr~D~9 -29- PCT/US~0/06008
the structure of an inner lay~r of the particle, for example,
1 from amorphous to crystalline or from crystalline to
amorphous. Figure 24 shows a particle 90 of this type, and
comprising core 90a and shells 90b and 90c. The~write beam
melts outside shell 90c and changes the structure of inside
shell 90b, producing a particle shown at 92 in Fisure 25,
comprising core 92a and shell 92b. The plasmon resonance
effect produced by particle 92 is different than that shown
by particle 90, and consequently, the light absorption and
reflection characteristics of these two particles differ.
A sixth class of par~icles includes a layer of a
magnetized material, and the energy of the write beam is used
to raise the temperature of this material above the curie
point of that material, where it is no longer magnetic. For
example, Figure 26 shows a particle 94 of this general type,
~ 15 and including core 94a comprised of a magnetic material, and
; shell 94b comprised of a metal. In op~ a~ion, the write beam
raisec the temperature of particle 94 to a level above the
curie point of core 94a, so that the core is no longer
magnetic. As another example, Figure 27 shows a part:~le 96
that includes core 96a and two shells 96b and 96c. Core 96a
consists of a dielectric material, shell 96b is a metal, and
shell 96c is a magnetic material. In recording media 50a or
50b, a write beam is used to raise the temperature of shell
96c to a point above its curie point so that the shell ceases
to be magnetic.
As will be understood by those of ordinary skill in
the art, reversible procedures may be used to alter the
particles of recording media 50a and 50~, producing a
write-many read-many system. For example, the coated spheres
could be used to enhance the fields only to increase the
.
.~ .
.~. .
.~, .
,, ,
-30-
W~91/06035 PCT/US90/06008
f ~
temperature to the curie point ~as low as 150C for some
1 magnetic materials used in optical discs) and a magnetic
field can be used to set the orientation of the magnetic
domains. This pro~edure could be reversiblP.
The above-discussed changes, and other similar
changes, produced by a write beam will alter the extent to
; which light is absorbed by or transmitted through a xecording
medium. The particles in recording media 50a or 50b may be
either located ~n a surface, such as in an optical disk, ar
dispersed throughout a volume. When the particles are
~ lO dispersed throughout a volume, it may be preferred to provide
-~ that volume with a low density of such particles. In
addition, layered particles in which a metal is reacted with
a dielectric to form another dielectric, such as particle 60,
may be particularly useful for forming volume phase
holograms. Particles in which the polymerization of a
monomer is initiated by the write beam may also be
` particularly useful for ~orming volume phase holograms.
- Sensitizers used with conventional photographic
emulsions may be used with the coated spheres employed in the
photographic emulsion of the present invention, either on the
outside of or on the inside of the silver halide layer.
Also, the silver-halide emulsion of this invention
may be treated by many known chemical sensitization methods.
For example, the emulsion may be treated by a sulfur
sensitization method using a sulfur-containing compound
capable of reacting with active gelatin and silver (e.g., a
thiosulfate, a thiourea, a mercapto ~ompoun~ or a rhodamine);
a reduction sensitization method using a reducing material
(e.g~, a stannous salt, an amine, a hydrazine derivative,
formamidinesulfinic acid or a silane compound); or a no~le
~j
~ 35
:.
~ .
W~91/06035 2~ 31
PCT/US90/0600
metal sensitization method using a noble metal compound
1 (e.g., a gold comple~ salt, c~mple~ s~lt5 of metals belongi~g
to group VIII of the periodic table, such as Pt, Ir or Pd~.
As a protective colloid for us~ in the~preparation
of the silver halide emulsions of this invention, gela~in may
be used bu~ other hydrophilic colloids may be used, such as,
for example, gelatin derivatives; graft polymers of gelatin
and other polymers; proteins such as albumin and casein;
cellulose derivatives such as hydroxyethyl cellulose,
carboxymethyl cellulose and cellulose sulfuric acid esters;
sugar derivatives such as sodium alginate and starch
derivatives; and various synthetic hydrophilic polymers or
copolymers such as polyvinyl alcohol, partial acetal of
polyvinyl alcohol, poly-N-vinylpyrrolidone, polyacrylic acid,
polymethacrylic acid, polyacrylamide, polyvinyl imidazole and
polyvinyl pyrazole.
As gelatin, iime~processed gelatin as well as acid-
processed gelatin, and enzyme-processed gelatin may be used,
- as well as th hydrolyzed products and enzyme-decomposition
products of gelatin.
Various compounds can be added to the silver halide
photo~raphic emulsions of this invention for stabilizing the
photographic properties of the emulsions and for preventing
the formation o~ fog during the production, storage, or
processing of the photographic materials containi~g the
silver halide emulsions. Examples of antifoggants and
- stabilizers include benzothiazolium salts; nitroimidazoles;
ni~roben~imidazoles; cholorbenzimidazoles;
bromobenzimidazoles, mercaptothiazoles; mercaptothiadiazoles;
aminotraizoles; benzotraizoles; nitrobenzotriazoles;
1 3O mercaptotetrazoles; mercaptopyrimidines; mercaptotriazines;
.
~ 35
~'
., .
.
,':. ' ' ' ~ :, '
-32~-
~O9~/06~35 PCT/US90/06008
thioketo compounds such as oxazolinethione; azaindenes such
1 as triazaindenes, tetraazaindenes, pe~aazaindens;
benzenethio-sulfonic acid; benzenesulfinic acid; and
benzenesulfonic acid amide.
The silver halide photographic emulsions of this
invention may further contain polyalkylene oxides or the
derivatives thereof, such as the ethers, esters, amines,
thioether compounds; thiomorpholines; ~uaternary ammonium
salt compounds; urethane derivatives; urea derivatives;
imidazole derivatives; and 3-phyrazolidone derivatives for
increasing sensitivity and contrast or for accelerating the
development of the photographic materials containing the
silver halide emulsions.
The silver halide photographic emulsions of this
invention may be spectrally sensitized by methine dyes,
including cyanine dyes, merocyanine dyes, complex cyanine
dyes, complex merocyanine dyes, holopolar cyanine dyes,
hemicyannine dyes, styrl dyes, and hemioxonol dyes.
Particularly useful dyes are cyanine dyes, merocyanine dyes,
and complex merocyanine dyes. For these dyes, conventional
; 20 cyanine dye nuclei such as basic h~terocyclic nuclei can be
used, including a pyrroline nucleus, an oxazoline nucleus, a
thiazoline nucleus, a pyrrole nucleus, an oxazole nucleus, a
tetrazole nucleus and a pyridine nucleus. The foregoing
nuclei may he used to aromatic hydrocarbon rings, such as an
: 25 indolenlne nucleus, a benzindolenine nucleus, an indole
nucleus, a benzoxazole nucleus, a napththoxazole nucleus, a
benzoselenazole nucleus, a benzimidazole nucleus and a
quinoline nucleus.
~ S~ or 6-membered heterocyclic nucleus having a
ketomethylene structur~ such as a pyrazoline-5 one nucleus, a
'
" .
'. 35
.1
s
: ~
. .
W~ 91/06035 ~ n~,~ PCT/VSgO/06008
thiohydantoin nucleus, a 2-thioxazolidine~2,4-dione nucleus,
1 a thiazolidine-2,4-dione nucleus, rhodanine nucleus or
thiobarbituric acid nucleus can be used as a nucleus for the
merocyanine dyes or complex m~rocyanine dyes.
These sensitizing dyes may be used alone or in
combination, and a combination of sensitizing dyes is
fre~uently used for supersensitizationO
The silver halide photographic emulsions of this
invention may further contain dyes haviny a spectral
sensitizing action or materials which do not substantially
absorb visible light but which exhibit a supersensitizing
effect when used together with the foregoing sensitiziny
dyes.
The photographic materials using the silver halide
emulsions of this invention may contain water-soluble dyes as
-; 15 filter dyes or for various purposes such as irradiation
prevention. Examples of such dyes are oxonal dyes,
hemioxonol dyes, styryl dyes, merocyanine dyes, cyanine dyes,
and azo dyes. Among these dyes, oxonol dyes, hemioxonol dyes
and merocyanine dyes are useful.
The photographic materials containing the silver
halide emulsions of this invention may contain stilbene
series, triazine series, oxazole series, or cumarine series
; whitening agents in the silver halia~ emulsion layers and
other hydrophilic colloid layers. These materials may be
~, 25 water soluble or water insoluble and in the latter case, they
may be used as dispersions.
Known fading preventing agents may be used alang
` with color image stabilizers in this invention, alone or in
combination. The photographic materials using the silver
' 30 halide emulsions of this invention may further contain
,
..
.,
~,
"
. .
, .
. .
.. . .. . ..
. . . . . . . . .
-3~-
~Ogl/06035 PCT/US90/06008
hydroquinone derivatives, aminophenol derivatives, gallic
1 acid derivatives and ascorbic acid derivatives, as color
fogging preventing agents.
The silver halide photographic emulsions of this
invention can be used for both black and white photographic
5 materials and multilayer multicolor photographic materials.
A multilayer natural color photographic material ordinarily
has at least one red~sensitive layer, at least one
; green-sensitive layer and at least one blue-sen~itive silver
halide emulsion layer on a support. The red-sensitive layer
usually contains a cyan dye-forming coupler, the
green-sensitive layer contains a magenta dye~forming coupler,
and the blue-sensitive layer contai~s a yellow dye-forming
coupler, but if desired, other combinations may be employed.
As the yellow coloring couplers, known closed chain
ketomethylenic couplers can be used, including
benæoylacetoanilide series compound and pivaloylacetoanilide
series compounds. As magenta coloring couplers, pyrazolone
series compounds, indazolone series ~ompounds and cyanoacetyl
compounds can be used and pyrazolone series compounds are
particularly use~ul. As cyan coloring couplers, phenolic
compounds and naphtholic compounds and couplers having a
ureido group can be used. DIR couplers (development
inhibitor releasing couplers) can also be used in this
invention.
The photographic materials using the silver halide
emulsions of this invention may contain compounds capable of
releasing development inhibitors (apart from DIR couplers)
with the progress of the development. Also, couplers capable
of releasing development accelerators or fogging agents with
the process of development can be used in this invention.
:
.. . . . .
,:.~ ~ . . . .
~: : : ' ' ' ' - '
,
:, ~ . . , ~ . , :
W~9l/06035 ~?~ 9 35-
PCT/US90/0600B
The photographic materials containing the silver
1 halide emulsions of this invention may contain ultr~iolet
absorbents in the hydrophilic colloid layers, such as aryl
group-substituted benzotriazole compounds. 4-thiazolidone
compounds; benzophenone compounds, cinnamic acid ester
compounds, butadiene compounds and benzoxydol compounds. In
addition, ultraviolet absorbents can be used in this
; invention. Still further, ultraviolet absor~ing couplers
~e.g., a-naphtholic cyan dye-forming couplers) and
ultraviolet absorbing polymers may be used in ~his invention.
These ultraviolet absorbents may be m~rdanted in specific
layers of the photographic materials.
For processing the photographic materials
containing the silver halid- ~mulsions of this invention,
known processes and known p essing solutions can be used.
The processing temperatures are usually in the range ~f about
18C. to 50~C. but may be lower than 18 C. or higher than
50C. According to the purposes, a development processing
formlng silver image ~black and white development process) or
~ color photographic process composed of development process
for forming dye images can be used for developing the
photographic materials.
The colc~ developer which is used for developing
' the photographic materials of this invention is generally
composed of an alkaline aqueous solution containing a color
developing agent. Color developing agents include aromatic
;~ primary amino color developing agents such as
phenylenendiamines (e.g., 4~amino-N,N-d.iethylaniline,
3-methyl-4-amino-N,N-diethylaniline,
4-amino-N-ethyl~N-~-hydroxyethylaniline,
3-methyl-4-amino N-ethyl-N-B-hydroxyethylaniline,
. .
~, 35
~ . . . .
.
WO 91tO6035 ~36- . PCr/US~0/0600
ZC~ r`?,l ~.n,~ ,
3-methyl-4-amino~N-ethyl-N-B-methanesulfoamide-ethylaniline
1 and 4-amino-3-methyl-N-ethyl-N~-rnet~oxyethlaniline),
The photographic emulsion layers are usually
: bleached after color development. The bleach process may be
performed simultaneously with or separately from the fix
process. Bleaching agents include compounds of multivalent
metals such as iron(III), cobalt(III), chromium(VI) and
copper(II~; peracidsi quinones and nitroso compounds such as
ferricyanides; dichromates; organic complex salts of
iron(III) or cobalt(III); aminopolycarboxylic acids such as
ethylenediaminetetraacetic acid, nitrotriacetic acid and
1,3-diamino~2-propanoltetraacetic acid; complex salts of
organic acids such as citric acid, tartaric acid and malic
acid, persulfates; permanganates and nitrosophenol. Among
these materials, potassium ferricyanide,
ethylenediaminetetraacetic acid iron~III) sodium salt and
ethylenediaminetetraacetic acid iron(III) ammonium salt are
particularly useful. The ethylenediaminetetraacetic acid
iron~III) complex salts are useful for a bleach solution and
; for a fix solution~
~`. 20 Any suitable procedure may be used to prepare the
~, coated particles used in the recording media of the present
- invention. For example, with reference to Figure 28, silver
halide coated dielectric particles, such as particle 16 of
Figure 2, may be made by a process generally comprising the
steps o~ providing an aqueous solution including negatively
charged colloidal dielectric particles, positively charged
silver ions, and a halide, and reacting the halogen of the
halide with the silver ions to bond, or grow, coatings of
silver halide completely covering individual dielectric
particles. Preferably, the concentrations of dielectric
, . .
' 35
;.
~` :
... .
.i', ~ " '' . ` " " '' ~ ''` :," ' :' " ''" . '. ' "; " ' ' "' ' ' '.'. ' ' ` ' . .
W ~ l/06035 2 ~7 a ~,9 ~37~ Pcr/us9o/o6on8
may be dispersed in the solutlon, then the silver ions may be
l added, and then the halide may be added.
With a preferred process, after the dielectric
particles are added to the solution, the pH of that solution
is adjusted to and thereafter maintained at a level slightly
above 2, and even more preferably, between akout 3 and 5.
With this procedure, the dielectric particles do not have to
be negatively charged when they are added to the solution,
and, instead, the acidity of the aqueous solution causes the
~ dielectric particles to become negatively charged once the lO particles are in the solution. Further, with the preferred
process, the initial concentration of the silver ior~s in the
solution is relatively low, less than 10 4M; the initial
concentration of the halide in the solution is slightly
~reater than, such as about 10% greater than, the
concentration of the silver ions in the soiution; and also,
the solution is constantly stirred while the halide is being
added to it.
The silver ions may be added to the solution in any
suitable form, and for instance, these ions may ~e added in
the form of a soluble salt, e.g., silver nitrate. Likewise,
the halide that is added to the solution may be any suitable
halide, such as an alkali halide, e.g., sodium bromide,
, sodium chloride, potassium bromide, potassium bromide and the
`, like. In addition, any suitable dielectric may be used in
the above-discussed process, and the dielectric may be linear
- or non-linear and may have an~ suitable shape and size. For
example, the dielectric particles may be spherically shaped
silica particles. When, first, the dielectric particles are
these silica particles, second, the silver ions are added to
the solution in the form of silver nitrate, and third, the
. .
'`~'
; :
.
;~
., .
WO9l/06035 ~3B- PCT/IJS90/06008
2~
halide is sodium bromide, then the silver from the sllver
1 nitxate reacts with the bromide fxom the sodium bromide to
form silver bromide, which bonds to and forms layers over the
silica particles. ''
: Figure 29 generally outlinles a process for making a
5 metal coating on a dielectric particle, such as coating 20b
of particle 20, or coating 22b of pa:rticle 22. This process
generally comprises the steps of providing an a~ueous
solution including negatively charged colloidal dielectric
particles, metal ions, a secondary alcohol containing 3-7
lO carbon atoms, and an alkyl ketone containin~ 3-7 carbon
atoms; removin~ oxygen from the solution; and e~posing the
solution to ultraviol~t light to cause the metal ions to
attach to the dielectric particles and orm metal coatings
completely covering individual dielectric particles.
Preferably, the concentrations of the dielectric particles,
the metal ions, the isopropanol and the acetone, and the
length of time the solution is e~posed to the ultraviolet
light are selected so that coatings of a uniform, preselected
:~ thickness are formed on the dielectric particles.
~ 20 As used herein, the t~rm lower alk~l, when used
: alone or in combination, contain 1-7 carbon atoms. These
alkyl groups may be staight chained or branched and include
such groups as methyl, ethyl, propyl, isopropyl, butyl,
sec~butyl, isobutyl, t-butyl t pentyl, amyl, hexyl, heptyl,
and the like. As used herein, a secondary alkanol re~ers to
a lower alkyl alcohol in which the hydroxy group is attached
to a secondary carbon. Such groups include isnprop~nol,
sec-butanol and the like.
The pre~erred alkyl ketone is acetone.
` 35
,,
~'
~'
W ~ 1/06035 ~ ~9 PCT/US90/06~08
In the above-discussed procedure, without wishing
1 to be bound, it is believed that the ketone (acetone) absorbs
energy from the ultraviolet light and then reacts with the
secondary alcohol (isopropanol) to form alkyl secondary
~isopropyl) radicals. These radicals are powerful reducing
agents and cause metal ions that have become attached to the
dielectric particles to form metal molecules. The particular
` order in which the dielectric particles, the metal ions, the
; secondary alcohol and the ketone are added to the aqueous
solution is not critical; and, for instance, the secondary
alcohol and ketone may be added to the solution, the
~ dielectric particles may then be dispersed in the solution,
; and then the metal ions may be ~dded.
~ ith a preferred process, as with the process
outlined in Figure ~8, after the dielectric particles are
added to the solution, the pH of the solutio~ is adjusted to
and thereafter maintained at a level slightly above 2, and
even more preferably, between about 3 and 5. In this way,
the dielectric particles do not have to be negatively charged
when they are added to the solution and the acidity of the
aqueous solution causes the dielectric particles to become
negatively charged. In addition, the initial concentration
of the metal ions in the solution is relatively low, such as
2 x lO 4M; and the initial concentration of the acetone and
~i isopropanol in the solution are about equal to each other and
much greater than, such as about 400 times greate- than, the
initial oncentration of the metal ion in the solu~ion. In
addition, preferably the solution is stirred while exposed to
the ultra~iolet light.
; Numerous speci$ic types o~ metal coatings may ~e
made using a procedure as described above. ~he types o~
... ~
., .
~, .
~ 35
. ~
.~
.. :
~f~
,
...... . ...
:., ., : , ' ,, . . ' . ` . . . , ' , ' . , ' . , :' , . ' . ' .
-~o- l
WO91/06035 q PC~/US90/06008
z ~ 3
metals that can be used include transition metals, the
1 lanthanides and the Group IIIA metals. The especially
preferred metals include the Group VIII and IB metals,
especially copper, silver, gold, iron, nickel, palladium,
platinum, cobalt, rhodium, iridi~, ruthenium, aluminum and
the like. Especially preferred metals include copper,
silver, gold, nickel, palladium, platinum and nickel.
It is most preferred that the process may be used
to form silver coated dielectric particles, gold coated
particles or palladium coated particles. In addition, the
metal ions may be provided in the solution in any suitable
manner; and, for example, these ions may be provided by
adding a water soluble metal salt such as silver nitrate, to
the solution~
Moreover, any suitable dielectric may be used in
the above-discussed process, and the dielectric may be linear
or non-linear and may have any suitable shape and size. For
instance, the dielectric particles may be spherically shaped
silica particles. When such dielectric particles are used,
and the metal ions are added to the solution in the form of
silver nitrate, then the ultraviolet light, in combination
with the acetone and the isopropanol, causes the silver ions
to bond to and form metal silver coatings over the silica
particles.
.,
.
~ 3o
.
~ 35
:
... , , . : , :
, . , , . ,, : .. :. . : , .: . . . ..
W~ ?1/06035 -4:L- ~cr/US90/06008
~r~n~
, .
1 EXAMPLE 1
The followiny example illustrates this process for
forming metal coated dielectric particles.
An aqueous solution is prepared by mixing the
following solutions in a 50 ml beaker:
(1) 0.5 ml of 0.01 M AyN03,
(2) 0.5 ml of O.S0 M of low poroslty SiO2
particles. ~ .
The particle diameter is chosen to be between 5 to 20
- nanometers, although other sizes can be readily substituted,
13) 1.5 ~1 of pure isopropanol,
(4) 1.5 ml o~ pure acetone.
~- All chemicals used axe of reagent grade quality,
15 unless otherwise specified. The above mixture is diluted
with 16 ml of distilled water, and the ~H adjusted to be
: between 4 to 5 by dropwise addition of a 0.01 M nitric acid
solution. In this pH range, the silica particles are
negatively charged, causing the positively charged silver
ions to be bound to the surf~ce. After thorough mixin~ by
stirring for o~e minute using a ma~netic stirre~, the sample
is transferred ~o a W photolysis vessel, equipped with a
quartæ window and provision for careful deoxy~enation by
bubbling nitrogen gas for one h--~r. It is important that no
oxygen be present in the solutiorl. The sample is irradiated
by a 450 Watt Hg-Xe lamp ~or one hour, with ~entle stirring
, continued by means of a magnetic stirrer. The solution
: color, and conse~uently the thickness of the coat, can be
;~ controlled by adjusting the period o~ illumi~atio~ ~y W
~,
~,1
.~ :
..;~ 35
~ : :
.,
i~'
-42-
W09l/06035 PCr/US90/06008
~ 7.~ ~ '3
light. This forms the basis for the preparation of the
1 silver coated silica particles in the present ex~mple.
Silver coated dielectric particles may also be made
by a process employing photoreduction of silver halide, and
one such process is outlined in Figure 30. In this process,
silver halide coated die7ectric particles are made, for
example, by the process discussed above in connection with
Figure 28, and then the coated particles are exposed to light
to change the silver halide coatings over the individual
particles to metal silver coatings.
Preferably, though, a more integrated process,
generally outlined in Figure 31, is used to ~orm silver
coated dielectric particles. In accordance with this
process, dielectric particles are dispersed in a solution
including silver ions, a halide and an electron hole
scavanger, and the silver ions react with the halogen of the
halide to form silver halide coatings completely covering the
` dielectric particles. The solution is then exposed to
ultraviolet light, and this light changes the silver halide
coatings to silver coatings. Preferably, the concentrations
of the dielectric particles, the silver ionsp the halide and
the electron hole scavanger in the solution, and the len~th
o~ time the solution is exposed to the ultraviolet light are
selected so that coatings of a uniform, preselected thickness
are formed on the dielectric particles.
Preferably, with this process, the initial
concentration o~ silver ions in the solution is greater than
the initial concentration of the halide in the solution; and
for instance, the former concentration may be about 5 times
the latter concentration. The silver ions may be in the
solution in any suitable form, and for instance, these ions
': .
'~
.i ~
.,'. ~ ' ' : ' ' . , '
Wr~??]/06035 rcT/us~o/06oo8
may be added to the solution in the form of a salt that is
1 soluble in aqueous solutiGn, e.g., silver nitrate.
Similarly, the halide that is added to the solution may be
any suitable halide such as an alkali halide, e.g., sodium
bromide, sodium chloride, potassi~n bromide, potassium
chloride, and the like. Further, any suitable dielectric may
be used in this process, and the dLelectric may be linear or
non-linear and have any suitable shape and size. For
example, the dielectric particles may be spherically shaped
silica particles. When (i~ the dielectric particles are the
silica particles, (ii) the silver :Lons are added to the
solution in the form of silver nitrate, and (iii) the halide
is sodium bromide, then the silver from the silver nitrate
reacts with the bromide ~rom the sodium bromide to form
silver bromide; and the ultraviolet light, in the presence of
EDTA, then reduces the silver bromide coatings to metallic
silver.
In the above procedure, it is preferred that the
light source used contain ultraviolet light. It is pre~erred
that the light source contain wavelength ranging from 150-550
nm. The preferred wavelengths range fxom 200-400 n~.
Furthermore, it is preferred that the intensity of
light used ranges from 50 watts to 1~5 kilowatts, with the
preferred intensity ranging from 250-lOOQ watts. Especi~lly
preferred intensity ranges from 350-550 watts, with an
intensity of about 450 watts being the most preferred.
.
.i
,.
,,:
,
WO9l/0603S PC~/~S~0/060~8
2~
EXAMPLE ~
Metallic silver on SiO~ particles can be obtained
by photoreduction of silver halides, which are typically
prepared in the presence of excess Ag ions. A hole ~h~)
scavanger, EDTA, is added to the solution. One ml of a 0.002
M NaBr solution is added to 19 ml of a solution which is
prepared in a 50 ml beaker by mixing the following:
(1) 1 ml of 0.01 M AgNo3,
~2) 0.5 ml of 0.50 M of low porosity SiO2 particles. The
particle diameter was 12 nanometers, although other sizes can
~, be readily substituted,
: (3) 1 ml of 0.02 M EDT~,
t4~ 16 ml of distilled water.
After thorough mixing, the solution is transferred
to a 1 cm W quartz cuvette and exposed to a 375 Watt.
tungsten halogen light source. Under these conditions, very
little light is actually absorbed since the colloidal AgBr
has a very low absorbance above 350 nm. A possible mechanism
for the reduction process is given by-
AgBr ~ AgBr~e ~ h )
AgBr + e ----~ Ag ~ Br
: EDTA + h ~ product
Br + Ag (excess) --~ AgBr
The duration of illumination, which is in the order
of minutes~ determines the color of the silver coated silica
:- particles. This color is a result of the thickness of th~
~-~ silver layer, and can range from yellow to a purplish gray.
:`
,
3
.
,....................... .
. 35
''
., .
:
, . .
WQ ?1/06035 ~ A ~r~ PCT/US90/06008
Once the silver coated silica spher~s are prepa~ed,
1 they are purified by dialysis and then placed in a sodium
dodecyl sulfate micellar solution, or a ~,icroemulsion.
~ variation of the process described above may be
employed to form metal coatings other than silver on nano
5 particles, and this variation uti_izes the fact that metallic
s.ilver on the dielectric particles will act as a catalyst to
help grow metal coatings on those p,articles from other metal
ions in the solution. In accordance with this variation,
: which is outlined in Figure 32, a solution is provided
lO including dielectric particles, silver halide is formed on
those particles, the solution is exposed to light to chanye
i at least a portion of the silver halide to metallic silver,
and ions of a metal are added to the solution to form
coatings of that metal completely covering individual
. 15 dielectric particles, with th~ metallic silver on those
particles acting as a catalyst to accelerate the formation of
: the metal coatin~s. These metal ions may be added to the
solution in any suitable manner, and for instance,
conventional photographic developing solutions may be added
to the solution to reduce t~e metal ions.
Only minute amounts of metallic silver are needed
on the dielectric paxtioles to help grow the metal coatings
thereon; and hence, in the above-described process, it is
onIy necessary to form minute amounts of silver halide on the
d.electric particles~ Alternatively, complete coatings of
silver halid~ may be formed on th~ dielectric particles, with
only minute amounts of the silver halide on individual
~,
, 35
`''
:. ... : . . . , , .; . .: . , : . . : .
~' ` . ' . I . , , . ~ . ' . . , , !
~ , ,' :. ,
-~6-
W09l/06035 PCT/U~90/06008 ~.
~r~
particles ~eing changed to metallic silver. With another
1 variation, silver halide coatings may be made completely
covering dielectric particles, only minute amounts of the
silver halide may be changed to metallic silver on individual
particles, and then these minute amounts of metallic silver
5 may be used to help form metal coatings completely covering
the silver halide that remains on thLe dielectric particles.
The resulting product comprises a dielectric core, a first
coating of silver that substantially completely covers the
dielectric core, and a second coating of a metal that
lO completely covers the layer of silver halide.
The following example illustrates the coating of
silver on a dielectric core of silver bromide. The silver
bromide nanoparticles exposed briefly to intense W light in
the presence of EDTA have optical extinction spectra similar
15 to those computed f or distribution of silver-coated silver
bromide nanoparticles~ By intense~ it is meant that the
intensity of the light ranges from 50 watts to 1.5 kilowatts,
with the preferred range being 250-550 watts, and the most
preferred having a range of 350-55n watts.
As clearly shown by the following discussion, with
shorter exposure time, the plasmon resonance maximum is
- shifted to longer wavelengths, a result consistent with
theory so long as the coat thickness increases with exposure
to light. The resonance maximum of the distributions of
coated particles can be controllably shifted to 600 to 700
, nm.
~': ' ' . ' : . . '
WQ~1/06035 PCT/VS90/06008
~2~
EXAMPLE 3
i
Silver bromide colloids were prepared by rapidly
mixing equal volumes of AsNC3 and NaBr solutions~. A growth
- stabilizer (SDS) and an electron doner (EDTA) were added
immediately after precipitation. Typically the final
concentrations were 1 X 10 M Br, 4 X 10 mAg , 5 X 10 4 M
(SDS). and 5 X 10 M EDTA. The concentration of SDS was far
~ below the critical micellization concentxation ~10 2 M).
: Freshly prepared solutions were exposed to light from a 450
- 10 Watt Hg-Xe lamp for a few seconds. With the shortest
; exposures the spectra appeared blue. With longer e~posures
the solution appeared orange. When ammonia, which dissolves
AgBr by forming complexes with Ag was added to any of the
illuminated solutions the color changed to a yellow color
c~aracteristic of small metallic silver colloids.
` The particle siæe distributions were characterized
with transmission electron microscopy (JEOL 1200EX). A
typical micrograph is shown in (Fig. l9a). A size
distribution consistent with the limited micrograph data is
the log normal distribution.
1 N(r)=NOexp(-((ln(r)-ln(rm))/ln(s)~2), (1)
c
:~ with rm equal to 1 nm or less and s in the range of 4 to 4.5
~5 nm. The size distributions as determined by TEM did not
, appear to change markedly with e~posure to light.
- ~ After the addition of ammonia to any of the
illuminated samples only small particles having diamet2rs 5
nm or~le~s were observed in th~ T~ IFig l9b). The most
likely interpretation is that only part of the AgBr was
.1 .
;`' 35
';
.~ .
W O 91/06035 PC~r/U~90/06008
~C~ 3 . ~ ';
reduced to Ag during the illumination and that the larger
l particles are ~gBr/Ag composites.
Example optical extinction spectra measured shortly
after exposure are shown in Figs 20a) to d). The exposure
time and/or EDTA concentration and hence the reduction of
Ag+, increases in going from a) to d). The peak extinction
shif~s to shorter wavelengths as the illumination time is
increased. This result is consistent with theory so long as
the coat thic~ness increases with exposure. A spectrum of
the ammonia treated solution shown in Fig 20e), is typical of
homogeneous silver nanoparticles. The general shapes of the
above spectra are readily reproduci~le. At comparable
illumination times, in the absence of Br , the appearance of
color in a given sample is negligible.
Theoretical optical extinction spectra of
individual silver coated spheres are shown in Fig. 21. The
peak of the theoretical extinction sh.ifts from red to blue as
- the ratio of coat thickness to core radius increases. This
data is consistent with the measured spectra where the
absorption maxima shift toward the blue as the time o~
exposure increases since the coat thickness should increase
with exposure time. The compound spectra are very sensitive
to the coat thickness. The measured spectra are much more
broad than the spectra shown in Fig. 21 because of the
distributions of core diameters and coat thicknesses.
~, 25 The magnitudes of the extinc~ion spectra are also
i characteristic of silver coated particles. For example, at a
wavelength of 7G0 nm the extinction cross section per unit
~olume of silver is lOO's of times larger in a silver coated
nanoparticle having the appropriate ratio of core radius to
coat thickness than it is in a solid silver sphere. The fact
., .
W ~1/06035 ~'
P~/US90/06008
that the theoretical extinction is so large can be used to
1 help verify that the partic~es arE~ coate~ ~lt~ silver.
However, since there is a broad distribution of sizes care
must be taken in making the comparlson.
Here we started with the size disbribution of core
particles described by the above equation, then used trial
and error to determine the distributions of coat thicknesses
required to match the measured spectra, and then found that
the magnitudes of the spectra were within the range of values
expected from the initial concentrations of Ag~ and Br .
The assumptions made in computing the spectxa are
as follows:
1. The reduced silver is in the form of a smooth
coat on the surface of a spherical AgBr particle. The
- extinction efficiencies were computed using the separation of variables solution for concentric spheres based on
algorithms.
2. The size distribution of the core particles is
described by the log-normal distribution of the above
equation. The values of No were determined by setting the
, 20 total volume of all the particles prior to illumination in
- the distribution e~ual to the volume of AgBr. The initial
`j total volume of AgBr was determined by solving the ionic
equilibria equations including the Ag - EDTA complexO
3. The size distribution of the coat thicknesses
is a Gaussian, typically with a standard deviation of 2 to 8
nm .
4. The silver coat may be formed either ~rom the
reduction of the silver halide of the initial particle, or
from the reduction of Ag from solution. Computations have
been done for each of the two limiting cases.
.. . .
. . ~ : . . . . .. ; ., : .
~r~ 3 -SO-
WOsl/0~35 ` PCT/U~gO~06008
5. The total extinction i.s computed by numerically
l integrating over distributions of core radii and coat
thicknesses.
be(~)= Nn(rc)Ng(t)Q(rc,t,mc,mt,~ ~rlr2drcdt (2
where Nn is the size distribution of the cores, Ng is the
size distribution of the coats. Q is the extinction
efficiency, mc is the refractive inclex of the core, and mt is
the refractive index of the coat. Typically the integrations
over cores were from r - 2 to r = 18.
. The re~ractive index fo the silver was computed
from the data of Hagemann et al. in J. OP~ Soc. Am., 65,
742-744 ~1975) and Ker~er, in J. OP. Soc. Am. B., 1327-1329
~ (1985), either by itself, or combined with a Drude model in
- 15 which the increased electron scattering at the surfaces of
the very thin coat was taken into account. The refractive
index data of Johnson and Christy in ~y Rev~ B, 6, 4370~4379
(1972) was also used for some computations not shown. Linear
interpolation was used to obtain the values of refractive
index at points not in the data.
7. The refactive index of AgBr was o~tained by
combining the data from White, . Am., 62, 212
(1972), and James, "Theory of the Photographic Process",
MaMillan (1977) p. 216.
Fig. 41 shows a measuxed spectrum and two computed
spectra. In the topmost curve the Ag in the coat is assumed
to come only from the solution. i.e., the AgBr cores are not
reduced in size as the coat grows. In the bottom curve the
Ag in the coat is assumed to come only from the reduction of
~- 30 AgBr at the suxface of the particle an~ so the core shrinXs
., .
~ 35
'`
;,
:~, . ... . .. . .
~ 51-
W ~ l/06035 PCT/VS90/06008
as the coat grows. Since the measured curve lies between the
l two computed spectra, the magnitudes of the plasmon enhanced
extinction is in the range of values computed.
The main parameters that ccln be adjusted in fitting
the distributions to the spectra are: 1) the thickness and
standard deviation of the coats and t:he limits of the
numerical integration for the coats. 2) the size
distribution and limits of integration for the cores. 3) the
data for the refractive index of silver, the fraction of the
reduced silver that came rom solution. The computed spectra
lO are very sensitive to the distributions o cores and coats
chosen and to the limits of integration, which also deine
the size distri~utions. The computed spectra depend on the
refractive index of silver used. However, by varying the
size distributions, similar spectra can be obtained with the
15 different models for 5ilver. The effect of the different
assumptions about the source of the A~ for the coat can be
seen in Fig. 40. In a preliminary experiment without excess
silver a spectrum similar to that shown in Fig~ 39d was
generated.
Without wishing to be bound, it is ~elieved that
the silver coat is formed by the coalescing o~ ma~y small
~ silv~r particles. The coat may also contain some AgBr or
-i voids, but it is homogeneous enough to have a refractive
index similar to that of bulk silver. The bonds between the
25 particles may be relatively weak because the coat breaks into
many small particles when the solution is treated with
ammonia.
It mi~ht have been thought that the spectra could
be accounted for by nonsherical silver particles. The fact
that ammonia, which dissolves AgBr but not Ag, reduaes the
~ 1
., :
Wo9lJ0603s ~ 3 -52- PCT/US90/060~8
spectrum to that of small solid particles, and the ~act that
l the particles in the TEM do not have large eccentriclties,
argue against *his h~potheses. Also, the particle shapes do
not seem to be related to the colors of the sol~tions.
In summary, the predicted tunability of the
surface-plasmon resonance frequenc~y and enhanced extinction
at longer wavelengths was experimentaly con~irmed with
Ag-AgBr colloidal composites. The particles scatter as if
the Ag is smoothly coated on the Ag~r.
; Silver coated dielectric particles may also be
formed by a process utilizing chemical reduction of silver
.~ ions by hydroquinone at elevatecl temperatures. The followirlg
example, generally outlined in Figure 33, illustrates this
process.
.
:,.
, ;
:.
.,
3o
: I
., .
...
.
W Q 91/06035 PC~r/US90/06008
EX~MPLE 4
1 100 ml of a silica solutioll (particle diameter 7
nm) which had been purified by overnight dialysis was
transEerred to a 250 ml beaker, and t:he pH adjust~d to 4.0 by
dropwise addition of 0.01 M AgN03 solution added dropwise
5 under gentle stirrin~ to achieve the final concentration
shown in the table below. After about 2 minutes~ sufficient
quantity of 0.01 M hydroquinone was added in a similar
manner. The reduction to metallic silver takes place
gradually over a time period of about five minutes,
10 accompanied by a color change from pale yellow to dark brown.
Th~ rate of silver deposition by this method can be
controlled by varying the temperature hetween 8S to 95DC. A
- transparent solution is obtained in every case, and is
allowed to cool and then purified by dialysis.
: 15 EXAMPLE 5
The following table summarizes the experimental
conditions, including final concentrations (in molar), which
were used in oux different sets:
I II III IV
Sio2 1% 196 1% 1%
AgN03 5.0xlO 4 l.OxlO 3 1.5xlO 3 2.0xlO 3
, Hydro- 5.0xlO 5 l.OxlO 4 l.SxlO 4 2.0xlO 4
quinone
i 25 The amount o~ silver deposited increases from I to
IV, and is evident from the color of the solutions (light
yellow to dark brown). Electron microscopy also provided
evidential support. The optical absorption specra show the
presence of a single peak maximum at about 400 nm.
3o
, . .
~. 35
.
.
.' ~
W0 91/0603g 2~7D~ "9 I'C~/IJS90/06008
ELECTRON MICROSCOPIC RESULTS:
1 Solution I consists of particles which are smaller
and better defin~d, appear darker, and were in the size range
of 10 to 30 nm. In solution II, III, and IV, the particle
size range was found to be between 40 to 100 nm, the
particles were simllarly dark, but contained elongated as
well as spherical shapes. The final size distribution may be
due in part to the non uniform size of the silica core
particles, found to be between 7 to 11 nm by electron
microscopy.
With all of the processes described above, after
the coated particles are prepared, they may be removed from
the solution in which they were prepared by dialysis, and
then placed in a sodium dodec~1 sulfate micellar solution or
~: a micro emulsion. Additional coatings of either silver
halide, a metal or a polymer, may then be added until the
desired final con~iguration is reached. Polymer coating of
any of these particles may be readily achieved in a solution
by the well known emulsion polymerization method, in which a
`. suitable amount of monomer and initiator have been addedO
For instance, the following process, outlined in
Figure 34, shows how a polymer coating may be made on a
silver coated particle.
The following aqueous stock solutions were
prepared:
(I) 0.1 M KH2P04,
0.1 M NaOH,
(III) 2 ~ solution of sodium salt of styrene sulfonic acid,
, NaSS (co-monomar),
~. (IV) 3 % solution o~ K2S2O~
.~ 30 All solutions were prepared in doubly distilled
water, and all chemicals were reagent grade.
., .
.. . . . . . . . , . ~. ... .. .
W~l/0603S ~ 55 PCT/US90/~6008
131.6 ml of a 1~ solution of the silver coated
l silica particles were transferred to a three necked flask. 8
ml of solution IV, followed by 6.4 ml of solution II, were
added with constant stirring using a magnetic stirrer. The
flask was equipped with a condenser, and a platinum
thermometer, which, in combination with a thermoregulator and
a heating mantle, allowed regulation o~ the temperature of
the flask to 65 + 1 C. At this temperature, nitrogen gas
was bubbled through the mixture continuously, and 30 ml o~
styrene was added. After 15 minutes, 10 ml of solution III
were added, and after another 20 minutes 4 ml of the
; initiator, solution IV, were added. Depending upon the
thickness of the polymer film desired~ the reaction can be
terminated by addition of 25 ml of a 1 % solution of
hydroquinone, and cooling the reaction mixture to room
temperature. The particles are filtered, wa~hed several
times with doubly distilled water, resuspended in water, and
. further purified by dialysis.
:
- 25
, .
. .
. 30
",
.. ~ .
.
,
.,
. . ~ . ~ . .
: : ~ . . .
-56-
WO9l/DS03S PCT/US90/06008 ,~
1 EXAMPLE 6
Coating of carbon fibres with copper was carried
out by photochemical reduction of Cu using highly reductive
short lived l-hydroxy-1-methylethy:L radicals. ~hese radicals
were produced in situ by illuminatLng a mixture of 1 M
acetone and 1 M propanol-2 with an UV source of Hg Xe lamp
operated at 45G watt. The reactioII can be presented by
(CH3)2CO-~ (CH3)~CO
(CH3)2C ~ (CH3)2CH0~2(cH3~2cOH
; 2(CH3)2COH + Cu 2_~ 2~CH3~2CO + Cu +2H
nCu ~ Cun
Two different solutions of Cu (1 x 10 M and 1 x 10 3 M~
were used to achieve two different coating thic~nesses. Both
solutions contained 1 M acetone, 1 M propanol-2, and carbon
fibers. The illumination time was two hours.
~ These coated fibres, washed with distilled water
^~, and observed under an optical microscope, show a very fine
q and smooth coating and visibly exhibit a metallic lustre of
~:1 25 copper. The amount of copper on these fibres was detected
using atomic absorption spectroscop~ after removing the coat
with 1 M nit~ic acid. The presence of copp~r on these fibres
; was also confirmed using Energy Dispersive Spectroscopy
EDS), which shows a peak ~or copper. ~he thickness of the
; 30 coat can be controlled by the copper concentration in
,., :
,i .
. . .
.1 .
,q
'~1
., :
. , .
W ~ 1~0603~ 9 PCT/US90/06008
solution and the duration of illumination. It can be readily
l varied in the range of tens of nanometers to microns.
The processes discussed above may be used i21
various combinations to form paxticles of a desired
configuration. For example, Figure 35 generally outlines a
procedure to make particle 20 of Figure 3. First, metal
coatin.g 20b is formed over dielectric core 20a, for example
using the method illustrated in Fiyure 31; and then silver
halide coating 20c is made over metal layer 20b, ~or instance
by generally following the method shown in Figure 2a.
Similarly, Figure 36 generally illustrates a procedure to
make particle 22 of F.igure 4. In this procedure, first,
metal coating 22b is formed over dielectric core 22a, ~or
example by the process described above in connection with
Figure 29, then polymer coating 22c is applied over coating
22b, and then silver halide layer 22d is formed ov~r coating
22c, for example by generally following the procedure
discussed above in connection with Figure 28.
While it is apparent that the invention herein
disclosed is well calrulated to fulfill the objects
previously stated, it will be appreciated that numerous
modifications and embodiments may be devised by those skilled
. in the art, and it is intended that the appended claLms cover
- all such modifications and embodiments as fall within the
- true spirit and scope of the present inv~ntion.
~ 25
.,
.
.
~ 3O
''
. 35
.,
y