Note: Descriptions are shown in the official language in which they were submitted.
-1- 2070697
BACKGROUND OF THE INVENTION
This invention is concerned with a microbial process for
the production of compositions of matter containing octalactone
having the generic structure:
Considerable time and effort have been expended by
microbiologists in the search for better processes for the
production of saturated lactones; and more generally, lactones
per se. U.S. Patent No. 3,076,750 discloses a method for
preparing certain optically active lactones and the
corresponding hydroxycarboxylic acids by microbial reduction of
ketocarboxylic acids. The metabolism of ricinoleic acid by
some Candida strains was investigated by Okui, et al (J. Bio-
chemistry, 54,536-540, 1963) who showed that gamma hydroxy
decanoic acid was an intermediate in the oxidative degradation
of ricinoleic acid. However, only trace amounts of gamma
hydroxydecanoic acid were recovered from the fermentation
medium due to the metabolism of gamma hydroxydecanoic acid upon
completion of the fermentation, and the toxicity of ricinoleic
acid to the microorganism, which limits the amount of substrate
that can be used.
-2- 2070697
U.S. Patent No. 4,560,656 provided a method of producing
optically active gamma hydroxydecanoic acid comprising
culturing or incubating a microorganism capable of hydrolyzing
castor oil, and effecting beta-oxidation of the resulting
hydrolysate in the presence of castor oil, to produce gamma
hydroxydecanoic acid.
U.S. Patent No. 4,560,656 also provided a method of
producing optically active gamma hydroxydecanoic acid
comprising enzymatically hydrolyzing castor oil using lipase to
form an enzymatic hydrolysate and culturing or incubating a
microorganism capable of effecting beta-oxidation of the
enzymatic hydrolysate in the presence of said hydrolysate to
produce gamma hydroxydecanoic acid.
U.S. Patent No. 4,560,656 also provided a method of
producing optically active gamma hydroxydecanoic acid
comprising culturing or incubating a microorganism capable of
hydrolyzing castor oil and a microorganism capable of effecting
beta-oxidation of castor oil hydrolysate in the presence of
castor oil to produce gamma hydroxydecanoic acid.
European Published Patent Application 258993 published on
April 9, 1988 discloses a process for the production of
optically active gamma hydroxydecanoic acid suitable for con-
version to optically active gamma decalactone. The process
covers steps of:
(a) culturing Sporobolomyces odorous; and/or
Rhodotorula ~lutinis on a medium containing
a ricinoleic acid source at 15°-35°C at a pH
of 3-9 and, optionally;
(b) lactonizing the resulting gamma hydroxydecanoic
acid to gamma decalactone.
-3-
2Q70697
Lion Corporation, European Published Patent Application
No. 269,351 filed on November 17, 1987 discloses a method for
producing a fat containing gamma linolenic acid comprising the
steps of culturing a microorganism belonging to the genus
Absidia, the genus Mortierella, the genus Mucor, the genus
Rhizopus or the genus Syncephalastrum with a fatty acid or an
ester thereof as the carbon source, and converting the fatty
acid or the ester thereof to gamma linolenic acid. The
microorganisms exemplified are Absidia corymbifera, IFO 4010,
Mortierella isabellina, IFO 7873, Rhizopus oryzae, IFO 5418 and
_Syncephalastrum racemosum, IFO 4816. Fatty acids or esters
exemplified are set forth in paragraph 5, on page 3 of European
Application 269,351, to wit, fatty acids having 8 to 22 carbon
atoms, particularly 8 to 18 carbon atoms, as exemplified by
n-capric acid, n-caprylic acid, lauric acid, myristic acid,
palmitic acid, stearic acid, oleic acid and linoleic acid.
PCT Application 89/12104 (BASF Corporation) discloses a
process for the preparation of gamma and delta lactones from
organic carboxylic acids or derivatives thereof by means of
cultivating, under aerobic conditions, a fungus of the genus
Mucor in a suitable medium containing the carboxylic acid or a
derivative thereof. Examples of the Mucor fungus are the
following strains: M, subtillissimus, M. mucedo, M. miehei,
M. circinelloides, M. luteus, M. flavus, M. corticolus and
M. albo-ater. Furthermore, Example 2 of PCT Application
No. 89/12104 indicates that when using Mucor circinelloides and
using an ethyl octanoate substrate, the resulting product
recovered is 49.4% gamma octalactone having the structure:
v
(.
r.
-4- 2070fi97
having a specific rotation (alphad of -39.8).
Kutney, et al, Helvetica Chimica Acta., Volume 65, Fasc. 5
(1982) No. 127, at pages 1343-1350, discloses the use of
Mortierella isabellina in carrying out a gamma hydroxylation of
the compound having the structure:
to form the compound having the structure:
!'Ia~,
a
0
~V
~0
-5-
X070697
according to the reaction:
U.S. Letters Patent 4,960,597 issued on October 2, 1990
described a process for the preparation of compositions of
matter containing both saturated and unsaturated lactones
including the saturated gamma decalactone. Thus, U.S. Patent
4,960,597 described a process for the preparation of
compositions of matter defined according to the generic
structure:
X
wherein R represents C6 alkyl or alkenyl, and X represents
C2, C4 or C6 alkylene or alkenylene; with the proviso
that R is C6 alkyl when X is alkenylene and R is C6 alkenyl
when X is alkenylene by means of the sequential steps of (i)
fermentation of castor oil or ricinoleic acid using a micro-
organism selected from the group consisting of:
-6-
207069
Candida petrophilum, ATCC 20226;
Candida oleophila, ATCC 20177;
Candida sue.., ATCC 20504; and
Candida sake, ATCC 28137
whereby gamma hydroxydecanoic acid and a mixture of other acids
defined according to the generic structure:
y c
OOH
is formed wherein Y represents an oxo-saturated,
oxo-unsaturated or di-unsaturated C9, C11 or C13 moiety
according to the reaction:
/ 1/
OH
H
O
~' ~ ~ H
\oH
-'- 2070697
(ii) lactonization of the resulting gamma hydroxydecanoic acid
by means of simultaneous acidification and heating according to
the reaction:
X
~o
y e~~H ~ o 0
and (iii) lactonization (via distillation) of one or more of
the resulting acids defined according to the structure:
0
C~oH
according to the reaction:
X
Y c\~H K o 0
2Q7 Q~97
wherein the sum of the number of carbon atoms in the X moiety
and in the R moiety is equal to the number of carbon atoms in
the Y moiety minus 1.
In the flavor and fragrance art, a need has arisen for the
development and efficient production of naturally occurring
lactones which have heretofore been found to be useful and
necessary in the creation of flavor formulations used in
augmenting or enhancing the aroma or taste of foodstuffs,
chewing gums, toothpastes, medicinal products, chewing tobaccos
and smoking tobaccos and also useful in augmenting or enhancing
the aroma of perfume compositions, colognes and perfumed
articles (e.g., solid or liquid anionic, cationic, nonionic or
zwitterionic detergents, perfumed polymers, fabric softener
compositions, fabric softener articles, hair preparations,
cosmetic powders and the like).
Gamma octalactone defined according to the structure:
~~o' ~o
particularly its optical isomers having the structures:
' 1 O
x,474697
and
I
1
i
~y
is useful particularly for forming butter flavors for use in
flavoring products which cannot contain any natural butter due
to health reasons. Thus, compounds having the structures:
' 1 0
0
v
and
I
I
9
~y
-1°- 2070697
are found to be highly useful in producing butter flavored
margarine. Furthermore, the combination of the compounds
having the structures:
' 1 O
and
~\
O \O
taken further together with other naturally occurring materials
produced via fermentation including but not limited to, for
example, the compounds having the structures:
t
I
-11-
Image
_12_ 2070697
has been found to be highly useful in the production of butter
flavors as well as in the production of perfumery materials,
colognes and perfumed articles.
Nothing in the prior art however discloses the ability by
means of fermentation to create novel mixtures of lactones
together with other furan derivatives for use in augmenting or
enhancing the organoleptic properties of consumable materials.
-13- 207097
SUMMARY OF THE INVENTION
The present invention is directed to a microbiological
process for the production of compositions containing a major
proportion of gamma octalactone generically defined according
to the structure:
" 'a' ~o
containing the stereoisomers having the structures:
1 O
a nd
I
1
1
-14- '~070~97
According to this process, a culture of one of the
following fungi is incubated with a substrate, caprylic acid or
ethyl caprylate defined according to the generic structure:
0
bR
wherein R is ethyl or hydrogen to form the gamma hydroxy acid
defined according to the structure:
using a microorganism selected from the group consisting of:
Mortierella isabellina, ATCC 44583;
Mortierella isabellina, ATCC 38063;
Syncephalastrum racemosum, NRRL A-5889;
Mortierella isabellina, IFO 7884;
Mortierella ramanniana var. angulispora, IFO 8187;
Mortierella isabellina, CBS 221.29;
Mortierella isabellina, IFO 7873;
Mortierella ramanniana var. ramanniana, CBS 112.08;
Mortierella ramanniana var. ramanniana, CBS 478.63;
Choanephora cucurbitarum, NRRL 2744;
Mortierella isabellina, IFO 8183;
Mortierella isabellina, IFO 8309;
Mortierella nana, IFO 8794;
Mortierella isabellina, IF0 7824; and
Mortierella vinacea. IFO 6738
-14a- X070697
or an active mutant thereof capable of producing the compound:
OH
O~
in recoverable amount s
15 ~07p~97
according to the reaction:
\ O \ O
oR ~ ~.oH
O/,~
The resulting carboxylic acid is then heated in the
presence of acid (as by distillation and/or evaporation) to
yield the desired lactone according to the reaction:
o
\oN
0 0
ON
The resulting acid is usually an optical isomer defined
according to one of the structures:
-ls- 2070697
y ,
y v
0
and/or
O
\; .
off
H
or a mixture of such isomers with either the dextro or laevo
rotatory isomer being the predominant isomer. Such stereo
isomers yield stereoisomers of the resulting octalactones
defined according to the structures:
1
1 .
VN
9
_1~_ 2070697
and
I
1
y
Along with the gamma octalactone, minor amounts of other
products are also produced when using the above-mentioned
organisms, to wit, the compounds having the structures:
J
o ~o
-18-
Image
x
r
-19- 2070697
a nd
The nutrient broth used according to the process of this
invention includes the usual sources of nitrogen, carbo-
hydrates, minerals and oxygen. Incubative fermentation
conditions used according to the process include any pH,
temperature, substrate concentration and substrate feed rate
which will maintain the viability of the culture.
The inventive process may be conducted in a batch or
continuous mode of operation. In a batch fermentation, the
nutrient broth, culture and substrate are combined and
fermented until the lactone concentration becomes constant. In
a continuous process, the substrate in the nutrient broth may
be continuously recirculated through a fermentation reactor
with the provision that substrate and product are respectively
added and removed from the recirculating broth.
In carrying out the present invention, cultivation and
fermentive incubation of the fungus are accomplished in an
aqueous medium in the presence of the usual nutrient
a
i r
-2~- zo~oss~
substances. A suitable medium is one which contains carbon
sources, nitrogen sources, inorganic salts and growth factors.
Among the suitable carbon sources are, for example, glucose,
fructose, xylose, sucrose, maltose, lactose, mannitol,
sorbitol, glycerol, corn syrup and corn syrup solids. Examples
of suitable nitrogen sources include organic and inorganic
nitrogen-containing substances such as peptone, corn steep
liquor, meat extract, yeast extract, casein, urea, amino acids,
ammonium salts, nitrates, enzymatic digest of soy, and mixtures
the reof .
Examples of inorganic salts include the phosphate and
sulfate salts of magnesium, sodium, calcium and potassium.
These nutrients may be supplemented with, for example, one
or more vitamins of the ~B~ group and one or more trace
minerals such as iron, manganese, cobalt and copper as desired.
For the nutrient broth, it is preferred to utilize dextrose
at a concentration of from about 2 up to about 20 weight
percent, preferably at about 10 weight percent. It is also
preferred to employ ~B~ vitamins either as a separate
supplement or in the form of a yeast extract. The kind and
amounts of the above-mentioned additives can be determined by
applying the general knowledge in the art for the cultivation
of microorganisms.
In a typical procedure, one of the fungi as set forth below:
Mortierella isabellina, ATCC 44583;
Mortierella isabellina, ATCC 38063;
Syncephalastrum racemosum, NRRL A-5889;
Mortierella isabellina, IFO 7884;
Mortierella ramanniana var. angulispora, IFO 8187;
Mortierella isabellina, CBS 221.29;
Mortierella isabellina, IFO 7873;
_21_ 2070697
Mortierella ramanniana var. ramanniana, CBS 112.08;
Mortierella ramanniana var. ramanniana, CBS 478.63;
Choanephora cucurbitarum, NRRL 2744;
Mortierella isabellina, IFO 8183;
Mortierella isabellina, IFO 8309;
Mortierella nana, IFO 8794;
Mortierella isabellina, IFO 7824; and
M~rtierella vinacea. IFO 6738
is first cultivated in inoculum quantities to produce a mature
culture in nutrient broth. The culture is inoculated into a
fermentor nutrient broth and allowed to establish itself. The
substrate is then added and fermentation continued until a
steady concentration of lactone is present.
The cultivation and fermentative incubation of the fungus
can be carried out as a stationary culture or as a submerged
culture (e. g., shake-flask, fermentor), preferably under
aerobic conditions. Cultivation and incubation may proceed in
a pH range of from about 3 up to about 9, preferably in the
range of about 5 to about 7. The pH may be regulated by the
addition of an inorganic or organic acid or base such as hydro-
chloric acid, acetic acid, sodium hydroxide, calcium carbonate,
ammonia, ion-exchange resins, or by the addition of a buffer
such as a phosphate or phthalate. The incubation temperature
is suitably maintained at between about 18°C up to about 31°C,
with a range of from about 26-28°C being preferred.
In accordance with another typical procedure of the present
invention, the process is conveniently carried out by adding
the substcate to the culture medium at the onset of culti-
vation, under aerobic conditions. Alternatively, the sub-
strate may be added either alone or in combination with another
carbon source, such as glucose, during fermentative incubation,
or when cultivation is complete. It is preferable to add the
-22- 2 0 7 0 6 9 7
substrate to the culture medium during the period of from 4 up
to 24 hours after the growth of the culture in the fermentative
broth has commenced. Desirable results can be obtained when
the substrate is added continuously over the entire fermenta-
tion after an initial fungal cultivation period of from 3 up to
12 hours. A preferred feed rate for this continuous addition
is from about 0.01 up to 1 gram per hour per liter with a
preferred range of from 0.6 up to 0.8 gram per hour per liter.
The concentration of the substrate in the medium may vary
depending on the conditions employed. In practice, the
concentration of the substrate in the medium may conveniently
vary from 0.01% up to about 10% with a preferable concentration
of about 1% by weight, consistent with the manner in which it
is added to the culture.
Under the usual conditions, mixtures of optically active
lactones having the structures:
' 1 O ~~O
and
I
1
1
are produced.
23 2Q 7 ~ ~ 9 7
Depending on the pH, oxygen flow rate, nutrients and whether R
is ethyl or hydrogen, the concentrations of side products will
vary, these side products being the compounds having the
structures:
t
O J
0
p O
t
-24-
Image
-25- 20 7 0 6 9 ~
The reaction period for carrying out the reaction, to wit:
ON
H
varies according to the specific incubation parameters, such as
the strain of microorganism employed, the composition of the
culture medium and whether the substrate used is the ethyl
caprylate ester having the structure:
U
or caprylic acid having the structure:
H
-26-
~474697
In general, shake flask cultures require between 100 and 200
hours, preferably between 120 and 150 hours, depending upon the
microbial strain and the substrate utilized. However, when a
fermentor is used, the fermentation period may be reduced to
40-50 hours.
The incubation is carried out under aerobic conditions,
wherein the dissolved oxygen content in the incubation broth is
from 20 to 100% of saturation by weight, preferably 30% to
80%. Also, preferably, the substrate is maintained in
~Icontinuous contact with the aqueous phase and the
i microorganism. Generally, vigorous stirring or shaking is
i satisfactory, but if desired a surface active agent, such as
TWEEN~80, can be added to aid in the dispersion of the
substrate. Conventional antifoam agents such as silicone oils,
polyalkylene glycol derivatives, or soya oil can be used to
,control foaming.
The form in which the microorganisms are used for the
fermentation is not critical. The fermentation may be carried
out using the cells of the microorganism isolated from the
culture solution, or with an enzyme extract isolated from the
cells in a known manner. In the latter case, the reaction can
be conveniently carried out in an aqueous solution, for
example, in a buffer solution, in a physiological saline
solution, in a fresh nutrient solution, or in water. The
isolated cells or an enzyme extract thereof may be immobilized
on a solid support and the desired transformation conducted
separately. It will be convenient to employ the immobilized
form of the enzyme extract in a continuous process. The
fermentation of the substrate may also be effected by mutants
of the fu ngus .
2070697
The progress of the fermentative production of the hydroxy-
carboxylic acid or hydroxy carboxylic acid ester having one or
both of the structures:
.\
VH
and/or
.\
~ ~i
off
. . ~
can be monitored by assaying for hydroxy acid or hydroxy ester
concentration using standard analytical techniques such as
chromatography (gas-liquid, thin layer or high pressure liquid)
and spectroscopy such as IR and NMR. The fermentation can also
be followed by measuring consumption of substrate, glucose,
oxygen or by measuring pH changes. The fermentation is gen-
erally terminated when all of the substrate has been consumed
i
r
-2$- 2070697
or when no further increase in the hydroxy acid or hydroxy
ester concentration is observed.
When the resulting material is sterilized as by heating,
the hydroxy acid or hydroxy ester is formed into one or both of
the lactones defined according to the structures:
1
1
and/or
I
1
1
'y
-29- 2Q70697
Necessarily, the reaction itself will cause in situ formation
of lactones in a lower concentration. Resultant sterilization
and subsequent distillation gives rise to complete conversion
of the hydroxy acid and hydroxy ester to the lactones having
the structures:
- , o
o
and/or
~\
0 ~O
~y
The present invention produces unexpectedly high yields of
the octalactones defined according to the structures:
r
-30- 2070697
O
O
and/or
1
1
1
~y
compared with prior art methods which yield products having a
much lower yield.
Hereinafter, the term "lactone derivative(s)" will be under-
stood to mean the reaction products subsequently purified con-
taining substantially all of the compounds having the
structures:
~. \ O
O
-31- 2070697
and/or
'O
~y
together with one or more of the side products having the
structures:
o J
0
-32-
Image
-33- 2070697
and
The lactone derivatives) and one or more auxiliary perfume
lingredients, including, for example, hydrocarbons, alcohols,
ketones, aldehydes, nitriles, esters other than the lactone
derivatives of our invention, ethers, synthetic essential oils,
and natural essential oils may be admixed so that the combined
odors of the individual components produce a pleasant and de-
~sired fragrance, particularly and preferably in the fruity area
a , g. , peach and apr icot a romas ) . Such per fume compo si tions
usually contain (a) the main note or the ~bouquet~ or founda
tion stone of the composition; (b) modifiers which round off
i and accompany the main note; (c) fixatives which include
odorous substances which lend a particular note to the perfume
throughout all stages of evaporation and substances which
retard evaporation; and (d) topnotes which are usuaally low-
boiling, fresh-smelling materials.
In perfume compositions, it is the individual compositions
which contribute to their particular olfactory characteristics,
however, the overall sensory effect of the perfume composition
will be at least the sum total of the effects of each of the in-
gredients. Thus, one or more of the lactone derivatives) of
-34- 2 0 7 0 6 9 7
our invention can be used to alter, modify or enhance the aroma
characteristics of a perfume composition, for example, by
utilizing or moderating the olfactory reaction contributed by
another ingredient in the composition.
The amount of lactone derivatives) of our invention which
will be effective in perfume compositions as well as in per-
fumed articles and colognes depends upon many factors including
the other ingredients, their amounts and the side effects which
are desired. It has been found that perfume compositions con-
taining as little as 0.005% of lactone derivatives) or even
less (e. g., 0.002%) can be used to impart sweet, fruity (peach
and apricot) aromas to soaps, cosmetics, detergents including
anionic, cationic, nonionic and zwitterionic solid or liquid
detergents, perfumed polymers and other products. The amount
employed can range up to 70% of the fragrance components and
will depend upon the consideration of cost, nature of the end
product, the effect desired on the finished product and the
particular fragrance sought.
The lactone derivatives) of our invention are useful
(taken alone or taken together with other ingredients in
perfume compositions) in detergents, soaps, space odorants and
deodorants, perfumes, colognes, toilet waters, bath pre-
parations, hair preparations such as lacquers, brilliantines,
pomades and shampoos; cosmetic preparations such as creams,
deodorants, hand lotions and sun screens; powders such as
talcs, dusting powders, face powders and the like.
As little as 0.25% of the lactone derivatives) will
suffice to impart an intense, sweet, fruity (peach and apricot)
aroma to floral perfume formulations. Generally no more than
5% of the lactone derivatives) based on the ultimate end
product is required to be used in the perfume compositions.
35 ~0 ~ 0 fi 9 7
Furthermore, as little as 0.25% of the lactone deriva-
tive s) will suffice to impart such aromas to perfumed articles
per se, whether in the presence of other perfume materials or
whether used by themselves. Thus, the range of use of the
lactone derivatives) of our invention in perfumed articles,
e.g., perfumed polymers and solid or liquid anionic, cationic,
nonionic or zwitterionic solid or liquid detergents, may vary
from 0.25% up to about 5% by weight based on the total weight
of the perfumed article.
in addition the perfume composition or fragrance
composition of our invention can contain a vehicle or carrier
for the lactone derivative(s). The vehicle can be a liquid
such as a non-toxic alcohol, e.g., ethanol, a non-toxic
glycol, e.g., propylene glycol, or the like. The carrier can
also be an absorbent solid such as a gum (e.g., gum arabic or
xanthan gum or guar gum) or components for encapsulating the
composition by means of coacervation (such as by gelatin) or by
means of formation of a polymer around a liquid center (as by
using a urea formaldehyde prepolymer to form a polymeric
capsule around a perfume composition center).
It will be appreciated from the present disclosure that the
lactone derivatives) according to the present invention can be
used to alter, vary, fortify, modify, enhance or otherwise
improve the flavor of a wide variety of materials which are
ingested, consumed or otherwise organoleptically sensed.
The terms "alter" and "modify" in their various forms will
be understood herein to mean the supplying or imparting of a
flavor character or note to an otherwise bland, relatively
tasteless substance, or augmenting an existing flavor char-
acteristic where the natural flavor is deficient in some regard
or supplementing the existing flavor impression to modify its
organoleptic character.
-3s- 2Q74697
The term "enhance' is intended herein to mean the
intensification (by use of the lactone derivative of our
invention) of a flavor or aroma note or nuance in a tobacco
flavor or foodstuff or perfume composition or perfumed article
without changing the quality of said note or nuance.
A "flavoring composition" is taken to mean one which con-
tributes a part of the overall flavor impression by supple-
menting or fortifying a natural or artificial flavor in a
material or one which supplies substantially all the flavor
and/or aroma character to a consumable article.
The term "foodstuff" as used herein includes both solid and
liquid ingestible materials for man or animals which materials
usually do, but need not, have nutritional value. Thus, food-
stuffs include meats, gravies, soups, convenience foods, malt,
alcoholic and other beverages, milk and dairy products,
seafoods, including fish, crustaceans, mollusks and the like,
candies, vegetables, cereals, soft drinks, snacks, dog and cat
foods, other veterinary products, and the like. The lactone
derivatives) of our invention are also useful in tobacco
flavorants and flavor enhancers.
The term "tobacco" will be understood herein to mean
natural products such as, for example, burley, Turkish tobacco,
Maryland tobacco, flue-cured tobacco and the like including
tobacco-like or tobacco-based products such as reconstituted or
homogenized leaf and the like as well as toacco substitutes in-
tended to replace natural tobacco such as lettuce and cabbage
leaves and the like. The tobaccos and tobacco products in
which the lactone derivatives) of our invention are useful
including those designed or used for smoking such as in
cigarettes, cigar and pipe tobacco, as well as products such as
snuff, chewing tobacco and the like.
-3'- 2070697
When the lactone derivatives) of this invention are used
in a flavoring composition, they can be combined with
conventional flavoring materials or adjuvants. Such
co-ingredients or flavor adjuvants are well known in the art
for such and have been extensively described in the
literature. Requirements of such adjuvant materials are: (1)
that they be non-reactive with the lactone derivatives) of our
invention; (2) that they be organoleptically compatible with
the lactone derivatives) of our invention whereby the flavor
of the ultimate consumable material to which the lactone
derivatives) are added is not detrimentally affected by the
use of the adjuvant; (3) that they be ingestibly acceptable and
thus non-toxic or otherwise non-deleterious. Apart from these
requirements, conventional materials can be used and broadly
include other flavor materials, vehicles, stabilizers,
thickeners, surface active agents, conditioners, and flavor
I intensifers.
Such conventional flavoring materials include saturated
fatty acids, unsaturated fatty acids and amino acids; alcohols
including primary and secondary alcohols, esters, carbonyl
compounds including ketones and aldehydes; lactones; other
cyclic organic materials including benzene derivates, alli-
cyclic compounds, heterocyclics such as furans, pyridines,
pyrazines and the like; sulfur-containing compounds in-
cluding thiols, sulfides, disulfides and the like; proteins;
lipids, carbohydrates; so-called flavor potentiators such as
monosodium glutamate; magnesium glutamate, calcium glutamate,
guanylates and inosinates; natural flavoring materials such as
cocoa, vanilla and caramel; essential oils and extracts such as
anise oil, clove oil and the like and artificial flavoring
materials such as vanillin and the like.
-38- 207p697
Specific preferred flavor adjuvants are as follows:
anise oil;
ethyl-2-methyl butyrate;
vanillin;
cis-3-heptenol;
cis-3-hexenol;
trans-2-heptenol;
cis-3-heptenal;
butyl valerate;
2,3-diethyl pyrazine;
methyl cyclopentenolone;
benzaldehyde;
valerian oil;
3,4-dimethoxyphenol;
amyl acetate;
amyl cinnamate;
gamma butyryl lactone;
furfural;
trimethyl pyrazine;
phenyl acetic acid;
isovaleraldehyde;
ethyl maltol;
ethyl vanillin;
ethyl valerate;
ethyl butyrate;
cocoa extract;
coffee extract;
peppermint oil;
spearmint oil;
clove oil;
anethol;
cardamom oil;
-39-
wintergreen oil;
cinnamic aldehyde;
ethyl-2-methyl valerate;
gamma hexenyl lactone;
2,4-decadienal;
2,4-hep tadienal; and
butylidene phthalide.
2070697
According to another aspect of our invention, an organolep-
tically improved smoking tobacco product and additives therefor
as well as methods of making the same which overcome specific
problems heretofore encountered in which specific Turkish,
oriental-like aromas prior to smoking and improved Turkish,
oriental aromas on smoking in the main stream and the side
stream are created or enhanced or modified or augmented and may
be readily controlled and maintained at the desired uniform
level regardless of variations in the tobacco components of the
blend. In particular, low grade Virginia-type tobaccos may be
upgraded using the lactone derivatives) of our invention.
This invention further provides improved tobacco additives
and methods whereby various desirable natural aromatic Turkish
tobacco flavoring characteristics with oriental notes may be
imparted to smoking tobacco products and may be readily varied
and controlled to produce the desired uniform flavoring
characteristics.
In carrying out this aspect of our invention, we add to
smoking tobacco materials or a suitable substitute therefor
(e. g., dried lettuce leaves) an aroma and flavor additive
containing as an active ingredient one or more of the lactone
derivatives) of our invention.
t
2070697
In addition to the lactone derivatives) of our invention,
other flavoring and aroma additives may be added to the smoking
tobacco material or substitute therefor either separately or in
admixture with the lactone derivatives) of our invention as
follows:
I. Synthetic Materials
Beta-ethyl-cinnamaldehyde;
Eugenol;
Dipentene;
Beta-damascenone;
Maltol;
Ethyl maltol;
Delta undecalactone;
Delta decalactone;
Benzaldehyde;
Amyl acetate;
Ethyl butyrate;
Ethyl valerate;
Ethyl acetate;
2-Hexenol-1;
2-Methyl-5-isopropyl-1,3-nonadiene-8-one;
2,6-Dimethyl-1,6-undecadiene-10-one;
2-Methyl-5-isopropyl acetophenone;
2-Hydroxy-2,5,5,8a-tetramethyl-1-(2-hydroxyethyl)-
decahydronaphthalene;
Dodecahydro-3a,6,6,9a-tetramethyl naphtho(2,1-b)
furan;
4-Hydroxy hexanoic acid, gamma lactone; and
Polyisoprenoid hydrocarbons defined in Example V of
U.S. Patent No. 3,589,372 issued on ,Tune 29, 1971.
-41- 2o~oss~
II. Natural Oils
Celery seed oi;
Coffee extract;
Bergamot oil;
Cocoa extract;;
Nutmeg oil; and
Origanum oil.
An aroma and flavoring concentrate containing one or more
of the lactone derivatives) of our invention and, if desired,
one or more of the above indicated additional flavoring addi-
tives may be added to the smoking tobacco material, to the
filter or to the leaf or paper wrapper. The smoking tobacco
material may be shredded, cured, cased and blended tobacco
material or reconstituted tobacco material or tobacco substi-
tutes (e. g., lettuce leaves) or mixtures thereof. The propor-
tions of flavoring additives may be varied in accordance with
taste but insofar as enhancement or the imparting of oriental
i and/or Turkish tobacco notes, we have found that satisfactory
results are obtained if the proportion by weight of the sum
total of lactone derivatives) to smoking tobacco material is
~ between 50 ppm and 1,500 ppm (0.005%-0.15%) of the active in-
gredients to the smoking tobacco material. We have further
found that satisfactory results are obtained if the proportion
by weight of the sum total of lactone derivatives) used to
flavoring material is between 500 and 15,000 ppm (0.05%-1.5%).
Any convenient method for incorporating the lactone deri-
vative s) into the tobacco product may be employed. Thus, the
lactone derivatives) taken alone or along with other flavoring
additives may be dissolved in a suitable solvent such as
ethanol, diethylether, and/or volatile organic solvents and the
resulting solution may either be spread onto the cured, cased,
-42-
2070697
and blended tobacco material or the tobacco material may be
dipped into such solution. Under certain circumstances, a
solution of the lactone derivatives) taken alone or taken
further together with other flavoring additives as set forth
above may be applied by means of a suitable applicator such as
a brush or roller on the paper or leaf wrapper for the smoking
product, or it may be applied to the filter by either spraying
or dipping or coating.
Furthermore, it will be apparent that only a portion of the
tobacco or substitute therefor need be treated and the thus-
treated tobacco may be blended with other tobaccos before the
ultimate tobacco product is formed. In such cases, the tobacco
treated may have the lactone derivatives) in excess of the
amounts or concentrations above indicated so that when blended
with other tobaccos, the final product will have the percentage
within the indicated range.
In accordance with one specific example of our invention,
an aged, cured and shredded domestic Virginia tobacco is
sprayed with a 20% alcohol solution of the compound having the
structure:
' ~ 0
7
on a dry basis. Thereafter, the alcohol is removed by
evaporation and the tobacco is manufactured into cigarettes by
the usual techniques. The cigarette, when treated as
indicated, has a desired and pleasing aroma which is detectable
in the main stream and the side stream when the cigarette is
smoked. The aroma is described as being sweeter, with pro-
nounced Turkish/oriental characteristics and with improved body
and enhanced tobacco character in the main stream and side
stream. In addition, interesting amber nuances are imparted.
While our invention is particularly useful in the manufac-
ture of smoking tobacco such as cigarette tobacco, cigar
tobacco and pipe tobacco, other tobacco products formed from
sheeted tobacco dust or fines may also be used. Likewise the
lactone derivatives) of our invention can be incorporated with
materials such as filter tip materials, seam paste, packaging
materials and the like which are used along with tobacco to
form a product adapted for smoking. Furthermore, the lactone
derivatives) can be added to certain tobacco substitutes of
natural or synthetic origin (e. g., dried lettuce leaves) and,
accordingly, by the term 'tobacco" as used throughout this
specification, is meant any composition intended for human
consumption by smoking or otherwise when composed of tobacco
plant parts or substitute material or both.
The lactone derivatives) of our invention can be used to
alter, vary, fortify, modify, enhance or otherwise improve the
organoleptic properties, including flavor and/or aroma, of a
wide variety of materials which are ingested, consumed, or
otherwise organoleptically sensed.
i
i
The term "alter" in its various forms will be understood
herein to mean the supplying or imparting of a flavor character
or note to an otherwise bland, relatively tasteless substance,
or augmenting the existing flavor characteristic where the
natural flavor is~deficient in some regard, or supplementing
the existing flavor or aroma impression to modify the organo-
leptic character. The materials which are so altered are
generally referred to herein as consumable materials.
The lactone derivatives) of our invention are accordingly
useful in flavoring compositions. Flavoring compositions are
hereinafter taken to mean those which contribute a part of the
overall flavor impression by supplementing or fortifying a
natural or artificial flavor in a material, as well as those
which supply substantially all the flavor and/or aroma charac-
ter to a consumable article.
The term "foodstuff" as used herein includes both solid and
liquid ingestible materials for man or animals, which materials
usually do, but need not, have nutritional value. Thus,
foodstuffs include meats, gravies, soups, convenience foods,
malt and other alcoholic or non-alcoholic beverages, milk and
dairy products, (including but not limited to margarine and
butter) nut butters such as peanut butter and other spreads,
seafoods including fish, crustaceans, mollusks and the like,
candies, breakfast foods, baked goods, vegetables, cereals,
soft drinks, snack foods, dog and cat foods, other veterinary
products, and the like.
When the lactone derivatives) produced according to this
invention are used in a food flavoring composition, they can be
combined with conventional flavoring materials or adjuvants.
Such co-ingredients or flavoring adjuvants are well known in
the art for such use and have been extensively described in the
literature. Apart from the requirement that any such adjuvant
w _ __. - .
_ ____ _~.-
-45- 2 0 7 0 6 9 7
material is ingestibly acceptable, and thus non-toxic or
otherwise non-deleterious, conventional materials can be used
and broadly include other flavor materials, vehicles,
stabilizers, thickeners, surface active agents, conditioners
and flavor intensifiers.
Examples of preferred co-flavoring adjuvants are:
Methyl thiazole alcohol (4-methyl-5-beta-hydroxyethyl thiazole;
2-Methyl butanethiol;
4-Mercapto-2-butanone;
3-Mercapto-2-pentanone;
1-Mercapto-2-propanone;
Benzaldehyde;
Furfural;
Furfuryl alcohol;
2-Mercapto propionic acid;
Alkyl pyrazine;
Methyl pyrazine;
2-Ethyl-3-methyl pyrazine;
Tetramethyl pyrazine;
Polysulfides;
Dipropyl disulfide;
Methyl benzyl disulfide;
Alkyl thiophenes;
2-Butyl thiophene;
2,3-Dimethyl thiophene;
5-Methyl furfural;
Acetyl furan;
2,4-Decadienal;
Guiacol;
Phenyl acetaldehyde;
B-Decalactone;
d-Limonene;
Acetoin;
Amyl acetate;
E.
_ _ . _. . .._ _ . . _. __ . _.:; _. . _ .. . . .._. _ _ . . _ _ . _ _ _ . _
-46-
2070697
Maltol;
Ethyl butyrate;
Levulinic acid;
Piperonal;
Ethyl acetate;
n-Octanal;
n-Pentanal;
n-Hexanal;
Diacetyl;
Monosodium glutamate;
Monopotassium glutamate;
Sulfur-containing amino acids, e.g., Cysteine;
Hydrolyzed vegetable protein;
2-Methylfuran-3-thiol;
2-Methyldihydrofuran-3-thiol;
2,5-Dimethylfuran-3-thiol;
Hydrolyzed fish protein;
Tetramethyl pyrazine;
Propylpropenyl disulfide;
Propylpropenyl trisulfide;
Diallyl disulfide;
Diallyl trisulfide;
Dipropenyl disulfide;
Dipropenyl trisulfide;
4-Methyl-2-[methylthio)-ethyl)-1,3-dithiolane;
4,5-Dimethyl-2-[methylthio)ethyl]-1,3-dithiolane;
4,5-Dimethyl-2-(methylthiomethyl)-1,3-dithiolane; and
4-Methyl-2-(methylthiomethyl)-1,3-dithiolane.
20706g'~
The lactone derivatives) of our invention or compositions
incorporating them, as mentioned above, can be combined with
one or more vehicles or carriers for adding them to the parti-
cular product. Vehicles can be edible or otherwise suitable
materials such as ethyl alcohol, propylene glycol, water and
the like. Carriers include materials such as gum arabic,
carrageenan, xanthan gum, guar gum and the like.
The lactone derivatives) prepared according to this in-
vention can be incorporated with the carriers by conventional
means such as spray-drying, drum-drying and the like. Such
carriers can also include materials for coacervating the
lactone derivatives) of our invention to provide encapsulated
products. When the carrier is an emulsion the flavoring
composition can also contain emulsifiers such as mono- and
diglycerides or fatty acids and the like. With these carriers
or vehicles, the desired physical form of the composition can
be prepared.
The quantity of lactone derivatives) utilized should be
sufficient to impart the desired flavor characteristic to the
product, but on the other hand, the use of an excessive amount
of the lactone derivatives) is not only wasteful and uneconom-
ical, but in some instances too large a quantity may unbalance
the flavor or other organoleptic properties of the product
consumed. The quantity used will vary depending upon the
ultimate foodstuff; the amount and type of flavor initially
present in the foodstuff; the further process or treatment
steps to which the foodstuff will be subjected; regional and
other preference factors; the type of storage if any to which
the product will be subject; and the preconsumption treatment,
such as baking, frying, and so on, given to the product by the
ultimate consumer. Accordingly, the terminology "effective
amount" and "sufficient amount" is understood in the context of
the present invention to be quantitatively adequate to alter
the flavor of the foodstuff.
-48- 2 0 7 0 6 9 7
It is accordingly preferred that the ultimate composition
contain from about 0.1 parts per million (ppm) to about 500 ppm
of the lactone derivative(s).
The lactone derivatives) of our invention when utilized in
flavoring compositions can be varied over a wide range
depending upon the particular flavor nuances desired to be
added to the foodstuff. Thus amounts of the lactone
derivatives) of our invention may be contained in flavoring
materials from about 1 ppm up to about 50% by weight of the
flavoring composition. Indeed, the compounds having the
structures:
- , o
o
and
('
I
I
~o
o
~y
-49- 2 Q 7 p 6 9 7
as well as the hydroxy acids having the structures:
OH
UH
and
70
\ ~i
off
may be utilized in margarine flavors at levels of between about
1% and about 50%.
,:
-50- 2070697
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a block flow schematic diagram setting forth
apparatus used in carrying out a process for the production of
the novel compositions of matter of our invention including the
lactone having the structure:
0
Figure 2 is a GLC profile for the reaction product of
Example I (a) containing the compound having the structure:
v \ o' \o
(Conditions: Ov-1 column (50 meters x 0.31 mm programmed from
75-225°C at 2.0°C per minute).
-51- 207097
Figure 3 is the GLC profile for the reaction product of
Example I (b) containing the compound having the structure:
\ o' \o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 4 is the GLC profile for the reaction product of
Example I (c) containing the compound having the structure:
v
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 5 is the GLC profile for the reaction product of
Example I (d) containing the compound having the structure:
I
7
-52- 2070697
~'o' ~o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 6 is the GLC profile for the reaction product of
Example I (e) containing the compound having the structure:
v \o' \o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 7 is the GLC profile for the reaction product of
Example I (f) containing the compound having the structure:
53 2070697
~'o' ~o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 8 is the GLC profile for the reaction product of
Example I (g) containing the compound having the structure:
v \o' \o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
I75-225°C at 2.0°C per minute).
Figure 9 is the GLC profile for the reaction product of
Example III containing the compound having the structure:
'a'
-54- GO 7 0 ~ 9 7
Figure 10 is the GC mass spectrum for the combined product
of Examples II and III containing the compound having the
structure:
~'o' ~o
Figure 11 shows in detail the peaks indicated by reference
numerals 252 and 254 of the GC mass spectrum of Figure 10.
Figure 12 is a mass spectrum for the combined product of
Examples II and III.
Figure 12a is a detailed section of the mass spectrum of
Figure 12.
Figure 13 is a mass spectrum of the combined product of
Examples II and III.
Figure 13a is a detailed section of the mass spectrum of
Figure 13.
Figure 14 is the GLC profile for the first and second
extraction of the reaction product of Example N containing the
compound having the structure:
d
-55- 20 7 0 6 9 7
v
(Conditions: 50 m x 0.31 mm OV-1 column .programmed from
75-225°C at 2.0°C per minute).
Figure 15 is the GLC profile for the third extraction of
the reaction product of Example N containing the compound
having the structure:
'a' ~o
(Conditions: 50 m x 0.31 mm oV-1 column programmed at 200°C
isothermal; and from 200-250°C at 10°C per minute after a ten
minute period).
Figure 16(a) is the GLC profile for distillation Fraction 2
of the reaction product of Example VI.
207097
Figure 16(b) is another GLC profile for distillation
Fraction 2 of the reaction product of Example VI.
Figure 17 is the GC mass spectrum for distillation Fraction
2 of Example VI containing the compound having the structure:
v \ o' \o
Figure 18 is the mass spectrum for distillation Fraction 2
of Example VI.
Figure 19 is the GLC profile for distillation Fraction 4 of
the reaction product of Example VI.
Figure 20 is the GC mass spectrum of distillation Fraction
4 of the reaction product of Example VI containing the compound
having the structure:
v
r
'S'- 2070697
Figure 21 is the GLC profile for distillation Fraction 5 of
the reaction product of Example VI.
Figure 22 is the GC mass spectrum of distillation Fraction
5 of the reaction product of Example VI containing the compound
having the structure:
~'o' ~o
Figure 23 is a detailed section of the peak indicated by
reference numeral 284 of the GC mass spectrum of Figure 22.
Figure 24 is the GLC profile for the reaction product of
Example VII(a) containing the compound having the structure:
~~o' ~o
i
Figure 25 is the GLC profile for the reaction product of
Example VII(b) containing the compound having the structure:
-58-
2474697
U\0~0.
Figure 26 is the GLC profile for the reaction product of
Example VII(c) containing the compound having the structure:
~\o' \o
Figure 27 represents a cut-away side elevation m ew of
apparatus used in forming perfumed polymers which contain
imbedded therein at least one of the lactone-containing
compositions of our invention.
Figure 28 is a front view of the apparatus of Figure 27
looking in the direction of the arrows.
-59-
2070697
DETAILED DESCRIPTION OF THE DRAWINGS
Figure 1 is the block flow diagram setting forth in
schematic form the apparatus used in carrying out the process
for producing the lactone derivatives) of our invention. Into
fermentor _10, medium _12 is passed through line 14 using pump 16
and control valve _18. The addition of the medium into the
fermentor 10 is followed by the addition of prepared culture
from location _26 through line _32 past pump 28 using control
val~~p _30. The addition of the prepared culture to the medium
is followed by the addition of the substrate from location 20
through line 22 using control valve 24.
The fermentation is carried out for a period of, for
example, 48 hours.
The fermentation broth is then passed through line 34 using
pump 36 past control valve _38 through line 42 into extractor 40
wherein extractant from location _44 and from feed tank 52 is
passed through line _54 using pump 58 past control valve 56
into the extractor _40. The extraction takes place in extractor
40 and the lactone-containing extraction solvent is evaporated
in evaportor _68 after being fed into the evaporator through
line 64 using control valve _66. The extraction solvent from
location _44 is passed through line _50 using pump 48 past
control valve _46 into feed tank _52. Solvent evaporated from
the evaporator _68 passes through line _70 past control valve 72
into the feed tank _52 recycled and original extraction solvent
from feed tank _52 passes through line 54 using pump 58 past
control valve 56 into the extractor 40.
s
-6°- 2070697
The resulting product containing a high proportion of gamma
octalactone having the structure:
v \o' \o
is passed through line 74 into distillation column 76 wherein
the octalactone is distilled overhead using reflux head 88
having therein control valve _86 and _92 and recycle line 90.
The octalactone passes through line 94 into holding tank 96
where it may further be distilled or extracted and used for its
organoleptic properties. The bottoms from distillation column
_76 passes through line _78 using control valve 80 into reboiler
_98 where reboiled product passes through line 84 using control
valve _82. The bottoms from the distillation column 76 are held
in holding tank 99.
Figure 2 is the GLC profile for the reaction product of
Example I(a). The peak indicated by reference numeral 200 is
the peak for methyl caprylate having the structure:
o
~pCH~
y
-61- 2070697
The peak indicated by reference numeral 202 is the peak for
ethyl caprylate having the structure:
p
J
The peak indicated by reference numeral 204 is the peak for
gamma octalactone having the structure:
~\o/ \o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 3 is the GLC profile for the reaction product of
Example I(b). The peak indicated by reference numeral 206 is
the peak for methyl caprylate having the structure:
C"3 .
-62- 20 7 0 ~ 9 7
The peak indicated by reference numeral 208 is the peak for
ethyl caprylate having the structure:
C.'~oJ .
The peak indicated by reference numeral 210 is the peak for
gamma octalactone having the structure:
a 'O
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 4 is the GLC profile for the reaction product of
Example I(c). The peak indicated by reference numeral 214 is
the peak for ethyl caprylate having the structure:
C~oJ .
-63- 2070697
The peak indicated by reference numeral 212 is the peak for
methyl caprylate having the structure:
D
pcH3
The peak indicated by reference numeral 216 is the peak for
gamma octalactone having the structure:
~'o' ~o
(Conditions: 50 m x 0.31 mm OV-1 column programmed from
75-225°C at 2.0°C per minute).
Figure 5 is the GLC profile for the reaction product of
Example I(d). The peak indicated by reference numeral 218 is
the peak for methyl caprylate. The peak indicated by reference
numeral _220 is the peak for ethyl caprylate. The peak
indicated by reference numeral _222 is the peak for gamma
octalactone. (Conditions: 50 m x 0.31 mm OV-1 column
programmed from 75-225°C at 2.0°C per minute).
64 2070697
Figure 6 is the GLC profile for the reaction product of
Example I(e). The peak indicated by reference numeral 224 is
the peak for methyl caprylate. The peak indicated by reference
numeral _226 is the peak for ethyl caprylate. The peak
indicated by reference numeral _228 is the peak for gamma
octalactone. (Conditions: 50 m x 0.31 mm OV-1 column
programmed from 75-225°C at 2.0°C per minute).
Figure 7 is the GLC profile for the reaction product of
Example I(f). The peak indicated by reference numeral 230 is
the peak for methyl caprylate. The peak indicated by reference
numeral _232 is the peak for ethyl caprylate. The peak
indicated by reference numeral 234 is the peak for gamma
octalactone. (Conditions: 50 m x 0.31 mm OV-1 column
programmed from 75-225°C at 2.0°C per minute).
Figure 8 is the GLC profile for the reaction product of
Example I(g). The peak indicated by reference numeral 236 is
the peak for methyl caprylate. The peak indicated by reference
numeral _238 is the peak for ethyl caprylate. The peak
indicated by reference numeral _240 is the peak for gamma
octalactone. (Conditions: 50 m x 0.31 mm OV-1 column
programmed from 75-225°C at 2.0°C per minute).
Figure 9 is the GLC profile for the reaction product of
Example III. The peak indicated by reference numeral 242 is
the peak for methyl caprylate. The peak indicated by reference
numeral _244 is the peak for ethyl caprylate. The peak
indicated by reference numeral 246 is the peak for gamma
octalactone.
-65- ~p70697
Figure 10 is the GC-mass spectrum for the reaction products
of Examples II and III, combined. The peak indicated by
reference numeral 248 is the peak for the compound having the
structure:
0
The peak indicated by reference numeral 250 is the peak for the
compound having the structure:
o J~o
The peak indicated by reference numeral 252 is the peak for
caprylic acid having the structure:
H
66 2p7U697
The peak indicated by reference numeral 254 is the peak for the
compound having the structure:
Ho
~7
The peak indicated by reference numeral 256 is for gamma
octalactone having the structure:
v ~o' ~o.
The peak indicated by reference numeral 258 is for the compound
having the structure:
2070697
-67-
Figure 11 is an enlargement of peaks 252 and 254 of Figure
10. The peak indicated by reference numeral 252 is for
caprylic acid having the structure:
t
The peak indicated by reference numeral 254 is for the compound
having the structure:
Ho
~7
hydroxy methyl furfural.
Figure 14 is the GLC profile for the first and second
extraction of the reaction product of Example IV. The peak
indicated by reference numeral 260 is the peak for gamma
octalactone.
X07 p697
Figure 15 is the GLC profile for the third extraction of
the reaction product of Example IV. The peak indicated by
reference numeral _262 is for gamma octalactone. (Conditions:
50 m x 0.31 mm OV-1 column programmed at 200°C isothermal for
10 minutes followed by 200-225°C at 10°C per minute).
Figure 17 is the GC-mass spectrum for distillation Fraction
2 of the distillation product of the reaction product of
Example VI. The peak indicated by reference numeral 264 is the
peak for the compound having the structure:
/\
o \U
The peak indicated by reference numeral 266 is for the compound
having the structure:
0
n
I
-69- 2070697
The peak indicated by reference numeral 268 is the peak for the
compounds having the structures:
0 0
.y
and
Ho
The peak indicated by reference numeral 270 is the peak for
gamma octalactone having the structure:
O 0
-7°- 2070697
The peak indicated by reference numeral 272 is for the compound
having the structure:
Figure 20 is the GC-mass spectrum for distillation Fraction
4 of the distillation product of the reaction product of
Example VI. The peak indicated by reference numeral 274 is the
peak for the compound having the structure:
J,
o
The peak indicated by reference numeral 276 is the peak for the
compound having the structure:
o ~o
-71- 2070697
The peak indicated by reference numeral 278 is the peak for the
compound having the structure:
'O
The peak indicated by reference numeral 280 is the peak for
gamma octalactone having the structure:
v
The peak indicated by reference numeral 282 is the peak for the
compound having the structure:
~' o' ~o
-'2- 2o~oss~
Figure 22 is the GC-mass spectrum for distillation Fraction
5 of the distillation product of the reaction product of
Example VI. The peak indicated by reference numeral 284 is for
gamma octalactone having the structure:
v
The peak indicated by reference numeral 286 is for the compound
having the structure:
Figure 23 is an enlargement of peak 284 of Figure 22. Peak
284 is for gamma octalactone having the structure:
'o' ~o.
4:
:a
i r'
2070697
Figure 24 is the GLC profile for the reaction product of
Example VII(a). The peak indicated by reference numeral 288 is
the peak for gamma octalactone having the structure:
~'o' ~o
Figure 25 is the GLC profile for the reaction product of
Example VII(b). The peak indicated by reference numeral 290 is
the peak for gamma octalactone having the structure:
v \ o' \o
Figure 26 is the GLC profile for the reaction product of
Example VII(c). The peak indicated by reference numeral 292 is
the peak for gamma octalactone having the structure:
O O
Y
-74-
~~7Q69~
Referring to Figures 27 and 28, there is provided a process
for forming scented polymer elements (wherein the polymer may
be a thermoplastic polymer such as low density polyethylene or
polypropylene or copolymers of ethylene and vinyl acetate or
mixtures of polymers and copolymers such as copolymers of
ethylene and vinyl acetate and polyethylene) such as pellets
useful in the formation of plastic particles useful in fabricat-
ing certain articles which may be perfumed. This process com-
prises heating the polymer or mixture of polymers to the
melting point of said polymer or mixture of polymers, e.g.,
250°C in the case of low density polyethylene. The lower most
portion of the container is maintained at a slightly lower
temperature and the material in the container is taken off at
such location for delivery through the conduit. Thus, refer-
ring to Figures 27 and 28, in particular, the apparatus used in
producing such elements comprises a device for forming the poly-
mer containing perfume, e.g., polyethylene or polyethylene-poly-
vinyl acetate of mixtures of same or polypropylene, which
comprises a vat or container 1212 into which the polymer taken
alone or in admixture with other copolymers and the perfuming
substance which is at least one of the lactones of our in-
vention or mixtures of lactones and other compatible perfumes
is placed. The container is closed by means of an air-tight
lid 1228 and clamped to the container by bolts 1265. A
stirrer 1273 traverses the lid or cover 1228 in an air-tight
manner and is rotatable in a suitable manner. A surrounding
cylinder 1212A having heating coils which are supplied with
electric current through cable 1214 from a rheostat or control
1216 is operated to maintain the temperature inside the con-
tainer 1212 such that the polymer in the container will be
maintained in the molten or liquid state. It has been found
advantageous to employ polymers at such a temperature that the
viscosity will be in the range of 90-100 sayboldt seconds. The
heater 1218 is operated to maintain the upper portion of the
container 1212 within a temperature range of, for example,
20~0~~~
-75-
220-270°C in the case of low density polyethylene. The bottom
portion of the container 1212 is heated by means of heating
coils 1212A regulated through the control 1220 connected
thereto through a connecting wire 1222 to maintain the lower
portion of the container 1212 within a temperature range of
220-270°C.
Thus, the polymer or mixture of polymers added to the con-
tainer 1212 is heated from 10-12 hours, whereafter the perfume
composition or perfume material which contains one or more of
the lactones of our invention is quickly added to the melt.
Generally, about 10-45 percent by weight of the resulting
mixture of the perfumery substance is added to the polymer.
After the perfume material is added to the container 1212,
the mixture is stirred for a few minutes, for example, 5-15
minutes and maintained within the temperature ranges indicated
previously by the heating coil 1212A. The controls 1216 and
1220 are connected through cables 1224 and 1226 to a suitable
supply of electric current for supplying the power for heating
purposes.
Thereafter, the valve "V" is opened permitting the mass to
flow outwardly through conduit 1232 having a multiplicity of
orifices 1234 adjacent to the lower side thereof. The outer
end of the conduit 1232 is closed so that the liquid polymer in
intimate admixture with one or more of the lactones of our in-
vention or mixture of perfume substance and one or more of the
lactones of our invention, will continuously drop through the
orifices 1234 downwardly from the conduit 1232. During this
time, the temperature of the polymer intimately admixed with
the perfumery substance in the container 1212 is accurately
controlled so that a temperature in the range of from about
240-250°C, for example, (in the case of low density poly-
ethylene) will exist in the conduit 1232. The regulation of
~I zo~os~~
-7 6 -
the temperature through the controls 1216 and 1220 is essential
in order to insure temperature balance to provide for the con-
tinuous dropping or dripping of molten polymer intimately ad-
mixed with the perfume substance which is all or which contains
one or more of the lactones of our invention, through the
orifices 1234 at a rate which will insure the formation of
droplets 1236 which will fall downwardly onto a moving con-
veyor belt 1238 caused to run between conveyor wheels 1240 and
1242 beneath the conduit 1232.
When the droplets 1236 fall onto the conveyor 1238, they
form pellets 1244 which harden almost instantaneously and fall
off the end of the conveyor 1238 into a container 1250 which is
advantageously filled with water or some other suitable cooling
liquid to insure the rapid cooling of each of the pellets
1244. The pellets 1244 are then collected from the container
1250 and utilized for the formation of other functional
products, e.g., garbage bags and the like.
The following examples are given to illustrate embodiments
of the invention as it is preferred to practice it. It will be
understood that these examples are illustrative and the
invention is not to be considered as restrictive thereto except
as indicated in the appended claims.
All parts, proportions, percentages and ratios hereinafter
referred to are by weight unless otherwise indicated.
2070697
EXAMPLE I
PRODUCTION OF GAMMA OCTALACTONE IN SHAKE FLASKS
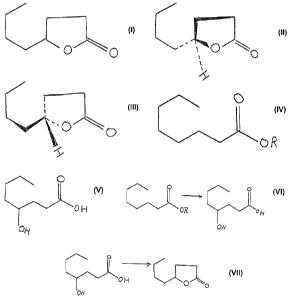
Reactions:
c~o~
U ti
end
~ oN
ON
o ~o ~ .
2070697
100 ml Shake flasks operating at 25°C and 150 rpm (starting
pH 4.5) are filled with medium and substrate as set forth below.
Each of the flasks is inoculated with a different organism
as is shown for Examples I(a), I(b), I(c), I(d), I(e), I(f),
I(g), I(h), I(i), I(j), I(k), I(1), I(m), I(n) and I(o):
-79-
207097
a
t
Ip 'O
N t ~ I
N ro
o a. c
~
~ ~ W ~ ~ ~
A
O
L (, v I
~
..~o O c j I
o vo
w a~
v u, L o y n co n ~c
~n .~ t w
ro
G N U ri ~ V' d' rl e1 N rl tl1
rf W t . .
V O
I 1.
o ro ~ . .
a 1
o
v
~
a a '~ '"' oo '' N -1 ~ v
a ~ ' ~
a
~ p
o
ro
x a +~
v d
x c
a
a
N N ~xwv~n
a
ro w
.- a 1 c j
~ ~ ~
o w
a~
w ro.~ oaH.t~o I 1
c
L. 4 1
iJ
..1
v E
v
ro.~
x A.
L
a
ro E . ~ I
o .~
ro
v
rnu
~n N
.t i
In
_..
_..
.u E E E E E E 6 N
(
a ro ro ro ro ro ro ro
1 .
~ a w a a N a a a ro
~ .o rr a~ m a~ a~ o~ rn N
I
o ( .u
....-~ ~ '
_ y N N h N 01 1l1 r1 ri .N
.
y v wv h .w o ao vo .-v .a. .O
W I
p a rn .-t h N .y. m ~r o
.
ro . >. ( N
w 0 0 0 0 0 0 0
U o
~
>' a . o
'
a I
a, w
.
a
ro .
o
ro y
1
G
?. N i "r
.G
b
U
.
.c U rt7 t b
~ G
?G
~ .~,~svro ro
~
' ro ~ v u N U N N u ::
W ~
I N
a a a a a a a N
ro U ~ ~ ~ F C Q
L a ~ R'H ~ . . C . .
O C C . G
w a ~r v w v d. b
v N,
v
~
N N N N d N N
H ' >>
fn fO. ro
~
~
v
"
~ ~ ro
w N v
'
o ro ro o ro ro ro ro
G C E C C c G >,
.,, .,~ v .~, ro
U .-i ~ U .-i .a r-i .-t
G! N v N. N c d N
,a .p ~o ro ,o .4
~ ~ N o
V w .. ~ a N a .. ..
~ . . i ~
..
a 3
v
o ut ro ro N ovro ro ro ro ..ro
a +~ .-1r~.i roao.--~ .-1a ~ .-v
ao
'
v '~ .-~oo~ .-ieo.1..,-~o... ,-1.. x
v ,.-1
O
N
G o E v tnv roanv av hv v M N
o
O N N a eha .C1 N arka Na0a a h
a~
2
a
W Ol .r1 v V'v ~ N a0N .1rid v OD
yt
p' c ., "'~ a~ ..,h..t ao..~ ..~h w
W
c ro ~ au ua s .~ +~ a
r
v
v
P. o tT a a c P4a ON Oa Na O
r o ~o ~> o o aw o w
A
N >a ~ ,Q~ .2~nx ~ w~ roHo m~ H .
p Z H ~ U
E ~ ._...___ .- ... _.. ._...... __~... ........ .._...
a eo
eo
m
en
-.~otn
o0
v
v o
00
'-'' ro ro i~ v ro v w in
_. x _, ,. ~ ... ,. _ _
W N H H H H H N
~
'~,1
2070697
i I
~ ro I
0 0. C i
' ~ ~
..,a vwAO .
U V '
C ~
.,i( O C ~ 1
o O ~ L
V1 ~
w ~n a~ o ~ I ; I
..~
ro
.
N ~ U .~ , m ~ ; ~I ; n m n
~r w ~ ;
a
i , ro a.i W m p o a c In m
~ o ~D . I ~
.u
U II .--1 , , ,
N II U aC m ~
II 14
~ N c m ''' .-I .W n o0
~ ~
x N x a ~
a ~
roo ~ ~
c
I
.-1L O I C .
> ~ a.~
w w
w ro on H ! ~ ;
~., .~
C ~ p
~ a a.l 1
.~
v ~ v
ro
~
x A.
a
N
roa ..r
o ~o ' ~
.cv a~ ,
o m
cnN .~ I , i
tn i
.....
a.~ ~ ~ E ~ E ~ ~ ~ ai
i
. ro a a a a ro ro
p l
~ ..l L >.
f l .,
g 3 'o tr~ o~ o~ o~ o~ u~ o~ d, N
I
o I
___ O
_ ~ p
(y~ ~ ~ 1D m O n N rl
p ' , , ~ ow n , o w n 0
ro y j o 0 0 ~-I O O
p
"' ~
.u w
a N .. I o
;
p, o ., ,
.
t C
ro
a . i I o
~ ~ I
.-, N v N I
G
7, N .C 'd
~ U ri
c U TI
i G ?G
. ~ ~ ~C I
N
. . w N ,~ N N ul N N Ua N b
ro ~
~
N N ~ ' N ~ N N ~ N
N
U O F O F ~ X C O
N ,~C C . . .
N . C C
0
a ~ +1 N
a. ~r m ~ ~r oo o m ro
f ~ A' N N.
N ~
N N d' N N d'
.Oas N c V
'
t ~ ~ ~ ~ w
~ N
~N cn.
~cC
~
_1 -_.__ ... 2
. .
_..
N
w
t
O 5 > 1~~'-
,C ro ro N ro ro ro
rooro i ~ ~ ~
-.
I
--~ v .,~..~, . .-I ,.., .., ro
' i
A
C N~ ~ . rl r-1~ .-I N
ro~. a v p v ro
~
r ro
.l
~ ~'~ .~ ~ .~ a
ro ou v ~ .. ~ '
o ~
N U
.
v ~ ~ O rl ~ ~ N
v ~ r-1 .-n a . .-i i
~ ~ W a -i .-
N
G N N v ~;a ~ m v m v ~ v ~ v
o N ro ao I
o~ N w N.~ N n N m N o N o,N N Nm
zu
L N N n N N N .-IN m N n N aoNn
N ~ C G 4
W
~
p. .~I .~w p ~1m .i ao.~ao.in W
ro C o
W
7S
~ 0 ~ O f
A .C N O O G f O m 0 W O W O W O
~ 9 ' t W
'
H p .F. .'F.,U , Z..F..FI,. H ,i,H 1",H ,E,,
N U H
......_.., ~,-~ _... ._ _...... ~ ......_...... .._...
7 0V
d~'
IJ1
~N
~ O V1
p
O
U .-1
O ~ .~ '~ x o
O
O
.-, ro . .i ~ v ~ a ~1
_. ~e ~ ~-I ..1 .-1 .i
.-~
w
B .I
207097
-ai-
L~YDMDT.G' TT
PREPARATION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA IFO 7873
Reactions:
C~oJ
and
OH
o .
2o70s97
_82_
The following medium is prepared:
1% Peptone
0.5% Yeast extract
0.05% TWEEN 0 80
10.0% Dextrose
Mortierella isabellina, IFO 7873 is placed into 100 ml of the
above medium and a culture is produced. 500 ml of the above-
medium is then inoculated with 2% of the above-formed culture.
A production medium is prepared as follows:
1% Peptone
0.5% Yeast gxtract
0.05% TWEEN ~R 80
10.0% Dextrose.
The production medium is placed into a 9 liter fermentor
equipped with a 500 rpm agitator and operating at an aeration
rate of 0:5 v/v/m and a pH of 4.5. The production medium is
sterilized at 121°C for 30 minutes,
The fermentor is inoculated with 0.5 liters of the prepared
inoculum. 25 Hours after the fermentor is inoculated, ethyl
caprylate having the structure:
a
0
2070697
-83-
addition is commenced. The pumping of the ethyl caprylate is
carried out at a rate of 0.22 grams/hour/liter. The pumping of
the ethyl caprylate is carried on for a period of 48 hours.
At the end of the fermentation the pH of the broth is
adjusted to between 2 and 4 using 85$ phosphoric acid, and then
sterilized for 15 minutes at 121°C. The broth is extracted 3
times with 1/3 volume of ethyl acetate. The combined extracts
are washed twice with saturated sodium chloride solution
(aqueous) and the solvent is evaporated, The crude is analyzed
as methyl esters and is then distilled on a 12" Goodloe column
at a vapor temperature of 92°C and a vacuum of 1 mm/Hg. The
yield is 1.72 grams per liter of gamma octalactone. The
percent octalactone recovered is 15.64. The crude weight of
the gamma octalactone is 11 grams per liter.
_._ .. ... . _ . ' _.. ..._.. _ _ ..
207097
-t34-
EXAMPLE III
PREPARATION OF
GAMMA OCTALACTONE USING
MORTIERELLA ISABELLINA, IFO 7873
T7nanhinnc.
c~o~
A I1a
Vl'i
oN
o
2074697
-85-
A medium is prepared containing the following ingredients:
1% Peptone
0.5% Yeast extract
0.05% TWEEN 0 80
10.0% Dextrose
A slant of Mortierella isabellina, IFO 7873 is placed into
100 ml of the above medium. The inoculum is cultured for a
period of 3 days. Two percent of the thus-obtained culture is
placed into 500 ml of the above medium and the inoculum is
prepared by growing the culture in the medium at a pH of 4.5
for a period of 24 hours.
A production medium is prepared in a 9 liter fermentor as
follows:
1% Peptone
0.5% Yeast extract
0.05% TWEEN O BO
10.0% Dextrose
and maintained at 27°C with agitation of 500 rpm, aeration of
0.5 v/v/m and maintained at a pH of 4.5,
The fermentor medium is sterilized at a 121°C for 30
minutes. The fermentor is then inoculated with 0.5 liters of
the above-prepared culture. The fermentor is maintained at
27°C and operated at 500 rpm for a period of 5 hours after the
inoculation.
a
.-._.~_..... '.~ ...-'. __..........___....__. ~ __._._. ...'.~ . ._...
._. .... :-...,._.-.__ ... .._..._._.......~ -r~_.-~r.~,-
.~._____.~s.:.~.~_::._ .. ...~.
_...~ __ .._' __._~ ~. __ .. : .. . ~_.__ . ... _ . ; :.. ___.. ._. _ ..... __
.
207067
At the 5 hour time interval, addition of the substrate, the
compound having the structure:
0
is commenced at the rate of 0.25 g/hr/L.
The ethyl caprylate addition is continued for a period of
48 hours.
At the end of the 48 hour period, addition of ethyl
caprylate ceases. The pH of the fermentation broth is adjusted
to between 2 and 3 using 85% phosphoric acid. The broth is
then extracted three times with one-third volume of ethyl
acetate each time and the solvent is evaporated.
The crude extract is then fractionally distilled at a vapor
temperature of 92°C and a pressure of 1 mm/Hg. yielding the
product having the structure:
~~ o' ~o.
The reaction product contained 34.72% gamma octalactone and
the yield was 2.62 grams per liter. The crude weight of the
gamma octalactone was 7.56 grams per liter.
_87_ 2070697
EXAMPLE IV
PREPARA'PION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA, IFO 7873
Reactions:
c~o~
O
Vn
ON. ,
and
v~
OH
~/\O
~ y-L....~.1') r -.i ' T ~1-.. t .t ' ~ .'j -.r
.- - - -~ _w , ---. ...- - _ _-__, _. "' ~. _. -- . -s ~-r-~.,cs.'~ ' _.... ".
.
.__ _.. ' _-..._ .. _._ _. ..:.. _ . --_. .._ .._ _... __._. . .__.,. _. ..-_ -
._.,rr_ __,.._ _..=-r.
2070697
_a8_
The following medium is prepared:
1.0% Peptone
0.5% Yeast extract
0.05% TWEEN 0 80
10.0% Dextrose.
To 100 ml of the above medium, a slant of Mortierella
isabellina, IFO 7873 is added. An inoculum is cultured for a
period of 3 days. At the end of the 3 day period, the
resulting culture is added to 500 ml of the above medium at the
rate of 2%. A culture is grown in the 500 ml batch for a
period of 24 hours at a pH of 4.5.
The medium:
1.0% Peptone
0.5% Yeast xtract
0.05% TWEEN~80
10.0% Dextrose
is added to a 9 liter fermentor operating at 27°C, 500 rpm
agitation; an aeration rate of 0.5 v/v/m and a pH of 6.5.
The above 500 ml of culture is then inoculated into the
medium in the fermentor with stirring and aeration.
Five hours after inoculation, pumping of ethyl caprylate
having the structure:
0
0
II
2070697
is commenced into the fermentor at the rate of 0.25 g/hr/L.
The pumping of the ethyl caprylate is continued over a period
of 3 days while maintaining the above conditions in the fer-
mentor.
At the end of the.pumping of the ethyl caprylate, the pH of
the broth is adjusted to between 2 and 3 using 85% phosphoric
acid and the broth is then sterilized for 15 minutes at 121°C.
The broth is extracted three times with one-third volume of
ethyl acetate. The combined extracts are washed twice with
saturated aqueous sodium chloride solution and the solvent is
evaporated.
The crude extract is then distilled at a vapor temperature
of 92°C and a pressure of 1 mm/Hg. yielding gamma octalactone
having the structure:
'0
The yield of gamma octalactone is 7.56 grams per liter; the
percentage of gamma octalactone is 58.96%; and the crude weight
is 12.88 grams per liter.
II 20~~69~
_g0_.
EXAMPLE V
PREPARATION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA, IFO 7873
w
OH
off
o~
and
U
'ON O \
A medium is prepared containing the following ingredients:
1.0% Peptone
0.5% Yeast Extract
0.05% TWEEN 0 80
10.0% Dextrose.
Bu
207Q~97
-91-
Into 100 ml of the above medium, a slant of Mortierella
isabellina, IFO 7873 is added. The inoculum of the Mortierella
isabellina, IFO 7873 is cultured for a period of'3 days, At
the end of the 3 day period, the culture of the Mortierella
isabellina, IFO 7873 is added to 500 ml of the medium:
1.0% Peptone
0.5% Yeast xtract
0.05% TWEEN ~80
10.0% Dextrose.
500 ml Of the above culture is grown for a period of 24 hours
at a pH of 4.5.
Into a 9 liter fermentor operated at 27°C, 500 rpm
(agitation), an aeration rate of 0.5 v/v/m and a pH of 6.5 is
added the medium:
1.0% Peptone
0.5% Yeast extract
0.05% TWEEN~80
10.0% Dextrose,
500 ml Of the Mortierella isabellina, IFO 7473 culture prepared
above is added to the fermentor medium with stirring and
aeration as set forth above.
Five hours after inoculation with.Mortierella isabellina,
IFO 7873, caprylic acid having the structure:
Y
oN
2070697
-92-
is pumped into the fermentor batch at the rate of 0.27 g/hr/L.
The pumping of the caprylic acid is continued for a period of
98 hours.
At the end of the 48 hour period, the pH of the broth is
adjusted to between 2 and 3 using 85% phosphoric acid and then
sterilized for 15 minutes at 121°C. The broth is extracted
three times with one-third volume of ethyl acetate, The
combined extracts are washed twice with aqueous saturated
sodium chloride solution and the solvent is evaporated. The
crude product is then fractionally distilled on a 12" Goodloe
column at a vapor temperature of 92°C, a liquid temperature of
104°C and a pressure of 1 mm/Hg, yielding gamma octalactone
having the structure:
a
The yield of gamma octalactone is 31.94%; 4.82 grams per
liter. The crude weight of the gamma octalactone is 15.32
grams per liter.
207pg97
-93-
EXAMPLE VI
PREPARATION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA, IFO 7873
Reactions:
fl
-T
\dN pN
O~
and
U
~ OOH O \Q
Oy
The following medium is prepared:
1.0% Peptone
0.5% Yeast extract
0.05% TWEEN 0 80
10.0% Dextrose.
B II I
2~A7p697
-94-
To 100 ml of the above medium, a slant of Mortierella
isabellina, IFO 7873 is added and an inoculum is grown for a
period of 3 days. At the end of the 3 day period, 500 ml of
the medium:
1.0% Peptone
0.5% Yeast extract
0.05% TWEEN ~80
10.0% Dextrose
is inoculated with 2% of the above culture.
The 500 ml culture is grown for a period of 29.hours at a
pH of 4.5.
Into a 9 liter fermentor operated at 27°C, an agitation of
500 rpm, an aeration rate of 0.5 v/v/m and a pH of 6.5, the
medium:
1.0% Peptone
0.5% Yeast extract
0.05% TWEEN O BO
10.0% Dextrose
is added. With agitation and aeration, the medium in the
fermentor is inoculated with the 500 ml of the above culture
containing the Mortierella isabellina, IFO 7873.
Twenty four hours after inoculation, pumping of the
substrate, caprylic acid having the structure:
N
2070697
-95-
is commenced at the rate of 0.32 g/hr/L (grams per hour per
liter). The pumping of the caprylic acid into the fermentor is
continued for a period of 72 hours.
At the end of the 72 hour period, the pumping ceases and
the pH of the broth is adjusted to between 2 and 3 using 85%
phosphoric acid; and then the broth is sterilized for 15
minutes at 121°C. The broth is extracted 3 times with one-
third volume of ethyl acetate. The combined extracts are
washed twice with saturated sodium chloride solution (aqueous)
and the solvent is evaporated.
The crude product is then fractionally distilled on a 12"
Goodloe column yielding the following fractions:
Vapor Liquid Vacuum
FractionTemp. Temp. mm/Hg.
No. (C) (C) Pressure
1 98/ 101/ 3.0
2 98 105 3.0
3 91 103 1.0
4 92 104 1.0
92 104 1.0
6 102 115 1.0
The distillation product has a percentage of gamma octalactone
of 55.76%; and a yield of 7.81 grams per liter. The crude
weight of the gamma octalactone is 14 grams per liter.
The gamma octalactone, containing a mixture of the isomers
having the structures:
207097
-96-
. . 1 O "'~~
;.
and
I
1
y
has an optical rotation of minus 28.22 degrees.
ii
207097
-97-
EXAMPLE VII
PRODUCTION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA, IFO 7873
Reactions:
ON ~N
~N
and
U
~ OOH O \
O
The following medium is prepared:
1.0% Enzymatic digest of soy
0.5% TASTONE 0 900
0.05% TWEEN 0 80
10.0% Dextrose.
. ~:...~s
X474697
.98_
The enzymatic digest of soy is obtained from the Deltown
Chemurgic Corporation and is identified as SE-50 BT and
contains:
5.1% moisture
10.59% ash
2.35% amino nitrogen
8.83% total nitrogen
55,21% protein
7.10 pH.
(SE-50 MK can be used as a replacement for SE-50 BT).
The TASTONE 0 900 is a brand of bakers yeast extract,
spray-dried; a high clarity water soluble bakers yeast extract,
spray-dried to a fine yellow tan powder having the following
analysis:
65-70% protein
3-6% moisture.
6.4-7.0 pH.
It is manufactured by the Universal Foods Corporation,
Fermentation Division, Milwaukee, Wisconsin.
100 ml Of the above medium is admixed with a slant of
Mortierella isabellina, IFO 7873 and an inoculum is grown for a
period of three days. The resulting inoculum is then added to
500 ml of the above medium at a rate of 2% and the resulting
inoculum is grown for a period of 24 hours at a pH of 4.5.
a
207097
-99-
The following medium is added to a 9 liter fermentor
operated at 27°C, agitation rate: 500 rpm and aeration rate:
0.5 v/v/m at a pH of 6.5:
1.0% enzymatic digest of
0.5% soy
0.05% TASTONE 0 900
TWEEN 0 80
10.0% Dextrose.
With agitation and aeration, 500 ml of the above inoculum,
grown for a period of 24 hours at a pH 4.5, is added to the
fermentor. The fermentor is then operated for a period of 5
hours after which time addition of caprylic acid having the
structure:
H
is commenced at the rate of 0.32 g/hr/L. The pumping of the
caprylic acid is continued for a period of 48 hours.
At the end of the 48 hour period, the pH of the broth is
adjusted to between 2 and 3 using 85% phosphoric acid. The
broth is then extracted three times with one-third volume ethyl
acetate and the solvent evaporated, The crude product is then
fractionally distilled at a vapor temperature of 92°C and a
pressure of 1 mm/Hg. yielding a composition of matter
containing gamma octalactone having the structure:
2Q7~~97
-100-
Examples VII(a), VII(b) and VII(c) are repeat runs of the
same experiment. The following table shows the example, crude
weight, percent gamma octalactone and the distilled product and
yield:
Example Crude Wt. % g-Octalactone Yield
VII(a) 12.44 g/L 59.97 7.46 g/L
VII(b) 11.76 g/L 86.92 10.22 g/L
VII(c) 17.04 g/L 61.14 10.42 g/L.
aii
2474697
-i0i-
EXAMPLE VIII
PREPARATION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA. IFO 7A84
Reactions:
C~o~
O
and
o
~o
oN
o ~o .
~u
2070697
-l02-
The following medium is prepared:
1.0% Peptone
0.5% Yeast E tract
0.05% TWEEN ~80
10% Dextrose.
A slant of Mortierella isabellina, IFO 7884 is added to 100
ml of this medium and the medium is cultured for a period of
three days. The resulting culture i.s added to 500 ml of the
above medium at the rate of 2%. The Mortierella isabellina,
IFO 7884 is then cultured for a period of 24 hours at a pH of
4.5.
The medium:
1.0% Peptone
0.5% Yeast Extract
0.05% TWEEN 0 80
10% Dextrose
is placed in a 9 liter fermentor operated at 27°C with an
agitation rate of 500 rpm and an aeration rate of 0.5 v/v/m
while being maintained at a pH of 6.5. To this medium is added
500 ml of the above inoculum of Mortierella isabellina,
IFO 7884.
Five hours after inoculation, pumping of ethyl caprylate
having the structure:
~u
~07Q697
-103-
U
w
~0
is commenced. The pumping of the ethyl caprylate is carried
out at a rate of 0.31 g/hr/L and is continued for a period of
72 hours.
The pH of the fermentation broth is adjusted to between 2
and 3 using 858 phosphoric acid and the broth is then
sterilized for 15 minutes at 121°C. The fermentation broth is
extracted three times with one-third volume of ethyl acetate.
The combined extracts are washed twice with saturated aqueous
sodium chloride solution and the solvent is then evaporated.
The crude product is then distilled on a 12" Goodloe column
yielding a product rich in gamma octalactone having the
structure:
\o
~"~''~
..'k
,. A,..-~
2070697
-104-
The distillation is carried out at a vapor temperature of 92°C
and a pressure of 1 mm/Hg. The yield of gamma octalactone is
11.7%; the crude weight is 8.08 grams per liter and the yield
is 0.95 grams per liter.
2070697
-105-
F'YSMDT L~ TY
PREPARATION OF
GAMMA OCTALACTONE USING MORTIERELLA ISABELLINA, IFO 7884
Reactions:
O
aN
~H
o~
and
O
~ SON
O
The following medium is prepared:
1 . 0 % Pep tone
0.5% Yeast Extract
0.05% TWEEN 0 80
10% Dextrose.
II
2070697
-lU6-
A slant of Mortierella isabellina, IFO 78x4 is placed into
100 ml of the above medium and the resulting mixture is
cultured for a period of 3 days. At the end of the 3 day
period, the resulting culture is added to 500 ml of the above
medium at a rate of 2%. The resulting inoculum is cultured for
a period of 24 hours at a pH of 4.5.
The resulting inoculum is then added to a 9 liter fermentor
which also contains the medium:
1.0% Peptone
0.5% Yeast~tract
0.05% TWEEN R 80
10% Dextrose.
The fermentor is operated at a temperature of 27°C, an
agitation rate of 500 rpm and an aeration rate of 0.5 v/v/m and
is operated at a pH of 6.5.
Five hours after inoculation with the Mortierella
isabellina, IFO 7884, pumping of caprylic acid having the
structure:
0
oN
ii
207097
-l07-
is commenced at the rate of 0.32 g/hr/L. Pumping of the
caprylic acid into the fermentor continues for a period of
48 hours,
At the end of the 48 hour period, the pH of the broth is
adjusted to between 2 and 3 using 858 phosphoric acid, and the
broth is then sterilized at 121°C for 15 minutes. The broth is
then extracted three times with one-third volume ethyl acetate
(each time) and the solvent is evaporated. The crude product
is then fractionally distilled at a temperature of 92°C (vapor)
and a pressure of 1 mm/Hg, to yield gamma octalactone.
The yield of gamma octalactone is 33.978 and 2.63 grams per
liter. The crude weight is 7.75 grams per liter.
a
207497
-108-
EXAMPLE X
In a churn or premixer 900 kilograms of fat mixture were
mixed with the aqueous phase described below. The fat mixture
consisted of 258 rape seed oil, 458 coconut oil, 208 hardened
whale oil having a melting point of 40-42°C and 108 hardened
rape seed oil having a melting point of 40-92°C. In the fat
mixture was dissolved 4 kilograms of monoglyceride and 3 kilo-
grams of lecithin. The temperature of the fat mixture when
introduced into the churn was about 45°C.
The aqueous phase had a temperature of about 15°C and con-
sisted of 115 kilograms of pasteurized and cultured milk, 55
kilograms of water, 17 kilograms of salt 2.8 kilograms of
potato meal), 1.1 kilograms of sodium benzoate, 0.150 kilograms
of sodium bicarbonate, 1.0 Kilograms of diacetyl, and 0.350
kilograms of the gamma octalactone composition of bulked dis-
tillation Fractions 2-5 of Example VI, supra, containing gamma
octalactone.
After completion of the mixing there was added 0.750 kilo-
grams of vitamin oil containing 40,000 I.U, of vitamin A and
2,300 I.U, of vitamin D2 per gram, and 0.560 kilograms of
carotene oil containing 7,000 I.U. of carotene per gram. After
mixing there was added an aroma preparation consisting of
2.5 kilograms of decalactone and 10 kilograms of stearolactone
dissolved in 200 kilograms of oil. The mixing was continued
for a few minutes and the batch was then pumped to a chilled
roll. The chilled emulsion was then supplied to a complector
and then put up in packets.
The result was a product having excellent taste and flavor
similar to that of butter. The flavor developed only after a
few days.
2070697
-109-
TYaMDT T~ YT
The procedure described in Example X was repeated except
that as aromatizing substances were used 0.9 kilograms of
nonyllactone, 3.0 kilograms of gamma octalactone, bulked distil-
lation Fractions 2-5 of Example VI, supra, 1.2 kilograms of un-
decalactone, and 12 kilograms of stearolactone, per ton of the
finished product.
T.Y~MDT.I: YTT
The procedure described in Example X was repeated except.
that as aromatizing substances were used 1.5 kilograms of deca-
lactone, 3.5 kilograms of the gamma lactone composition of
Example IX (distilled), 1.0 kilograms of dodecalactone, and
kilograms of stearolactone, per ton of finished product.
EXAMPLE XIII
The procedure described in Example X was repeated except
that as aromatizing substances were used 0.25 kilograms of
nonyllactone, 3.5 kilograms of the composition of Example
VII(b) containing gamma nonyllactone, 1.6 kilograms of
decalactone, 0.50 kilograms of undecalactone, and 10 kilograms
of stearolactone per ton of the finished product.
~u
llo- 2070697
FY~MDT L~ YTtI
The procedure described in Example X was repeated except
that as aromatizing substances were used 12 kilograms of stearo-
lactone, and a kilograms of the gamma octalactone containing
composition of Example IV, supra, per ton of finished product.
The taste was excellent and the frying flavor agreeable. As in
the preceding examples it was found that pastry made with this
margarine had an agreeable butter flavor and retained this
flavor also after storing.
EXAMPLE XV
Artificial cream was made in the following way.
One kilogram of margarine, to which had been added emulsi-
fying agents for cream whipping, was melted and had added
thereto 0-.25 milligrams of nonyllactone, 2.5 milligrams of
decalactone, 3.5 grams of the gamma octalactone-containing
composition of Example III, supra, 0.5 milligrams of undeca-
lactone and 10 milligrams of stearolactone. Two liters of milk
were then added and mixed with the margarine and the mixture
was then passed through a homogenizes. The artificial cream
thus obtained had good taste and was free from the extraneous
taste characteristic of the ordinary artificial cream.
~' .. .~
207069
-111-.
EXAMPLE XVI
PATCHOULI PERFUME FORMULATION
The following mixture is prepared:
Ingredients Parts by Weight
Orange oil.............................. 50
Bergamot oil............................ 20
Lime oil...................... ........ 100
Neroli oil.............................. 5
R
LYRAL
(registered trademark.......,.,. 100
of " "
International Flavors
Fragrances Inc.)
~
GALAXOLIDE
(registered trademark.,. " " " "
o " "
international Flavors
&
Fragrances Inc.)
Gamma methyl ionone,...".."""""", 20
1-Acetyl-2,5,5-trimethyl-
ycloheptane ,..,. " " " 150
" ,
amma octalactone
prepare
according to Example....,.. " " 150
VI "
(bulked distillation
Fractions 2-5)
Bulked distillation Fractions 2-5 imparts to this patchouli
formulation a sophisticated coconut topnote and tonka bean-like
undertone. Accordingly, the perfume composition of Example XVI
can be described as "patchouli, with coconut-like topnote and
tonka bean-like undertone".
2070697
-ll2-
EXAMPLE XVII
PREPARATION OF SOAP COMPOSITIONS
One hundred grams of soap chips are produced according to
Example V of U.S. Patent No. 9,058,487 issued on November 5,
1977. '
'Thesodium salt"of" ari equal mixture of C10-Cl4 alkane
sulfonate (958 active), 40 pounds, is dissolved in a mixture of
80 pounds of anhydrous isopropanol and 125 pounds of deionized
water at 150°~F,. In this mixture is dissolved 10 pounds of
partially hydrogenated coconut oil fatty acids and 15 pounds of
sodium mono-C14 alkyl maleate, and the pH of this solution is
adjusted to 6,0 by the addition of a small amount of 508
aqueous solution of sodium hydroxide. The isopropanol is dis-
tilled off and the remaining aqueous solution is drum dried.
The resulting solid actives are then blended in a chip mixture
with 10 pounds of water, 0.2 pounds of titanium hydroxide and
0.7 pounds of one of the perfume ingredients set forth in
Table I below. The chips are then plodded into logs, cut to
size and finally stamped into bars having a pH of approximately
6.9.
Each of the perfumed soaps produced by means of the fore-
going procedure manifests an excellent aroma as set forth in
Table I, infra.
TABLE I
Ingredient- Fragrance Pro a
Imxcure of compounds A coconut aroma w t Tonca
,produced according to bean-like undertones,
Example VI, bulked
distillation Fractions
2-5.
Perfume. composition patchouli, with coconut-like
of Example XVI, topnote.and Tonka bean-like
undertone.
. '
. .a:
wfa
2070697
-113-
EXAMPLE XVIII
PREPARATION OF DETERGENT COMPOSITION . ,
A total of 100 grams of~a detergent powder prep~r,e.~lr
according to o.S. Patent No. 4,058,472 and containing 5% by '
weight of the sodium salts of a miXt4rxa of sulfonated ,'
w C14 Clp alkyl catechol as a surface aclive.component, the
mixture being 60 parts.by. weight of mono-C14-C18 alkyl
catechol and 40 parts by weight of di-C14-C16 catechol, 35%
sodium tetrapyrophosphate, 30% sodium silicate, 20% of sodium
carbonate, 3% of sodium carboxymethyl cellulose and 7% of
starch is mixed with 0.15 grams individually with each of the
aroma ingredients set forth in Table I of Example XVII until a
substantially homogeneous composition is obtained. Each of the
compositions has an excellent aroma as set forth in 'P.able L of
Example XVII.
cvsMnr c vTv
PREPARATION OF A COSMETIC POWDER COMPOSITION
A cosmetic powder is prepared by mixing in a ball mill, 100
grams of talcum powder with 0.25 grams of each of the perfume
materials of Table I of Example XVII. Each of the powders has'
an excellent aroma as set forth in Table I of Example XVII.
.. ,
'?r. ., °;
. ~~ Sn
~; '~.y
2070697
-114-
cvnMOr r vv
PERFUMED LIQUID DETERGENT
Concentrated liquid detergents with aromas as set forth in
Table T of Example XVII are prepared by adding 0.10%, 0.15% and
0.20% of each of the ingredients set forth in Table I of
Example XVII. They are prepared by adding and homogeneously
mixing the appropriate quantity of perfume substance of Table I
of Example XVII in the liquid detergent. The detergents in-
dividually possess aromas as set forth in Table I of Example
XVII, the intensity increasing with greater concentrations of
perfume substance set forth in Table I of Example XVII.
EXAMPLE XXI
PREPARATION OF A COLOGNE AND HANDKERCHIEF PERFUME
Each of the ingredients of Table I of Example XVII is
incorporated individually into colognes of several strengths at
concentrations of 2.0%, 2.5%, 3.0%, 3.5%, 4.0% and 5.0% in 75%,
80%, 85%, 90% and 95% aqueous ethanol; and into several con-
centrations of handkerchief perfumes at the rate of 15%, 20%
and 25% (in 80%, 85%, 90% and 95% aqueous ethanol). Distinct
and definite aromas as set forth in Table I of Example XVII are
imparted to the colognes and to the handkerchief perfumes at
the several concentrations set forth above.
;:
._.._...._..... . .. ' . ____.___... ... .......
. . ,:
2070697
-115-
~vnrenr c~ vvrr
PREPARATION OF SOAP COMPOSITIONS
One hundred grams of soap chips (IVORY U produced by the
Proctor 6 Gamble Company of Cincinnati, Ohio) are admixed with
one gram of each of the substances set forth in Table I of
Example XVII, supra, until homogeneous compositions are obtain-
ed. In each of the cases, the homogeneous compositions are
heated under 3 atmospheres pressure at 180°C for a period of
three hours and the resulting liquids are placed into soap
molds. The resulting soap cakes, on cooling, manifest
excellent aromas as set forth in Table I of Example XVII.
EXAMPLE XXIII
PREPARATION OF SOLID DETERGENT COMPOSITIONS
Detergents are prepared from the following ingredients
according to Example I of Canadian Patent No. 1,007,998;
Ingre ents Parts y Weiq t
EODOL v 45-11
(a C14-C15 alcohol . . 12
ethoxylated with 11 mole
of ethylene oxide)
Sodium carbonate.....,.. " " ,. " " , 55
Sodium citrate...,..,. " " " " " " , 20
Sodium sulfate, water brighteners.. q.s,
2070697
-116-
This detergent is a ~phosphate-free~ detergent, A total of
100 grams of said detergent is admixed with 0.10, 0.15, 0.20
and 0.25 grams of each of the substances set forth in Table I
of Example XVII, supra. Each of the detergent samples has an
excellent aroma as indicated in Table I of Example XVII.
~i --~'
s '~;;ist.
..,; .
2070697
-117- .
FYtvMDT L~ vv TV
PREPARATION OF DRIER-ADDED FABRIC SOFTENER ARTICLE
Utilizing the procedure of Example I at column 15 of U.S.
Patent No. 3,632,396 the specification for which is incor-
porated by reference herein, a non-woven cloth substrate
useful as a drier-added fabric softening article of manu-
facture is prepared Wherein the substrate, substrate coating
and outer coating and the perfume material are as follows:
1. a water dissolvable" paper ('Dissolvo Paper") as
the substrate;
2. ADOGEN 0 498 (melting point about 140°F) as the
first substrate coating; and
3. an outer coating having the following formulation
(melting point about 150°F);
57% C-20 C22 RAPS;
22% isopropyl alcohol; -
20% antistatic agent; and
1% of one of the perfumery substances set forth
in Table I of Example XVII, supra.
Fabric softening compositions containing the substances as
set forth in Table I of Example XVII, supra, essentially con-
sist of a substrate having a weight of about 3 grams per 100
square inches; a substrate coating weighing about 1.85 grams
per 100 square inches of substrate; and an outer coating
weighing about 1.5 grams per 100 square inches of substrate are
prepared thereby providing a total aromatized substrate and
outer coating weight ratio of about 1:1 by weight of the sub-
strate.
.,''f
'._,.~ ~~. '»,.r.
"~ii'i:
r
~07p697
-1 a-
The aromas as set forth in Table I of Example XVII, supra,
are imparted in a pleasant manner to the head space in a drier
on operation thereof using the said drier-added fabric soften-
ing non-woven fabric by adding to the drying cycle.
As stated above in the case of fabric softener articles,
the entire U.S. Patent No. 3,632,396 is incorporated by re-
ference herein. Thus, all of the articles of U.S. Patent No.
3,632,396 acting as fabric softening articles in said U.S.
Patent may be perfumed in their outer coating with from 0.25%
up to 5% by weight of each of the perfuming substances of Table
I of Example XVII, supra.
vi".,r;
'u'~i
207097
-119-
EXAMPLE XXV
HAIR PREPARATION
A "soft-feel, good-hold" hair spray is produced containing
the following ingredients:
Ingredients Parts by Weight
o yvinylpyro i one mn~
cetate "E-735 Copolymer"
anufactured by the GAF .....,... 4.00
or oration of New York N.Y.
Any rous et ano ."".." """.""" 70.90
Dioctyl sebecate,. " " " " . " " " " " " " 0.05
Benzyl alcohol......,..... " " ,. " " " " , 0.05
"Prope ant A- " manu acture
by the GAF Corporation of .,.,.,. " , 24.95
New York, N.Y.
Fragrance ngre ent as set
forth in Table I of Example XVII,I .... " , 0.05
supra.
The PVP/VA copolymers are first dissolved in alcohol and
all other ingredients are added until uniform. The propellant
is then pressuri2ed and used as an aerosol. The resulting hair
sprays each have pleasant aromas as set forth in Table I of
Example XVII, supra.
';..,_,:
i '~t~r.
,;
2070697
-120-
ovnranr x~ vvVT
SCOURING CLEANSER COMPOSITION
A scouring cleanser composition is prepared in accordance
with Example I at columns ll and 12 of U.S. Patent No.
4,193,888 issued on March 18, 1984,. To this composition, the
substances set forth in Table I of Example XVII, supra, are
added at the level of 0.258 as set forth in the table in said
Example I of U.S. Patent No. 4,193,888 yielding an aroma on
using said cleanser in ordinary circumstances which is quite
pleasant and described in Table I.of Example XVII, supra.
EXAMPLE XXVII
A fabric softening article prepared substantially as set
forth in Example VIII of Canadian Patent No. 1,069,260,is
prepared containing 0.21$ by weight of a perfuming substance as
set forth in Table I of Example XVII, supra, and yielding on
use in a drier, a faint aroma as set forth in Table I of
Example XVII, supra.
2070697
-121-
EXAMPLE XXVIII
TOBACCO FLAVOR FORMULATIONS
Cigarettes are produced using the following tobacco
formulations:
Ingredients Parts by Weight
Bright..............................40.1
Burley..............................24.9
Maryland............................1.1
Turkish.............................11.6
Stem (flue cured)..................,14.2
Glycerine...........................2.8
H20.............. ............... 5.3
At the rate of 0.2%, the following
tobacco formulation is
applied to all of the cigarettes with the above
produced
tobacco formulation:
Ingredients by Weight
Parts
Ethylbutyrate....................Ø05
Ethylvalerate " " .,.... " " " 0.05
" " ,
Maltol.............................2.00
Cocoaextract............,... " 26.00
" "
Coffeeextract............... " 10.00
" "
Ethylalcohol (95%)...........,...,20.00
H20..............,............... 41.900
'
g v ~t~l'~.1
v
2A70~97
-122-
To portions of 508 of the cigarettes at levels of 10 and 20
ppm, the octalactone-containing composition of Example V
(distilled) is added. These cigarettes are hereinafter called
'experimental' cigarettes. The cigarettes without the octa-
lactone composition are hereinafter called "control"
cigarettes. The control and experimental cigarettes are then
evaluated by paired comparison and the results are as follows:
(a) In aroma, the experimental cigarettes are all
found to be more aromatic with Turkish tobacco-like
nuances;
(b) In smoke flavor, the experimental cigarettes are
all found to be more aromatic, more sweet with Turkish
tobacco, oriental-like nuances than the control cigar-
ettes.
The experimental cigarettes containing the mixture of
lacto nes are found to be fruity and have pleasant aesthetically
pleasing fruity notes in addition.
.._\
~2Q70697
-123-
EXAMPLE XXIX
PUDDING
At the rate of 0.8 ppm, the composition containing gamma
octalactone of Example VI, bulked distillation Fractions 2-5 is
added to a ROYAL ~ butterscotch pudding composition.
Pleasant aesthetically pleasing coconut nuances were added to
the butterscotch pudding as a result of the use of the octa-
lactone composition. Without the octalactone composition no
such coconut nuances are added. A~.panel of 10 individuals
prefers the octalactone-containing butterscotch pudding.
,... a:
'G'~~i