Note: Descriptions are shown in the official language in which they were submitted.
f:" W092/06479 ~ 2 0 ~ O ~ ~ 8 PCT/us9l/0743l
~ . .
METHOD OF MAKING PERMANENT MAGNETS
Field of the Invention
The present invention relates to a method of
making rare earth-transition metal alloy permanent
magnets characterized by isotropic microstructures and
magnetic properties.
Back~round of the Invention
A large amount of technological interest has
been focu~ed on rare earth-iron-boron alloyR (e.g.,
26.7 weight % Nd-72.3 weight % Fe-1.0 weight ~-B) as a
result of their promising magnetic properties for
permanent magnet applications attributable to the
magnetically hard Nd2Fe14B phase. Commercial permanent -
magnets of these alloys having anisotropic, aligned
structure exhibit high potential energy products
(i.e., BH~ax) of 40-48 MGOe while those having an
isotropic, non-aligned structure exhibit potential
energy products of 5-10 MGOe. Such energy product
levels are much higher than those exhibited by Sm-Co
alloys (e.g., SmCo5 and Sm2Co17) previously regarded as
having optimum magnetic properties. The rare
earth-iron-boron alloys are also advantageous over the
SmCo alloys in that the rare earth (e.g., Nd) and Fe
- . : . .
W092/0~79 2 0 7 0 8 0 ~ PCT/US91/07431 ~
are much more abundant and economical than Sm and Co.
As a result, rare earth-iron-boron permanent magnets
are used in a wide variety of applications including,
but not limited to, audio loud speakers, electric
S motors, generators, meters, scientific instruments and
the like.
Two different approaches are currently in
use to produce isotropic permanent magnets from rare
earth-iron-boron alloys (e.g., Nd-Fe-B). one approach
involves rapidly solidifying the Nd-Fe-~ alloy by melt
spinning to produce a near-amorphorous, fine grained
ribbon material, mechanically comminuting the ribbon
to form flake particulates, and then vacuum hot
pressing the flakes in a die cavity to consolidate the
material. This approach suffers from numerous
disadvantages such as microstructural inhomogenetics
induced by non-uniform quenching, contamination and
non-ideal particle shape (e.g., thin platelets) for
further magnet fabrication operations. The vacuum hot
pressing operation typically requires at least brief
exposure to a partial liquidification (melting)
temperature to enhance interparticle bonding.
The second approach involves mechanical
W O 9~/06479 ?;!~ 2 7 ~ ~ ~ 8 PCT/US91/07431
.~ 3
comminution of a chill cast ingot and "powder
metallurgy" consolidation of the resulting fine
comminuted alloy powder wherein the fine comminuted
powder is pressed and sintered using liquid phase
sintering and long time anneals (e.g., total anneal
times up to 25 hours) to consolidate the powder. This
latter approach has traditionally been used to
fabricate SmCo, ferrite and other types of magnets.
This latter approach suffers from numerous
disadvantages such as explosibility hazards,
contamination, microstructural inhomogeneities,
excessive grain growth and, as mentioned, long
processing times.
Both of the aforementioned fabrication
approaches thus are disadvantageous in that they
involve difficult-to-process, irregular-shape alloy
particulates using complex, time consuming and high
cost particulate processing and heat treatment
techni~ues. The magnets fabricated in these ways from
such alloy particulates are prone to inhomogeneities
in microstructure and composition that can adversely
affect the desired isotropic magnetic properties of
the magnet.
: - .. ., . . ,: :
. : -: . .
~V092J0~79 2 0 7 ~ 8 0 8 PCT/US91/07431 ~
It is an object of the present invention to
provide a method of making isotropic permanent magnets
from rare earth-transition metal (e.g., iron) alloy
particles in a manner that overcomes the disadvantages
of the fab~ication approaches described hereinabove.
It is another object of the present
invention to provide a method of making isotropic
permanent magnets from rare earth-transition metal
alloy particies wherein prc.-essing times ~nd steps are
reduced ~ ad simplified and wherein the excessive heat
treatment requirements of the fabrication approaches
described hereinabove are eliminated so as to reduce
the cost of producing the isotropic magnets.
It is still another object of the present
invention to provide a method of making isotropic
permanent magnets from rare earth-transition metal
alloy particles wherein the microstructures and
compositions of the fabricated magnets exhibit
improved homogeneity as compared to isotropic
permanent magnets f abricated by the approaches
described hereinabove.
It is still a further object of the present
.. . . . : . : - - . .
. . ~ -. . -: : .
l~ 2,~o~u~
W092/0~79 PCT/US91/07431
invention to provide a method of making isotropic
permanent magnets from rare earth-transition metal
alloy particles wherein the magnets have dramatically ;
improved mechanical strength.
Summary of the Invention
The present invention involves a method of
making isotropic permanent magnets by providing
generally spherical, rapidly solidified rare earth-
transition metal alloy particles exhibiting desirable
magnetic properties for the particular alloy
composition and magnet service application involved.
Preferably, the particles are provided by atomizing a
melt of a rare earth-transition metal alloy under
conditions to produce a majority of particles falling
within a given particle size range (thus a given grain
size range) exhibiting desirable (e.g., near optimum)
magnetic properties in the as-atomized condition. The
particles in the given size range are then subjected
to concurrent elevated temperature and elevated
isotropic pressure for a time to yield a densified
magnet compact or body having improved magnetic
properties (e.g., energy product, coercivity,
remanence) as compared to the as-atomized particles
., . , . - . , .
. . . . . . .
.. . . . , . . . : . .
.. ..
, ~ . - : . : . . .
W092/0~79 2 Q ~ ~ ~ Q ~ PCT/US91/07431
and dramatically improved strength as compared to rare
earth-transition metal alloy magnets available
heretofore.
In one embodiment of the invention, the
majority of the atomized particles have a particle
size (diameter) less than a given particle diameter.
Particles having a particle diameter greater than the
given diameter are initially selectively removed from
the particle batch by scree~ning or other size
classifying techniques and the remaining particles are
then subjected to the elevated temperature/pressure
step to form the magnet compact or body.
lS In another embodiment of the invention, the
particles are subjected to one or more size
classifying techniques to provide multiple individual
particle size fractions that each exhibit a grain size
in a relatively narrow range in the as-atomized
condition. A particular particle size fraction can
then be subjected to the elevated temperature/pressure
step.
In still another embodiment of the
invention, the particles are treated to form an
h ~ ~ V ~) V (~
W092t0~79 PCT/US91/0743t
environmentally protective coating thereon that
facilitates handling and fabrication of magnets and
also limits grain growth beyond the particle
boundaries during the consolidation/annealing step.
In an exemplary working embodiment of the
invention to make rare earth-iron-boron alloy
isotropic permanent magnet compacts, a melt of the
rare earth-iron-boron alloy is high pressure inert gas
atomized to provide a batch of particles in an
environmentally stable form (e.g., protectively
coated) wherein a majority of the particles in the
batch are less than about 44 microns in diameter,
preferably in the range of about 5 to 40 microns, to
achieve optimum, as-atomized magnetic properties
(e.g., maximum energy product of about 9-lO MGOe in
the as-atomized condition). The alloy composition is
preferably enriched in rare earth and boron to promote
formation of particles having an equiaxed, bloc~y
microstructure with a large volume percentage of the
hard magnetic phase (Nd2 Fe14B) while substantially
avoiding formation of the ferritic iron (Fe) phase.
Following a preliminary screening operation
to substantially remove particles greater than about
.... . . . .. .
. . . . . . '' : ~ ~ -.
.
W092t06479 2~7;~8` PCr/US91/07q31
44 microns diameter from the particle batch, the
remaining portion of the particles (i.e., less than
about 44 microns diameter) are hot isostatically
pressed at a temperature of at least about 600C and
pressure of at least about 20 ksi for a time to
produce a densified magnet compact having improved
magnetic properties as compared to the as-atomized
particle magnetic properties and significantly
enhanced mechanical properties as compared to other
Nd-Fe-B magnet compacts heretofore available. The
method of the invention combines the heretofore
separate particulate consolidation and annealing steps
into a single, shorter duration step that avoids
partial particle melting and grain growth and also
15 yields a permanent magnet compact having improved ~ -~
homogeneity of microstructures and composition.
:
The method of the invention can be used to
economically produce isotropic permanent magnet
compacts of desired microstructure and des~red
magnetic properties, such as energy products in the
range of about 4 to about 10 MGOe. Isotropic
permanent magnet compacts having transverse rupture
strength of at least about 200 MPa are provided.
-
- . . . : . : . ~ .,
,~. W 0 92/06479 2~`7~ PC~r/US91/07431
Brief Descri~tion of the Drawinas
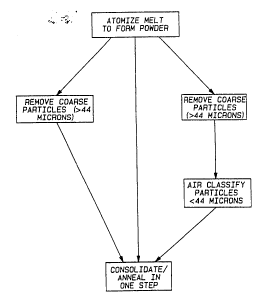
Figure l is a flow sheet illustrating the
sequential method steps of one embodiment of the
invention.
Figure 2 is a schematic view of apparatus
for practicing one embodiment of the invention.
Figure 3 is a photomicrograph ~ lOOOX of a
batch c ~ -apidly solidified powder particles
classified into a particle size fraction of less than
38 microns diameter.
Figure 4 is a photomicrograph at lOOOX of a
section of an isotropic permanent magnet made in
accordance with Example 1 and exhibiting a homogeneous
microstructure and isotropic magnetic properties.
Figure 5 is a bar graph illustrating the
distribution in weight ~ of particles as a function of
particle size (diameter).
Figure 6 is a bar graph illustrating the
magnetic properties of as-atomized Nd-Fe-B alloy
- .
. . . :
.: :
W092/06479 2 o!~ ;8 PCT/US91/07431
particles as a function of particle size.
Figure 7 is a similar bar graph for Nd-Fe-B-
La alloy particles.
5~
Figure 8 is a bar graph for Nd-Fe-B alloy
particles illustrating particle grain size as a
function of particle size.
Figure 9 is a bar graph illustrating the
magnetic properties of alloy particles as-atomized and
as-HIP'ed for different times.
Figure lO is a side elevation of a modified
atomizing nozzle used in the Examples.
Figure ll is a sectional view of a modified
atomizing nozzle along lines ll-ll.
Figure 12 is a view of the modified -
atomizing nozzle showing gas jet discharge orifices
aligned with the nozzle tube surface.
Figure 13 is a bottom plan view of the
modified nozzle.
"
W092/06479 2 0 ~ ~ 8 0 8 PCT/US91/07431
Detailed DescriDtion of the Invention
Referring to Figure l, the various steps
involved in practicing one particular embodiment of
the method of the invention are illustrated. In this
particular embodiment of the invention, a melt of the
appropriate rare earth-transition metal alloy is
atomized by a high pressure inert gas atomization
process of the type described in copending commonly
assigned U.S. patent application (Attorney Docket No.
l250) entitled "~nvironmentally Stable,Reactive Alloy ~-
Powders And Method Of Making Same", to produce fine,
environmentally stable, generally spherical, rapidly
solidified powder particles of the rare earth-
transition metal alloy. The rapid solidification rate
that is achieved during this inert gas atomization
process is similar to that achieved in melt spinning
in so far as there is a beneficial reduction in alloy
constituent segregation during freezing, particularly
as compared to the coarse segregation patterns evident
in chill cast ingots.
Referring to Figure 2, a gas atomization
apparatus is shown for atomizing the melt in
accordance with the aforementioned high pressure inert
.
.
-.. ... , .,,, . ,.. - ..... ,. i ~, .. . .
20`~08~
W092/0~79 PCT/US91/07431
12
gas atomization procsss. The apparatus includes a
melting chamber 10, a drop tube 12 beneath the melting
chamber, a powder separator/collection chamber 14 and
a gas exhaust cleaning system 16. The melting
chamber 10 includes an induction melting furnace 18
and a vertically movable stopper rod 20 for
controlling flow of melt from the furnace 18 to a melt
atomizing nozzle 22 disposed between the furnace and ~-
the drop tube. The atomizing nozzle 22 preferably is
of the general supersonic inert gas type described in
the Ayers and Anderson U.S. Patent 4,619,845, the
teachings of which are incorporated herein by
reference, as-modified in the manner described in
Example 1. The atomizing nozzle 22 is supplied with
an inert atomizing gas (e.g., argon, helium) from a
suitable source 24, such as a conventional bottle or ;
cylinder of the appropriate gas. As shown in Figure
2, the atomizing nozzle 22 atomizes the melt in the
form of a spray of generally spherical, molten
droplets D discharged into the drop tube 12.
Both the melting chamber 10 and the drop
tube 12 are connected to an evacuation device (e.g.,
vacuum pump) 30 via suitable ports 32 and conduits 33.
Prior to melting and atomization of the melt, the
- . . -. . .
V092/0~79 PCTJUS91/0743
13
melting chamber 10 and the drop tube 12 are evacuated
to a level of 10 4 atmosphere to substantially remove
ambient air. Then, the evacuation system is isolated
from the chamber 10 and the drop tube 12 via the
valves 34 shown and the chamber 10 and drop tube 12
are positively pressurized by an inert gas (e.g.,
argon to about 1.1 atmosphere) to prevent entry of
ambient air thereafter.
The drop tube 12 includes a vertical drop
tube section 12a and a lateral section 12b that
communicates with the powder collection chamber 14.
The drop tube vertical section 12a has a generally
circular cross-section having a diameter in the range
of 1 to 3 feet, a diameter of 1 foot being used in the
Examples set forth below. As will be explained below,
the diameter of the drop tube section 12a and the
diameter of the supplemental reactive gas jet 40 are
selected in relation to one another to provide a
reactive gas zone or halo H extending substantially
across the cross-section of the drop tube vertical
section 12a at the zone H.
The length of the vertical drop tube section
12a is typically about 9 to about 16 feet, a preferred
.~ ......
~ , .
~ , . . . .
` 2 0 ~ $
W092/0~7914 PCT/US91/0743
length being 9 feet being used in the Examples set
forth below, although other lengths can be used in
practicing the invention. A plurality of temperature
sensing means 42 (shown schematically), such as
radiometers or laser doppler velocimetry devices, may
be spaced axially apart along the length of the
vertical drop section 12a to measure the temperature
of the atomized droplets D as they fall through the
drop tube and cool in temperature.
. ,:
The supplemental reactive gas jet 40
referred to above is disposed at location along the ~:
length of the vertical drop section 12a where the
falling atomized droplets D have cooled to a reduced
temperature (compared to the droplet melting
temperature) at which the droplets have at least a
solidified exterior surface thereon and at which the
reactive gas in the zone H can react with one or more
reactive alloying elements of the shell to form a
20 protective barrier layer (reaction product layer -:
comprising a refractory compound of the reactive
alloying element) on the droplets whose depth of
penetration into the droplets is controllably limited
by the presence of the solidified surface as will be
described below.
- .. , : - . . :.
- ~ .: ., .
, : , ' , . ~ ~, . . ,: .
.. 2.~ 8~
~V092/0~79 PCT/US91/07431
In particular, the jet 40 is supplied with
reactive gas (e.g., nitrogen) from a suitable source
41, such as a conventional bottle or cylinder of
appropriate gas, through a valve and discharges the
reactive gas in a downward direction into the drop
tube to establish the zone or halo H of reactive gas
through which the droplets travel and come in contact
for reaction in-situ therewith as they fall through
the drop tube. The reactive gas is preferably
discharged downwardly in the drop tube to minimize gas
updrift in the drop tube 12. The flow patterns
established in the drop tube by the atomization and
falling of the droplets inherently oppose updrift of
the reactive gas. As a result, a reactive gas zone or
halo H having a more or less distinct upper boundary B
and less distinct lower boundary extending to the
collection chamber 14 is established in the drop tube
section 12a downstream from the atomizing nozzle in
Figure 1. As mentioned above, the diameter of the
drop tube section 12a and the jet 40 are selected in
relation to one another to establish a reactive gas
zone or halo that extends laterally across the entire
drop tube cross-section. This places the zone H in
the path of the falling droplets D so that
substantially all of the droplets travel therethrough
W0~2/0~79 2 ~ 7 0 ~ 0 ~ PCT/US91/07431 ~
16
and contact the reactive gas.
The temperature of the droplets D as they
reach the reactive gas zone H will be low enough to
form at least a solidified exterior surface thereon
and yet sufficiently high as to effect the desired
reaction between the reactive gas and the reactive
alloying element(s) of the droplet composition. The
particular temperature at which the droplets have at
least a solidifiéd exterior shell will depend on the
particular melt composition, the initial melt
superheat temperature, the cooling rate in the drop
tube, and the size of the droplets as well as other
factors such as the "cleanliness" of the droplets;
i.e., the concentration and potency of heterogeneous
catalysts for droplet solidification.
Preferably, the temperature of the droplets
when they reach the reactive gas zone H will be low
enough to form at least a solidified exterior skin or
shell of a detectable, finite shell thickness; e.g., a
shell thickness of at least about 0.5 micron. Even
more preferably, the droplets are solidified from the
exterior surface substantially to the droplet core
(i.e., substantially through their diametral
r;; wo 92/0~,9 2 ~ 7 0 8 0 ~ PCTtUS91/07431
17
cross-section) when they reach the reactive gas zone
H. As mentioned above, radiometers or laser doppler
velocimetry devices, may be spaced axially apart along
the length of the vertical drop section 12a to measure
the temperature of the atomized droplets D as they
fall through the drop tube and cool in temperature,
thereby sensing or detecting when at least a
solidified exterior shell of finite thickness has
formed on the droplets. The formation of a finite
solid shell on the droplets can also be readily
determined using a physical sampling technique in
conjunction with macroscopic and microscopic
examination of the powder samples taken at diffPrent
axial locations downstream from the atomizing nozzle
in the drop tube 12. This technique is disclosed in
aforementioned copending U.S. patent application
(attorney docket no. ISURF 1250), the teachings of
which are incorporated herein by reference.
Referring to Figure 2, prior to atomization,
a thermally decomposable organic material is deposited
on a splash member 12c disposed at the junction of the
drop tube vertical section 12a and lateral section 12b
to provide sufficient gaseous carbonaceous material in
the drop tube sections 12a,12b below zone H as to form
wo 92/n~79 2 ~ ~ ~ 8 PCT/~S91/0~431 f~1
a carbon-bearing (e.g., graphite) layer on the hot
droplets D after they pass through the reactive gas
zone H. The organic material may comprise an organic
cement to hold the splash member 12c in place in the
drop tube 12. Alternately, the organic material may
simply be deposited on the upper surface or lower
surface of the splash member 12c. In any event, the
material is heated during atomization to thermally
decompose it and release gaseous carbonaceous material
into the drop tube sections 12a,12b below zone H. An
exemplary organic material for use comprises Duco
model cement that is applied in a uniform, close
pattern to the bottom of the splash member 12c to
fasten it to the elbow 12e. Also, the Duco cement is
applied as a heavy bead along the exposed uppermost
edge of the splash member 12c after the initial
fastening to the elbow. The Duco organic cement is .
subjected during atomization of the melt to
temperatures of at least 500C so that the cement is -.. ;-
thermally decomposed and acts as a source of gaseous
carbonaceou~ material to be released into the drop
tube sections 12a,12b beneath the zone H. The extent
of heating and thermal decomposition of the cement
and, hence, the concentration of carbonaceous gas
available for powder coating is controlled by the
.. . .. . . : . : : :: . .. . .
U U V U
r~WO 92/06479 PCr/US91/07431
`~s; 19
position of the splash member 12c, particularly the
exposed upper most edge, relative to the initial melt
splash impact region and the central zone of the spray
pattern. To maximize the extent of heating and
thermal decomposition, additional Duco cement can be
laid down ~deposited) as stripes on the upper surface
of the splash member 12c.
Alternately, a second supplemental jet 50
can be disposed downstream of the first ~upplemental
reactiv ~ ~as jet 40. The second jet 50 is provided to
receive a carbonaceous material, such as methane,
argon laced with paraffin oil and the like from a
suitable source (not shown), for discharge into the
drop tube section 12a to form the carbon-bearing
(e.g., graphitic carbon) coating or layer on the hot
droplets D after they pass through the reactive gas
zone H.
Powder collection is accomplished by
Reparation of the powder particles/gas exhaust stream
in the tornado centrifugal dust separator/collection
chamber 14 and by retention of separated powder
particles in the valved particle-receiving container,
Fig. 2.
'
`
2~8~
W092/0~79 PCT/US91/07431
In practicing the present invention using
the apparatus of Figure 2, the melt may comprise
various rare earth-transition metal alloys selected to
achieve desired isotropic magnetic properties. The
rare earth-transition metal alloys typically include
those described in the U.S. Patents 4,402,770;
4,533,408; 4,597,938 and 4,302,931, the teachings of
which are incorporated herein by reference, where the
rare earth is selected from one or more Nd, Pr, La,
Tb, Dy, Sm, Ho, Ce, Eu, Gd, Er, Tm, Yb, Lu, Y and Sc.
Lower weight lanthanides (Nd, Pr, La, Sm, Ce, Y, Sc)
are preferred. Rare earth-iron-boron alloys,
especially Nd-Fe-B alloys comprising about 26 to 36
weight % Nd, about 62 to 68 weight % Fe and about 0.8
to l.6 weight % B, are useful in practicing the
invention as a result of their demonstrated excellent
magnetic properties.
Nd-Fe-B alloys rich in Nd (i.e., at least
about 27 weight %) and rich in ~ (i.e., at least about
l.l weight %) are preferred to promote formation of
the hard magnetic phase, Nd2Fe~4B, in an equiaxed, --
blocky microstructure, and minimize, preferably avoid,
formation of the ferritic Fe phase in all particle
sizes produced. The Nd-Fe-B alloys rich in Nd and B
2~ 08~
~`~o W092/0~79 PCT/US91/07431
21
were found to be substantially free of primary
ferritic Fe phase, which was observed in some particle
sizes (e.g., lO to 20 microns) for Fe rich and near-
stoichiometric alloy compositions. Alloyants such as
Co, Ga, La and others may be included in the alloy
composition, such as 31.5 weight % Nd- 65.5 weight %
Fe- l.408 weight % B- l.592 weight % La and 32.6
weight % Nd- 50.94 weight % Fe- 14.l weight % Co- l.22
weight % B- l.05 weight % Ga.
In the case of the rare earth-transition
metal-boron alloys, the rare earth and boron are
reactive alloying elements that must be maintained at
prescribed concentrations to provide desired magnetic
properties in the powder product.
The reactive gas may comprise a nitrogen
bearing gas, oxygen bearing gas, carbon bearing gas
and the li~e that will form a stable reaction product
comprising a refractory compound, particularly an
environmentally protective barrier layer, with the
reactive alloying element of the melt composition.
Illustrative of stable refractory reaction products
are nitrides, oxides, carbides, boride~ and the like.
The particular reaction product formed will depend on
.: :
,.. ,., ;.1 .',
W092/0~79 2 o7 083 8 22 PCTtUS91/07431
the composition of the melt, the reactive gas
composition as well as the reaction conditions
existing at the reactive gas zone H. The protective
barrier (reaction product) layer is selected to
provide protection against environmental constituents,
such as air and water in the vapor or liquid form, to
which the powder product will be exposed during
particle size classifying operations, during
subsequent fabrication to an end-use shape, and during
use in the intended service application.
The depth of penetration of the reaction
product layer into the droplets is controllably
limited by the droplet temperature (extent of exterior
shell solidification) and by the reaction conditions
established at the reactive gas zone H. In
particular, the penetration of the reaction product
layer (i.e., the reactive gas species, for example,
nitrogen) into the droplets is limited by the presence
of the solidified exterior shell so as to avoid
selective removal of the reactive alloying element (by
excess reaction therewith) from the droplet core
composition to a harmful level (i.e., outside the
preselected final end-use concentration limits) that
could substantially degrade the end-use properties of
-
: :
.
,~ W092/06479 20~0~a~ PCT/US91/07431
3' 23
the powder product. For example, with respect to the
rare earth-transition metal-boron alloys, the
penetration of the reaction product layer is limited
to avoid selectively removing the rare earth and the
boron alloyants from the droplet core composition to a
harmful level (outside the prescribed final end-use
concentrations therefor) that would substantially
degrade the magnetic properties of the powder product
in magnet applications. The thickness of the reaction
product layer formed on rare earth-transition
metal-boron alloy powder is limited so as not to
exceed about 500 angstroms, preferably being in the
range of about 200 to about 300 angstroms, for powder
particle sizes in the range of about 1 to about 75
microns, regardless of the type of reaction product
layer formed. Generally, the thickness of the
reaction product layer does not exceed 5% of the major
coated powder particle dimension (i.e., the particle
diameter) to this end.
The reaction barrier (reaction product)
layer may comprise multiple layers of different
composition, such as an inner nitride layer formed on
the droplet core and an outer oxide type layer formed
o~ the inner layer. The types of reaction product
- ~ ~
:,
W092/0~79 2 0 ~ 8 24 PCT/US91/07431
layers formed again will depend upon the melt
composition and the reaction conditions present at the
reactive gas zone H.
.
~s mentioned above, a carbon-bearing
(graphite) layer may be formed in-situ on the reaction
product layer by various techniques. Such a layer is
formed to a thickness of at least about l monolayer
(2.5 angstroms) regardless of the technique employed.
The carbon-bearing layer provides protec~ion to the
powder ~ -duct against such environmental constituents
as liquid water or water vapor as, for example, is
present in humid air. Importantly, the carbon-bearing
layer also facilitates wetting of the powder product
by polymer binders, such as polyolefins (e.g.,
polyethylenes) in injection molding of binder/alloy -
powder mixtures to form complex, end-use magnet
shapes.
The invention is not limited to the
particular high pressure inert gas atomization process
described in the patent and may be practiced using
other atomization nozzles, such as annular slit
nozzles, close coupled nozzles or conventional free-
falling nozzles that yield rapidly solidified powder
.. . . . .
.. . . . .
- :
.. . . . . .. . . .
- . ~ . . ~ . .
u u v --
092/0~79 PCT/US91/07431
having appropriate sizes for use in the fabrication of
isotropic permanent magnets.
Referring to Fig. 1, one embodiment of the
invention invo;ves producing environmentally stable,
generally spherical, rapidly solidified powder
particles using the high pressure inert gas
atomization process/apparatus described in Example 1
such that the rare earth-transition metal alloy
particles fall within a given particle size (diameter)
range (and thus within a given grain size range)
wherein the majority of the particles exhibit particle
diameters less than a given diameter determined to
exhibit desirable magnetic properties for the
particular alloy composition and magnet service
application involved. For example, in practicing the
invention to make Nd-Fe-B alloy magnets, the powder
particles produced using the high pressure inert gas
atomization process/apparatus typically fall within a
particle size (diameter) range of about 1 micron to
about lO0 microns with a majority (e.g., 66-68% by
weight) of the particles having a diameter less than
about 44 microns, typically from about 3 to about 44
microns. Preferably, a majority of the particles are
less than about 38 microns in diameter, a particle
., .
W092/0~79 2 0 7 8 8 ~ 8 PCT/US91/07431
26
size found to yield optimum magnetic properties in the -
as-atomized condition as will become apparent below.
Figure 5 illustrates in bar graph form a typical
distribution in weight % of two batches of Nd-Fe-B-La
alloy particles as a function of particle size. The
composition (in weight %) of the alloys before
atomization is set forth below in the Table:
Table
Nd Fe B La
Alloy BT-1-190 31.51 65.49 1.32 1.597
Alloy BT-1-216 33.07 63.93 1.32 1.68
Both alloys BT-1-19o and BT-1-216 were atomized under
like conditions similar to those set forth in Example
1. With Nd-Fe-B type alloys, the Nd content of the
alloy was observed to be decreased by about 1-2 weight
% in the atomized powder compared to the melt as a
result of melting and atomization, probably due to
reaction of the Nd during melting with residual oxygen
and formation of a moderate slag layer on the melt
surface. The iron content of the powder increased
relatively as a result while the B content remained
generally the same. The initial melt composition can
be adjusted to accommodate these effects.
. .
... . .
.
~lu~u~
'"'i`; W 0 92/06479 ~, . PC~r/US91/07431
27
Figure 5 reveals that a majority of the as-
atomized powder particles fall in the particle size
(diameter) range of less than 45 microns, even more
particularly less than 38 microns (i.e.,-38 on the
a~scissa). In particular, greater than 60% (about 66-
68%)by weight of the particles exhibit particle
diameter of less than 38 microns found to exhibit
optimum magnetic properties in the as-atomized
condition as will become apparent. These weight
distributions were determined by hand sifting
(screening) an entire batch of powder through a full
range of ASTM woven wire screens and by an automated
size analysis technique based on laserlight scattering
by an ensemble of particles dispersed in a transparent
fluid.
The advantage of producing the alloy powder
particles in the manner described above is evident in
Figs. 6 and 7. In Figs. 6 and 7, the magnetic
properties (namely, coercivity, remanence and
saturation) of as-atomized powder as a function of
particle size is set forth for alloy BT-1-162 (32.5
weight % Nd-66.2 weight % Fe-1.32 weight % B, Fig. 6)
and the aforementioned alloy BT-1-190 (Fig. 7). The
alloys were atomized under like conditions similar to
W092/0~9 2 0 7 0 ~ 0 8 PCT/US91/07431 ~
28
those set forth in Example 1. The Figures demonstrate
that coercivity and, to a lesser extent, remanence
appear to vary as a function of particle size in both
alloys. Elevated levels of coercivity and remanence
are observed in both alloys as particle size
(diameter) is reduced below about 38 microns. On the
other hand, saturation magnetization of both alloys
remains relatively constant over the range of particle
sizes. For alloy BT-1-162, the coercivity falls
significantly as particle size is reduced below about
5 microns. These results correlate with grain size
measurements which reveal a continuous decrease in
grain size with reduced particle size; e.g., from a
grain size of about 500 nm for 15-38 micron particles
to about 40-70 nm for less than 5 micron particles;
for example, as shown in Fig. 8 for alloy BT-1-162.
Magnetic property differences between powder size
classes were due to differences in the
microcrystalline grain size within each particle.
From Figs. 6 and 7, it is apparent that the
magnetic properties, particularly the coercivity, of
the alloy powder increase with decreased particle size
to a maximum of about 10-11 Koe for powder particles
of about 15-38 microns diameter, and then decrease for
,,
W092/0~79 PCT/US91/07431
29
particles of further reduced size. Moreover, it is
apparent that near optimum overall magnetic properties
are exhibited by the as-atomized alloy particles in
the general particle size (diameter) range of about 3
microns to about 44 microns and, more particularly,
about 5 to about 40 microns where the majority of the
particles are produced by the high pressure inert gas
atomization process described above. Thus, the yield
of as-atomized powder particles possessing useful
magnetic properties is significantly enhanced in
practic _ ~ the invention as described above.
Typically, in the above-described embodiment
of the invention, each batch of alloy particles
produced using the high pressure inert gas atomization
process of Example 1 is initially size classified by,
for example, sifting (screening) through an ASTM 44
micron woven wire mesh screen. This preliminary size
classifying operation substantially removes particles
greater than 44 microns diameter from the batch and
thereby increases the percentage of finer particles in
each batch. This preliminary screening operation is
conducted in a controlled atmosphere (nitrogen) glove
box after the contents of the sealed powder container,
Fig. 2, are opened in the glove box.
,: ~. -:
- : ,
W092/06479 ~ !~ PCI/US91/07431
The remaining alloy particles having
particle sizes less than 44 microns diameter can then
be further processed (i.e., hot isostatically pressed)
in accordance with the invention in a manner to be
described below.
Those skilled in the art will appreciate
that the initial size classifying (screening)
operation may be employed using other than the
lO aforementioned 44 micron woven wire screen depending ~-
upon the particular alloy involved and the variation
of magnetic properties of the as-ato~ized alloy
particles as a function of particle size. In
particular, an appropriate screen size can be used for
each batch of alloy particles to remove particles
greater than the given size range exhibiting near
optimum magnetic properties, thereby increasing the
weight percentage of particles in each batch having
particle sizes below the given size.
2~
Referring to Figure 1, in another embodiment
of the invention, the generally spherical, rapidly
solidified pcwder produced by the high pressure inert
gas atomization process is subjected to the
preliminary size classifying (screening) operation
. , . . : . . .
. . .
.
: .. . . . . . . .
: . . .
:.~ W092/0~79 2 ~ 7 b ~ n~ ~ PCT/US91/07431
... 31
described above and also to one or more additional
size classifying operations to form multiple particle
size fractions or classes wherein each fraction or
class comprises powder particles having a particle
size (diameterj range in a given relatively narrow
range. For example, for a typical batch of high
pressure inert gas atomized Nd-Fe-B powder (e.g., BT-
1-162 described above), the following particle size
fractions or classes having the listed range of
particle sizes (diameters) are provided by carrying
out an air classifying operation on the batch:
Fraction #l- about 15 to about 38 (diameter)
Fraction t2- about lO to about 15 microns (diameter)
Fraction #3 -about 5 to about lO (diameter)
Fraction #4- about 3 to about 5 (diameter)
In particular, the rapidly solidified powder particles
were air classified using a commercially available
air classifier sold as model A-12 under the name Majac
Acucut air classifier by Hosok.awa Micon International
Inc., lO Chantham Rd., Summit~ N.J. The air
classifier was operated at a blower pressure of 13.5
inch water, an ejector pressure of 50 psi with rotor
speeds of 507 rpm, 715 rpm, 1145 rpm, and 1700 rpm to
Q~Q~
W092/0~79 PCT/US9l/07431
32
produce the particle size fractions #1, #2, #3 and #4,
respectively.
As is apparent, in any given particle size
fraction or class, the powder particles fall within a
given narrow range of particle sizes (diameters). As
a result, the powder particles in each particle size
fraction or class exhibit a rapidly solidified
microstructure, especially grain size, also within a
very narrow range and provide isotropic magnetic
properties upon consolidation/annealing in accordance
with the next step of the invention. For example the
following grain size ranges were observed for each
particle size fraction:
Fraction #1- about 49Onm to about 500nm grain size
Fraction #2- about 210nm to about 220nm grain size
Fraction ~3- about 115nm to about 130nm grain size
Fraction ~4- about 60nm to about 75nm grain size
A plurality of particle size fractions or
classes having quite uniform particle microstructures
(grain sizes) within each fraction or class are .
thereby provided by the size (air) classifying .
operation depicted in Figure 1. Depending upon the
. :. . . . . .
. ,, ~ -. . . . . . ..
.
:. . . .: . ~
.
- .
, ~ ......... . . .
" . ~ . .
~` W092/06479 2 0 ~ 0 8 ~ ~ PCT/US91/07431
~. ~
33
particular magnetic properties desired in the magnet,
a particular particle size fraction or class having
the appropriate microstructure can then be selected to
this end for further processing in accordance with the
invention-to produce the desired magnet. A different
particle size fraction or class can be chosen for
further processing in accordance with the invention in
the event slightly different magnetic/mechanical
properties are specified by the magnet user or
manufacturer.
Referring again to Figure 1, the size
classified powder particles, either as initially size
classified (screened) in accordance with the first
embodiment of the invention, as air classified in
accordance with the second embodiment of the invention
or as-atomized, are subjected to a co~bined
consolidation and annealing step using an elevated
temperature and elevated isotropic pressure for a time
2~ to densify the powder particles to a desired magnet
body or compact configuration and enhance the magnetic
and mechanical properties. Generally, the magnet
compacts resulting from the combined
consolidation/annealing operation will exhibit a
density between 85% and 99%, preferably 100%, of -
- : . - . . , ,; :
- . , ~ , . , . . > .~ . . . .. . . . .
20`7`~8`~
W092/0~79 PCT/US91/07431
34
theoretical, although the invention is not limited to
any particular density. The isotropic magnetic
properties achieved will depend upon the particular
rare earth-transition alloy composition, the selected
particle size (grain size) and the hot isostatic
pressing cycle conditions, such as temperature,
pressure and cycle time. Use of the alloy particles
having generally uniform particle size and thus
microstructures as-atomized and screened and/or air
classified yields a ~ompact having isotr_pic magnetic
proper~_ 3 after the consolidation/annealing step.
Moreover, the magnetic properties of body or compact
will improve beyond those exhibited by the as-atomized
alloy particles. For example, referring to Figure 9,
the magnetic properties of as-atomized alloy BT-1-174
(34.7 wei~ht ~ Nd-63.89 weight ~ Fe-1.31 weight % B)
as atomized and after hot isostatic pressing for
different times at 700C and 300 MPa (44 ksi) are
shown. The magnetic properties of as-atomized
particles were determined on appropriate samples
wherein the particles were bonded in epoxy. A SQUID
magnetometer (saturation field strength of 4.5 Tesla)
was used to measure magnetic properties at ambient
temperature.
.
, . .. .
.
. , . . .
2~7~8~8
W092/0~79 PCT/US91/07431
As is apparent in Fig. 8, HIP processing of
the alloy particles notably improved magnetic
properties for both HIP times involved. For example,
after 1.5 hours, significant increases occurred in
intrinsic magnetic saturation and remanence. This
might be attributed to the solid state transfor~ation
of a metastable phase(s3 to the equilibrium hard
magnetic phase, Nd2Fe14~, which exhibits higher
saturation and remanence. Although only a modest
increase in coercivity was observed after l.5 hours,
the total energy product increased about 67%. The 2.5
hour HIP cycle resulted in a further enhancement in
coercivity with little further improvement in
saturation and remanence. This behavior suggests the
growth of the fine, overquenched (120nm) grains of
Nd2Fel~B, to a more optimum size. The total energy
product displayed an 83% improvement after the 2.5 HIP
cycle compared to as-atomized particles. The improved
magnetic properties appear to result from grain growth
within the prior particle boundaries to achieve an
optimum grain size and magnetic domain distribution.
Since the initial grain size of the as-atomized
particles is near optimum as a result of the atomizing -
conditions and screening/air classifying operations,
only minor exposure to elevated temperature is
,
I ` : J "
W092/0~79 2 0 ~ O ~ ~ 8 36 PCT/US91/07431
required to achieve optimum grain size and can be
combined with particle consolidation into a one step
treatment in accordance with the invention. The
maximum grain size of the densified compact appears to
be limited by ~he environmentally stable particle
coating so as not to exceed the dimensions of the
prior particle boundaries.
Typically, in conducting the combined
consolidation/annealing step, the powder particles are
packed in a suitable container, such as a cleaned,
outgassed, end-capped tantalum foil sleeve. The
packed tantalum foil sleeve is then placed in an
annealed thin wall, copper outer container which is
hermetically sealed under vacuum by welding. The
container assembly is then subjected to an elevated
temperature of at least about 600C, preferably about
675 to 800C, and elevated isotropic pressure of at
least about 20 ksi, preferably about 25 to about 44 -
ksi, for a time of at least about 30 minutes,
preferably about 60 to about 180 minutes, in the case
of the Nd-Fe-B alloy powder described hereinabove. Of
course, other temperature and pressure parameters can
be used for other rare earth-transition metal alloy
particles as necessary to achieve the desired
- ~ , . ~,
- ~ :
,
8 0 8
J~ WO92t0~79 PCT/US91/07431
:F~ 37
densification and development of magnetic properties
for the compact. The powder filled container assembly
is typically subjected to the elevated temperature and
isotropic pressure in a conventional hot isostatic
pressing apparatus.
In practicing the method of the invention,
the powder particles are subjected to concurrent
consolidation and annealing of the powder particles to
develop desired density, improved magnetic properties
(intrinsic coercivity, magnetic remanence and maximum
energy product) and improved mechanical properties in
the compact. The magnet compacts produced exhibit
improved homogeneity of microstructure (grain size),
15 composition and properties (magnetic and mechanical) -~
than achievable by the aforementioned prior art
fabrication approaches. In addition, as will become
evident from the Examples which follow, magnet
compacts produced in accordance with the method of the
2~ invention exhibit levels of coercivity and energy
products competitive with those achieved heretofore by
the more complex, time consuming and costly prior art
fabrication approaches. Moreover, the mechanical
properties of the magnet compacts of the invention are
dramatically improved as compared to tho6e produced by
W092/06479 2 ~ 7 0 8 0 8 38 PCT/US91/07431
the prior art fabrication approaches as a result of
the beneficial effect of particle sphericalness on
interparticle bonding.
Following the combined consolidation/
annealing step, the copper container and tantalum
sleeve are removed from the resulting magnet compact
by cutting under oil-cooled conditions. The magnet
compact can then be subjected to machining or other
shaping operations as necessary for the intended
service application.
ExamDle
The melting furnace of Fig. 2 was charged
with an Nd-16 weight % Fe master alloy as-prepared by
thermite reduction, an Fe-B alloy carbo-thermic
processed and obtained from Shieldalloy Metallurgical
Corp. and electrolytic Fe obtained from Glidden Co.
The quantity of each charge constituent was controlled
to provide a melt composition of about 33.0 weight %
Nd-65.9 weight S Fe-l.l weight ~ B. The charge was
melted in the induction melting furnace after the
melting chamber and the drop tube were evacuated to 10
4 atmosphere, and then pressurized with argon to 1.1
.
2~`3~
; ` W092/0~79 PCT~US91/07431
atmospheres. The melt was heated to a temperature of
1650C. After a hold period of 10 minutes to reduce
(vaporize) Ca present in the melt (from the thermite
reduced Nd-Fe master alloy) to melt levels of 50-60
ppm by weight, the melt was fed to the atomizing
nozzle by gravity upon raising the boron nitride
stopper rod. The atomizing nozzle was of the type
described in U.S. Patent 4,619,845 as modified (see
Figs. 10-13) to include (a) a divergent manifold
expansion region 120 between the gas inl~t 116 and the
arcuate ~ nifold segment lla and (b) an increased
number (i.e., 20) of gas jet discharge orifices 130
that are NC (numerical control) machined to be in
close tolerance tangency T (e.g., within .002 inch,,
preferably .001 inch) to the inner bore 133 of the
nozzle body 104 to provide improved laminar gas flow
over the frusto-conical surface 134 of the two-piece
nozzle melt tube 132 (i.e., inner boron nitride melt
supply tube 132c and outer Type 304 stainless steel
tube 132b with thermal insulat1ng space 132d
therebetween. The divergent expansion region 120
minimizes wall reflection shock waves as the high
pressure gas enters the manifold to avoid formation of
standing shock wave patterns in the manifold, thereby
maximizing filling of the manifold with gas. The
".' ' . '' ' :, , : '. ~ :~ . :,' ': .. ' :
W092/0~79 ~7a~0~ PCT/US91/07431 ~
manifold had an rO of 0.3295 inch, r1 f 0.455 inch and
r2 f 0.642 inch. The number of discharge orifices 130
was increased from 18 (patented nozzle) to 20 but the
diameter thereof was reduced from 0.0310 inch
(patented nozzle) to 0.0292 inch to maintain the same
gas exit area as the patented nozzle. The modified
atomizing nozzle was found to increase the percentage
of particles falling in the desired particle size
range (e.g., less than 38 microns) for optimum
magnetic properties for the Nd-Fe-B alloy involved
from about 25 weight % to about 66-68 weight %. The
yield of optimum particle sizes was thereby increased
to improve the efficiency of the atomization process.
The modified atomizing nozzle is described in
copending U.S. patent application entitled "Improved
Atomizing Nozzle And Process" (attorney docket no.
ISURF 1250-A), the teachings of which are incorporated
herein by reference.
Argcn atomizing gas at 1050 psig was
supplied to the atomizing nozzle in accordance with
the aforementioned patent. The reactive gas jet was
located 75 inches downstream of the atomizing nozzle
in the drop tube. Ultra high purity (99.95%) nitrogen
gas was supplied to the jet at a pressure of 100 psig
.. . . - . - . .
~ ~ 7 ~
W092/0~79 ` `~ PCT/US91/07431
41
for discharge into the drop tube to establish a
nitrogen gas reaction zone or halo extending across
the drop tube such that substantially all the droplets
traveled through the zone. At this downstream
location from the atomizing nozzle, the droplets were
determined to be at a temperature of approximately
1000C or less, where at least a finite thickness
solidified exterior shell was present thereon. After
the droplets traveled through the reaction zone, they
10 were collected in the collection container of the --
collection chamber (see Figure 2). The solidified -~
powder product was removed from the collection chamber ~ -~
when the powder reached approximately 22C.
The powder particles comprised a core having
a particular magnetic end-use composition, an inner
protective refractory layer and an outer carbonaceous
(graphitic carbon) layer thereon. The refractory
layer thickness is limited so as not to exceed about
500 angstroms, preferably being in the range of about
200 to about 300 angstroms. Auger electron
spectroscopy (AES) was used to gather surface and near
surface chemical composition data on the particles
using in-situ ion milling to produce a depth profile.
The AES analysis indicated an inner surface layer
: . ~ ' . '. -
.. . .
- .
.. . . , . -; . . . . .
W092/06479 2 0 i a 8 0 8 pcr/us91/o743l
42
enriched in nitrogen, boron and Nd corresponding to a
mixed Nd-B nitride (refractory reaction product). The
first inner layer was about 150 to about 200 angstroms
in thickness. A second inner layer enriched in Nd,
5 Fe, and oxygen was detected atop the nitride layer. ~-
This second layer corresponded to the mixed oxide of
Nd and Fe (refractory reaction product) and is
believed to have formed as a result of decomposition
and oxidation of the initial nitride layer while the
powder particles were still at elevated temperature.
The second layer was about lO0 angstroms in thickness.
An outermost third layer of graphite was also present
on the particles. This outermost layer was comprised
of graphitic carbon with some traces of oxygen and had
a thickness of at least about 3 monolayers. This
outermost carbon layer is believed to have formed as a
result of thermal decomposition of the Duco~ cement
(used to hold the splash member 12c in place in the
drop tube 12) and subsequent deposition of carbon on
the hot particles after they passed through reactive
gas zone H so as to produce the graphitic carbon film
or layer thereon. Subsequent atomizing runs conducted
with and without excess Duco cement present confirmed
that the cement was functioning as a source of gaseous
carbonaceous material for forming the graphite outer
,
W092/0~79 2 ~ 7 a (~ ~ g PCT/US91/07431
43
layer on the particles. The Duco cement is typically
present in an amount of about one (1) ounce for
atomization of a 4.5 kilogram melt to produce the
~raphite coating on the particles.
The rapidly solidified, spherical Nd-Fe-B
powder particles produced in this way exhibited
particle sizes (diameters) in the range of about 1 to
about 100 microns with a majority of the particles
being less than about 42 microns in diameter. The
powder particles were initially size classified
(screened) under a nitrogen atmosphere glove box using
an ASTM 44 micron woven wire mesh screen and then air
classified into several particle size fractions or
classes by using the commercially available air
classifier referred to above operated at rotor speeds
of 570 rpm, 715 rpm, 1145 rpm, for size fractions #2
to #4, respectively, with a blower pressure at 13.5
inches water and ejector pressure at 50 psi. Particle
size fraction~ or classes were thereby provided
wherein the particles of each fraction or class
exhibited the following particle sizes (diameters) and
~rain sizes:
. :. . : . , . , :, ~.... . , : .. . .
XU` 7~-$~8
W092/0~79 PCT/US91/07431
Particle Size Grain Size
(Diameter)
Fraction #1-38 to 44 microns >500 nm
Fraction #2-15 to 38 microns 500 nm
Fraction #3-10 to 15 microns 215 nm
lo Fraction #4-5 to 10 microns 125 nm
Fraction #2 was selected for further
processing in accordance with the method of the
invention. A portion of the powder part _ les of this
fractio-~ 2 are shown in Figure 2. In particular, -
powder particles from fraction #2 were packed into a
tantalum foil sleeve (0.010 inch wall thickness). The
sleeve had been previously chemically cleaned in a
solution of 90 volume % HN03/10 volume ~ HF for 10
minutes, washed in water, rinsed in an ultrasonic
alcohol bath and, after drying, outgassed at 1400C
for 2 hours in a vacuum of 10-6 torr. The powder
filled tantalum foil sleeve was then placed into an
annealed thin wall (0.0625 inch wall thickness) copper
~grade 101) container of cylindrical shape. The
copper container was then hermetically sealed in a
vacuum of 105 torr by welding the container closed.
The powd~r filled container assembly was
..
~.
- ~
W092/0~79 ~ 7~ PCT/US91/07431
then subjected to a combined consolidation/annealing
operation at 700OC and isotropic pressure of 44 ksi
(303 MPa) in a conventional hot isostatic pressing
apparatus for 1.5 hours.
After the consolidation/annealing operation,
the container/sleeve were machined off to yield a
cylindrical shaped magnet compact. A metallographic
section of tne resulting magnet compact is shown in
Figure 3 wherein it is apparent that a homogenous
microstructure (grain size) is present. A 3/16 inch
t4.76 mm) diameter x 1/4 inch (6.35 mm) long sample
(designated Sample 1) was machined from the resulting
magnet compact and was tested in a vibrating sample
magnetometer to determine saturation (M~), energy
product (BHmax), remanence (Br)and coercivity (Hci).
The measured magnetic properties are shown in Table 1
set forth hereinbelow following the remaining
Examples.
The following additional samples were made
in accordance with the procedures described in Example
1 but using, in some cases, different alloy
compositions, particle sizes and hot isostatic
pressing conditions as set forth in each following
- . : .
W092/0~79 2 ~ ~ Q ~ Q ~ PCT/US91/07431
46
Example.
- ExamDle 2
Particle size fraction #4 of Example l was
hot isostatically pressed under the same conditions as
Example l to determine the influence of particle
size/grain size on magnetic properties. Sample 2 was
prepared from the HIP'ed fraction #4 as set forth in
Example l. From Table II, it is apparent that the
particles in the size range 15 to 38 microns exhibited
the better magnetic properties.
ExamDle 3
A batch of alloy powder particles was
produced as in Example l from a melt comprising 32.5
weight % Nd-66.2 weight % Fe-l.32 weight % B in a size
range of about l to about lO0 microns diameter with a
majority of the particles falling in the particle size
range of about 15 to 20 microns diameter. The batch
was initially screened using an ASTM 400 mesh woven
wire screen to provide particle sizes less than 38
microns. Powder from the screened batch (without
further air classification) were hot isostatically
- :~ ?
-
~ u ~
W092/0~79 PCTtUS91/07431
47
pressed at 750C and 44 ksi for 1.5 hours. Sample 3
was prepared from the HIP'ed particles as set forth in
Example 1.
ExamPle 4
A portion of the batch of alloy powderparticles of Example 3 was air classified as in
Example 1 to below 15 micron diameters. The air
classified particles were HIP'ed in the manner set
forth in Example 3. Sample 4 was prepared from the
HIP'ed particles as set forth in Example 1. The
resulting magnetic properties were lower for the
finer-sized particles having the finer grain size. -
ExamPle 5
Alloy powder was atomized from a melt
comprising 34.7 weight %Nd-63.89 weight ~ Fe-1.31
weight % B (i.e., enriched in rare earth and ~oron),
air classified to 15 to 38 micron diameters and HIP'ed
in a manner similar to Example 1. Sample 5 was
prepared from the HIP'ed particles as set forth in
Example 1. Excellent magnetic properties were
observed.
.
. .
- : . . .
2070~
W092/0~79 . PCT/US91/07431
48
Exam~le 6
The procedures of Example 5 were repeated to
produce Sample 6 determine reproducibility of the
process.
Example 7
The procedures of Example 5 were repeated
except that the HIP time was increased to 2.5 hours.
Sample 7 was prepared from the HIP'ed particles as set
forth in Example l. Sample 7 shows increased
coercivity with the longer HIP time.
TABLE
Magnetic Performance of HPGA Powders and Con~olLdated Samples
Sample Saturation B~max Remanence Coerci~ity
(kGauss) ~MGOQ) (kGauss)(kOe)
1. 12.4 10.0 6.9 10.0
2. 12.4 5.0 6.3 5.5
3. 12.0 6.9 6.3 7.5
4. g.5 4.6 5.2 7.2
5. 11.9 8.. 0 6.5 9.0
6. 12.2 7.8 6.5 9.0
7. 11.73 8.8 6.6 11.5
The magnetic properties set forth in Table l
illustrate that the method of the invention produced
isotropic permanent magnets exhibiting a range of
. . . , ~ -
. ~:
:
. ,~
. . .
W092/0~79 2 ~ 7 ~ PCT/US91/07431
49
moderate levels of coercivity and other magnetic
properties competitive with those exhibited by
commercially available magnets produced by the
aforementioned prior art approaches which are more
time consuming, complex and costly. The magnetic
properties set forth in Table l were isotropic for
each compact produced.
The mechanical properties of the magnet
compacts of the invention are isotropic ~nd
dramati~lly superior to those achievable by the
aforementioned prior art approaches. For example, the
transverse rupture strength of magnet compacts of the
invention was detérmined using the known three point
bend test method (ASTM B528-76). The magnet compacts
of the invention typically exhibited a transverse
rupture strength of at least about 200 MPa. In
particular, transverse rupture strengths of 239, 300
and 421 MPa were measured for three compacts prepared
by Examples l, 4 and 6. These results are quite
remarkable considering that most of the commercially
available rare-earth-iron-boron magnets are too
brittle to be extensively handled as well as too
brittle to be machined into a transverse rupture bar
and to bear even minor applied testing load. The
- . : . ,: -
,. , ~
wo gvn64~9 2 ~ 7 ~ ~ a ~ PCT/US91/07431
dramatic improvement in mechanical strength of the
magnet compacts of the invention appears to result
from the enhanced interparticle bonding due to the
sphericalness of the particles in conjunction with HIP
treatment, which is achieved without the occurrence of
excessive grain growth.
While the invention has been described in
terms of specific embodiments thereof, it is not
intended to be limited thereto but rather only to the
extent set forth hereafter in the following claims.
.: ,::
- ,