Note: Descriptions are shown in the official language in which they were submitted.
2 ~'~ 0~.~~S~~o~204
This invernion relates to electronic articles having a fluorinated
poly(arylene ether)
dielectric.
Polymer films and coatings are often used in the electronic industry as
insulating
to materials and passivatbn layers, especially in irnegrated arcuit devices
such as muftichip
modures. Polymers having a bw dielectric aonstarn s are preferred, because
components
insulated with them can be designed with higher arcuit densities and can
operate at higher
speeds and with less signal broadening. The effect of s on the performance of
multilayer
irnegrated circuit articles is discussed in "Microelectronics Packaging
Handbook," Tummala et al.
~5 (eds.), pp. 687-692 (van Nostrand Reinhoid); Watari et al., U.S. Pat.
4,744,007 (1988); and
Budde et al., U.S. Pat. 4,732,843 (1988).
Polyimide is an insulator of choice for many electronic applications, because
of its
superior mechanical and thermal properties and its fabricabiGty irno thin
films and coatings.
2o However, polyimide has a relatively high e, a imitation acoenhrated by
polyimide's tendency to
absorb water (up to 3-4 %) in humid environments. Water absorption causes s to
rise,
compromising performance. One commercially available polyimide has an a of
about 3.2 at 0
relative humidity (%RH), Which rises t0 about 3.8 at 80 ~eRH. As noted by
Denton et al. in,),
~gl,14(2),119 (1985), polyimide moisture absorption can also adversely affect
25 performance through increased insulator conductivity, bss of adhesion, or
corrosion. Further,
some polyimides are susceptible to hydrolysis and/or attack by solverns (often
manifested by
crazing or cracking upon exposure to a soNern).
It has been proposed, in Mercer, U.S. Pat. 4,835,197 (1989), to improve the
solvent
3o resistance of polyimide by curing with an acetylene, maleimide, or vinyl
tertHnated curing agent.
However, a polyimide so cured would still have the relatively high dielectric
oonstarn of polyimides
and their tendency to absorb moisture.
One of us has proposed using fluorinated polymers having a binaphthyl moiety
as
3s dielectric materials.
Polyquinoxalines, polyquinozabnes, potybenzoxazoles, and copolymers thereof
with
polyimides have also been proposed as polymers for microelectronic app~cations
by Labadie et
al., in SAMPE J. vol . 25, pp.18-22 (Nov.IDec. 1989).
Kellman et al., ACS Symp. Ser. 326, Phase Transfer Catalysis, p. 128 (1987)
discloses
the preparation of polyethers from diphenols and hexatluorobenzene and
decatluorotHphenyl.
atlhough no particular utility is discbsed for the polymers so prepared.
Similar disclosures are
made in Keliman et al., Polym. Prepr. 22(2), 383 (1981) and Gerbi et al., J.
Polym. Sci. Polym
Letters Ed. 23, 551 (1985).
2~'~~8~~
WO 91/09071 PCT/US90/07204
2
This invention provides electronic articles having a fluorinated poly(arylene
ether)
dielectric material. This dielectric material has a low dielectric constant
which isllttle affected by
increases in the ambient hurt>ic~ty, can be made solvent resistant, and
exhibits excellent adhesion
to itself and other adherends.
This invention provides an electror>ic article having a dielectric material
comprising a
fkrorinated poly(aryiene ether) comprising a repeal unit ~ the stnraure
to
wherein -W- is
P
-o-~ ~ ~ ~ o-
15 ~P HøPJ m
-O ~ O- -U-
or ~ ~ ,
wherein each -A is independently -F. -CI. -Br, -CFg, -CH3, -CH2CH=CH2, or -
CgHS: P is
20 0.1, or 2; -Z- is a diced bond. -C(CH3)2-. -C(CF3)g-, -0-. -S-. -S02-, -CO-
, -P(C6H5)-, -
C(CH3)(C8H5), -C(CgH5)2'. '(CF2)1-8'. or
Y
-C
25 wherein -Y- is -O- or a direct bond;
and m is 0,1, or 2:
each -X is independently -H, -CI, -Br, -CF3, -CH3, -CH2CH=CH2, or -CgHS; q is
0, 1, or 2; and n
is 1 or 2.
dV0 91/09071 PCT/US90/07204
3 2Q~0~26
Preferably, -V~- is
AP AP
°~ ~ t ~ °~
m
H4-P ~PJ
corresponding to a fluorinated poly(arylene ether) having the repeat unit
AP AP Xq
-°~ ~ t
H4.P H4-P J m t..F49 n
wherein -A, p, -Z-, m, -X, q, and n are as previously defined. Further, the
group -Z- is preferably
t o para-bonded to each ether oxygen in the benzene rings.
In one embodiment, the electronic article is a multichip module comprising a
substrate, a
plurality of semiconductor chips carried on the substrate, and a multilayer
interconnect connecting
the semiconductor chips; the muftllayer interconnect comprising plural layers
of conductive
is material and plural layers of a dielectric material made of a fluorinated
poty(arylene ether) as
aforesaid.
In another err~~odiment, the electronic article is an integrated circuit chip
having thereon a
mutGlayer interconnect comprising p~ral layers of cor>ducUve material and
plural layers of a
2o dielectric material made of a fluorinated poly(arylene ether) as aforesaid.
In yet another embo~ment, the electronic article is an integrated circuit chip
having
thereon a protective coating comprising a fluorinated poly(arylene ether) as
aforesaid. In still
another embodiment, the electronic article is a circuit board in which the
substrate is a fluorinated
2s poty(arylene ether).
Fig. 7a depicts a mu~ichip module having a muftilayer interconnect in which
the interlayer
3o dielectric is a fluorinated poly(arylene ether) of this invention. Fig. 1b
shows in cross-section the
muhllayer interconnect.
Fig. 2 shows in cross-section an integrated arcuit chip having thereon a
multilayer
interconnect in which the interiayer dielectric is a fluorinated poly(arylene
ether) of this invention.
WO 91/09071 O ~ ~ ~ ~ ~ PtrT/US90/07204
4
Frg. 3 shows in cross-section an integrated arcuit chip protected by a coating
of a
fluorinated poly(arylene ether) of this invention.
Fg. 3a shows in cross-sect'ron a arcu'tt board in which the substrate is made
from a
fluorinated poly(arylene ether).
Fg. 4 compares the dielectric constants of polymers of this invention and of
comparison
polymers not according to this invention.
to Fig. 5 shows a substrate carrying a plurality of multilayer devices having
as an interlayer
dielectric a crossknked fluorinated poly(arylene ether) of this invention.
t5 The fluorinated poly(arylene ethers) of this invention can be made by the
condensation
polymerization of a diphenol (I) with a fluorinated monomer (II):
X9
H-W-H + F ~ ~ -~- w ~ ~ + z HF
'F4.ct n ~..F4-q n
(I) (B)
In the equation above, -W-, -X, q, and n have the same meaning as defined
earner.
Suitable diphenols (I) inckrde 4,4'-(hexatluoroisopnopyndene)drphenol, 4,4'-
isopropybdene-di(2,6-
dimethylpheral), 4,4'~(1-phenylethyidene) bisphenol, 4,4'-
isopropyWdenediphenol, 9,9'-bis(4-
hydroxyphenyl)fluorene,1,5~iihydroxynapthalene, 2,7~ihydroxynaplhalene,
resorcinol, and 4,6-
dichbroresorcinol, corresponding to fluorinated poly(arylene ether) repeat
units in which -W- is:
H3C CH3
~3
-O ~ ~ C ~ ~ O , -O ~ ~ C
t'y'F3 CH3
H3C CH3
_ CH3 CH3
O ~ / C ~ / O ' -O ~ / C ~ / O '
Ct>H5 CH3
WO 91/09071 PCT/IJ590/07204
207U~3~
wl wl _
-o / \ ~ ~ o_ .
\ /
/ \
o i ~ ~
w i \ l
-o ~ o- _o I ~ o_
I , or -
C1 ~ C1
Preferred diphenols (I) inck~de 4,4'-(hexalluoroisopropypdene)c~phenol, 9.9'-
bis(4-hydroxyphenyl)-
fluorene, and 1,5-dihydroxynaphthalene.
Suitable fluorinated monomers (II) inckide hexafluorobenxene.
decafluorobiphenyl,
pentalluorobenzene, octalNiorotoluene,1,4-~bromotetrafluorobenzene,
chbropentatiuoro-
. benzene. allylpenfatluorobenzene, and 2,2'.3.3',5,5',8,8'-
oGafworobiphenyl.oorresponding to
IIuoAnated poly(arylene ether) repeat units in which
\I
F~
is
p F F F F F F3
-I1
H
F F F
Br F
\ / ' ~ ~~~ ' ' ,
F2 Cl ~2~°~2
BI
WO 91/09071 ~ ~ ~ ~ PCT/US90/07204
6
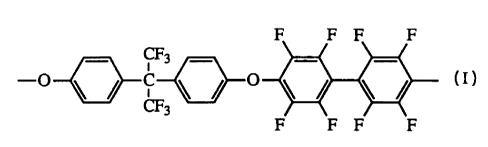
or H~! ~ ~ I~~ .
~F3 F3
Preferred fluorinated monomers includes hexafluorobenzene and
decafluorobiphenyl.
s Contrary to what has been taught in the art, it has been discovered that
complete fluorine
substitution of the aromatic ring in monomers (II) is not necessary for
effective polymerization,
monomers such as pentafluorobenzene, odafk~orotoluene,1,4-
dibromotetratluorobenzene, and
chloropentafluorobenzene being suitable.
to The two monomers are used in substantially stoichiometric amounts if high
molecular
weight polymer is desired. Aitematively, 'rf bwer molecular weight material is
desired, for example
to faalitate the preparation of solutions for spin or other solvent coating
operations, a slight
stoichiometric excess of either monomer can be used to control the molecular
weight.
t5 A base such as an alkali metal carbonate, bicarbonate, or hydroxide is
added to the
polymerization mixture to convert the phenoxy groups to the corresponding
phenoxides. Sodium
and potassium carbonate are preferred. A polar aprotfc solvent, such as N,N-
dimethylacetamide,
N,N-dimethyitormamide, or 1-methyl-2-pyrrolidinone is used. The use of such
solvents is r
advantageous compared to other solvents such as nitrobenzene, which are more
toxic and which
2o are not soluble in water, thereby requiring work-up of the polymerization
mixture in an organic
solvent as opposed to water. The reac8on is carried out at an elevated
temperature, although
such temperature should not be excessively high. A temperature between about
50 °C and about
125 °C is generally suitable, with a temperature between about 60 and
about 90 °C being
especially preferred. Reaction times are typically between about 10 and about
72 hours.
The folbwing repeat units are preferred:
F F F F
~3
~ / ~ ~ / ~ / (A)
F F F~F
F F
~3
~3
ao F F
WO 91/09071 PCT/US90/07204
2~'~0~~6
H3C , CH3 F F F F
~3
I - -
~~ / ~ ~ / ~
~3 F 'F F
(D)
F F
F F
F3
~3
~ ~
H
F3
O (G)
~3
(H)
WO 91/09071 PCT/US90/07204
2~7~~36
s I ~ I _.
I
-° \ / c \ / °~ ~ (I)
i
ci
-° \ / ~ \ / °~ ~ ('')
cH2c~I=c~I2
~3
-° t\ / C \ /
cF3 H~~ /
F3 F3
(L)
F F F F
~3
° \ / ~ \ / \ / \ / ("")
~s
F F F F
io
F F F F
_° ~3 / \ ~3 Q (M')
\ / t~t \ / \ / \ /
~3 ~3
F F F F
The polymers can be homopolymers, consisting essentially of a single repeat
unit such
as one of the aforementioned ones. Or, they can be copolymers comprising a
repeat unit of this
~5 invention in comf~ination with another repeat unit o1 this invention or
with a different type of repeat
unit. Fluorinated poly(arylene ether) copolymers can be made for example by
using two different
diphenols (I) as comonomers, or two different fluorinated monomers (II) as
comonomers. A
preferred copolymer comprises repeat units (A) and (N):
WO 91/09071 PCT/US90/07204
9
F F F F
O ( \ O ~ ~ ~ / (N)
F F F F
Another preferred copolymer comprises the repeat units (A) and (D). Yet
another
preferred copolymer comprises repeat un'tts (A) and (O)
F F F F
(O)
F F F F
Still other preferred copolymers comprise repeat unit (A) and either repeat
unit (P) or (O) or
repeat unit (D) with repeat unit (O):
to
F F F F
O I ~ O ~ ~ ~ ~ (P)
F F F F
F F F F
)
-O ~ ~ F F F F
15 In a copolymer where a repeat unit of this invention is combined with a
repeat unit of
another type of polymer, it is preferred that at least 60 mole %, more
preferably at least 80 mole
%, of the repeat units area fluorinated aromatic ether repeat unit according
to this invention. A
capolyrrrer can be altematirp, random, or block.
2o Fig.1a shows a multlchip module 1 of this invention. Substrate 2, typically
made of
silicon, glass, or ceramic, supports high density mulUlayer interconnect 3 in
which the dielectric
material providing insulation between the various layers is a fluorinated
poly(arylene ether). On
interconnect 3 are mounted semiconductor chips 4a-d, which are connected to
each other by
electrical conductors in interconnect 3. Substrate 1 may also contain
electrical conductors, for
25 example for power and ground. Lead frames 5 (only one labeled for
simplicity) provide
connections to external arcuitry.
Fg. tb shows a partial cross-section of muftilayer interconnect 3 supported on
substrate
2. layers of electrical connections t0a-c are separated from each other by a
fluorinated
CA 02070836 2001-04-18
t
WO 91 /0907 t ~ PCT/US90/07204
poiy(arylene ether) dielectric 12. Via 11 provides connectic>ns between the
various layers as
necessary. Interconnect 3 is connected to an integrated circuit chip (not
shown) by bond pad 13.
Via 1 i is shown here in the stacked pillar design, although it is to be
understood that other
designs conventional in the art, such as the stair-stepped or nested via
designs, can be used.
5 Other muitichip module designs in which the fluorinated po~(aryiene ethers}
of this invention can
be used as interlayer dielecirics is disclosed in Balde, 'Overview of
Muftichip Technology";
Electronic Materials Handbook, vol. 1, Packaging ASM International, p. 297-312
(1989),
1 o The fluorinated poly(arylene ethers} can also be used as interlayer
dietectrics in an
interconnect associated with a single integrated circuit chip. Fig: 2 shows
this embodiment in
cross-section. Integrated arcuit chip 15 has on a surface thereof plural
layers 16 of poly(arylene
ether} dielectric and multiple layers of metal conductors 17.
i s The fluorinated poly(arylene ethers) of this invention can further be used
as protective
coatings on integrated crcuit chips, for protection against alpha particles.
Semiconductor devices
are susceptible to soft errors when alpha particles emittedl from radioactive
trace contaminants in
the packaging of other nearby materials strike the active surface. Fg. 3 shows
schematically an
integrated circuit having a protective coating of f~rorinatecl poly(aryiene
ether): Integrated circuit
2o chip 25 is mounted on substrate 26 and held in ptaae with the assistance of
adhesive 27. A
coating of fluorinated poly(arylene ether) 28 provides an alpha particle
protection layer for the
active surface of chip 25. Optionally, additional protection is provided by
erxapsutant 29, made of
for example epoxy or silicone. Conductor 30 provides connections between chip
25 and
conductors (not shown) an substrate 26 and thence to external arcuiiry.
The fluorinated poly(aryiene ethers) can also be used as a substrate
(dielectric material)
in circuit boards (also referred to as printed wiring boards or PWB's). Figure
3a shows in cross-
section a circuit board 35 made of a substrate 36 having on a surface thereof
a pattern of
conductors 37. Substrate 36 is made of a fluorinated poty(arylene~ether) of
this invention.
Substrate 36 may be reinforced with woven noncondfuairg fibers, such as glass
Both. Although in
Figure 3a the cirwit board is shown as single sided, those skilled in the art
will appreciate that
other constructions, such as double sided or multitayer, can also be made with
a f~orinated poly-
(arylene ether} substrate.
3s Films or coatings of fluorinated poiy(arylene ethers) can be formed by
solution techniques,
such as spraying, spin coating, or casting; with spin coatic~g being
preferred. Preferred solvents
are 2-ethoxyethyl ether, cyclohexanone, N,N-cfimethylfonmamide. N,N-
dimethytacetamide, methyl
isobutyl ketone, 2-methoxyethyl ether, 5-methyl-2-hexan'~ne, y-butyrotactone,
and mixtures
thereof. Typically the coating thidkness is between about 3 to about t5 p.
Additives can be used to enhance or impart partiicular target properties, as
is
conventionally known in the polymer art; including siatciii,zers, flame
retardants, pigments,
plastidzers, surfactants, and the like. Compatible or non-compatible polymers
can be blended in
to give a desired property.
WO 91/09071 PCT/US90/07204
2~70~~~
Polymers for electronic applications desirably contain k~r levels (generally
less than 20
ppm) of ionic impurities. If a polymer is made by a synthetic route which
requires the use of a
tran,Sitiort metal reagent or catalyst, the effed'rve removal of transition
metal residues may be a
ditficuR task. An advantage of the install polymers is that they can be made
by a route which
does not involve transition metal species, and the potassuum (or sodium)
carbonate reagent and
potassium (or sodium) fkioride by-product can be easily removed.
The fluorinated poly(arylene ethers) show good high temperature stability. For
example,
polymer (A) shows by TGA an initial weigh loss in air at 500 °C.
However, they also unexpectedly
t o crosslink when heated in air at temperatures above 300 °C, as
sftown by the torrnation of large
amounts of gelled material. Preferably, the crossliNdng temperature is between
about 300 and
about 425 °C. It appears That the presence of oxygen is required for
the crosstinking reaction to
take place, as similar heating cycles in nitrogen do not lead to the formation
of gel. This
characteristic makes the fluorinated poly(arylene ethers) particularly useful
in the hereindescribed
15 electronic appNcations, because they can be really applied from solution,
and then be converted
into a solvent resistant coating by heating in air.
The fluorinated poly(arylene ethers) can also be crossanked by bistriazene
compounds at
the formula
Rt N-N=N~~I r ~='/N=N-NRs
I I
I'~d.r Hd-r
wherein
-R~ , -R2, -R3, and ~R4 are independently -H, -CgHS, -CgH4Y', or C~-C4 alkyl;
-R5- is -O-, -S02-, .
~3
O / ~ / O ~ O ~ / C ~ / O-
v v
3
p CF3
-O ~ / S ~ / O- ~ -O ~ ~ C ~ / O- ,
~ ~3
Br Br Br
-O ,_~_ _~~ O- -O ~I_
/ ~~ . ~/ o_ ,
I I i
F4.r Fd.r F4.r
WO 91/09071 t'CT/US90/07204
20'~0~36
-° \ / \ / °- , ~ s ~ ~ , -
l \ i i
\ /
°\ / °- '
-B is -F, -CI, -Br, -CHg, or -CFg; r is 0,1, 2, 3, or 4; and -Y' is haiogen, -
NCB, -CgHS, or Ct-C4
alkyl.
Preferably, each of -R~, -R2, -Rg and -R4 is methyl and r is 0. Also
preferably, -R5- is
~3
-o \ / \ / O- or ° \ / C \ / O-
,o
It is also preferred that the tMStriazene groups be located para- to the -R5-
group.
Particularly preferred bistriazene crosslinldng agents are
and
These bis~rtazene crosslinking agents can be prepared by treating a solution
(in a solvent
such as tetrahydroturan or methanol) of a c~amine of the formula
H2N~/=) r ~=~/NH2
H4-r H4-r
wherein -RS-, -B, and r are as defined hereinabove, with hydrochloric acid
(added gradually, with
stirring). Theri a solution of sodium nitrite is added gradually, with cooNng.
After a reaction perior
of at~out 1 hour, the solvent is removed under reduced pressure. The residue
is neutralized to pH
6-7 and treated with the dihydrochloride of a diamine such as dimethylamine.
WO 91/09071 ~ ~ ~ ~ ~ ~ ~ pOI'/US90/07204
13
The bistriazene crosslinking agent is used in an amount effective to cresslink
the
fluorinated polymer, preferably between about 10 and about 40, more preferably
between about
15 and about 30 weight %, based on the combined weights of the polymer and
bistriazene
s compound. The fluorinated poly(arylene ether) and the bistriaaene compound
are intimately
mixed, preferably by solution mixing. A film of the mixture is fom~ed, for
example by spin coating,
and the solvent is removed. Crosslinking is effected by heating to a
temperature above the
decomposition temperature of the bistriazene compound, typically between 300
and 400 °C,
optionally with a stepped or stagewise heating profile, typically for between
about 15 and 90
~ o minutes total time.
It is beNeved that, when heated up to or above a threshokl temperature, the
triazene
groups decompose to form phenyl radcais. These then insert into aromatic
groups in the
fluorinated poly(arylene ether) to form aryl-aryl cross~nkages, as illustrated
by the folbwing
15 equations: , ,
Me' ._ Me
M6 N ~ ~ 5 ~ ~ ~ a
2 P
~~Rs~ ~ + 2 NZ + 2 ~NMe2
PO = rest of polymer
chain
P ~ s ~ p + 2 HNMez
As a matter o1 convenience, in the equations the trlazene groups have been
depicted as
decomposirp simultaneously to give a d~radical. H is possible, if not pkely,
that the decomposition
is not enUrely simultaneous, so that monoradicals are also fom~ed, which,
however, would react in
a similarlaafaort, albeit sequentially. A noteworthy aspect is that the
cross~nks are via aryl-aryl
bonds. Compared to their aNphatic oounlerpaAs, these are much less vulnerable
to
thertnooxidaUve or other chemical attack and hence stabler.
3o Another method of crossiitwdng fluorinated poly(arylene ethers) is with a
peroxydic
compound, such as dicumyl peroxide, cumene hydroperoxide, or benzoyl peroxide.
An intimate
mixture of the polymer and the peroxydic comound is heated to a temperature of
between about
350 °C and about 425 °C, preferably about 400 °C, under
nitrogen. Typically, the peroxydic
compound is used in an amount of between about 5 and about 20 % by weight,
based on the
combined amounts of polymer and peroxy~c compound, with about 10 wt. % being
preferred. In
some fluorinated poly(arylene ethers), peroxide crosslir>klng may be
faci~tated by their
possessing reactive side chains, such as the allyl groups in polymer (J),
although the presence of
such tunctiona~ties is not required for effective peroxide crossrnking.
WO 91/09071 ~ ~ ~ ~ ~ ,~ ~ PCT/US90/07204
14
Fluorinated poly(arylene ethers) are also useful as adhesives and matrix
resins for
composite applications. Further, they are also useful as solvent resistant,
cross~nked films for a
variety of appications, such as wires having a wrapped insulation, espeaally
after crossGnking.
The practice of our invention can be further urxierstood by reference to the
foibwing
examples, which are provided by means of illustration, not imitation.
E~m~ 1
1 o This example describes the preparation of a polymer having repeat unit
(A): To a 500 mt_
round bottom tlaisk was added 15.01 g (x.0447 mole) of 4,4'-
(hexafluoroisopropyGdene)diphenol
('8F-diphenol~,15.29 g (0.0458 mote) of decalkarobiphenyl, 240 g of
dimethylacetamide
('DMAc'~, and 16.85 g (0.125 mole) of potassium carbonate. The mixture was
heated with stirring
under r>itrogen at about 80 °C for 23 hours. The mixture was filtered
hot to remove the unreacted
t 5 potassium carbonate and potassium fluoride by-product. About 75 mt_ of
DMAc was removed by
rotary evaporatbn. The solution was cooled to room temperature and poured into
water to
precipitate the polymer. The polymer was filtered, washed three times with
water, suspended in
200 mL of ethanol for 2 hours, fihered, and dried at 100 °C for 2 hours
to yield a white powder. A
solution of 2 grams of polymer in 8 grams of a 50/50 mixture of 2-ethoxy ethyl
ether and
2o cycbhexanone was spin coated onto a ceramic substrate and dried 15 minutes
at 100 °C, 20
minutes at 180 °C, end 45 minutes at 400 °C. The resul~np
polymer film was tough and flexible,
insoluble in 2-ethoxy ethyl ether, and had a Tg of 189 °C by DSC (192
°C by TMA).
~Cem~l9 2
This example describes the preparation of a polymer having repeat unit (B): To
a 100 mL
round bottom flask was added 2.20 g (0.0118 mole) of hexafluorobenzene, 3.90 g
(0.0116 mole)
of 6F-diphenol, 4.0 g (0.030 mole) of potassium carbonate, and 50 g of DMAo.
The mixture was
heated with stirtfng under nitrogen at about 70 °C for 48 hours. The
mixture was then worked up
3o as descrbed in Example 1 b yield a white powder. A film of the polymer
obtained was tough and
flexible, ir>8oluble in 2-ethoxy ethyl ether, and had a Tg of about 185
°C by DSC.
This example describes the preparaUon of a polymer having repeat unit (C): The
reaction
of F~cample 1 was repeated except that 12.7 g of 4,4-isopropyliderte bis(2,8-
dimethylphenol)
(~tetramethyl Bispheral A'~ was used in place of the 6F-phenol and the
reaction was heated to
80 °C for 72 hours. 22.3 g of polymer was obtained. A film of the
polymer had a moisture
absorpUon of 0.15% alter immersion in 50 °C water for 16 hours.
E~RIe 4
This example describes the preparation of the copolymer having repeat units
(A) and (N):
The reaction of tacample 1 was repeated except that a mixture of 7.51 g of 6F-
diphenol and 2.458
g of resorcirwl was used in place of the 6F-diphenol. 19.8 g of polymer was
obtained. A film of the
polymer had a moisture absorption of 0.10% after immersion in 50 °C
water for 16 hours.
wo 91/0907t ~ o ~ o ~ c~ ~ PCT/US90/07204
The polymer having the repeat unit (D) was prepared as follows: To a 250 mL
round
5 bottom flask was added 10.15 g (0.029 mole) of 9,9~bis(4-
hydroxyphenyl)fluorene, 9.97 g (0.0298
mole) of decafluorobiphenyl,115 g of DMAc, and 10.0 g (0.074 mole) of
potassium carbonate.
The mixture was heated with stirring under nitrogen at 75 °C for i 6
hours. The mixture was
cooled to room temperature, poured into rapidly stirring water to preapitate
the polymer, filtered,
washed twice with water, flitered and dried. A white fluffy powder was
obtained. Two grams of the
1 o white polymer powder were ckssolved in 8 grams of a 50150 mixture of
cydohexanone and 2-
ethoxy ethyl ether. About 1.5 mL of the polymer solution was spin coated onto
a glass substrate
and dried 10 min at 100 °C,15 min at 200 °C, and 30 min at 400
°C. The resulting polymer film
was released from the glass substrate by irtwnersion in water to yield a
tough, flexible, transparent
film. The film had a electric constant of 2.62 at 0 % RH and a dielectric
constant of 2.68 at 58
15 RH. The polymer had a Tg of about 258 °C by DSC.
fi
This example describes the preparation of the polymer having the repeat unit
(E): The
2o procedure of Example 5 was repeated, except that 5.54 g (0.0298 mole) of
hexafluorobenzene
was used in place of the decatluorobiphenyl and the reaction was allowed to
run for 42 hours.
The resulting polymer film had a dielectric constant of 2.65 at 0°~ RH
and of 2.73 at 58 % RH.
F~camr?le Z
This example describes the preparation of the copolymer having repeat units
(A) and (D):
To a 250 ml. round bottom flask was added 5.07 g (0.0145 mole) of 9,9-bis(4-
hydroxyphenyl)tluorene, 4.87 g (0.0145 mole) of 8F,~diphenol, 9.97 g (0.0298
mole) of
decafluorobiphenyl,115 g of DMAc, and 10.0 g (0.074 mole) of potassium
carbonate. The mixture
3o was heated with stirring under nitrogen at 75 °C for 16 hours. The
mixture was cooled to room
temperature, poured into rapidly stirring water to predpitate the polymer,
filtered, washed twice in
300 mL of water, li8ered and dried. A white fktfty powder was obtained. Two
grams of the white
polymer powder were dissolved in 8 grams of a 50/50 mixture of cyclohexanone
and 2-ethoxy
ethyl ether. About 1.5 mL of the polymer solution was spin coated onto a glass
substrate and
dried 10 min, at 100 °C,15 min, at 200 °C, and 30 min. at 400
°C. The resulting polymer film was
released from the glass substrate by immersion in water to yield a tough,
flexible, transparent film.
The film had a dielectric constant Of 2.60 at 0 % RH and 2.68 at 58 % RH.
This Example describes the preparation of a polymer having repeat unit (F). To
a 100 mt.
round bottom flask was added 3.50 g (0.0208 mol) of pentafluorobenzene, 7.00 g
(0.0208 mot) of
6F-diphenol, 4.2 g of potassium carbonate, and 50 g of DMAc. The mixture was
heated to 80 °C
for 24 hours under nitrogen with stirting, then heated to t 20 °C for
an additional 36 hours. The
mixture was allowed to cool to room temperature and poured into water to
precipitate the polymer
as a lightly colored powder. The polymer was washed three times with water and
dried at room
WO 91/09071 PCT/US90/07204
,s 20'~0~?6
temperature for 18 hours and at 100 °C for 4 hours. One gram of polymer
was dissolved in 4
grams d a 1:1:1 mixture of OMAC, 2-ethoxy ethyl ether, and cycbhexanone. The
mixture was
spin coated on to a glass substrate and erred 15 min at 100 °C, 15 min
at 200 °C, and 15 min at
400 °C to yield an amber film. The polymer had a moisture absorption of
0.150. Based on model
studies with similar fluorinated benzenes, discussed in rtare detail bebw, and
the expected
mechanism for the polymerization reaGion, it is believed that in the
pentafkrorobenzene two
tluorines are cosplaced, with the hydrogen being retained. Polymer (F) had a
Tg of 120 °C by
osc
to
This example describes the preparation of a polymer having repeat unit (G).
The
procedure in Example 8 was repeated except that 4.99 g (0.0211 mol) of
odafluorotoluene was
used in place of pentafluorobenzene and 7.38 g (0.0211 mol) of 9,9-bis(4-
hydroxyphenyl)tluorene
15 was used instead of the 6F-diphenol. The reaction was run at 80 °C
for 24 hours and then at 120
°C for an additional 24 hours. A white powder was obtained. Again, it
is believed that two ring
fluorines are displaced, with the trttluoromethyl group remaining intact. The
polymer had a Tg of
260 °C by DSC.
20 iQ
This Example describes the preparation of a polymer having repeat unit (H).
The
procedure in Example 9 was repeated except that 6.40 g (0.0208 mol) of 1,4-
dibromotetralluorobenzene was used in place of ocfafluorotoluene. A white
powder was obtained.
25 One gram of the powder was dissolved in 4 grams of DMAc and spin coated on
to glass substrate
and cured as described in Example 8 to yield an amber flim. The polymer had a
dielectric
constant of 2.8 and a moisture absorplbn of 0.15%. Hs Tg was 199 °C as
measured by DSC.
CiC-MS analysis of the products from the model rescoon between phenol (2
ecMivalents)
splaced with the two
3o and 1,4-dibromotetraikiorobenzene showed that two fiuorines were di ,
brominss beirp retained and a mixture of isomeric products being obtained.
Thus, it is believed
that in poiyrrrer (H), the two bromines were also retained.
a
This Example describes the preparation of a polymer with repeat unit (i). To a
100 ml.
round bottom flask was added 5.05 g (0.0249 moQ of chbropenlafluorobenzene,
9.10 g (0.0260
mol) of 9,9-bis(4~hydroxyphenyl)tluorene, 65 g of DMAc, and 11.5 g of
potassium carbonate. The
mixture was heated 10100 °C for 27 hours under nitrogen with sorting.
The mixture was allowed
4o to cool to room temperature and poured into water with stirring to
predpitate the polymer. The
polymer was washed with three times with water and dried at room temperature
for 18 hours and
at 100 °C for 5 hours to yield a whHe powder. Two grams of the polymer
were dissolved in 8 m~
of a 1:1 mixture of 2-ethoxy ethyl ether and cycbhexanone, spin coated onto a
glass substrate
and dried as described in Example 8. An amber film was oMained. The polymer
had a moisture
absorption of 0.1 %.
WO 91/09071 PCT/US90/07204
17
CC-MS analysis of the product from the model reaction between phenol (2
ec~ivalents)
and chbropentafluorobenzene showed that two fluorines were displaced, w'tth
the chbrine being
retained and a mixture of isomeric products being obtained. Thus, it is
bekeved that, in polymer
(I), the chlorine was also retained.
X18 ~
This Example describes the preparation of a polymer with repeat unit (J). To a
100 mt_
round bottom flask was added 4.20 g (0.0202 mot) of allyipeMafluorobenzene,
6.85 g (0.0204
t o mot) of 8F-dipherwl, 45 ml of OMAc, and 8.0 g of potassium carbonate. The
mixture was heated
to t t 0 °C under nitrogen with stirtirp for 72 hours. The mixture was
aNowed to cool to room
temperature and was poured into water to preapitate the polymer. The polymer
was washed with
100 ml_ of debnized water and 100 mL of denatured ethanol and dried in air for
3 days to yield a
IigM yelbw powder. Three grams of the powder and 0.15 g of t-butyl
peroxybenzoate were
dissolved in 8.5 ml of DMAc and spin coated onto a glass substrate and dried
10 min at 110 °C
and 20 min at 200 °C to yiekf an amber film that was insoluble in OMAc.
GC-MS analysis of the product from the reaction between phenol (2 equivalents)
and
allylpentatluorobenzene showed that two fluorines were displaced, with the
allyl group being
2o retained and a mixture of isomeric products being obtained. Thus, it is
believed that, in the
polymer described above, the aNyl group was also retained.
~R181~
This example describes the preparation of a polymer with repeat unit (K); To a
100 ml
round botbm flask was added 1.25 g (0.0042 mot) 2,2',3,3',5,5',8,8'-
octalluorobiphenyl ("OFB"),
1.41 g (0.0042 mot) of 8F-dipheral,19 g of OMAc, and 2 g of potassium
carbonate. The mixture
was treated to 120 °C for 72 hours under Ntroqert with sUrting. The
mixture was albwed to cool to
room temperature and poured into water b predpifate the polymer. The polymer
was collected by
fiHratbn, washed with 75 ml of a 50/50 mixture of ethanol and water, and dried
over night at room
temperature, folbwed by 1 hour at 100 °C b yield a white powder. The
polymer had a Tg of 147
°C by DSC.
aC/MS analysis of the product from the reaction between 4-methoxyphenol (2
equivalents) and OFB showed that two fHiorines were displaced, with retention
of the two
hydrogens, and a mixture of isomeric products beirp obtained. Thus, it is
believed that, in the
polymer described above, the two hydrogens were also retained.
E~RIB 14
This example describes the preparation of a polymer with repeat unit (L). The
procedure
of Example 12 was repeated with the exception that 8.22 g (0.0202 mot) of 1,4-
dibromotetrafluorobenzene. 7.07 g (0.0202 mot) of 9,9-bis(4-
hydroxyphenyl)fluorene,10 g
potassium carbonate, and 55 mt- of DMAc were used. The polymer was obtained as
a white
powder, Tg 291 °C by OSC.
WO 91/09071 2 0 ,~ o g ~ ~ PCT/US90/07204
18
This example describes the preparation of a polymer with repeat unit (M). To a
250 ml
round bottom flask was added 10.2 g (0.0354 moQ of 4,4'-(1-phenylethylidene)
bisphenol, 11.6 g
(0.0347 moi) of decafluorobiphenyl, 12 g of potassium carbonate, and 135 g of
DMAc. The
mixture was heated to 80 °C under nitrogen with sorting for 16 hours.
The mixture was albwed to
cool to room temperature and poured iMo water to precipitate the polymer. The
polymer was
filtered. washed wRh water, and dried. Two grams of the polymer were dissohred
in 8 g of a
mixture of 2-ethoxy ethyl ether and ctn5ohexanone (ratio 8 : 2, resped'rvely)
and spin coated
onb a glass substrate, and dried 15 min at 100 °C,15 min at 200
°C, and 15 min at 400 °C to
yield a flexible, transparent film. The polymer had a Tg of 208 °C by
DSC and a dielectric
constant of 2.64 at 0 %RH.
Ex~ 16
~.
This example describes the preparation of a copolymer having repeat units (A)
and (O),
in a molar ratio of 1:4. To a 100 ml. round bottom Ilask was added 3.75 g
(0.021 mot) of 4,6-
dichbroresorcinol,1.76g (0.0053 mol) of 6F-diphenol, 8.80 g (0.026 mol) of
decafluorobiphenyl,
62 g of OMAC, and 10 g of potassium carbonate. The mixture was heated under
nitrogen for 8
2o hours at 110 °C. The rtaxture was poured without oootirp into water
to precipitate the polymer.
The polymer was oolteded by flKratbn, washed with water, and dried to yield a
light pink powder.
The polymer had a Tg of 149 °C by DSC.
a
This example describes the preparation of a polymer (referred to hereinafter
as BPA-
OFB) from 4,4'-isopropylidsr>ediphsral and decalWofobiphenyl: The reaction of
6cample 1 was
repeated except that 10.20 g of 4,4'-iaopropyWdsnedfphenol (°8isphenol
A'~ was used in place of
the 6F<liphenol. 21.5 g of polymer was obtained from the reaclbn. A 81m o1 the
polymer had a
3o bulk moisture absorption of 0.2 % atler irtwr>erabn in 50 °C water
for 16 hours.
E~m~ 18
This is a comparative example in which a polymer not according to this
irnention, having
the repeat uNt
O O
_ _ I
O ' / N I / N- (PMDA-ODA)
I - II
O O
is prepared and compared against the polymers of this invention.
CA 02070836 2001-04-18
wo 9vo9oW PCT/US90/07204
19
To a 100 m~ round bottom flask was added 1.80 (0.009 moles) g of 4,4'-
oxydianiline
('ODA"y and 30 m~ of dry 1-methyl-2-pyrroGdinone ('NMP'~. The solution
was~cooled in an ice
bath arxi 2.006 g (0.0092 moles) of pyromellitic dianhydridie (PMDA) was added
with stirting
under nitrogen. A viscous, amber solution resulted. The polymer so~rtion was
spin coated onto a
4 by 4 inch (ca. 10 by 10 cm) glass substrate and dried for 10 min at 100
°G, 15 min at 200 °C.
and 30 min at 350 °G to yield an amber film. The film showed a bulk
moisture absorption of 2.55%
after immersion in 50 °C water for 16 hours.
to
This is another comparative example in which another prior art polyirr>ide,
referred to
herein as Pi7, is prepared and compared against the polymers of this
invention.
O F3C'C~~3 O
~ ~ N-
(/ (/
. O O
(PIE
To a 100 mL round bottom flask was added 3.35 c~ (0.009 mole) of 4,4'-bis(4-
aminophenoxy)biphenyi and 17 g of NMP. After stirring at room temperature
under nitrogen for 45
minutes, a solution of 4.00 g (0.009 mole) of 2,2-bis(3,4-
dicarboxyphenyl)fiexafluoropropane
2o dianhydride ('6FDA") in 14 g of NMP was added dropwise with stirring over
10 minutes. After
stirring an additional 24 hours at room temperature a visa>us sohrtion (2650
cps) resulted. The
solution was spin coated on to a 4 by 4 inch (ca. 10 by 10 cm) glass substrate
at 2000 rpm and
dried 30 min at 100 °C, 20 min at 200 °C, and 30 min at a50
°C to yield an amber film. This
polyimide film showed a bu~c moisture absorption of 0.85°~e after
immersion in 50 °C water for 16
hours. The properties of PI7 are compared against those of polymers of this
invention in Table 1.
below.
Table I compares the dielectric properties of the polymers of this inver~diort
against the
properties of the comparative polymers. The dielectric carrstants were
measured at 25 °C and 10
~Iz by the method described in U.S. Patent No. 5,108,840 of Mercer. -w,
tt can be seen from Table I that floe polymers of .this invention have
sigr>ifrcantly bwer dielectric constants (g), bebw 2.80 at 0 %RH ancf as bw as
2.50, compared to
s's above 2.80, up to 3.16, for the cor~artson potymerslyurther, the ~'s of my
polymers are less
sensitive to variations in the ambient hum'ufrtyr. At about 61) %RH, their e's
increase only slightly.
as evidenced by a smati sbpe of between about 10 and about 30, while the
comparison polymers
have a sbpe ~f between about 60 and about 100. In a mH:roeiedronic article, it
is very important
that the dielectric material have a low e, preferably below 3 in both dry and
wet environments.
These differences between my polymers and the comparison polymers are shown
graphically ~n
Figure 4.
WO 91/09071 ~ ~ ~ a ~ ~ ~ PL''1'/US90107204
TABLE
1
Dielectric
Constant
of
Polymers
-
Dielectric Constanta at 25 C and
10 KHz
Ex. PO mer ( a[~ O~o RH) ev, (%RH) SIO
a
'
1 A 2.504 2.62 65.3 17.8
2 B 2.50 2.63 72.3 18.0
3 C 2.78 2.89 65.1 16.9
4 A/N mer 2.62 2.68 54.1 11.1
5 D 2.62 2.68 58 10.3
6 E 2.65 2.73 58 13.8
7 AID co mer 2.60 2.66 58 10.3
8 BPA-DFB 2.617 2.787 65.3 26.1
9 PMDA-ODA 3.16 3.76 58.2 103.2
10 PI-7 2.85 3.223 59.4 63.0
. Sbpe ~ ewet - edrv x 10,000
%RH (Wet)
This example describes the preparation of a mufGlayer high density
interconnect article
suitable for use in a multichip module or in combination with a single
integrated circuit chip.
A solution of 22.5 g of polymer (A) in 77.5 g of a 50/50 mixture of 2-ethoxy
ethyl ether and
cycbhexanone was prepared. A polymeric insulating layer was applied to a
ceramic substrate in
1 o the tollowirp manner: (1 ) a 5 mL aliquot of the polymer solution was spin
coated onto a clean 125
mm diameter ceramic substrate. (2) The coatitp was dried at t00 °C for
15 min and at 200 °C for
another 15 rNn. (3) A second 5 mL aliquot was applied and cured as above with
an additional
cure for 60 min at 400 °C. A conductive material was formed by blanket
sputtering 200 A of
chromium followed by 5 miaons of copper, and finally another 500 b of
chromium. The metal was
15 photo~thographically patterned.
A aeoortd polymeric insulating layer was applied over the patterned metal
layer by the
three~atep process described above. An aluminum metal layer was blanket
sputtered onto the
dielectric, photolithographically patterned, and vias were generated in the
second dielectric layer
2o so that electrical contact could be made with the first metal layer. The
aluminum layer was
removed. A second metal layer of chromiumcopper-chromium was applied as
described above
and electrical contact was made with the first layer by means of the vies. The
second metal layer
was then also photoAthographically patterned.
All metal layers in the device showed excellent adhesion to the fluoropofymer
and there
was no observable delamination of metal to polymer or polymer to polymer.
WO 91/09071 2 ~ ~ O ~ ~ ~ PCT/US90/07204
21
A sample of polymer (A) was aossGnked by curing in air, for the times and at
the
temperatures indicated in Table II, below, to produce the indicated gel
contents (detemnined by
Sohxlet extraction with DMAc for 24 hrs):
Table II
Crosslinking of Polymer (A)
300 30 55
400 15 79
400 30 80
400 60 86
However, when polymer (A) was cured in nitrogen for t3 min at 300 °C
and then 27 min
at 400 °C, no gel was detected.
to
This comparative example demonstrates the preference for f~orination in the
bisphenol
moiety for the preparation of fluorinated poly(arylerte ethers) having
enhanced ihertnal stability.
The thermal stability of polymer (A) (derived from 8F-diphenol and having two -
CF3 groups) was
compared to that of the polymer BPA-OF8 (derived Irom Bispheral-A and
consequently having
two -CH3's in the oorreaporxiirp position) by thentagravimetrlc analysis
(TQA), under isothermal
conditions in air. The resuas are provided in Table III.
Table III
CompaAson of Themtal StabiAty
After 3 hr at
0 °C 2.5 3.0
~ 425 °C 5.0 20.0
This example describes the deposition of layers of polymer (D), crosslinked
with a
bistriazene crosswnWng agent. A solution of polymer (D) (about 23 weight per
cent sods) in a
solvent system of 1:1:1 bis(2-ethoxy ethyl)ether, DMAc, and 5-methyl-2-
hexanone (WJW/W) was
prepared. To this was added 16.7 weight % of the bistriazene (1)
WO 91/09071 ~ ~ ~ ~ ~ ~ ~ PCT/US90/07204
22
This sokatbn was then coated oMO a substrate (ceramic or silicon) by spin
coating. The coated
substrate was heated in a nitrogen purged oven having a conveyor bait which
ran the substrate
through the oven axordng to a temperature profile of 300 °C for 6.5
min, 400 °C for 13.5 min,
and then cooNng to room temperature over 20 min. This procedure produced a
clear coating of
cross~nked polymer (D) which dd not crack upon subsequent processing (e.g,,
during the
deposition of addtbnal polymer layers) and did not cause oxidation of metal
conductors thereon.
1o It was found that, to improve the adhesion of the crossinked polymer (D) to
the substrate,
it is desirable 1o use a thin layer (about 1 p thick) comprising acetylene
terminated polyin>ide
(Thermid IP-615) and ~raminopropyllrimethoxysilane coupling agent, between
polymer (D) and
the substrate as an adhesion promoting inteAayer. The polyimidelooupling agent
layer is
deposited onto the substrate and cured at 150 °C for 10 min and then
200 °C for 15 min. The
polymer (D) layer is then coated on top and cured as described above.
This example describes the various steps of metallizatbn, patterning, etching,
and via
2o formation on a substrate coated with polymer (D) as described in the
previous example.
Metal condudor traces were deposited on a cured polymer (D) coatirp by
sputtering. The
conductor was a chromium-oopper~chromium sarxlwich, with 200 Jl thick layers
of chromium
ailing as tle-down layers for the copper (5 w thick). This oondudor
construdion is preferable 10
the more conver>tional aluminum, which does rat adhere as well to crosslinked
polymer (D).
The metal was covered with a ph~oresist, which was then covered with a mask
and
exposed ~ ulraviolet light. The exposed portions of the photoresist were
rertaved by washing
with sodium hydroxide sokrbbn, IeaNng poAlons of the metal exposed. The
exposed metal was
3o removed by etcftirp with CRE-473 (tradename for a hydrochbric add etchant,
available from
T~ar>ser~) and ferric chbrlde to remove respectivvely the top chromium layer
and the copper layer.
The bolbm chromium layer was etched away with CE 8001~N (tradename for a ceric
ammonium
nitrate-nitric add etd>aM, available irom Chemlech Industries). Laser ablation
can also be used
for rertaving the bottom chromium layer, but CE 8001-N is prelerred because n
is fasterand less
harsh on the polymer.
After etching of the metal, the unexposed photoresist was removed by iboding
the entire
substrate wafer with ubraNolet light and devebping otl the remaining
photoresist w'tth sodium
hydroxide. An alternative method is to strip off undevebped photoresist with
a~ 7:3:1 (VNN)
ao mixture of NMP, debnized water, and methanol.
The patterned metal is overcoated with more crosslinked polymer (D). A metal
layer or
via mask about 3 p thick is sputtered oMo the polymer coating and
photo~thographically
patterned as described above, to forth holes in the metal where vias are
desired. The entire water
WO 91/09071
23 ~ ~ ~ ~ ~ '~ ~j PCT/US90/07204
was ablated with a 308 nm laser, with polymer being removed wherever there was
a hole in tfie
metal until bottom metal was reached. The mask was then removed by etching -
(To avoid etching
the metal conductors abng with the via mask, the via mask should be made of
dfferent,
selectively etchable metal, such as aluminum).
Using the above procedures, a substrate wafer cartying a plurality of
muftilayer units was
prepared. This substrate and the units thereon is shown schematically in Fg. 5
(where kke
numerals depict ike elements). Substrate 40 has thereon a plurality of
muttilayer units 41 (also
shown in magnified ovefiead and crosssedion views). Each unit 41 has layers of
metal
t o conductors 43a and 43b isolated by a dielectric 42 of crossliNced polymer
(0). has 44a and 44b
provide inlerfayer connectivity. Each unit 21 can be viewed as a parallel
plate capaator. Twenty
units 41 were tested by measuring their capadtances. Each had a capaatance
which agreed with
that predicted by the ecpration
t s C ~ DEpA/l
where C is the capaatance. D is the dielectric constant of the polymer
iMerlayer, eo is the
permittivity of tree space, A is the area of the capaator plates, and L is the
distance separating
the capaator plates. (The distance between the capadtor plates (i.e., the
layers of conductors)
20 was determined to be 35 p by scanning electron microscopy.)
In this example, the dielectric properUes of polymer (D) crosspMced with a
bistriazene in
25 the manner of Example 23 are compared whh those of a benzocyclobutene
("BCB") resin
(XU13005.02L available tram Dow Chemical Company), proposed as a dielectric
for electronic
packaging applications. Capacitors were made from crosspnked polymer (D) and
the BCB resin
aocordirp to the procedure of Example 24. The capacilances of strips of five
capadtors of made
from each polymer were measured as a function of yeRH, before and affer aging.
The results are
3o provided in Table IV.
WO 91/09071 PCT/US90/07204
24 2070836
Table
IV
Comparison
of Aging
Effects
on Dielectric
Constant
of BCB XUt3005.02L
and &striazene
CrossGnked
Polymer
(D)
f3Cf3 Pol mer
D
Aging in Dielectric Dielectric
air
200 C hrs foRH Constant % RH Constant
0 0 2.485 0 2.656
21 2.494 30 2.690
42 2.503 - -
78 2.522 69 2.734
24 0 2.747 0 2.623
34 2.817 34 2.662
71 2.897 71 2.702
96 0 2.891 0 2.649
33 3.008 33 2.687
76 3.152 76 2.737
336 0 3.198 0 2.614
28 3.401 30 2.635
82 3.603 73 2.673
These results show that the dielectric properties of crossHnked polymer (D)
compare
favorably to those of the BCB resin. Although the BCB resin has a lower
initial c~electric constant,
upon exposure to elevated temperatures, as might occur in the course of the
normal service life of
an electronic article, the BCB resin's dielectric canstanl increases at a
fairly sharp rate, with the
increase befrp particularly noticeable at high °~RH's. In contrast, the
dielectric constant of
polymer (D) remains bw, below 2.8 at all aging time-relative humidity
combinations.
F~mg(B 26
to
In this example, the crosslinWng of a variety of fluorinated poly(arylene
ethers) by a
variety of bistriazene crosspNdng agen~s is illustrated.
A sample of fluorinated poly(arylene ether) (2 g) was combined in a 30 mL vial
with
f 5 cycbhexanone (4 g), T-butyrolactone (4 g), bistriazene compound (ca. 0.4
g) and a surfactant
(Fluorad FC-431 from 3M, 2 drops). The mixture was stirred until all the
solids had dissolved. The
solution was albwed to sit until all bubbles formed by agitatbn had dispersed.
A majority of the
solution was deposited o0.a ceramic substrate and spin coated at 250 rpm to
form a thick coating.
The sample was soff-baked at 100 °C for 15 min, then at 200 °C
for another 15 min. The sample
2o was then baked in a nitrogen-purged zone furnace accorckng to the folbwing
cycle: 300 °C for 6.5
min, 400 °C for 13.5 min, and room temperature for 20 min, to yield a
sample of approximately
1.5 g.
This cured sample was removed from the ceramic substrate and divided into
three equal
25 sections. Each section was cut into small pieces and placed inside a pre-
weighed gauze tube.
WO 91/09071 PCT/US90/07204
25 207086
The gauze tube was sealed and re-weighed. All three sections were placed
inside a Soxhlet
extraction apparatus and extracted with DMAc for 24 hr. After drying in a
vaaram oven at 100 °C
ovemigM, the samples were cooled and weighed again to detemwne the gel
oornent. The results
provided in Table V show that bistriazene compounds are generally effective
crosslinking agents
for fluorinated poly(arytene ethers):
Table V
Crosslinfdng of Fluorinated Poly(arylene Ethers)
by &striazene Compounds
Men R ~~ Me
N_N=N-~ s~N=N_IV
Me ~j Me
..(a lane ether)R~ / BistriazenePer cent
~ Gel
D ooMrol 0.00 3.3 0.2
D p, p'_ .O ~ / / o_ 64.1
4.76 0.8
D " 9.1 78.8
1.0
D " 13.04 86.4 t
2.7
p " 16.67 93.7
2.2
p 4.76 46.6 2.4
p, p,_ _o~C~o_
CF'
D ~ " 9.1 62.5
2.4
p 13.04 68.6
t 1.6
D " 16.87 86.6
2.6
p P. p'_ _o~o._ 9.1 87.1
5.8
p " 16.67 94.9
0.7
p P. p'_ _p ~ 16.67 81.2
/ .I / o. 2.1
F, F4
p 16.67 85.7
~ t 0.5
I I
p m, m'_ -S02- 16.67 52.9
t 2.2
A control 0.00 0.8
0.8
A P. P~- -'o 18.67 65.3
~ / / O- 2.9
~x~m~lB ZZ
1 o In this example, fluorinated poly(arylene ether) 0 is cross~nked with a
peroxydic
compound. Samples of polymer D containing 10% of dicumyl peroxide or cumene
hydroperoxide
were heated at 300 °C for 6.5 min and then at 400 °C for 13.5
min under n'ttrogen in an infra-red
oven to produce crosslinked polymer having gel content of 94.0% and 81.4%,
respectively. In
comparison, a sample of polymer D similarly heated in the absence of any
peroxide had a gel
WO 91/09071 ~ n ~ ~ ~ ~ ~ PCT/US90/07204
26
content of only 3.3%. The crosslinked polymers retained their low moisture
absorption and
dielectric constant characteristics. The sample crosswnked with diarmyl
peroxide had a moisture
absorption of 0.2°~ and a dielectric constant of 2.6 at 0% RH.
In additional peroxide crosslinking experiments, polymers (A) and (D) were
each
crossanked with 10 wt. % benzoyl peroxide to produce crosskrwced polymers
having gel content of
about 51 and 49 ~°, respectively. Polymer (A) was also crossYnked with
10 wt. % dicumyl
peroxide to a gel content of 69%. In comparison, a control sample of polymer
(A), similarly
heated in the absence of any peroxide, had a gel content of about 0.8
~°.
to
This example describes the preparation of a polymer with the repeat unit (M').
To a 100
mL round bottom flask was added 3.21 g (0.0093 mol) Bisphenol P, 3.12 g
(0.00934 mol)
decafluorobiphenyl, 4.2 g of potassium carbonate, and 22 g DMAc. The reaction
mixture was
heated at 100 °C for 6 hours under nitrogen with sliming. The polymer
was isolated as described
in Example 5 to yield a white powder. The polymer t>ad a Tg of 162 °C
by DSC. A film of the
polymer had a dielectric constant of 2.58 at 0% RH and 2.71 at 66.45% RH.
This example describes the preparation of the copolymer having repeat units
(A) and (O).
To a 100 mL round bottom flask was added 3.75 g (0.026 mol) of 4,6-
dichbroresorcinol,1.76 g
(0.0052 mol) of 6F-diphenol,10.45 g (0.031 mol) of decafluorobiphenyl,l2 g
potassium
carbonate, and 39 g of OMAc. The reactbn mixture was healed to 110 °C
for a hours under
nitrogen with stirring. The gelled reaction mixture was albwed to cool to room
temperature and
added to water and digested in a blender to isolate an oH-white powder. The
powder was
washed wHh water and dried. The polymer had a Tg of 149 °C by DSC.
~ppp~ ~Q
This example describes the preparation of the copolymer having repeat uMts (A)
and (P)
To a 100 mL round bottom flask was added 5.70 g (0.017 mol) of
decafluorobiphenyl,1.34 g
(0.0083 mon of 2,7-dihydroxynaphthalene, 2.82 g (0.0083 moQ o16F-diphenol. The
reaction
mixture was heated to 90 °C for 18 hours under Ntrogen with sliming and
albwed to cool to room
temperature The polymer was isolated by the procedure described in Example 5
to yield a white
powder. The polymer had a Tg of 190 °C by DSC. A film of the polymer
had a dielectric constarn
Of 2.54 at 0% RH and 2.84 at 65.4% RH.
~,mRle ~1
This example describes the preparation of the copolymer having repeat units
(A) and (C
The procedure described in the Example irtxnedrateiy above was repeated except
chat 1.34 g
(0.0083 mop of 1,5-dihydroxynaphthalene was used instead of 2,7-
s~hydroxynaphthalene. An o.'
a5 white powder was obtained. The-polymrer had a Tg of 203 °C by DSC.
WO 91/09071 ~ ~ ~ ~ ~ ~ ~ PCT/US90/07204
27
This example describes the preparation of a copolymer having repeat units (D)
and (Q)
and its subsequent crosslinking w'tth a peroxide. To a 250 mL round bottom
flask alas added 3.32
g (0.0207 mol) of 1,5-~hydroxynaphthalene, 7.26 g (0.0207 mon of 9,9-bis(4-
hydroxyphenyl)fluorene, 14.22 g (0.0427 mol) of decafluorobiphenyl, t7 g of
potassium
Carbonate, and 127 g of OMAc. The mixture was heated to 85 °C for 16 hr
under nitrogen with
stirring and then poured while still hot into a blender containing 300 mL of
water to precipitate the
polymer. The polymer was collected by filtration and washed twice more with
300 mL of water
t o and dried. Two grams of polymer and 0.22 g of dicumyl peroxide were
dissolved in 8.5 g of a 1:1
mixture of y-butyrolactone and cyclohexanone. The solution was spin coated
onto a ceramic
substrate and cured as follows: 30 min at 130 °C, heat to 400 °C
at a rate of 5 °C/min, hold at 400
°C for 15 min, and cool to room temperature at a rate of 3
°C/min. An amber film was obtained,
which did not stress crack or dissolve when exposed to the aforementioned
~butyrolaclone-
cycbhexanone mixture. A control film of the copolymer, similarly heated under
nitrogen but
without the added of dicumyl peroxide, showed solvent induced stress cracking
when exposed to
the same solvent mixture.