Note: Descriptions are shown in the official language in which they were submitted.
2~7~ 1~2
H~LS ARTI~NG~S~LLSCHAFT - 1 -
- PAT%NT DEPARTMENT - O.Z. 4594
Process for the preparation of L-alkoxy-2-dialkylamino-
ethanes
The invention relates to a process for the preparation of
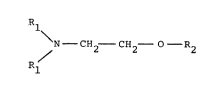
1-alkoxy-2-dialkylaminoethanes of the general formula
S (III) from ~-dialkylaminoethano:L (I) and alkyl hal~de~
F~-X (II)
R~ Rl
N - CH2~ - CH2 - 0~ ~11 - C~2 - C~2 - - Q2
Rl
(~) (III)
Rl = alkyl C1-C10, preferably Cl to C3,
R~ = alkyl Cl-C10, identical to or different from Rl,
preferably C1 to C3, and
X = Cl, Br or I,
characterised in that
a) ~-dialkylaminoethanol (I) is reacted with sodium
alkoxides or potassium alkoxides of simple alcohols,
preferably methanol, with total removal of the
simple alcohol,
b) the dialkylaminoethanol (I) is used in an excess of
0.1 to 30, preferably 5 to 20 mol%, relative to the
alkali metal alkoxide,
c) the total removal of the simple alcohol is carried
out by distillation with inert solvents such as
aliphatic or aromatic hydrocarbons, but preferably
with the 1-alkoxy-2-dialkylaminoethane itself to be
prepared,
d) the reaction of the alkali metal ~-dialkylamino-
ethoxide formed according to a) to c) is carried out
with alkyl halides, preferably with alkyl chlorides,
at elevated temperatures and at atmospheric pressure
or elevated pressure,
e) the distillation of the pure target product
1-alkoxy-2-dialkylaminoethane is carried out with
prior addition of Na/R alkoxide,
f) these Na/R alkoxides according to e) are added in
2~7~ 1~2
23443-480
amounts which are at least stoichiometrically equivalent to the
residual dialkylaminoethanol, but preferably are employed in an
excess of up to 15 mol~,
g) this alkali metal ~-dialkylaminoethoxide remaining
in the bottom of the distillation of the pure product is converted
in the alkylation reaction of the following batches.
l-Alkoxy-2-dialkylaminoethanes have been known for a
long time. They were prepared by reaction of 2-dimethylamino-
ethyl chloride with sodium methoxide. The reaction and working-up
conditions without yield data found therein are difficult and
result in low purity.
According to U. S. Patent 4,588,843, the alkali metal
alkoxide formation from ~-dialkylaminoalkanol is carried out
using NaOH/KOH and the subsequent reaction using alkyl halides.
Working-up from an aqueous phase by extraction with ether is
troublesome and results in only 37~ yield.
The object for the preparation of l-alkoxy-2-dialkyl-
aminoethane was a technically simple process by which the target
product is obtained in high yields and purities.
Thus, according to one aspect, the invention provides
a process for the preparation of a l-alkoxy-2-dialkylaminoethane
of the formula ~III):
\ N - CH2 - CH2 R2 (III)
Rl
wherein Rl and R2 represent a Cl-Cl0-alkyl, which process
comprises:
~7~:~a~
- 2a -
23443-480
a) reacting ~-dialkylaminoethanol of the formula (I):
\ N - CH2 - CH2 - OH (I)
wherein Rl is as defined above, with an alkali metal alkoxide of
a simple alcohol, with total removal of the simple alcohol,
b) reacting the alkali metal ~-dialkylaminoethoxide
so obtained with an alkyl halide of the formula (II):
R2 - X (II)
wherein R2 is as defined above and X is Cl, Br or I, and
c) recovering the l-alkoxy-2-dialkylaminoethane so
obtained.
According to this invention, the process starts from
~-dialkylaminoethanol, a product which is simple to prepare on a
large scale from ethylene oxide and dialkylamines.
This ~-dialkylamino alcohol must be converted
quantitatively into its alkali metal alkoxide, which is carried
out according to the invention using alkali metal alkoxides. The
sodium alkoxides and potassium alkoxides of short-chain alcohols
having 1 to 3 C atoms are preferred, particularly sodium
methoxide. The alkoxide as a solution in the respective alcohol
or alternatively as
2~ 71 l 0~?
- 3 - O.Z. 4594
the isolated salt i~ additionally preferred.
The alkali metal alkoxides are employed in a sub-equiva-
lent amount to the ~-dialkylaminoethanol (I), at most in
stoichiometrically equivalent amounts. The formation of
the alkali metal salt of th~e ~-dialkylaminoethanol
thereby takes place more rapidly and more simply, and no
residual alkali metal alkoxide solution remains, which
would Lmmediately form low-boiling alkyl ethers with the
alkyl halide.
The molar excess of (I) is 0.1 to 30~, preferably 5 to
( 20%, and is later bound in the distillation of the pure
product as alkali metal salt, as i~ still to be
explained.
The difficulty in this process is the total removal of
the short-chain alcohol, i.e. in the quantitative conver-
sion into the sodium ~-dialkylaminoethoxide. Thi~ is
achieved by entraining the methanol by distillation with
an inert solvent, it also being possible for this solvent
to form an azeotrope with methanol, for example toluene,
xylene, alkanes having 5 to 8 C atoms such as hexane,
heptane, cyclohexane or t-butyl methyl ether. Because of
the difficulties of recovering this solvent, i.e. the
separation of the methanol, the target product, i.e. the
respective l-alkoxy-2-dialkylaminoethane is preferably
employed, in particular after the second or third batch.
The use of this not 80 pure final product has the great
advantage that no independent solvent subsequently has to
be worked up, i.e. separated from methanol, and the
purity of the final product cannot be contaminated by
traces of the independent solvent.
Furthermore, the solubility of the alkali metal
~-dialkylaminoethoxide therein is better than in an
extraneous solvent.
2 ~
- 4 - O.Z. 4594
When the suspension of the alkali metal ~-dialkylamino-
ethanol is present, the reaction with alkyl halides, for
example ethyl chloride, methyl bromide and the like, is
subsequently carried out, the alkyl chlorides being
preferred here as higher yields can be achieved with them
than with the alkyl bromides.
The alkyl halides are employed in stoichiometric amount~
or in a slight excess of up to 15 mol%. Excess unreacted
alkyl halide can be returned to the alkali metal salt of
(I) of the following batch by release of pressure. The
reaction is carried out at temperatures from 30 to 160'C,
preferably between 50 and 120C, at normal pres~ure or
alternatively at pressures up to 20 bar. In this process,
the alkyl halide is metered in at the reaction tempera-
ture in a period of 50 to 80 minutes, partly using ametering pump against the pressure of the reaction
solution. The reaction i8 slightly exothermic snd ths
reaction time therefore depends on the size of the
batches and on the possibility of heat dissipation. The
post-reaction time is another 2 to 6 hours.
After the end of the reaction, the alkali metal halide,
for example NaCl, is to be filtered off or all of the
volatile fraction is to be di~tilled off from the ~alt.
~`
Distillation of the pure product follows as the last
working-up step. Unfortunately, the starting material
~-dialkylaminoethanol forms with many solvents such as
toluene and cyclohexane the respective azeotropes which
have solvent contents of only a few per cent. ~ven from
the target product, for example 1-dimethylamino-2-ethoxy-
ethane, ~-dimethylaminoethanol can only be distilled off
in a highly complicated distillation through columns
having a theoretical number of 6tages of more than S0. It
is therefore expedient to lower the dialkylaminoethanol
in the crude target product as far as possible. This is
possible if alkali metal alkoxide corresponding to the
low content of ~-dialkylaminoethanol ~s added and thus
2~71~ ~
- S - O.Z. 4594
the dialkylamino alcohol is bound as the alkoxide ~alt in
the distillation bottom. A S to lS mol% strength excess
of alkali metal alkoxide is prefarably employed. In the
distillation of the pure product, the simple alcohol is
S therefore first distilled off as a forerun, before the
target product distils over as the main fraction. This
di~tillation of pure product can be carried out at normal
pressure or under a ~light vacuum and does not require a
highly complicated separation.
In this manner, purities of 1-alkoxydialkylamino-
2-ethanes of 99.9% are achieved.
(
The alkali metal salt of the dialkylaminoethanol remains
in the distillation bottom in addition to some alkali
metal alkoxide suspended in the target product, which i~
returned to the synthesis reactor. No losses of starting
material thus result. For this reason, the yields of
l-alkoxy-2-dialkylaminoethane are also very high at 95
of theory.
Example 1 (for comparison)
l-Etho y-2-dimethyl~minoethane (KDE)
359 g of 30% strength sodium methoxide solution (2.0 mol)
are introduced into a glass autoclave which is equipped
( with a stirrer, temperature measuring device, pressure-
tight distillation section and alkyl halide metering pump
and the methanol is removed by distillation up to a
bottom temperature of 130C. 214 g of ~-dimethylamino-
ethanol DMAE (2.4 mol) and 700 ml of cyclohexane are then
added and all the methanol is removed by distillation up
to a bottom temperature of 85C.
After cooling to 2SC, 22 g of ethyl bromide (2.04 mol)
are to be pumped in in 1 h and the reaction i~ to be
completed at 65C in 4 to S h. After cooling to 20C,
NaBr i8 filtered off, washed with cyclohexane and dried:
219 g (106% of theory by quaternary ammonium salts). The
filtrate is rectified in a 1 m long laboratory column.
~1 7` ~
- 6 - O.Z. ~59~
Cyclohexane initially di~til~ off, then the mRin fraction
and ~ubsequently a fraction rich of dimethylaminoethan
(b.p.: 95 to 98C at 500 mbar).
Main fraction: 196.7 g containing 94.1% EDE, 5.4% D~AE
Subsequent 30.3 g containing 3.3% BDB, 89.3% DMAE
fraction:
Yield: 186.1 g of EDE (79.5~ of theory)
DMAE recovery:
as an azeotrope with cyclohexane, altogether 60 g of DMAE
(0.67 mol) distil off in the main and subsQquent fraction
(determined from final weight and G.C. purity), which
doe~ not correspond completely to the excess (35.6 g) and
unreacted product.
B~ample 2 (for comparison)
1-Btho~y-2-dimethylaminoethanQ (EDE)
359 g of 30% strength by weight methanolic sodium
methoxide solution (2.0 mol) are concentrated to dryness
in the autoclave (as described in Example 1) and then
172 g of p-dimethylaminoethanol and, from preliminarr
experiments, 800 g of c~lohe~anQ which still cont~ins
42 g of D~AB dissolved (altogother 214 g of nY~,
2.4 mol~ are introduced. All the methanol i8 di~tilled
off as an azeotrope, then the autoclave i~ sealed and, at
about lOO-C, 142 g of ethyl chloride (2.2 mol) are to be
pumped in at a pressure of 2 to 3 bar in 1.5 h. The post-
reactlon time i~ 3 h. After cooling, the NaCl is filtered
off, washed and dried (110 g, 94.1% of theory).
All the filtrates are rectified through a 1 m long
laboratory column in a vacuum of 500 mbar and under
reflux. After removal of cyclohexane, an EDE main
fraction and a smaller DMAE subsequent fraction distil.
~ain fraction: 222 g containing 94.7~ EDE, 4.9% D~AB
Subsequent lS.5 g containing 64.4% EDE, 35% DMAE
fraction:
Yield: 220.2 g of EDE (94.0% of theory).
2~7~
- 7 - O.Z. 4594
The excess of DMA~ (O.4 mol) ~ distributed in the two
distillation fractions (16.3 g) and in the cyclohexane
forerun.
~xample 3 (invention)
1-Ethoxy-2-dimethylaminoethane (EDE)
361 g of 29.9% strength by weight methanolic NaOCH3
(2.0 mol) are evaporated to dryness in the apparatus
described above and 188 g of DMAE containing 800 g of
EDE, which still contains 8 g of DMAE (together DMA~:
196 g, 2.2 mol) are introduced.
All the methanol is distilled off. At 100C, 142 g of
ethyl chloride (2.2 mol) are pumped into the sealed
apparatus at a pre~sure of about 1.5 bar in the course of
1.5 h. The reaction is complete after a post-reaction
time of 3 h at lOO-C.
All the EDE is distilled off from the NaCl in a vacuum of
500 mbar.
(NaCl: 117 g, 100% of theory).
1106 g of distillate are obtained having a DMAE content
of 2.9% (corresponding to 0.36 mol). For binding as the
Na salt, 72 g of 30% strength by weight methanolic NaOCH3
solution (0.40 mol) are added and the whole mixture is
distilled through a column. After removing all the
Ci methanol and a small intermediate fraction, very pure EDE
distils over at 120C. The Na salt of DNAE containing
some EDE remains in the distillate bottom, and is added
to the following batch during the formation of Na DMAE.
Yield: 1015 g (le~s 800 g of solvent)
215 g of EDE (91.9% of theory, 95% including
bottom)
G.C. purity: 99.9%.
Yield after four batches with return of EDE and Na DMAE
gives 96% yield.
, S, ~ IJ J
- 8 - O.Z. 4594
~ample 4 (.inv~ntion)
I-~thoxy-2-d.imethyl.~m;.noethane (EDE~
The distillation hottom from Example 3, which contains
0.36 mol of Na salt of D~AE ancl 0.04 mol of NaOCH3, i8
treat~d with 1.96 mol ~f fresh NaOCH3 solution and the
me~hanol is di~tilled off. 1.84 mol of DMAE (164 g) ar.d
800 g of ED~ are then added and the Na DMAE i8 formed
with removal of all the methanol by di~tillation.
The additional reaction ~equence proceeds a~ de~cribed in
Ex~mple 3.
The yield of EDE is 222 g (95% of theory)
After four batches with return of EDE and Na DMAE, a
yield of ~7~ and a puri-ty of 99.8% re~ults.
Example 5 (invention)
1-Ethoxy-2-diethylaminoethane (EDEE)
359 g of 30% strength by weight NaOCH3 solution t2.0 mol)
are concentrated to dryness (130C) in the autoclave
described in Example 1, 281 g of 2-diethylaminoethanol
(DEAE, 2.4 mol) and lO00 ml of t-butyl methyl ether are
introduced and all the methanol is removed by azeotropic
distillation. At a temperature of 100C, 142 g of ethyl
chloride (2.2 mol) are pumped in in the course of 1.5 h
and the reaction is then completed in a further 3 h
(pressure: 1 to 3.5 bar). After cooling, the NaCl i8
filtered off, washed with t-butyl methyl ether and dried
(114 g, 97.5% of theory)
The collected filtrates are distilled through a column;
EDEE containing 5% DEAE as the main fraction distils over
after removal of the solvent. A small DEAE-rich fraction
remains in the distillation bottom. The main fraction is
treated with 1.1 times the equivalent amount of NaOCH3
solution - relative to residual DEAE - and again sub-
jected to fractional distillation. After distilling off
the methanol forerun, very pure EDEE distils over at
710C/40 mbar. The bottom remaining from this distilla-
tion of pure product, which contains the Na salt from
DEAE, is employed again for subsequent batches, in which
EDEE is then used as the solvent (instead of t-butyl
_ g _ o.z. 4594
methyl ether); the yields rise above 90~ on frequently
repeated reuse of the distillation bottom.
Yield: 240.5 g (82.9~, relative to Na methoxide; includ-
ing 95% bottom).
After returning four times, yields of 95~ and purities of
over 99.5~ are obtained.
Examples 6 to 9
Example 6: 1-Propoxy-2-dimethylaminoethane (PDM~)
Example 7: 1-Butoxy-2-dimethylaminoethane (BDME)
Example 8: 1-Propoxy-2-diethylaminoethane (PDEE)
Example 9: 1-Butoxy-2-diethylaminoethane (BDEE)
In accordance with the procedure of Example 5, both
~-dimethyl- and ~-diethylaminoethanol are reacted in the
solvents cyclohexane or t-butyl methyl ether with
n-propyl chloride and n-butyl chloride to give the
corresponding aminoalkyl ethers.
Under a pressure of 1 to 3 bar, the reaction temperatures
in the autoclave are 100 to 115C.
The maximum yields and purities are only obtained after
two to four batch cycles using the target product as
solvent and returning the Na dialkylaminoethoxide from
the distillation bottom.
- 10 - O. 8 . ~59~
,., .
,~ a~ ~ co co
___ _ ~
~u ~ 8 u~ u~ ~ d'
O ~ ~
_ __
~ ~ U~
~J ~ ~ N N N
~17 ~
_I ~ er ~ ~O'
~3 ~ ~J N ~ N
._ ~ ~ ~ ~
o o c~ o
r~
-
u~