Note: Descriptions are shown in the official language in which they were submitted.
WOQl/09030 PCT/EP90/02147
1- 207~97
;-- j, - ..
2-substituted 4,5-diphenyl-imidazoles :
This invention relates to therapeutically useful
imidazole derivatives, to processes ~or their
production, to pharmaoeutical compositions containing
them, and to their use in a method of treatment of the
human or animal body.
The present invention provides compounds of the
general formula:-
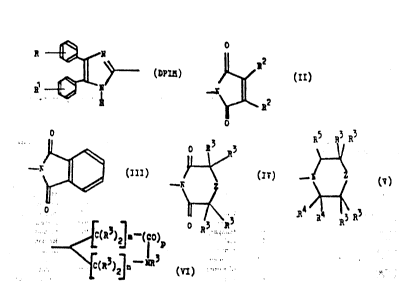
[DPIM]-S-W-Y (I)
wherein ~DPIM] is as hereinafter depic~ed, R and R1 are
the same or different and each represents a hydrogen or
halogen atom or a straight- or branched-chain alkyl
group containing from 1 to about 5 carbon atoms, W
represents a methylene group or a straight a~kylene
chain containing from 2 to about 5 carbon atoms and is
optionally substituted with one or more straight- or
branched-chain alkyl groups containing from 1 to about
4 carbon a oms, Y represents a group of formula ~II),
(III~, (IV), (V) or (VI) hereinafter depicted, wherein : .
the symbols R2 axe the same or different and each
represents a hydrogen atom or a methyl group, the
symbols R3 are the same or differènt and each .
represents a hydrogen atom or a straight- or branched-
~chain~alkyl qroup containing from 1 to about 4 carbon
atoms, ~he s~mbols R each represent a hydrogen atom or
-else together the two symbols R~ and the carbon atom to
.. . ..
-- '.: .
' ~ .
W091/09030 PCT/EP90i~2147
which they are both joined form a carbonyl group, R5
represents a hydrogen atom or a hydroxy group, æ
represents an oxygen or sulphur atom or a group of
formula [C(R3)2]~ or NR3, m represents zero or an
irlteger from 1 to about 5, n represents zero or an
integer from 1 to about 5, and p represents zero or i,
such that m ~ n + p = about 3, 4 or 5, g represents 0,
1 or 2, and pharmaceutically acceptable acid addition
salts thereof, for use in the preparation of a
pharmaceutical composition for the treatment of.
conditions which can be ameliorated by the
administration of an inhibitor of acyl coenzyme-A~
cholesterol-O-acyl transferase (ACAT; EC Z.3.1.26),
such as atherosclerosis, hyperlipidaemia, cholesterol
ester storage disease and atheroma in vein grafts.
The invention also provides a method for the
treatment of a human or animal patient suffering from,
or subje~t to, conditions,whi~h can be ameliorated by :
the admlnlstration of an inhibitor of acyl coenzyme-A:-
cholesterol-O-acyl transferase, such as
atherosclerosis, hyperlipidaemia, cholesterol ester
storage disease or atheroma in vein ~rafts,-which
comprises administering to the patient an effective
amount of a eompound of formula ~I), or a
.. . . . . ... . .
pharmaceutically accept~ble acid additi~n salt thereof,
as hereinbefore defined, to se~ure an improvement in
the ~ondition of the patient.
WO 91/09030PCI/EP90/02147
-` 207149~
~, ` ,7 ' ` ( ;
- 3
The invention also provides a compound of
formula (I~, or a pharmaceutically acceptable acid
addition salt thereof, as hereinbefore defined, for use
in a new method of treatment, of the human or animal
bo~y, by therapy, of conditions which can be
ameliorated by the administration o~ an inhibitor of
acyl coenzyme-A:cholesterol-O-acyl transferase, such as
atherosclerosis, hyperlipidaemia, cholesterol ester
istorage disease or atheroma in vein grafts. `.
The present invention also provides
pharmaceutical formulations which contain at least one
of the compounds of formula (I), or a pharmaceutically
acceptable acid addition salt thereof, as hereinbefore
defined, provided that when W represents a methylene
group or a straight alkylene chain containing from 2 to
4 carbon atoms, Y represents other than a group of
formula (V), as hereinafter depicted, wherein the
symbols R4 represent hydrogen atoms and Z represents an
oxygen atom or a group of formula [C~R3)2]q~ wherein g
represents O or 1, or NR3, or the symbols R4 and the
carbon..atom to which they are both joined form a
carbonyl group and Z represents a group of formula
~C(R3)2]g~ wherein q represents 0, R, Rl and R3 being
as hereinbefore defined, in association with a.
pharmceutically acceptable carrier or coating.
' .
.
.- . . :
.,.. , .. ,,~'.,'~. 'i ,",., ~ ""; ,",,,,";"",~ "~,
wos1/09030 PCT/EP90/02147
~0.7~497
`
The present invcntion also provides new
imidazole derivatives of general formula (I), and
pharmaceutically a~ceptable acid addition salts
thereof, as hereinbefore de~ined, subject ~o the
proviso that when W represents a methylene group or a .: .
straight alkylene chain containing from 2 to 4 carbon
atoms, Y represents other than a group of formula (V),
as hereinafter depicted, wherein the symbols ~4
represent hydrogen atoms and Z represents an oxygen : :
atom or a group of formula lC(R3)2]q~ wherein g
represents 0 or 1, or NR3, or the symbols ~4 and the
carbon atom to which the~ are both joined form a
carbonyl group and Z represents a group of formula ~ .
[C(R3)2]~, wherein g xepresents 0, R, Rl and R3 being
as hereinbefore defined. ~ .
Compounds within the scope of general formula
~I), whe_ein R and Rl are the same or different and
each represents a hydrogen or halogen atom or a
straight- or branched-chain alkyl group containing from : :
- ... .
l to 5 carbon atoms, W represents a methylene group or :: :
: a straight alkylene.chain containing from 2 to 4 carbon -::
., . . . .. i -
........ , , . . , ... . . . . . . .. ~ .. . .. . .
, . . , . . ... . , .. . . ., . , , ~ , .
: , . .
- '.,;, :.
''' ' !
i ...'.';:' ~
WO91/09030 PC~/EP90/02147
~ ~ ~07;1 ~ 9 7
.
atoms, Y represents a ~roup of formula (V~, as
hereinafter depiGted, the symbols R3 are the same or
dif~erent and each represents a hydrogen atom or a
straight- or branched-chain alkyl ~roup containing from
1 to 4 carbon atoms, and wherein the symbols R4
represent hydrogen atoms and Z represents an oxygen
atom or a group of fonmula [C(R3)2]q~ whereln q
represents 0 or 1, or NR3, or the symbols R4 and the
carbon atom to which they are both joined form a
~arbonyl group and Z represents a ~roup of formula
EC(R )2]q~ wherein ~ represents 0, and their
pharmaceutically acceptable acid addition:salts, alone
and in association with pharmaoeutically acceptable
carrLers or coatings, have been disclosed in the
specification of Japanese Patent O.P.I. No. 64-40467
(Application ~o. 62-196117).
However, in that specification, those compounds
and their pharmaceu~ical compositions are described as
having anti-ul~er and anti-inflammatory activity.
. .. . . . .. , . . ~
Nowhere in that specification is there any suggestion
.. .
that the compounds and their pharmaceutical
compositions could be useful as inhibitors of acyl
coenzyme-A:cholesterol-O-acyl transferase or in the
treatment of conditions such as atherosclerosis,
- ; ~ . ...
~ hyperlipidaemia, cholesterol ester storage disease and
.. - . -, ... ,~ , .. . .. , . , ~ .
atheroma in vein graf~s.
',
;:~
WO91/09030 PCT/~P9QtO2147
;; 2~:71~97
- 6 -
Thus, the utilities disclosed in the present
specification are entirely unexpected.
Especially important features of the present
invention are, or involve, compounds of general formula
(I) wherein at least one of the symb91s has a value
selected from the following~
(i) R represents a hydrogen or chlorine atom or
a methyl group;
(ii) Rl represents a hydrogen or chlorine atom
or a methyl group;
(iii) W represents a methylene group or an
alkylene chain of 2, 3 or 4 carbon atoms;
(iv) Y represents a phthalimido grou~ or an
optionally substituted maleimido, pyrrolidinyl,
piperidyl, perhydroazepinyl, piperazinyl, or morpholino -
group; and/or
(v) R3 represents a hydrogen atom or a methyl or
ethyl group; ' ''
the'other symbols bein~ as hereinbefore defin~d, and
. , . , . .. . .i . .~ , . ,.... , . , : .
'thelr pharmaceutically acceptable acid addltion salts.
'' ''' Important compounds according to the present ~'' ''
~' invention inciudé''''' ~' ' ''~'''~~~ ' '
'' ' 'iA- ' N-[2-('4,5-diphenylimidazol-2-yi ~ ethyl]~
'~'~-'' ~''`'~''''m'or'pholinë''"' ''`~'''~=~~~~ ~~ ''~~~'`~~~`~ ~ -
~.~ ...... . . ................ . .... .. , .
'`'' '''''''' -'lAA ''N-t2-(4,50diphenylimida'zol-2-ylthio)ethyl]-
morpholine hydrochloride
~ I
1 :'
WO91/09030 pcT/Epso/o2l47
2 ~ 7 1 ~ 9 7
- 7 - .
lB N-~3-(4,5-diphenylimidazol-2-ylthio)propyl]-
morpholine
lBA N-13-(4,5-diphenylimidazol-2-ylthio)propyl]-
morpholine dihydrochloride
lC N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
piperidine
lCA N-[2-(4,5-diphen~limidazol-2-ylthio)ethyl]-
piperidine dihydrochloride
lD N-[3-~4,5-diphenylimidazol-2-ylthio)propyl]-
N'-methylpipera~ine
lE N-t2-(4,5-diphenylimidazol-2-ylthio)ethyl~-
pyrrolidine-2-one .
lF N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
- pyrrolidine
lG N-[3-(4,5-diphenylimidazol-2-ylthio)propyl]-
piperidine
lGA N-~3-(4,5-diphenylimidazol-~-ylthio)propyl3-
piperidine dihydrochloride
lH N-t2-[4,5-diphenyllmidazol-2-ylthio)ethyl]- ~- .
- pexh~droazepine ~ ~
:~ ~ lH~ N-~2-(4,5-diphenylimidazol-2-ylthio)ethyl3-
erhydroazepine~dihydrochloride -
lI cis- and~trans-2,6-dimethyl-N-[4-(4,5-diphenyl-
r ~ imidazol-2.-ylthio)butyl]morph~Iine~
A cis- and.5E3~-2,6-dimethyl-N-[4-[4,5-diphenyl-
imidaz~l-2-ylthio)butyl]morpholine
Wosl/09030 PCT/EP90/02147
- ` 20.~ 8 -
dihydrochloride
2A N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
phthalimide . ~ :
2B N-~3~(4,5-diphenylimidazol-2-ylthio)propyl]-
phthalimide ~ .
2C N-[4-(4,5-diphenylimidazol-2-ylthio)butyl]-
phthalimide
2D N-[3-(4,5-diphenylimidazol-2-ylthioJpropyl]-
morpholine-3,5-dione ;
2E 3~5-dimethyl-N-~3-(4,5-diphenylimidazol-2-yl-
thio)propyl]piperidine-2,6-dione . :
2F N-[2-(4,~-diphenylimidazol-2-ylthio)ethyl]-
maleimide
2G N-~3-(4,5-diphenylimidazol-2-ylthio)propyl~-
4-methylmaleimide
2H N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]- .
pyrrolidine-2,5-dione ~ :
2I N-t3-(4,5-diphenylimidazol-2-ylthio)propyl]-
-- pyrrolidine-2,~-dione :`.
2J N-r3 (4,5-diphenylimidazol-2-ylthio)propyl~
. piperidine-2,6-dione .
2K 4,4-dimethyl-N-[2-~4,~5-diphenylimidazol-2-yl- .
-, ~3 ~ thio ) ethyl ]pip2ridine-2, 6-dione ~
2L 4,4-dimethyl-N-[3-t4,5-diphenylimidazol-2-yl-
thio)propyl~piperidine-2,6-dione -.
~; ~ ' .. '".
'''
WO91/09030 PCT/EP90/02147
`; 2~71497
_ 9 _ i . ;'T ~
2M N-[3-(4,5-diphenylimidazol-2-ylthio~propyl]-4-
methylpiperidine-2,6-dio~e
2N N-~3-(4,5-diphen~limidazol-2-ylthio)propyl~-4-
ethyl-4-methylpiperidine-2,6-dione
~-~3-(4,5-diphenylimidazol~2-ylthio)propyl]-
thiomorpholine-3,5-dione
2P 4,4-dimethyl-N-[3-t4,5-di-p-tolylimidazol-2-yl-
thio1propyl]piperidine-2,6-dione
2Q N-[3-(4,5-di-p-~olylimidazol-2-ylthio)propyl]-
morpholine-3,5-dione
2R N-[3-(4,5-di-2-chlorophenylimidazol-2-ylthio)-
propyl]-4,4-dimethylpiperidine-2,6-dlone
2S N-~4-(4,5-diphenylimidazol-2-ylthio)butyl]~
morpholine-2,6-dione
2T 3,3-dimethyl-N-~3-(4,5-diphenylimidazol-2-yl-
thio)propyl]piperidine-2,6-dione
3A [3-R,5]-3-~(4,5-diphenylimidazol-2-ylthio1-
methyl]-l-methylpiperidine
3AA [3-R,5]-3-~(4,5-diphenylimid~zol-2-ylthio)-
methyl]-l-methylpiperidine hydrochloride
3B [5-R,S]-5-[(4,5-diphenylimidazol-2-ylthio)-
methyl-2-pyrrolidinone - ~
3C r 6-R,S~-6-[(4,5-diphenylimidazol-2-ylthio)-
. methyl]-2-piperidin~ne - -
- 3D t6-R~S]-4~4-dimethYl-6-~(4~5-diphenylimidazol-
2-ylthio~methyl~-2-piperidinone .
W091~09~30 PCT/EP90/02147
`~ 2~
-- 10 --
3E [4-R,5]-4-[2-(4,5~diphenylimidazol-2-ylthio)-
ethyl]-l-methylpiperidine
3F ~2-R,S]-2-[S4,5-diphenylimidazol-2-ylthio)-
methyl]-1-methylpiperidine
4A N-~3-(4,5-diphenylimidazol-2-ylthio)propyl]-
5-hydroxypyrrolidin-2-one `
4B N-[3-(4,5-diphenylimidazol-2-ylthio)propyl]-
5-hydroxymorpholin-3-one
The codes lA to 4B are allocated to compounds
for easy reference later in the specification.
Compounds within the scope of the present
invention exhibit positive pharmacological activities
as demonstrated by the following in vivo and in vitro
tests which are believed to correlate to
pharmacological activity in humans and other mammals.
In assays performed in vitro, microsomes,
prepared from the livers of rats fed a diet
supplemented with 0.5%w/w cholesterol and 0.25%w/w -
cholic acid for 7 days, were incubated with
radiolabelled oleoyl-CoA in the presence of compounds
according to the invention at a concentration of
0.5, 1 or lO~g/ml. The de~ree of`ACAT inhibition
produced is~shown~in~Table I. ~~ ` - ,
In in ~ivo tests, using rats fed on a similar
diet to that above and ~urther ~suppIemented by 0.03%
w/w of test compound, the compounds according to the
:: -
., .; . . , ' ' , . ; . , . . ` , .
WO91/09030 PCT/EP90/02147
2 0 714 9 7
invention inhibited increases in ~lasma cholesterol
concentrations, measured after 3 days, relatlve to :
control animals fed on the cholesterol supplemented
diet without the drug, by amounts as sho~,~n, for
example, in Table I~.
., ~ . . . . i, , . .. . , ... : ;... : . - ~ .
WO 91/09030 PCr/EP90/02147
~ `"` 2071~97 ---
- 12 -
Table I
Compound Concentration % Inhibition
llg/ml
lA 10 74
lAA 10 90 ''
lB 10 91
lC 10 go -
lD 10 88
lE 10 86
lF 10 72 .
lGA 10 66 .:
lHA 10 77 ~
2A 1 6 8 ;.:
2B 1 86 . :
~' 2C 1 86 -
2D 1 88
2E 1 94
2F 1 69
: 2G 1 90 `
: . . .
2H 1 6 9
contd. ;.
~; , . . .
.. .
wo ~1/09030 pcr~Ep9o/o2l47
;2 0~ 7 1 4 9 7
-- 13 --
Table I ( cont~)
Compound Corlcentration % Inhibition
lls/ml
2I 1 86
2~ 1 ~6
2K 1 87
2L 1 93
2M l 83
2N 0.5 87
0 5 70
2P 0.5 88
2Q 0. 5 75
2R 0.5 70
2S 0. 5 78
2T o.5 9O
3A 1 94
3B 1 87
3C 1 86
3D ~ 1 82
3E 0.5 70
3F ~ 0.5 84
., . . . . , .. :
.
- ~ Table.. II ~
~ = .
- . Compound .. . - ~ -. ~ % Inhibition
3AA ~ 88
-,
'
WO 91/09030 PCI~/EP90/02147
207i~97
Compounds of formula (I) can be prepared by the
application or adaptation of known methods, by which is
meant methods used heretofore or des~ribed in the
literature. -
Thus, according to a feature of the present
invention, compounds of for~ula tI), as hereinbefore
defined, are prepared by the rea~tion of a salt of
the general formula:-
[DPIM]-S M (VII) -
wherein ~DPIM] is as hereinbefore defined and N is an
alkali metal, preferably a sodium or potassium, atom,
with a ~ompound of the general formula:-
X-W-Y (VIII)
or an acid addition salt thereof, wherein X is a group
displaceable by a thiolate salt, such as a halogen,
e.g. a chlorine, atom or an alkyl- or aryl-sulphonyloxy
group (e.g. methanesulphonyloxy or 4-toluene-
sulphonyloxy) and W and Y are as hereinbefore defined.
The reaction is carried out in an inert organic
.
solvent, such as dimethylformamide or tetrahydrofuran,
at from room temperature to 100C, optionally in the
presence of a proton acceptor, which may be organic,
such as an-amine (e.g. triethylamine), or i~organic,
. such as a carbonate of an alkali metal (e.g. potassium
-carbonate).
WO 91/09030 PCI`/EP90/02147
2~71497
- 15 -
According to a further feature of the present
invention, compounds of general formula (I) wherein Y
represents a group of formula (II), lIII) or (IV), the
other symbols being as herei~before defined, are
prepared by the reaction of a compound of the general
formula:-
. .,
1 DPIM ] S-W-NH ( IX )
wherein the symbols are as hereinbefore defined, with a
comp~und of the ~eneral formula (X), (XI) or (XII),
hereinafter depicted, wherein ~, R2 and R3 are as
hereinbefore defined. The reaction is generally
carried out in an inert organic solvent, f~r example
xylene, and optionally in the presence of an acidic
catalyst, suoh as 4-toluenesulphonic acid, at the
reflux temperature, with azeotropic removal of water.
According to a further feature of the present
invention, compounds of formula ~I), wherein Y
represents a group of formula (V3 and the other symbols
are as hereinbefore defined, are ~repared from the
correspanding compounds of formula (I) wherein Y
represents a group of formula (IV) and the other
sym~ols are as hereinbefore defined, by reduction with
a metal hydride reducin~ agent, such as lithium
aluminium hydride, in an inert solvent, such as an
ether, e.g. diethyl ether or tetrahydrofuran, at O~C
~o room temperature.
' : ' ;
' . ':
:: .. . . : .. .. ;, . . .- , , ~ . .
WO91/09030 PCTtEP90/02147
20``7~7 --
.. ~ ` . . ` ... ... .
- 1~
Acoording to a further feature of the present
invention, compounds of formula (IJ, wherein Y
represents a group of formula (V) in which the symbols
R represent hydro~en atoms and the other symbols are
as hexeinbefore defined, are prepared by reduction of
the corresponding compounds of formula (I) wherein Y
represents a group of formula (Y) in which the two
symbols R4 and the carbon atom to which they are both
joined form a carbonyl group and the other symbols are
as hereinbefore defined, in conditions similar to those :
described hereinbefore for the reduction of compounds ~ :
of formula I wherein Y represents a group of formula
. . .
(IV) to corres~onding compounds wherein Y represents a ~-~
group of formula (V).
According to a further feature of the present
invention, compounds of the general formula:-
~ DPIM]-S-Wl-CH2-Y (XIV)
within formula (I ), wherein Wl represents a methylene
group or a straight alkylene chain containing from 2 to
:~ .
,
- - . :
.
: ......... ' `'`'~`' '''''~ .
.
. --
W091/09030 PCT/EP90/02147
2071497
- 17 -
4 carbon atomsr op~ionally substituted with one or more
straight- or branched-chain alkyl groups containiny
from 1 to 4 ~arbon atoms, the other ~ymbols bein~ as
hereinbefore defined, are prepared by reduction of
compounds of the general ~ormula:-
[D~IM]-S-Wl-~O-Y ~XV)
wherein the symbols are as hereinbefore defined, in
conditions similar to those described hereinbefore for ~
the reduction of compounds of formula (I) wherein Y . :
represents a ~roup of formula (IV) to corresponding
compounds wherein Y represent~ a group of formula (V). ~
The starting materials and intermediates can be ~ .
prepared by the application or adaptation of known
methods.
Thus, compounds of formula (VII) can be prepared
by the reaction of a bai~e such as ~odium hydride with
compounds of the general formula:-
[DPIM~-SH (XVI)
wherein ~DPIM] is as hereinbefore defined. This
reaction is ~onveniently carried out ~n situ.
Compounds o~ formula lIX) can be prepared
by the reaction of a compound of formula-(VII), as
hereinbefore defined, with a compound of the general :: :
.. . . . .
formula:~
- - : X-W-N~2 - - (XIII)
.
- .
WO91/090~0 PCT/~P9o/02147
Z~7149 ~ - 18 -
or a salt thereof, wherein X and W are as hereinbefore
defined, under conditions similar to those described
above for the reaction of a compound of formula (VII)
with a compound of formula (VIII).
Compounds of formula (IX) can also be prepared
by the reaction of compounds of formula (I), wherein Y ;
represents a phthalimido group, with hydrazine, in a
solvent such as eth~nol at temperatures up to reflux.
C~mpounds of formula (XY) can be prepared by the
reaction of compounds of formula (~II) with compounds
of the general formula:-
X-Wl_co-y (XVII)
wherein the symbols are as hereinbefore defined, under
conditions similar to those described above ~or the .
reaction of a compound of formula (VII) with a compound
of formula (vIII).
By the term "pharmaceutically acceptable acid
addition salts" as used in this specification is meant .
acid addition salts the anions of which are relatively
innocuous to the animal organism when used in
therapeutic doses so that the beneficial pharmaceutical
properties of the parent compounds of general formula
(I).~.are not vitiated-by side-effects ascribable to
those anions.
suitable acid.addition salts for use in
phar~aceuticals may be selected from salts derived from
,, . . ~, . , ,: I . , , '
WO91/09030 PC~tEP9OtO2~47
,, ,` ` 20ql497
-- 19 --
inorganic acids, for example hydrochlorides,
hydrobromides, phosphates, sulphates and nitrates, and
organic acids, for example oxalates, lactates, ,
tartrates, acetates, salicylates, citrates,
propionates, succinates, fumarates, maleates,
methylene-bis-~-hydroxynaphthoates, gentisates and
di-~-toluoyltartra~es.
According to a fur~her feature of the invention,
acid addition salts of compounds of formula (I) are
prepared by reaction of the parent compounds of formula
(I) with the appropriate acid, by the application or
adaptation of known methods.
As well as being useful in themselves as active
compounds, salts of compounds of ~ormula (I) ar~ useful
for the purposes of purification of the parent
compounds of formula (I), for example by exploitation
of the solubility differences between the salts and the
parent compounds, by techniques well known to those :~
skilled in the ar~.
: The parent compounds of formula (I) can be
regenerated from their salts by the application or
adaptation of known methods.
For example, parent compounds of general
formula ~I~ can be reg~nerated from ~heir acid addition
salts by treatment with an alkali, e.g. aqueous sodium
bicarbonate solution or aqueous ammonia solution.
..
. :. : . . - .. :, ., ;: , , : ~. . ~ : -
.
WO91/09030 pcT/Epso/o2147
`` ;2~t71497 -`
. .... : .
~ 20 -
In this specification reference to compounds of
formula ~I) is intended to include reference to their
pharmaceutically acceptable acid addition salts, where
the context so permit~.
Compounds of formula (I) can be purified by ~he
usual physical means, ~or example by crystallisation or
chromatography.
The following Examples illustrate the
preparation of compounds according to the invention and
the Reference Examples illustrate the preparation of
intermediates.
N.M.R. spectra were recorded at 200MHz or
400MXz. Chemical shi~ts are expressed in ppm relative
to tetra~ethylsilane. Abbreviations have the following
significances:-
s = singlet, d = doublet, t ~ triplet, q = guartet,
quin = quintet, m = multiplet, dd = doublet of
doublets, dt - doublet of triplets, br = broad signal.
Infra-red spectra were recorded in potassium
bromide discs. The positions of the major absorption
peaks are given.
., . :
.
~: . , I '
WO 91/09030 PCI'/EP90/02147
o 21 ~ . ,` `. ~071~g7
--~ (D~)
~' ~L
)~R2
(II)
r ~Z -:
(III)
0 1~3 3
~Ly .
(n)
Ir /\ 3
o ~3
. ~ :
R3
(V)
R ~ R3
WO 91/09030 PCT/EP90/02147
`; ;` ` 2~7i"497 . :
-- 22 -- ~
~{C'R~ 'T~
~r( )2]n ;' ~ '
: . -
~,~1~ (X)
'
O
o~ (XI) '
~ .
: .
0 R3 ~3
2 (XII )
:: : , ; O -~
; ~ .
: : :
WOsl/09030 PCT/EP90/02147
.~ ~07~4~7
- 23 -
EXAMPLE 1
Com~ounds lA, lB, lC and lD
Sodium hydride (1.2g, 40mmol of an 80%
dispersion in oil) was added to a stirred suspension of.
4,5-diphenylimidazol-2-thiol (5.04~, 20mmol) in dry
tetrahydrofuran t180ml) at ambient temperature. After
stirring for 0.Shr, N-~2-chloroethyl)morpholine
hydrochloride ~3.72~, 20mmol) was added followed by
triethyl~mine (3ml). The mixture was heated under
reflux for 48hr, then poured into water (300ml) and
extracted with ether (2 x 200ml). The ethereal
solution was then extracted with aqueous 2N
hydrochloric acid (200ml). The acidic aqueous solution
was then basified (NaOH) and extracted with ether (2 x
150 ml). This ethereal solution was dried ~MgSO4), :
evaporated, and the residue recrystallised from toluene
to give N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]- .
morpholine (4.9g), as a colourless solid, m.p. ;
- 129-132~C;
EElemental analysis:- C, 68.9; H, 6.3; N, 11.
. Calculated for C21H23N3OS:- C, 69.0; H, 6.3;
- N, 11.5%;
-- ~ . N.M.R.(CDCl3 &.D2O): 2.55 ~(4H, br t, J=4Hz),
2.86 (2H, t, J=6Hz), 3~11 (2H,-~, J=6Hz), 3.5 (4H, br
- .t,:J=4Hz), 7.2-7.6 (10H, m)].~ . :
~.
.. . ~ . .. .... .. . . . . .. . .. . . .. .. . .
WO9l/09030 PcT/EP90/02l47
~071~97 - 24 - ~
By proceeding in the s~me manner, but replacing
~he N-(2-~hloroethyl)morpholine by:
1) N-(3-chloropropyl)morpholine;
ii) N-(2-chloroethyl)piperidine; and
iii) N-(3-chloropropyl)-N'-methylpiper~2ine
there were prepared:
i) N-[3-(4,5-diphenylimidazol-2-ylthio)propyl~-
morpholine, m.p. 135-1379C;
ii) N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
piperidine, m.p. 143-145C; and
iii) N-(4,~-diphenylimidazol-2-ylthio)propyl]-
N'-methylpiperazine, m.p. 175-178C.
EXAMPLE 2
Compound lAA
Acetyl chloride (2Oml) was added dropwise to
cold (10C) ethanol (90ml) with stirring, main~aining
the temperature below 30C. After stirring for 0.5hr,
a solution of N-[2-(4,5-diphenylimidazol-2-yl~hio)-
ethyl~morpholine (1.5g, 4.1mmol) in ethanol (lOml) was
added and stirring continued for 0.5hr. The mixture
was then evaporated and the residue recrystallised from
ethanol to give N-[2-(4~5-diphenylimidazol-2-ylthio)-
ethyl]morpholine dihydrochloride (1-46g~, as a
colourless~solid, m.p. 247C; ~ ~ 3
[Elemental analysis:- C, 55.1;-H, 5.95;
Clt 15.3; N, 8.9~;
..
.,. . .. . . . . . .. , . . ., .. , .;.. . . . ~ .. . , . , , ~ .. , . , . ;; ,, , .. ~ , , i
WO91~OgO30 pcT/Epso/o2l47
207..~497
.
- 25 -
Calculated for C21H23N3S.2HCl.H20:- C, 55.3;
H, 5.96; Cl, 15.5; N, 9.2~
N.M.R. (CD3SOCD3 & D2O) 3.23-3.95 (12H, m),
7.3~-7.56 (lOH, m)].
EXAMPLE 3
ompound lE
Sodium hydride ~ 0.48g, 16mmol of an 80%
dispersion in oil) was added to a stirred suspension of
4,5-diphenylimidazol-2-thiol (3.78g, 15mmol) in dry
tetrahydrofuran at ambient temperature. After stirring ~:
for 0.5hr, N-(2-tosyloxyethyl)pyrrolidin-2-one (5.66g,
20mmol) was added and stirring continued for 3hr. The ~ :
mixture was then poured into water (500ml) and
extracted with ether (2 x 200ml). The ethereal
solution was dried (~gS04), evaporated, and purified by
flash chromatography (19:1 dichloromethane / methanol
as eluent). The product was crystallised from 1:1 ethyl ~
ace~ate / petroleum ether ~60-80C) to give N-[2-(4,5~ :
diphenylimidazol-2-ylthio)ethyl]pyrrolidin-2-one
.
(4.2g), as a colourless solid, m.p. 128-130 C; :~
~Elemental analysis:- C, 69.4; H, 5.75; N, 11.7%
. .
.
C21~21N3S:-~C~ 69-4; H 5 82
- - N, 11.6~; ., : .~ ..... ; -
, . .
,~ f~
,. ~,, , . ~ ,, ,, . , . . ,:, . . . ` - , : ,, ', , ' .: ', ' , '. . ':; ', . ' ' ' ` '., ' ' :,
` ' ' ~ ' . '. . ' .. : ' . - ' . ` :':" ': .': -
WO 91/09030 PCI'/EP90/02147
~Q~4~7
2 ~
.
N.M.R. (CDCl3): 2.14 (2H, q, J=7Hz), 2.58 (2H,
t, J=7HzJ, 2.96 (2H, t, J=6Hz), 3.46 (2H, t, J=7Hz),
3.64 (2H, t, J=6Hz), 7.1-7.45 (6H, m), 7.46-7.74 (4~,
m~, 12.3 (lH, br s)].
EXAMPLE 4
ComPound lF
A solution of N-[2-(4,5-diphenylimidazol-2-yl-
thio)ethyl]pyrrolidin-2-one (3.2g, 9mmol ) in dry
tetrahydrofuran (20ml) was added to a stirred
suspension of lithium aluminium hydride (1.07g, 28mmol)
in diethyl ether (300ml) at ambient temperature under
an argon atmosphere. Stirring was continued for 0.5hr,
then ethyl acetate (50ml) was added slowly with ice
cooling followed by water (300ml) and stirring then
continued for 0.5hr. The organic layer was separated,
dried (MgS04), and evaporated to a gum which was
purified by flash chromatography (9:1 dichloromethane
/ methanol as eluent). The product was dissolved in
ethyl acetate (5ml) and petroleum ether (60-80~C; 50ml)
was added to give N-~2-(4,5-diphenylimidazol-2-ylthio)-
ethylJpyrrolidine (1.9g), as a colourless crystalline
solid, m.p. 102-103C; - - -
tElemental a~alysis:- C, 72.3; H, 6.4; N, 12.1% -
21H23N3S: C, 72.2; H, 6.6;
~, 12.0%
... .. . .; . .. .. . . . . .
WO 91/09030 PCI/EP90/02147
rl 2 0 7 1 4 9 7
- 27 -
N.M.R. (CDC13 & D20~: 1.53-1.64 (4H, m),
2.6-2.68 (4H, m), ~03-3.1 (4H, m)~ 7.2-7.33 (6H, m),
7.41-7.~5 (4H, m)~
S
Compounds lG and lGA
Potassium tert.-bu~oxide (4.44g, 39.6mmol) was
added to a stirxed suspension of 4,5-diphenylimidazol-
2-thiol (lOg, 39.Smmol) at ambient temperature to give
a yellow solution. Af~er stirring for O.Shr, N-(3-
chloropropyl)piperidine hydrochloride (11.77g,
59.4mmol) was added. The mixture was stirred for 18hr
and evaporated, and the residue mixed with ethyl
acetate (200ml) and washed with water (250ml),
saturated a~ueous sodium bicarbonate solution (200ml)
and water (200ml). The organic phase was dried (MgS04)
and evaporated and the orange residue was crystallised
from agueous ethancl to give N-[3-(4,5-diphenyl-
imidazol-2-ylthio)propyl~piperidine (3.98g), as a
colourless solid~ m.p. 118-120~C.
Acetyl chloride 125ml) was added dropwise to
cold ~<10C) ethanol (70ml) with stirring, maintaining :-.
the temperature below 10C. After stirring for O.Shr,
a solutio~ of ~-t3,.(4,5-diPhen~limidazol-2-ylthio)-
propyl]piperidine-(3.98g) in;etha~ol (lOml) was àdded
. .- .. . ... . .
Wo91/09030
PCT/EP~0/02147
`: 2071~7 -`
- 28 -
and stirring continued for 0.5hr. The mixture was then
evaporated and the residue crystallised ~rom ethanol /
ether to give N-r3-(4,5-diphenylimidazol-2-ylthio)-
propyl~piperidine dihydroc~.loride (2.62g~ as a
colourless solld, m.p. 180-185C;
~ Elemental ana~ysis:- C, 59.3; H, 6.5; Cl, 14.8;
N, 9.1; S, 6.6%
Calcul~ted for C23H27N3s.2Hcl~H2o - C, 59.0;
H, 6.67; Cl, 15.1; N, 8.97; S, 6.84%]
EXAMPLE 6
Co~ nds lH and lHA
Potassium tert.-butoxide (4.44g, 39.6mmol) was
added to a stirred suspension of 4,5-diphenylimidazol-
2-thiol (lOg, 39.6mmol) at ambient temperature to give
a yellow solution. Af~er stirring for 0.5hr, 2-(hexa-
methyleneimino)ethyl chloride monohydrochloride (11.9g,
60mmol) was added. The mixture was stirred for 18hr
filtered, and the solid washed with small portions of
dimethylformamide until the washings-were colourless.
The insoluble material was shaken with a ~ixture of
ethyl acetate (50Oml) and a~ueous potassium carbonate
solution 110%, 250ml) until complete solution occurred.
.. .. ~ ~ .
The organic;phase;was-washed-with water (2xiOOmlj,
dried (MgS04) and~evaporated to give-N-~2-(4,5-
diphenylimidazol-2-ylthio~ethyl]perhydroazepine
~10.8g~, as a colourless solid.
....
.: .: . ... . .. .
WO9l/09030 PCr/EP90/02147
.~ 207~97
- 29 -
Acetyl chloride (25ml~ was added dropwise to
cold (<lO~C) methanol (150ml) with stirring, :
maintaining the temperature ~elow 10C. After stirring
for O.Shr, a ~olution of N-[2-(4,5-diphenylimidazol-
2-ylthio)ethyl]perhydroazepine (10.8g) in methanol
(lOOml) was added and stirring continued for 5min.
Decolourising charcoal (lg) was added, stirring
conti~ued for 15min and the mixture was filtered. The
filtrate was evaporated to low volume and ether added
to give N-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]- :
perhydroazepine dihydrochloride (ll.Sg), as a
colourless solid, m.p. 225-230~C; : .
tElemental analysis:- C, 59.8; H, 6.7; Cl, 15.4; ~:-
N, 9.2; S, 7.4% :.
Calculated for C23H2~N3S.2HCl.~H20:- C, 60.1;
H, 6.58; Cl, 15.4; N, 9.15; S, 6.98%]
EXAMPLE 7
Compounds_2A, 2B and 2C
i) Sodium hydride (0.6g, 20mmol of an 80%
dispersion in oil) was added to a stirred suspension of
4,5-diphenylimidazol-2-thiol (4.45g, 17.7mmol) in dry :
tetrahydrofuran (130ml~ at ambient temperature. After
stirring . f or O.5hr, N-(2-bromoethyl~phthalimide (5.08g,
. ~ ~ .. .
20mmol) was added and the solution heated under reflux
for 6hr. The mixture was then poured into water
~500ml) and extracted with ethyl acetate (2 x 200ml). -:
: . .
:.
W091/09030 PCT/EP90/02147
2071~97
- 30 -
The extract was dried (MgS04) and evaForated to a gum
which was crystallised from toluene to give
N-~2-(4,5-diphenyllmidazol-2-yl hio)ethyl~phthalimide
~5.05g), as a very pale cream solid, m.p. 165-166C;
~Elemental analysis:- C, 70.8; H, 4.5; N, 9.8%;
Calculated ~or C25H1gN3O2S:~ C, 70.6; ~, 4.5;
N, 9.9%
N.M.R. (CDC13 & D2O): 3.22 (2H, t, J=5Hz), 3.98
(2H, ~, J=5Hz), 7.23-7.45 (6H, m), 7.54 (2H, d, J-7Hz),
7.62 (2H, d, J=7Hz), 7.73-7.78 (2H, m) 7.86-7.92 ~2~,
m)]. : :
By proceeding in a similar manner, but replacing
the N-(2-bromoethyl)phthalimide by N-(3-bromopropyl)-
phthalimide and N-(4-bromobutyl)phthalimide there were
prepared: :
- ii) N-[3-(4,5-diphenylimidazol-2-ylthio)-
propyl]phthalimide, a colourless solid, m.p. 188-190C;
~ Elemental analysis:- C, 70.7; H, 4.7; N, 9~2%
26 21 3O2S: C, 71.0~; H, 4.8;
N, 9.6% ..
.
N.M.X. (CDC13 & D2OJ: 2.03 (2H, guin, J=7Hz),
: 3.05 (2H, t, J=7Hz), 3.96 (2H, t, J=7Hz), 7.2-7.4 (6H,
m), 7.42-?.62 (4H, m), 7.:7~-(2H, dd, J-6 and 3Hz), 7.82
12H! dd, J=6 and 3HzJ~; and -~
iii) N-~4-(4,~-diphenylimidazol-2-yithio)butyl]-
phthalimide, a colourlsss solid, m.p. 170-171C;
W091/09030 PCT/EP90/02147 ~ ~
2~71~97
- 31 -
[Elemental analysis:- C, 71.7; H, 5.0; N, 9.3;
S, 7.4%
27 23N32S: C, 71.5; H, S.1;
N, 9.3; S, 7.1% i-~
N.M.R. (CDC?3 & D2O): 1.62-1.93 (4R, m~, 3.15
(2~, t, J=6Hz), 3.74 (2H, t, J=6Hz), 7.2-7.39 (6H, mJ,
7.4-7.6 (4H, m), 7.62-7.78 (4H, m~].
EXA~MPLE B
C~ , .:
Diglycolic anhydride (8.34g, 32.3mmol) was added~.
to boiling xylene (500ml) with stirring.
2-(3-Aminopropylthio)-4,5-diphenylimidazole (5.0g, ~:.
16.2mmol) was then added in lg portions at 45 minute
intervals with vigorous stirring to the boiling
mixture, removing any water formed azeotropically.
After the final addition, heating under reflux was
~on~inued for l.Shr and the mixture was allowed to cool
and decanted. The solution was evaporated and the :
residue puxified by flash chromatography on silica gel
~39:1 dichloromethane / methanol as eluent). The .
product was recrystallised from acetonitrile to give
N-~3-(4,5-diphenylimidaæol-2-ylthio)propyl3morpholine-
3,5-dione (3~7g), a~ a colourless solid, m.p. :
16~-166~C;
~ . .. ..
WOgl/09030 PCT/EP90/02147
~: ~d~ -~
- 32 -
~ Elemental analysis :- C, 64.6; H, 5.1; N, 10.2;
S, 8.0%
CalCulated for C22H21N33S:- C~ 64-9; H 5 2;
N, 10.3; S, 7.9~ ;
N.M.R. (CDCl3 & D2O): l.9B (2H, guin, J=7Hz),
3.09 (2H, t, J=7Hz), 4.02 (2H, t, J=7H2), 4.34 (4H, s),
7.2-7.37 (6H, m), 7.45-7.57 (4H, m)].
EXAMPLE 9
m~ounds ?E to 2T
2,4-Dimethylglutari~ anhydride (7.3~g, 51.7mmol)
was added to boiling xylene (500ml) with stirring.
2-(3-Aminopropylthio)-4,5-diphenylimidazole (4.0g,
12.9mmol) was added in lg portions at 1 hour intervals,
with vigoxous stirring, to the boiling mixture removing
any water formed azeotropically. Reflux was continued
$or 2hr and then toluenesulphonic acid monohydrate
(0.4g, 2.1mmol) was added and reflux continued for 8hr.
The mix~ure was allowed to cool and decanted and the
solu~ion was then evaporated. The ref~ulting residue
was purified by flash ~hromatography on silica gel
, .. .. .
.
.. ~ , . . . .. ; . .
. . . ~ , . .
' .
,.~ ~,-, .
WO91/09030 PCT/EP90tO2147
2 ~ 7 1 4 9 ~
- 33 -
(97:3 dichloromethane / methanol as eluent). The
product was recrystallised from acetonitrile to give
3,5-dimethyl-N-[3-(4,5-diphenylimidazol-2-ylthio)-
propyl]piperidine-2,6-dione (3.23g~, as a colourless
solid, m.p. 132-134DC;
~Elemental analysis :- C, 69.0; H, 6.2; N, 9.8;
S, 7.4% :~ :
Calculated for C25~27N325 - C~ 69.3; H~ 6-3;
N, 9.7; S, 7.4%
N.M.R. (CDC13 & D2O): 1.27 ~6H, d, J=7Hz), 1.49
(lH, q, J=12Hz), 1.73-2.04 14~, m), 2.02 (lH, dt, J-4
and 12Hz), 2.53-2.82 (2H, m), 2.93 (2~, t, J=~Hz), 4.13
~2H, t, J=7Hz), 7.22-7.34 (6H, m), 7.48-7.62 (4H, m)].
The N.M.R. spectrum indicated that this was a
9:1 mixture of syn and anti isomers.
By proceeding in a similar manner, but replacing
the 2,4-dimethylglutaric anhydride by the appropriate
anhydride and the 2-(3-aminopropylthio)-4,5-diphenyl-
imidazole by the appropriate amine, there:were
prepared:-
ii) N-~2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
maleimide, a yellow solid, m.p. 132-133C; ` -
. _ . . .
~ Elemental analysis:- C, 67.Q; H, 4.5,-N, li.1%
. Calculated for C21Hl~N3O2S:- C, 67-2; H, 4-53;
N, 11.2%]; .
' . -
WO91/09030 PCT/EP90/02147
` `- 2071497
- 34 -
N.M.R. (CDC13 & D20): 3.13 ~2H, t, J=6Hz), 3.82 ~ :
(2H, t, J=6Hz), 7.2-7.45 ~6H, m), 7.46-7.7 (4H, m)].
iii) N ~3-(4,5-diphenylimidazol-2-ylthio)- .
propyl]-4-methylmaleimide, a buff solid, m.p.
136-138C;
EElemental analysis:- C, 68.2; H, 5.15; N, 10.4;
S, 8.1%
23H21N3O2S: C, 68.5; H, 5.2;
N, 10.4; S, 7.94%];
N.M.R. (CDC13 & D2o~ 94 (2H, quin, J-7Hz),
2.06 (3H, d, J=4Hz), 3.02 (2H, t, J=7Hz), 3.78 (2H, t,
J=7Hz), 6.32 (lH, d, J=4Hz), 7.22-7.38 (6H, m),
7.45-7.56 (4H, m)~;
iv) N-r2-(4,5-diphenylimidazol-2-ylthio)-
e~hyl]pyrrolidine-2,5-dione, a colourless solid, m.p.
189-191C;
[Elemental analysis:- C, 66.8; H, 5.1; N, 11.1;
S, 8.5%
Calculated for C2lH1gN302S:~ C, 66.8; ~, 5.04;
N, 11.14; S, 8.5~
v) . N-~3-(4,5-diphenylimidazol-2-ylthio)- ~.
p~opylJpyrrolidine-2,5-dione, a colourless solid, m.p.
177-179C,~
Elementai-analysis:- C, 67.6; H, 5.4; N, 10.6
Calculated for C22H21N3o2s
, 10.7~];
:
- -:
WO 91/09030 PCI /EP90/02147
~ "
2~7~497
- 35 -
vi) N-[3-(4,5-diphenylimidazol-2-ylthio)propyl]-
piperidine 2,6-dione, a colourless solid, m.p. -
154-155C;
[Elemental anal~sis:-C, 68.2; H, 5.8; ~, 10.4%
C23H23N3O2S: C, 68 .1; H, 5 . 7;
N, 10.36%~;
vii) 4,4-dimethyl-N-[2-(4,5-diphenylimidazol- .
2-ylthio)ethyl]piperidine-2,6-dione, a buff solid, m.p.
86-88C;
[Elemental analysis:- C, 68.2; ~, 6.27; N, 9.6; :
S, 7.5% ~`
24 25N325 C, 68.7; H, 6.0
N, 10.0; S, 7.6%];
viii) 4,4-dimethyl-N-[3-(4,5-diphenylimidazol-
2-ylthio)propyl]piperidine-2,6-dione, a colourless :
solid, m.p. 1~5-157C;
[Elemental analysis:- C, 69.0; H, 6.24; N, 9.6;
S, ~.4% :
Calculate~ for C25H27N32S:- C~ 69-26; H~ 6-2~;
N, 9.7; S, 7.4%];
ix) N-[3-(4,5-diphenylimidazol-2-ylthio)-
propyl~-4-methylpiperidine-2,6-dione, a colourless
solid! m.p. 1~0-162C;
[Elemental analysis:- C, 68.9; H, 6.03; N, 9.9%
Calculated for C2~H25N3O25:- C, 6d.7; H, 6.0;
N, 10.0% ];
. j , , ~ ", ' i ' . . . ; . . , ' ~ ' ' ' ~ '
. . , ' . .
WOgl/09030 PCT/EP90/02147
2071497
- 36 -
x) N-~3-(4,5-diphenylimidazol-2-ylthio)-
propyl]-4-ethyl-4-methylpiperidine-2,6-dione, a buff
solid, m.p. 134-136C;
[Elemental analysis:- C, 69.6; H, 6.5; N, 9.3;
S, 7.3%
Calculated for C26H29N302S:- C, 69.77; H, 6.53;
N, 9.4; S, 7.16%];
xi) N-r3-(4,5-diphenylimidazol-2-ylthio)propyl]-
thiomorpholine-3,5-dione, a cream solid, m.p.
164-16~C.;
[Elemental analysi~:- C,62.3; H, 4.84; N, 9.8;
S, 15.0%
Calculated for C22H2lN3o2s2 :~ C, 62-4; H~ 5.0;
N, 9.9; S, 15.14%~;
xii) 4,4-dimethyl-N-~3-(4,5-di-p-tol~l-
imidazol-2-ylthio)propyl]piperidine-2,6-dione, a cream ~.
solid, m.p. 175-177C;
[Elemental analysis:- C, 69.8; H, 6.65; N, 9.1;
S, 6.95% . .
r C27H3l~3o2s:- C, 7~.25; ~, 6.77; -
N, 9.1; S, 6.95%];
xiii3 No[3-t4,5-di-p-tolylimidazol-2-ylthio)-
propyl]morpholine-3,5-dione, a buff solid, m.p.
148-149C; ~ - '
. ~ . . I .
- ~
. . :.. :.. :.. . ... : . .. , ..... :........ . .. .... .... . . - .
W091/09030 PCT/EPso/02147
.
I 2071497
37 - -
[Elemental analysis:- C, 66.4; H, 5.7; N, 9.5; :;
S, 7.4%; ~ :
~24H25~3035:- C, 66.18; H, 5.8;
N, 9.65; S, 7.36%];
xiv) N-[3-(4,5-di-2-chlorophenylimidazol-
2-ylthio)propyl]-4,4-dimethylpiperidine-2,6-dione;
xv) N-[4-(4,5-diphenylimidazol-2-ylthio)butyl]-
morpholine-3,5-dione, a colourless solid, m.p.
153-155C; ~-:
[Elemental analysis:- C, 65.1; H, 5.45, N, 10.0;
S, 7.5~
Calculated for C~3H23N3035:- C, 65.5; H, 5.5;
N, 9.97; S, 7.61~]; and
xvi) 3,3-dimethyl-N-t3-(4,5-diphenylimidazol- :
2-ylthio)propyl]piperidine-2,6-dione, a colourles~
solid, m.p. 168-170^C; ..
~Elemental analysis:- C, 69.3; H, 603; N, 9.7;
S, 7.~% . -
Calculated for C25H27N32S - C, 69 26; ~ 6 28; ~
N, 9.69; S, 7.496]. ~:
EXAMPLE 10 . .- .......................... ;
ComDounds 3A and 3AA
i) A suspension of 4,5-diphenylimidazole-.2-thiol
15.04g 20mmol) in anhydrous THF (180ml) was treated
with sodium hydride (80% dispersion in oil, 1.2g,. ~-
~ '
~, .. , ,~, , .
wosl/09030 PCT/EP90/02147
.. .
2 0 7 1 4 9 7 38
40mmol) and the mixture stirred at room temperature for30min. A mixture of (~)-3-~hloromethyl-1-methyl-
piperidine hydrochloride (3.68~, 20mm~1) and sodium
hydride (80% dispersion in oil, 0.6g, 20mmo1) in
anhydrous T~F (20ml) was also stirred at room
temperature for 30min. The two mixtures were mixed
together, triethylamine (3.0ml~ was added, and the
whole mixture stirred at reflux overnight. After
cooling to room temperature the reaction mixture was
poured onto iced water (300ml) and extracted with ether
(3x200ml). The ~ombined ethereal extracts were
extracted with 2M hydrochloric acid (3xlOOml). The
acidic solution was basified with 50% sodium hydroxide
solutio~ with ice cooling and the product extracted
into ether (3xlOOml). The combined extracts were dried
(MgS04) and evaporated to give a whi~e solid (2.0g).
Crystallisation from toluene gave [3-R,S]-
3-[~4,5-diphenylimidazol-2-ylthio)methyl~-1-methyl-
piperidine (1.5~), as a white crystalline solid, mp
167-169C;
~Found:-C, 72.6; H~ 6.9; N, 11.5; S, 8.$.%
CalCulated for C22H25N3S - C~ 72-7; H- 6-9;- ¦
N,~11.6;~ S,- 8.8%~
~ N M R (CDC13) 1.92~(3H-j s), 1.40-2.70, (9H, -
m~,-3.20 (2H, m), 7.20-7.50 (lOH, m)
IR (RBr): 696, 765, 1445, 1494, 2926 and ~,
, . .
` . 1 '
WO91/09030 PCT~EPgo/02147
. ~.
. . . ;.
- 39 - 20 71 ~ 9 7 -
3433 cm 1~.
ii~ A solution of [3-R,S]-3-~(4,5-diphenyl-
imidazol-2-ylthio)methyl]-1-methylpiperidine (4.1g,
11.3mmol) in ethanol (30ml) was ~dded with stirring and
ice cooling to a solution of hydrogen chloride in
ethanol [prepared by adding acetyl chloride (40ml)
dropwise to cold (10C) ethanol (180ml) with stirring,
maintainin~ the temperature below 30C]. The mixture
was allowed to warm to room temperature and s~irred ~or
2hr. Ether (200ml) was added and the white precipitate .
was collected by filtration and wash~d thoroughly with
fresh ether to give [3-R,S]-3-[(4,5-diphenyl-
imidaæol-2-ylthio)methyl]-1-methylpiperidine
hydrochloride ~4.6g), as a white powder, mp 175-177C;
[Found:- C, 59.7; H, 6.5; N, 9.0; Cl, 14.3;
S, 6 ~ 8 % - .
Calculated ~or c22H2sN35 l 8Hcl H2O - C~ 59-2;
H, 6.5; N, 9.4; Cl, 14.3; S, 7.2%~. -
~ - EXAMPLE 11
Compounds 3R, 3C and 3D
i) A mixture of 5-iodomethyl-2-pyrrolidinone
(1.83g, 8.1mmol), 4,5-diphenylimidazole-2-thiol (1.83g,
7 . 3mmol ) and anhydrous potassium carbonate ( O . 70g, ~ -
~ . .. . .
5.1mmol) in DMF (35ml) was stirred at room temperature
overnight. The mixture was evaporated to low bulk and
the residue suspended`in watèr (70ml). The product was
.
:~ '
.
WO91/09030 PCT/EPgo/02l47
,. 2071~97 .:
. .
- 40 -
collected b~ ~iltration, dried, and cr~6talli~ed from
ethanol / ether to give rs-R~s~-s-r(4~s-dip~e~vl-
imldazol-2-ylthio~methylJ-2-p~rrolldinone (O.g~g) as a
whi~e crystalline solld, mp 218C;
~Fo~nd:- C, 68.6~ H, 5.5; N, 12.0J S, 9.1%
20H19~30S:- C, 68.7) ~, g,5;
N, 12.0~ S, 9.2~ :
N.M.R. (CDCl3): 1.85 & 2.35 (4H, 2m), 3.00 ~ .
3.27 (2H, 2dd, J=14Hz and 4HzJ, 3.91 ~lH, m), 7.2-7.7
tlO~, m)
I~ (KBr): 69B, 762 and 1680 cm
~ i) By proceeding in the Eame mann~r, but
replacing the S-lodomethyl-2-p~rrolidin~ne by
6-lodo~ethyl-~-piperidinone 3nd puri~ylng t~e crude
prod~ct ~ flash chromatography (10% ethanol in ethyl
acetate) followed by crystallis~tion ~ro~ ethanol /
et~er, there was prepared ~6-R,S]-6-~(4,~-dlphenyl-
~midaziol-2-ylthio)methyl]-2-piperldinone, as a ~hite ~ .
~olid, mp 209-~10C:
[Founds- C, 69.4; H, 5.8~ N, ll.~t S, 8.~.%
- ~iouiated ~or~c2l.
~, 11.6, S, 8.8%
. .. . .... . . ~ . - - ; .. . . . ,. j
N.M.R. (CD3SOCD3)- 1.4-2.0 ~ 2.05-2.10 (6H, 2
3.24 (2H, m), 3.63 (1~, m), 7.20-7.55 (10~, m,)
IR (KBr~: 697, 7~3 and 1634 cm 1~.
.
, . .
WO91/09030 PCT/EPsO/02147
. ~ .
- 2071~97
- 41 -
iii) By proceeding in the same mannex, but
replacing the 5-iodomethyl-2-pyrrolidinone by
4,4-dimethyl-5-iodomethyl-2-piperidinone and purifying
the crude product by flash ~hromatography tethyl
acetate), there was prepared r6-R,S]-4,4-dimethyl-
6-[(4,5-~iphenylimidazol-2~ylthio)methyl]-2-
piperidinone as a white solid, mp 148-150C;
tFound:- c, 70.3; ~, 6.6; N, 10.4.%
Calculated for C23~25N~os - C, 70.6; H, 6.4;
N, 10.7%.
~.M.R. (CD3SOCD3): 0.94 ~ 0.98 (6H, 2s), 1.2-1.8
(2H, m), 1.96 (2H, m), 3.26 (2H, m), 3.73 (lH, m),
7.20-7.58 (lOH, m)
IR (RBr): 695, 763, 1488 and 1636 cm 1].
The iodomethyllactam starting materials were
prepared by the method of S. Knapp et al, -
J. org. Chem., 1988, 53, 4006.
EXAMPLE 12
Com~ounds 3E and 3F - .
. i) A suspension of 4,5-diphenylimidazole-2-thiol
(8g, 31,7mmol) in anhydrous THF (250ml) was-treated
... . , . ~ . i
..with.;sodium hydride 580%:in oil;.0,97g, 32.7mmol) and
.the.~mixture.stirredjat room temperature for 30min`. A
.mixture of (~)-4-~l2-(~-toluenesulPhonYloxy)ethyl]-
l-methylpiperidine t9.4g, 31.6mmol) in anhydrous THF
t20ml) was added and the mixture stirred at room
.' .
WO91/09030 PCT/EP90/02147
.;" 2'b7l~g7
- 42 -
temperature for 18hr, followed by refluxing for 24hr.
After ~ooling to room temperature, the reaction mixture
was poured into i~ed water (500ml) and extracted with
ether (4xlOOml). The combined ethereal extracts were
extracted with hydrochloric acid (2M, 4xlOOml~. The
a~idi~ solution was basified with 50% sodium hydroxide
solution with ice cooling and the product extracted
into ether (3x500ml). The combined extra~ts were dried
(MgS04) and evaporated and the residual solid (5.4g)
was purified by flash chromatography on silica gel (9:1
dichloromethane J methanol as eluent) to give [4-R,S]- ..
4-[2-(4,5-diphenylimidazol-2-ylthio)ethyl]-1-methyl-
piperidine (2.28g), as a colourless solid, m.p.
74-76C;
[Found:- C, 73.3; H, 7.4; N, 10.8; S, 8.6%
Calcula~ed for C23H27N35:- C~ 73 16; H 7 2;
N, 11.3; S, 8.5%J.
ii) By proceeding.-in a similar manner, but ; '
repla~ing the (l)-4-[2-(p-toluenesulphonyloxy)ethyl]-
l~methylpiperidine by (~ 2-(p-toluenesulphonyloxy)-
methyl-1-methylpiperidine and purifying the crude
product by crystallisation from toluene followed by .
conversion to the hydrochloride s'alt~''and':'s'ubséquént .
regeneration:of.-the.~ree base, there was prëpared' .
- 12-R,]-2-[~4,5-diphenylimidazol-2-ylthio~methyl~- :
, - :.: . ~ '
I
. .
WO91/09030 PCT/EP90/02147
-
2 0 71
1-methylpiperidine, as a colourless solid, m.p.
62-64C;
[Found:- C, 71.4; H, 6.9; N, 11.2; S~ 8.2%
Calculated for C2~H25N3S-~H2O:- C, 71-7;
H, 6.98; N, 11.42; S, 8.71%].
EXAMPLE 13
Com~ound lI
By proceeding in a manner similar to that
described in Example 4, but replacing the N-~2-(4,5-
diphenylimidazol-2-ylthio)ethyl~pyrrolidin-2-one, used
as a starting material, by a mixture of cis- and
trans-2,6-dimeth~l-N-~4-(4,5-diphenylimidazol-2-yl-
thio)butan-1-oyl]morpholine, carrying out the reaction
for 4 hours, followed by the addition of water and then
aqueous sodium hydroxide solution for the work-up,
there was prepared a mixture of cis- and trans-2,6-
dimethyl-N-[4-(4,5-diphenylimidazol 2-ylthio)butyl]-
morpholine, m.p. 50-60C (slowly melts). [Elemental
analysis:- C,70.0;~,7.4;N,9.8;S,7.6;H20,1.4%;
calculated for C25H31N35 -5~2 -
C,69.73;H,7.49;N,9.76;S,7.44;H2o,2.09%].: :
~ ; - EXAMPLE 14
Compounds 18A, lCA ~nd lIA - -
By proceeding in a manner similar to that
des~ribed in Example 2, but staxting with:-
i) N-~3-(4,5-diphenylimidazol-2-ylthio)propyl]-
""~ ,"" ~-"~ ,;; "
WO91/09030 PCT/EP90/02147
,
~; 20714~7 44
morpholine;
ii) N-[2-(4,5-diphenylimidazol-2 ylthio)ethyl]-
piperidine; ~nd
iii) a mixturP of cis- and trans-2,6-dimethyl-
N-~4-(4,5-diphenylimidazol-2-ylthio)butyl)-
morpholine;
there were prepared:-
i~ N-~3-(4,5-di~henylimidazol-2-ylthio)pro~yl]-
morpholine dihydrochloride, m.p. 173-175C ~Elemental
analysis:- C,54.7;H,6.02;Cl,14.9;N,9.1;S,6.41;H2O,6.4%;
calculated for C22H25N3Os:2~ .75H20
Cl,15.09;N,8.7;S,6.64;H2O,6.52%~;
ii) N-~2-(4,5-diphenylimidazol-2-ylthio)ethyl~-
piperidine dihydrohloride, m.p. 237-240C [Elemental
analysis:- C,60.06;H,6.4;Cl,16.0;N,9.6;S,7.3%;
calculated for C22H~5N3S:2HCl:- C,60.54;H,6.24;
Cl,16.25;N,9.63;S,7.35%]; and
iii) a mixture of cis- and trans-2,6-dimethyl-
N- r 4-(4,5-diphenylimidazol-2-ylthi~)butyl]morpholine ;
dihydrochloride, m.p. 208-210C [Elemental analysis:-
C,60,6;~,6.9;Cl,13.9;N,8.5;S,6.47i~; cal~ulated for ~ :
C252H31~5N305:2HCl:~ C,60.?2;H,6.73;Cl,14.34;N,8.5; . '
S,6.48%~. . ~ ....... .. . .~ i.:
. ~ ., . . ~ .. . .
... . ., .. - - :~:
. . .; ~ ~;
, ,:
! ~
j~ , ~ , , , , . : , . ~ , . : :
WO91/09030 pcT/Epso/o2l47
,. . '
~ 45 ~ ~ 2~71`-49~7
8~ ::
Com~ounds 4A and 4~
~ y proceeding in a manner similar to that
described in Example 4 but starting with:-
i) N-[3-(~,5~diphenylimidazol-2-ylthio)propyl]-
pyrrolidin-2,5-dione; and
ii) N-t3-(4,5-diphenylimidazol-2-ylthio)propyl]-
morpholin-3,5-dione;
there were prepared:-
i) N-[3-(4,5-diphenylimidazol-2-yl~hio)propyl]-
5-hydroxypyrrolidin-2-one, m.p. 185-187C; and
ii) N-t3-(4,5-diphenylimidazol-2-ylthio)propyl]-
5-hydroxymorpholin-3-one; m.p. 105C (shrinks at 87C).
. . .: . .
.
~:
; , , . ,. , ........... .: -
WO91/09030 PCT/EP90/02147
2071.49.~ 6 -
REFERENCE EXAMPLE 1
i) Sodium hydride (1.4g, 46mmol of an 80~
dispersion in oil) wa~ added to a stirred suspension of
4,5-diphenylimidazol-2-thiol (5.04g, 20mmol) in dry THF
(150ml) at ambient temperature. After stirring for
0.25hr, 3-bromopropylamine hydrobromide (4.38g, 20mmol)
was added. The mixture was then heated under reflux
for lhr then poured into water (lOOOml) and extracted
with ethyl acetate (2 x 500ml). The combined extract
w~s dried (~gSO4) and evaporatPd. The residue was
triturated with ethyl acetate / ether (1:1; 70ml) and
the solid collected to give 2-(3-aminopropylthio)-
4,5-diphenylimidazole (4.8g). An analytical sample was
prepared by recrys~allisation from toluene to give ;
colourless crystals, m.p 162-163C;
[Elemental analysis:- C, 70.1; H, 6.3; N, 13.5%
Calculated for C18H19N3S - C~ 69.9; H~ 6-2;
N, 13.6%
N.M.R (CDCl3 & D2O): 1.88 (2H, quin, J=7Hz~, :
2.93 (2H, t, J-7Hz), 3.2 ~2H, t, J-7~z), 7.2-7.32 (6H,
m), 7.47-7.53 (4H, m)].
ii) By proceeding in a similar manner, but ~ i
replacing the 3-bromopropylamine hyc~obromide by j~
2-bromoethylamine hydrobromide there was prepared
2-(2-aminoethylthio)-4,5-diphenylimidazole, ~ :
m.p.152-154C;
wosl/09030 PCT/~P90/02147
``: 2~1497
- 47 -
~ Elemental analysis:- C, 68.6; ~, 5.8; N, 14~1;
S, 10. 5%
17 17N3S C, 69.1; H, 5.8;
N, 14.2; S, 10.85%
N.M.R. (CDC13 & D2O): 3.1 (4H, br s), 7.16-7.36
(6H, m), 7.42-7.56 (4~, m)3.
REFERENCE EXAMPLE 2
N r 4-(4,5-Diphenylimidazol-2-ylthio)butyl~-
phthalimide (10.2g, 22.5mmol) [prepared as in Example
l(iii)~ was heated under reflux in a mixture o~ ethanol
(25Qml~ and hydrazine hydrate (1.25g, 25mmol) for 22hr.
An aqueous solution of hydrochloric acid (2N; 50ml) was
then added and boiling continued for 0.5hr. The mixtl~re
was then chilled (10C) and the solid filtered and
discarded. The filtrate was evaporated and the residue
dissolved in water (250ml) and filtered. The filtrate
was then basified (~pHlO) with a~ueous sodium hydroxide
solution (2N) and the mixture extracted with ethyl
acetate (3 x 200ml~. The combined extraot was dried
(MgSO4), evaporated and recrystallised from
ace oni~rile to give 2-(4-aminobutylthio)-4,5-diphenyl-
imidazole (4.5g), as colourless ~rystals, m.p.
138-139C;
[Elemèntal analysis :- C,70.7; H,6.6; N,13.0;
Calculated for C1gH21N3S :- C, 70.6; H, 6.5;
N, 13.0%~.
-: ~ ' ' ' , .;. ~ ' ~ '
Wosl/09030 PCT/EP90/02147
:2~7~97
- 48 -
REFEREN OE EXAMPLE 3
By proceeding in a manner similar to that
described in Example 1, but using a mixture of cis- and
trans-N-( 4-chlorobutanoyl) 2,6-dimethylmorpholine as a
starting material, there was prepared a nixture o~ cis- and
trans-2,6-dimethyl-N-t4-(4,5-diphenylimidazol-2-yl-
thio)butan-1-oyl]morpholine, m.p.147-149c.
.. ~ . .. . , ~ .
-: ~. .. ,. . ~
' ~ ' ~ '' , .... . , '. ' ' , ~ . .
WO91/09030 pcT/Epso/o2147
..20~1497
- 49 -
In clinical practice compounds of the pre~ent
invention may be administered parenterally, rectally or
orally.
Solid compositions for oral administration
include compressed tablets, pills, powders and
granules. In such solid compositions, one or more of
the a~tive compounds is, or are, admixed with at least
one inert diluent such as starch, sucrose or lactose.
The compositions may also ~omprise, as is normal
practice, additional substances other than inert
diluents, e.g. lubricating agents, such as magnesium
stearate.
Liquid compositions for oral administration -
include pharmaceutically acceptable emulsions,
solutions, suspensions, syrups and elixirs containing
inert diluents commonly used in the a~t such as water
and liguid paraffin. Besides inert diluents such
composi~ions may comprise adjuvants, such as wettin~
and-suspending agents, and sweetening, flavouring,
perfuming and preserving agents. The compositions
according to the invention ~or oral administration also
~include capsules of absorbable-material such as
gelatin, containing one or more of the active
substances with or without the addition of diluents or
excipients. ~ ~ ~
,
.
1.~
. ' ' ' , ' ' ~ ' , ,: .,
. .
wog1/oso30 PC~/EP90/02147
`2071497
- 50 -
Compositions according to the invention for
parenteral administration include sterile aqueous,
aqueous-organic~ and organic solutions, suspensions and
emulsions. Examples of organic solvents or suspending
media are propylene glycol, polyethylene glyool,
vegetable oils such as olive oil and injectable organic
esters such as ethyl oleate. The compositions may
also contain adjuvants such as stabilising, preserving,
wetting, emulsifying and dispersing agents. They may
be sterilised, ~or example, by filtration through a
bacteria-retaining filter, by incorporation in the -~
compositions o~ sterilising agents, by irradiation or
by heating. They may also be manufactured in the form
of sterile solid compositions, which can be dissolved
in sterile water or some other sterile injectable
medium immediately before use.
Solid compositions ~or rectal administration
include suppositories formulated in accordance with
known methods and containing at least one compo~1nd of
formula (I1.
The percen'age of active ingredient in the
compositions cf the invention may be varied, it being
necessary that it should constitute a proportion such
. . .
that a suitable dosage shall be obtained. Obviously,
several unit dosage ~orms may be administered at about
the same time. The dose employed will be determined
WO91/09030 PCT/EP90!02147
. .
~. ~';;i ~ / .
- 51 - ` 20~1497
by the physician, and depends upon the desired
therapeutic ef~ect, the route of administration and the
duration of the treatment, and the condition of the ¦
patient. In the adult, the doses are generally from
about O S to about 70 preferably about 1 to about 10
mg/kg body weight per day by oral administration.
The following Composition Examples illustrate
pharmaceutical compositions according to the present
invention.
- COMPOSITION EXAMPLE 1
No. 2 size gelatin capsules each containing:-
;~ N-~2-(4,5-diphenylimidazol-2-ylthio)ethyl]-
morpholine 20 mg
lactose 100 mg
~; starch 60 mg
dextrin- 40 mg
magnesium stearate 1 mg
were prepared in accordance with the usual procedure.
WO 91/09030 PCr/EP90/02147
2071497
-- 52 -- -
COMPOSITION EXAMPLE 2
NQ. 2 size gelatin capsules each containing:-
3,5-dimethyl-N-[3-(4,5-diphenylimidazol-2-yl- :
thio)propyl]piperidine-2,6-dione 20 mg
lactose 100 mg
starch 60 mg
dextrin 40 mg
magnesium stearate 1 mg .
were prepared in accordance with the usual procedure.
COMPOSITION EXAMRLE 3 :
No. 2 size gelatin capsules each containing:-
[3-R,SJ-3-[(4,5-diphenylimidazol-2-ylthio)-
methyl]-1-methylpiperidine 20 m~
lactose 100 mg
starch 60 mg
dextrin 40 mg
magnesium stearate .1 mg :
were prepared in accordance with the usual procedure. -
,:
.
'' . ,,.'.~,.. ,"
. ., i ., .
~. :