Note: Descriptions are shown in the official language in which they were submitted.
2~7~66
The present invention relates to the use of 6(7)-amino-
substituted quinoline-5,8-quinones for combating endo-
parasites, new 6(7)-amino-substituted quinoline-5,8-
quinones and processes for their preparation.
6(7)-Amino-substituted quinoline-5,8-quinones are already
known. ~owever, nothinq is known of their use against
endoparasites (compare DE-OS (German Published Specifica-
tion) 2,136,037; JP 58 067,672; and NL 6,505,524).
The present invention relates to:
1.the use of 6(7)-amino-substituted quinoline-5,8-
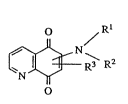
quinones of the general formula (I)
Rl
~ 3\ R2
in which
R1 represents substituted Cl-C6-alkyl or optionally
substituted cycloalkylalkyl, cycloalkyl,
aralkyl or aryl, substituents which may be
mentioned being halogen, nitro, alkyl, alkyl-
amino, aminoacyl, aryl, aryloxy, arylthio,
Le A 28 421 - i -
.. _ . _ ,, , ._, _ _,,, _ _ .. ~ _ . ......
~7~6
aralkyl, halogenoalkyl, alkoxy, halogenoalkoxy,
alkylthio, halogenoalkylthio, alkylsulphinyl,
halogenoalkylsulphinyl, alkylsulphonyl or
halogenoalkylsulphonyl,
R2 represents hydrogen or alkyl, or R1 and R2,
- together with the adjacent N atom, represent a
carbocyclic 5-, 6- or 7-membered ring, which
can optionally also be interrupted by 0, N or
S and is optionally substituted by C,~-alkyl,
R3 represents halogen or -S-R4 and
R4 represents alkyl,
for combating endoparasites in medicine and veterin-
ary medlcine.
The compounds of the formula (I) are known in some cases
and can be prepared by processes analogous to known
processes.
2. New 6(7~-amino-substituted quinoline-5,8-quinones of
the general formula (Ia)
O ~R
~ H \ R~ (Ia~
Le A 28 421 - 2 -
in which
R1 represents cycloalkylalkyl or aralkyl, which
are optionally substituted by halogen, nitro,
alkyl, alkylamino, am:inoacyl, aryl, aryloxy,
S arylthio, aralkyl, halogenoalkyl, alkoxy,
halogenoalkoxy, alkylthio, halogenoalkylthio,
alkylsulphinyl, halogenoalkylsulphinyl, alkyl-
sulphonyl or halogenoalkylsulphony].,
R2 represents hydrogen and
~al represents halogen, preferably chlorine or
bromine, in particular chlorine.
3. Process for the preparation of the new 6(7)-amino-
substituted quinoline-S,B-quinones of the formula
(Ia)
~ \ R2 (I~)
in which
R1, R2 and Hal have the meaning given under Item 2,
characterised in that
Le A 2B 42l - 3 -
.
~Q7 ~
a) compounds of the formula (II~
o
N ~ H~ (II)
in which
Hal has the abovementioned meaning,
are reacted with amino compounds of the ~ormula
(III)
Rl
H N/ (III)
in which
Rl and R2 have the abovementioned meaning,
if appropriate in the presence of metal salts
and if appropriate in the presence of adiluent, or in that
b) compounds of the formula (IV)
Le ~ ?8 421 - 4 ~
2~71~6~
o
OCH3
N ~ H~
in which
Hal has the abovementioned meaning,
are reacted with amino compounds of the formula
(III)
R1 -:
/ ~III)
H N
in which
R~ and R2 have the abovementioned meaning,
if appropriate in the presence of a diluent.
4. New6(7)-amino-su~stitutedquinoline-5,8-quinones of
the formula ~Ib
C ~ (Ib~
Le A 28 421 ~ S -
~ t . _ ~ _ _ , _ _ _ _ _ _ _ _ _ _ _ . ~ _ _, _ _
2~7~56~
in which
R1 represents substituted C1-C6-alkyl or optionally
substituted ~ycloalkylalkyl, cycloalkyl,
aralkyl or aryl, substituents which may be
mentioned being halogen, nitro, alkyl, alkyl-
amino, aminoaryl, aryl, aryloxy, arylthio,
aralkyl, halogencalkyl, alkoxy, halogenoalkoxy,
alkylthio, halogenoalkylthio, alkylsulphinyl,
halogenoalkylsulphinyl, alkylsulphonyl or
halogenoalkylsulphonyl,
R2 represents hydrogen or alkyl, or R1 and R2,
together with the adjacent N atom, represent a
carbocyclic 5-, 6- or 7-membered ring, which
can optionally also be interrupted by O, N or
S and is optionally substituted by C14-alkyl
and
R4 represents alkyl~
5. Process for the preparation of the new 6(7)-amino-
substituted quinoline-5,8-quinones of the formula
(Ib)
o ~Rl
~-R~ R2 (Ib)
Le A 28_421 - 6 -
2~7156~
in which
R1, R2 and R4 have the meaning given under Item 4,
characterised in that
a) the 6(7)-amino-substituted quinoline-5,8-
quinones, obtainable, for example, according to
process 3, of the formula (Ia)
N
o
in which
Rl, R2 and ~al have the meaning given under Item
2 and 4,
are reacted in a first reaction step with
alkali metal sulphides of the formula (V)
M2S ~ V ~
in which
M represents a monovalent alkali metal
cation, preferably potassium or sodium, in
Le A 28 421 - 7 -
2Q7~66
particular sodium,
and in a second reaction step the resulting
alkali metal salt of the formula (VI)
o ~Rl
- ~ R2 (VI)
in which
Rl and R2 have the abovementioned meani~g and
M represents one metal cation equivalent
bonded in salt form,
is then reacted with alkylating agents of the
formula (VII)
R~-E (VII)
in which
R4 has the abovementioned meaning and
E represents an electron-withdrawing leaving
group,
if appropriate in the presence of a diluent, or
in that
Le A 28 421 - 8 -
- :
2~7 1~6~
b) compounds of the formula (Ia)
O Rl
N / (Ia)
N
O
in which
R1, R2 and Hal have the meaning given under Item
2 and 4,
are reacted with thiols or alkali metal salts
thereof, of the formula (VIII)
R4-S Mt (VIII)
in which
M represents hydrogen, or represents one
metal cation equivalent bonded in salt
form and
R4 has the abovementioned meaning,
in the presence of a diluent and if appropriate
in the presence of a reaction auxiliary.
The compounds of the formula (I) are outstandingly
suitable for combating endoparasites, in particular in
Le A 28 421 - 9 ~
2 ~ 6
the field of veterinary medicine.
Formula (I) provides a general definition of the 6(7)-
amino-substitutad quinoline-5,8-quinone derivati~es
according to the invention.
Preferred compounds of the formula (I) axe those in which
Rl represents substituted Cl-C~-alkyl, or represents
optionally substituted C3-C6-cycloalkyl-Cl-C2-alkyl,
C3-C6-cycloalkyl, phenyl-Cl-Cz-alkyl, naphthyl-Cl-C2-
alkyl or phenyl, substituents which may be mentioned
being one or more identical or different radicals
from the group comprising Cl-C4-alkyl, in particular
methyl or ethyl, Cl-C4-alkoxy, in particular methoxy,
ethoxy, methylenedioxy or ethylenedioxy, which are
optionally substituted by fluorine or chlorine,
Cl-C4-halogenoalkoxy, in particular trifluoromethoxy
or fluorochloroethoxy, Cl-C4-alkylthio, in particular
methylthio, Cl-C4-halogenoalkylthio, in particular
trifluoromethylthioorfluorochloromethylthio, C1-C4-
alkylsulphonyl, in particular methylsulphonyl, Cl-C4-
halogenoalkylsulphonyl, in particular trifluoro-
methylsulphonyl, Cl-C~-halogenoalkyl, in particular
trifluoromethyl, halogen, in particular fluorine or
chlorine, NOz, CN, amino, Cl-C4-alkyl- or -dialkyl-
amino, Cl-C4-halogenoalkylamino, acylamino, in
particular acetylamino, and phenyl, phenylthio and
phenoxy, which are optionally substituted by one of
the abovementioned radicals,
Le A 28 421 - 10 -
2~71~
R2 represents hydrogen or C1-C6-alkyl, or R1 and R2,
together with the adjacent N atom, represent a
carbocyclic 5-, 6- or 7-membered ring, which can
optionally also be interrupted by 0, N or S and is
optionally substituted by C1-C4-alkyl, and
R3 represents halogen, preferably chlorine or bromine,
in particular chlorine, or Cl-C4-alkylthio.
Particularly preferred compounds of the formula (I)
O ~RI
~ R~ ~2 (I)
are those in which
R1 has the abovementioned preferred meaning,
R2 represents hydrogen and
R3 represents halogen, such as chlorine, or C1-C4-
alkylthio.
Especially preferred compounds of the formula (I) are
those in which
R1 represents substituted Cl-C6-alkyl, or represents
optionally substituted 1-cyclohexyl-ethyl, cyclo-
_e A 28 421 - 11 -
2~7~ ~6
propyl, cyclopentyl, cyclohexyl, benzyl, l-phenyl-
ethyl, l-naphthyl-ethyl or phenyl, substituents
which may be mentioned beincl hydrogen, halog0n, in
particular chlorine or fluorine, Cl-C4-alkyl, in
particular methyl or ethyl, C~-C4-halogenoalkyl, in
particular trifluoromethyl, C1-C4-alkylthio, in
particular methylthio, Cl-C4-halogenoalkylthio, in
particular trifluoromethylthio, Cl-C4-alkoxy, in
particular methoxy, C1-C4-halogenoalkoxy, in parti-
cular trifluoromethoxy, phenyl and NO2,
R2 represents hydrogen and
R3 represents chlorine or Cl-C4-alkylthio, in particular
methylthio, ethylthio or propylthio.
The following compounds of the formula (I) in which the
radicals R1, R2 and R3 have the following meaning may be
mentioned specifically:
(+)-6(7)-methylthio-7(6)-tl-14-methoxy-phenyl)-ethyl-
amino]-quinoline-5,8 quinone,
(+)-6(7)-methylthio-7(6)-[1-(4-methyl-phenyl)-ethyl-
amino]-quinoline-5,8-quinone,
(+)-6(7)-methylthio-7(6)-[1-(4-chloro-phenyl)-ethyl-
amino]-quinoline-5,8-quinone,
(+)-6(7)-propylthio-7(6)-[1-(2,4-dichloro phenyl)-ethyl-
amino]-quinoline-5,8-quinone,
(+)-6(7)-propylthio-7(6)-[1-(3,4-dichloxo-phenyl)-ethyl-
amino]-quinoline-5,8-quinone,
(+)-6(7)-propylthio-7(6)-[1-(4-fluoro-phenyl)-ethyl-
Le A 28 421 - 12 -
2~7~
amino]-quinoline-5,8-quinone,
R-(+)-7-propylthio-6-(1-phenyl-e~hylamino) q~inoline-5,8-
quinone,
S-(-)-7-propylthio-6-(1-phenyl-ethylamino)-quinoline-5,8-
S quinone,
R-(~)-7-methylthio-6-(1-cyclohexy:L-2thylamino)-quinoline-
5,8-quinone,
S-(-)-7-methylthio-6-(1-cyclohexyl-ethylamino)-quinoline-
5,8-quinone,
7-methylthio-6-cyclopropylamino-quinoline-5,8-quinone,
7-propylthio-6-cyclopentylamino-quinoline-5,8-quinone,
7-ethylthio-6-(4 trifluoromethoxy-phenylamino)-quiRoline-
5,8-quinone,
7-ethylthio-6-(4-trifluoromethylthio-phenylamino)-quino~
line-5,8-quinone,
7-methylthio-6-(4-chloro-3-trifluoromethyl-phenylamino)-
quinoline-5,8-quinone,
7-propylthio-6-(4-chloro-3-trifluoromethyl-phenylamino)-
quinoline-5,8-quinone,
7-methylthio-6-(2-trifluoromethylthio-phenylamino)-
quinoline-5,8-quinone,
7-ethylthio-6-(3-trifluoromethylthio-phenylamino)-quino-
line-5,8-quinone,
7-propylthio-6-(2,6-dichloro-4-trifluoromethylthio-
phenyl-amino)-quinoline-5,8-quinone,
7-methylthio-6-(2-chloro-4-trifluoromethylthio-phenyl-
amino)-quinoline-5,8-quinone,
7-methylthio-6-(3-chloro-4-trifluoromethylthio-phenyl-
amino)-quinoline-5,8-quinone,
7-methylthio-6-thiomorpholino-quinoline-5,8-quinone,
Le A 28 421 - 13 -
- 2~7~6~
7-methylthio-6-[1-(3-trifluoromethyl-4-chloro-phenyl)-
piperaziny1 ]-quinoline-5,8-quinone, and
7-propylthio-6-morpholino-quinoline-5,8-quinone.
The compounds of the formula (I~ are known in some cases,
and they can be prepared by processes a) to b) mentioned
above under Item 3 and 5 (compare, for example,
DE-OS (German Published Specification) 2,136,037;
JP 58,067,672; C.-W. Schellhammer et al., Liebigs Ann.
Chem. 624 ~1959), pages 108-119; O.S. Klimovich et al.,
Zh. Prikl. Khim. 49 (1976), pages 1823-1826; and
CA 86 (1976) 29,288).
If 6,7-dichloro~uinoline-5,8-quinone is employed as the
compound of the formula (II) and (+)-1-(3,4-dichloro-
phenyl)-ethylamine is employed as the compound of the
formula (III) in process 3a for the preparation of the
new 6(7)-amino-substituted quinoline-5,8-quinones and
regio-isomers thereof, the process can be represented by
the following reaction equation:
+ El2~3,~_~CI
~N~Cl + ~CI CH3 Cl
N ~ CH3 O H ~ Cl
o
Le A 28 421 - 14 -
- 2~7~6
Formula (II) provides a general definition of the 6,7-
dihalo-quinoline-5,B-quinones required as starting
substances for carrying out process 3a according to the
invention. In this formula, Hal preferably represents
those halogens which have already been mentioned as
preferred for these substituents in connection with the
dèscription of the substances of the formula (Ia) accord-
ing to the invention.
The compounds of the formula (II) used as starting
materials are known (compare, for example, T. Urbanski et
al., Roczniki Chem. tAnn. Soc. chim. Polonorum] 27
tl953), pages 390-394; CA 49 (1955) 1041; R. Long et al.,
J. Chem. Soc. [London] (1953), page 3919; and
Brit. 856,505), and can be obtained by the processes
described in these references.
The amino compounds of the formula (III) also to be used
as starting substances for carrying out process 3a
according to the invention are defined generally. In this
formula (III), R1 and R2 have the meanings which have
already been mentioned as preferred for these substi-
tuents in connection with the description of the substan-
ces of the formula (Ia) according to the invention. The
amino compounds of the formula (III) are commercially
obtainable in many cases or can be prepared in a manner
which is known per se by the "Leuckart-Wallach reaction"
(compare, for example, Houben-Weyl, Methoden der Organis-
chen Chemie (Methods of Organic Chemistry), Volume XItl,
4th edition 1957, G. Thieme Verlag, Stuttgart, page 648;
Le A 28 421 - 15 -
_ ~ __, . . . . . _ .. . _ . . _
2071~66
M.~. Moore in ~The Leuckart Reaction" in: Organic Reac-
tions, Volume 5, 2nd edition 1952, New York, John Wiley
& Sons, Inc. London: Chapman & Hall, Ltd. ~editor
R. Adams), page 301).
The reaction of the compounds of the formula (II) and
(III) is preferably carried out using diluents. Possible
diluents for carrying out process 3a according to the
invention are all the inert organic solvents.
Examples which may be mentioned are: halogenohydro-
carbons, in particular chlorohydrocarbons, such as
tetrachloroethylene, tetrachloroethane, dichloropropane,
methylene chloride, dichlorobutane, chloroform, carbon
tetrachloride, trichloroethane, trichloroethylene,
pentachloroethane, difluorobenzene, 1,2-dichloroethane,
chlorobenzene, dichlorobenzene, chlorotoluene and tri-
chlorobenzene; alcohols, such as methanol, ethanol,
isopropanol and butanol, ethers, such as ethyl propyl
ether, methyl tert.-butyl ether, n-butyl ethyl ether, di-
n-butyl ether, di-isobutyl etherr diisoamyl ether,
diisopropyl ether, anisole, phenetole, cyclohexyl methyl
ether, diethyl ether, ethylene glycol dimethyl ether,
tetrahydrofuran, dioxane and dichlorodiethyl ether;
nitrohydrocarbons, such as nitromethane, nitroethane,
nitrobenzene, chloronitrobenzene and o-nitrotoluene;
nitriles, such as acetonitrile, butyronitrile, isobutyro-
nitrile, ben~onitrile and m-chlorobenzonitrile;
aliphatic, cycloaliphatic or aromatic hydrocarbons, such
as heptane, hexane, nonane, cymene, benzine fractions
Le A 2~3 421 - 16 -
2~7~5~
-
within a boiling-point ran~e of 70C to 190C, cyclo-
hexane, methylcyclohexane, petroleum ether, ligroin,
octane, benzene, toluene and xylene; esters, such as
ethyl acetate and isobutyl acetate; amides, for example
formamide, N-methylformamide, N,N-dimethylformamide and
N-methylpyrrolidone; and ketones, such as acetone and
methyl ethyl ketone. Mixtures of the solvents and dilu-
ents mentioned are also possible.
Aliphatic alcohols are preferred.
Process 3a is carried out by bringing together and
heating compounds of the formula (II) and an excess of
the compounds of the formula (III) in one of the diluents
mentioned. The reaction time i5 about 2 to 10 hours. The
reaction is carried out at temperatures between +20C and
+200C, preferably between +50C and +150C, particularly
preferably at the boiling point of the diluent. The
reaction is preferably carried out under ~he pressure
which is established when the reactants are heated to the
required reaction temperature under the reaction condi-
tions.
For carrying out the process according to the invention,
in general 1.0 to 4.0 mol, preferably 2.S to 3.5 mol, of
amino compound of the formula (III3 are employed per mol
of 6,7-dihalo-quinoline-5,8-quinone of the formula (II).
When the reaction has ended, the reaction mixture is
cooled to 0C and the solid which has precipitated out is
Le A 28 421 - 17 -
~ Q 7 ~
filtered off, washed and dried. The regio-ismer mixture
obtained can be purified in the customary manner by
recrystallisation or separated into the particular
position isomer products by chromatography (compare also
the preparation examples).
Alternatively, this amination reaction can also be
carried out regio-selectively in the 6-position on an
activated metal complex formed in situ in the presence of
suitable salts of metals of sub-group I, III and VIII of
the periodic table of the elements (compare, for example,
Houben-Weyl, Methoden der Organischen Chemie (Methods of
Organic Chemistry), Volume VII/3a, 4th edition 1988, G.
Thieme Verlag, Stuttgart, page 580; Katsuhira Yoshida et
al., Bull. Chem. Soc. Jpn. 61 (1988), pages 4335-4340;
and JP 58,067,672). Chlorides of nickel and cerium are
particularly preferred here.
If 7-chloro-6-methoxy-quinoline-5,8 quinone is employed
as the compound of the formula (IV) and (_)-1-(4-fluoro-
phenyl)-ethylamine is employed as the compound of the
formula ~III) in process 3b, the process can be described
by the following reaction equation:
O
OCH3 H~N ~ -CH30H
~ N ~ F
Le A 28 421 - 18 -
.
2~7~6
In this ca~e, the compounds of the formula (Ia) are
formed regio-spe~ifically.
The compounds of the formula (IV) in which the radical
Hal has the preferred and particularly preferred meaning
S mentioned in the case of the compounds of the formula
(Ia) are preferably employed in process 3b.
The compounds of the formula (IV) required as starting
substances for carrying out process 3b according to the
invention are known ~compare Hsien-Saw Kuo et al., Tai-
wan Yao Hsueh Tsa Chih 33 (1981), pages 104-110; CA 97
(1982), 23,604; and T.R. Liao et al.~ J. ~eterocyclic
Chem. 13 (1976), pages 1063-1065).
The amines (III) also to be used as starting substances
for carrying out process 3b according to the invention
are defined generally. The amines of the formula (III)
are generally known compounds of organic chemistry.
Process 3b is carried out by bringing together and
heating compounds of the formula (IV) and an excess of
the compounds of the formula (III) in one of the diluents
mentioned. The reaction time is 10 to 40 hours. The
reaction is carried out at temperatures between +20C and
+200C, preferably between +50C and +150C, particularly
preferably at the boiling point of the diluent. The
reaction is carried out under normal pressure.
For carrying out the process according to the invention,
Le A 28 421 - 19 -
2~7~66
in general 1.0 to 4.0 mol, preferably 1.5 to 2.5 mGl, of
amino compound of the formula (III) are employed per mol
of 7-chloro-6-methoxy-quinoline-5,8-quinone of the
formula (IV).
When the reaction has ended, the mixture is cooled and
concentrated in vacuo and the solid which has precipita-
ted out is filtered off, washed and dried (compare also
. the preparation examples).
If 6-[3,5-bis-(trifluoromethyl)phenylamino]-7-chloro-
quinoline-5,8-quinone is employed as ~he compound of the
formula (Ia), disodium sulphide nonahydrate is employed
as the compound of the formula (V) and dimethyl sulphate
is employed as the compound of the formula (VII) in
process 5a, the process can be described by the following
reaction equation.
CF3
N ~ + Na2S-9H2O NaCI
~ ~ ~C~ CH O /
Le A 28 421 - 20 - -~
2Q71566
o ~ ,~
N ~\ ~
N SCH3 CF3
The compounds of the formula (Ia) in which the radicals
R1, RZ and Hal have the preferred and particularly pre-
ferred meanings mentioned in the case of the compounds of
the formula (Ia) are preferably employed in process 5a.
The alkali metal sulphides of the formula (V) alsQ to be
used as starting substances in carrving out process 5a
accordiny to the invention are generally known compounds
of inorganic chemistry.
The alkylating agents of the formula tVII) also to be
used as starting substances for carrying out process 5a
according to the invention are generally known compounds
of organic chemistry. In this formula (VII), R4 has the
meaning which has already been mentioned as preferred for
these substituents in connection with the description of
the substances of the formula (I) according to the
invention, and E has the meaning of an electron-withdraw-
ing leaving group.
Suitable leaving groups ~ are, for example, halogen, such
as chlorine, bromine or iodine, or a radical of the
formula O-SO2-C6H5, O-SO2-C6~4-CH3 or o-So2-oR4, wherein R4
has the abovementioned meaning.
Le A 28 421 - 21 -
~o7~6
The use of C1-Cb-dialkyl sulphates, in particular dimethyl
sulphate or diethyl sulphate, is preferred.
Process 5a is carried out by bringing together compounds
of the formula (Ia) and equimolar amounts of the com-
pounds of the formula (V) at room temperature in one ofthe diluents mentioned. The formation of the alkali metal
salts of the formula (VI) formed in situ takes about 1 to
30 minutesO
In a second reaction step, these salts are alkylated with
compounds of the formula (VII) during a reaction time of
about 10 to 60 minutes and a reaction temperature of
between +20 and +200C, preferably between ~50 and
+150C, particularly preferably at the boiling point of
the diluent. The reaction is carried out under normal
pressure. The reaction products are worked up and iso-
lated by generally customary methods (compare also the
preparation examples).
If a mixture of the regio-isomeric 6-chloro-7-(4-tri-
fluoromethyl-phenylamino)- and 7-chloro-6-~4-trifluoro-
methyl-phenylamino)-quinoline-5,8-quinones is used as the
compound of the formula (Ia) and sodium 1-propanethiolate
is employed as the compound of the formula (VIII) in
procesR Sb, the process can be described by the following
reaction equation:
Le A 28 421 - 22 ~
2~7i ~6~
O H ~ +N~ CH2-cH2-cH3 N
o H O
N ~ CF3 ~ S-CHrCH2-CH3
N S-CH2-CH2-CH3 o ' ~ CF3
Process 5b is carried out by bringing together and if
appropriate heating compounds of the formula (Ia)-and an
excess of the compounds of the formula (VIII) in one of
the diluents mentioned.
The reaction time is 10 to 40 hours. The reaction is
carried out at temperatures between 0C and +200C,
preferably between +20C and +150C, particularly prefer-
ably at room temperature. It is carried out under normal
pressure.
For carrying out the process according to the invention,
in general 1.0 to 3.0 mol, preferably 1.0 to 1.5 mol, of
alkali metal salts of the thiols of the formula (VIII)
are employed per mol of mixture of regio-isomeric 6-
chloro-7-(4-trifluoromethyl-phenylamino)- and 7-chloro-
6-(4-trifluoromethyl-phenylamino)-quinoline-5,8-quinones
of the formula (Ia).
Alternatively, this reaction can also be carried out with
Le A 28 421 - 23
_ . ._ ~,.,. . .. _ _ . .. . . .. ,,~.,.. _ _ _ _ . . _
2~7~66
thiols of the general formula (VIII) in which M repres-
ents hydrogen, in the presence of a basic reaction
auxiliary. Reaction auxiliaries which can be employed as
bases are all the suitable acid-binding agents, such as
amines, in particular tertiary amines, and alkali metal
and alkaline earth metal compounds. Examples of these
which may be mentioned are the hydroxides, oxides and
carbonates of lithium, sodium, potassium, magnesium,
calcium and barium, and furthermore other basic com-
pounds, such as trimethylamine, tribenzylamine, triiso-
propylamine, tributylamine, tribenzylamine, tricyclo-
hexylamine, triamylamine, trihexylamine, N,N-dimethylani-
line, N,N-dimethyltoluidine, N,N-dimethyl-p-aminopyri-
dine, N-methyl-pyrrolidine, N-methyl-piperidine, N-
methyl-imidazole, N-methyl-pyrrole, N-methyl-morpholine,
N-methyl-hexamethyleneimine, pyridine, quinoline, ~-
picoline, ~-picoline, isoquinoline, pyrimidine, acridine,
N,~,N',N'-tetra-methylenediamine, N,N,N',N'-tetraethyl-
enediamine, quinoxaline, N-propyl-diisopropylamine, N,N'-
dimethylcyclohexylamine, 2,6-lutidine, 2,4-luditine,
triethylenediamine, diazabicyclooctane (DABCO), diazabi-
cyclononene (DsN) or diazabicycloundecene (DBU).
Alkali metal compounds, such as, for example, sodium
hydroxide, potassium hydroxide or corresponding
carbonates, are preferably used.
When the reaction has ended, the reaction mixture is
concentrated in vacuo (to about 50~), aqueous acid is
added to the residue and the compounds of the formula
Le A 28 421 - 24 -
. , . _ .. . . . ~ _ . . . , . . ~ . _ _ _
2~71 ~6
(Ib) are isolated in a manner which is known per se by
extracting them with a suitablle solvent, for example
chloroform or methylene chloride. The compounds of the
formula (Ia) can then be purified or separated into the
particular position isomer products in the customary
manner, for example by chromatography.
The active compounds are suitable for combating patho-
genic endoparasites which occur on humans and in animal
keeping and animal husbandry on stock, breeding, zoo,
laboratory and experimental animals and pets, and have a
favourable toxicity to warm-blooded animals. They are
active here against all or individual stages of develop-
ment of the pests and against resistant and normally
sensitive species. Disease, fatalities and reductions in
output (for exanple in the production of meat, milk,
wool, skins, eggs, honey and the like) are to be reduced
by combating the pathogenic endoparasites, so that more
economic and easier keeping of the animals is possible by
usir~g the active compounds. The pathogenic endoparasites
include cestodes, trematodes, nematodes and Acantoce-
phalae, in particular:
From the order of the Pseudophyllidea, for example:
Diphyllobothrium spp., Spirometra spp., Schistocephalus
spp., Ligula spp., Bothridium spp., Diplogonoporus spp..
From the order of the Cyclophyllidea, for example:
Mesocestides sppO, Anoplocephala spp., Paranoplocephala
spp., Moniezia spp., Thysanosomsa spp., Thysaniezia spp.,
Le A 28 421 - 25 -
2~7 ~ ~$
.
A~itellina spp~, Stilea~ia spp., Cittotaenia spp., Andyra
spp., ~ertiella spp., Taenia spp., Echinococcus 9pp.,
~ydatigera spp., Davainea spp., Rilillietina spp., Hymeno-
lepis spp., Echinolepis spp., Echinocotyle spp., Diorchis
spp., Dipylidium spp., Joyeuxie;Lla spp., Diplopylidium
spp..
From the subclass of the Monogenea, for example: Gyro-
dactylus spp., Dactylogyrus spp., Polystome spp..
From the subclass of the Digenea, for example: Diplos-
tomum spp., Posthodiplostomum spp., Schistosoma spp.,
Trichobilharzia spp., Ornithobilharzia spp., Austrobil-
harzia spp., Gigantobilharzia spp., Leucochloridium spp.,
~rachylaima spp., Echinostoma spp., Echinoparyphium spp.,
~chinochasmus spp., Hypoderaeum spp., Fasciola spp.,
Fasciolides spp., Fasciolopsis spp., Cyclocoelum spp.,
Typhlocoelum spp., Paramphistomum spp., Calicophoron
spp., Cotylophoron spp., Gigantocotyle spp., Fischoede-
rius spp. Gastrothylacus spp., Notocotylus spp., Cata-
tropis spp., Plagiorchis spp., Prosthogonimus spp.,
Dicrocoelium spp., Eurytrema spp., Troglotrema spp.,
Paragoniums spp., Collyriclum spp., Nanophyetus spp.,
Opisthorchis spp~, Clonorchis spp., Metorchis spp.,
Heterophyes spp., Metagonimus spp..
From the order of the Enoplida, for example: Trichuris
spp., Capillaria spp., Trichomosoides spp., Trichinella
spp..
Le A 2a 421 - 26 -
2Q713~
.
From the order of the Rhabditia, for example: Micronema
spp., Strongyloides spp..
From the order of the Strongylida, for example: Stronylus
spp., Triodontophorus spp., Oesophagodontus spp., Tricho-
nema spp., Gyalocephalus spp., Cylindropharynx spp.,
Poteriostomum spp., Cyclicocercus spp., Cylicostephanus
spp., Oesophagostomum spp., Chabertia spp., Stephanurus
spp., Ancylostoma spp., Uncinaria spp., Bunostomum spp.,
Globocephalus spp., Syngamus spp., Cyathostoma spp.,
Metastrongylus spp., Dictyocaulus spp., Muellerius spp.,
Protostrongylus spp., Neostrongylus spp., Cystocaulus
spp., Pneumostrongylus spp., Spicocaulus spp., Elapho-
strongylus spp., Parelaphostrongylus spp., Crenosoma
spp., Paracrenosoma spp., Angiostrongylus spp., Aeluro-
lS strongylus spp., Filaroides spp., Parafilaroides spp.,Trichostrongylus spp., Haemonchus spp., Ostertagia spp.,
Marshallagia spp., Cooperia spp., Nematodirus spp.,
~yostrongylus spp., Obeliscoides spp., Amidostomum spp.,
Ollulanus spp..
From the order of the Oxyurida, for example: Oxyuris
spp., Enterobius spp., Passalurus spp., Syphacia spp.,
Aspiculuris spp., Heterakis spp..
From the order of the Spirurida, for example: Gnathostoma
spp., Physaloptera spp., Thelazia spp., Gongylonema spp.,
Habronema spp., Parabronema spp., Draschia spp., Dracun-
culus spp..
~e A 28 421 - 27 -
.. _ . . .. .. . . _ _ . .. _ _ .. . . . ~ .. . _ .. _ _ . _ , , _ , , _, , _
2~L3~6
From the order of the Filariida, for example:
Stephanofilaria 6pp., Parafilaria spp., Setaria spp., Loa
spp., Dirofilaria spp., Litomosoides spp., Brugia spp.,
Wuchereria spp., Onchocerca spp..
From the order of the Gigantorhynchida, for example:
Filicollis spp., Monoliformis spp., Macracanthorhynchus
spp., Prosthenorchis spp..
The stock and breeding animals include mammals, such as,
for example, cattle, horses, sheep, pigs, goats, camels,
water buffalo, donkeys, rabbits, fallow deer, reindeer
and fur-bearing animals, such as, for example, mink,
chinchillas and racoons, birds, such as, for example,
chickens, geese, turkeys and ducks, fresh- and salt-water
fishes, such as, for example, trout and carp, eels,
reptiles and insects, such as, for example, honey bees
and silkworms.
Laboratory and experimental animals include mice, rats,
guinea pigs, golden hamsters, dogs and cats.
Pets include dogs and cats.
Application can be either prophylactically or therapeuti-
cally.
The active compounds are used, directly or in the form of
suitable formulations, enterally, parenterally, dermally,
nasally, by treatment of the environment or with the aid
Le A 28 421 - 28 -
2~7~
.
of shaped articles containing the active compound, such
as, for example, strips, sheets, bands, collars, ear-
marks, limb bands and marking deYices.
Enteral use of the active compounds is effected, for
example, orally in the form of powders, tablets, cap-
sules, pastes, drinks, granules, orally administered
solutions, suspensions and emulsions, boli, medicated
feed or drinking wa'er~ Dermal use is effected, for
example, in the form of dips, sprays or pour-on and spot-
on formulations. Parenteral use is effected, for example,in the form of inject.ion (intramuscular, subcut~neous,
intravenous or intraperitoneal) or by implants.
Suitable formulations are:
solutions, such as injection solutions, oral solutions,
concentrates for oral administration after dilution,
solutions for use on the skin or in body cavities, pour-
on and spot-on formulations and gels;
emulsions and suspensions for oral or dermal use and for
injection; semi-solid formulations;
formulations in which the active compound is processed in
an ointment base or in an oil-in-water or water-in-oil
emulsion base;
solid formulations, such as powders, premixes or concen-
trates, granules, pellets, tablets, boli or capsules;
aerosols and inhalates, and shap~d articles containing
Le A 28 421 - 29 -
2~7~6~
active compound.
Injection solutions are administered intravenously,
intramuscularly and subcutaneously.
Injection solutions are prepared by dissolving the active
S compound in a suitable solvent and adding any additives,
such as solu~ilising ager.ts, acids, bases, buffer salts,
antioxidants and preservatives. The solutions are
subjected to sterile filtration and bottled.
Solvents which may be mentioned are: physiologically
tolerated solvents, such as water, alcohols, such as
ethanol, butanol, benzyl alcohol, glycerol, propylene
glycol, polyethylene glycols and N-methyl-pyrrolidone,
and mixtures thereof.
If appropriate, the active compounds can also be dis-
solved in physiologically tolerated vegetable or syn-
thetic oils which are suitable for injection.
Solubilising agents which may be mentioned are: solvents
which promote solution of the active compound in the main
solvent or prevent its precipitation. Examples are
polyvinylpyrrolidone, polyoxyethylated castor oil and
polyoxyethylated sorbitan esters.
Preservatives are: benzyl alcohol, trichlorobutanol, p-
hydroxybenzoic acid esters and n-butanol.
Le A 28 421 - 30 -
207~
Oral solutions are used directly. Concentrates are used
orally after prior dilution to the use concentration.
Oral solutions and concentrates are prepared as described
above for the injection solutions, in which case sterile
working can be omittedO
Solutions for use on the skin are dripped on, brushed on,
massaged in, sprinkled on or sprayed on. These solutions
are prepared as described above for the injection solu-
tions.
It may be advantageous to add thickeners during the
preparation. Thickeners are: inorganic thickeners, such
as bentonites, colloidal silicic acid and aluminium
monostearate, and organic thickeners, such as cellulose
derivatives, polyvinyl alcohols and copolymers thereof,
acrylates and methacrylates.
Gels are applied to or brushed on the skin or introduced
into body cavities. Gels are prepared by adding thick-
eners io solutions, which have been prepared as described
above for injection solutions, in an amount such that a
clear mass having an ointment-like consistency is formed.
The thickeners mentioned above are employed as the
thickeners.
.
Pour-on formulations are poured or sprayed onto limited
areas of the skin, the active compound penetrating the
skin and having a systemic action.
Le A 28 421 - 31 -
2~711 ~6~
Pour-on formulations are prepared by dissolving, ~uspend-
ing or emulsifying the active compound in suitable
solvents or solvent mixtures tolerated by the skin. Other
auxiliaries, such as dyestuffs, absorption-promoting
substances, antioxidants, light stabilisers and adhes-
ives, are added if appropriate.
. .
Solvents which may be mentioned are: water, alkanols,
glycols, polyethylene glycols, polypropylene glycols and
glycerol, aromatic alcohols, such as benzyl alcohol,
phenylethanol and phenoxyethanol, esters, such as ethyl
acetate, butyl acetate and benzyl benzoate, ethers, such
as alkylene glycol alkyl ethers, such as dipropylene
glycol monomethyl ether and diethylene glycol monobutyl
ether, ketones, such as acetone and methyl ethyl ketone,
aromatic and/or aliphatic hydrocarbons, vegetable or
synthetic oils, dimethylformamide, dimethylacet~mide, N-
methylpyrrolidone and 2,2-dimethyl-4-oxy-methylene-1,3-
dioxolane.
Dyestuffs are all the dyestuffs which are permitted for
use on animals and can be dissolved or suspended.
Absorption-promoting substances are, for example,
dimethylsulphoxide, spreading oils, such as isopropyl
myristate and dipropylene glycol pelargonate, silicone
oils, fatty acid esters, triglycerides and fatty alco-
hols.
Antioxidants are sulphites or metabisulphites, such as
Le A 28 421 - 32 -
2 ~ 7 ~
potassium metabisulphite, ascorbic acid, ~utylhydroxy-
toluene, butylhydroxyanisole and tocopherol.
Light stabilisers are, for example, novantisol acid.
Adhesives are, for example, cellulose derivatives, starch
S derivatives, polyacrylates and naturally occurring
polymers, such as alginates and qelatine.
Emulsions can be used orally, dermally or as injections.
Emulsions are either of the water-in-oil type or of the
oil-in-water type.
They are prepared by dissolving the active compound
either in the hydrophobic or in the hydrophilic phase and
homogenising this ~ith the solvent of the other phase
with the aid of suitable emulsifiers and if appropriate
other auxiliaries, such as dyestuffs, absorption-promot-
ing substances, preservatives, antioxidants, lightstabilisers and viscosity-increasing substances.
Hydrophobic phases (oils) which may be mentioned are:
paraffin oils, silicone oils, naturally occurring veget-
able oils such as sesame oil, almond oil and castor oil,
synthetic triglycerides, such as caprylic/capric acid
biglyceride, a triglyceride mixture with plant fatty
acids of chain length C8l2 or other specifically selected
naturally occurring fatty acids, partial glyceride
mixtures of saturated or unsaturated fatty acids, which
may also contain hydroxyl groups, and mono- and
Le A 2~ 421 - 33 -
2Q7~66
diglycerides of the C8/C10-fatty acids.
Fatty acid esters, such as ethyl stearate, di-n-'outyryl
adipate, hexyl laurate, dipropylene glycol pelargonate,
esters of a branched fatty acid of medium chain length
with saturated fatty alcohols of chain length C16-C1~,
isopropyl myristate, isopropyl palmitate, caprylic/capric
acid esters of saturated fatty alcohols of chain length
C12-C10, isopropyl stearate, oleyl oleate, decyl oleate,
ethyl oleate, e~hyl lactate, waxy fatty acid esters, such
as synthetic duck uropygial gland fat, dibutyl phthalate,
diisopropyl adipate, ester mixtures related to the latter
and the like.
Fatty alcohols, such as isotridecyl alcohol, 2-octyl-
dodecanol, cetyl stearyl alcohol and oleyl alcohol.
Fatty acids, such as, for example, oleic acid and its
mixtures.
Hydrophilic phases which may be mentioned are:
water, alcohols, such as, for example, propylene glycol,
glycerol and sorbitol, and their mixtures.
Emulsifiers which may be mentioned are: non-ionic surfac-
tants, for example polyoxyethylated castor oil, polyoxy-
ethylated sorbitan monooleate, sorbitan monostearate,
glycerol monostearate, polyoxyethyl stearate and alkyl-
phenol polyglycol ethers;
Le A 28 421 - 34 -
_ . . .. . _ _ .. . . . . .. . _ _ . . ~
2~71~66
ampholytic surfactants, such as di-Na N-lauryl-~-iminodi-
propionate or lecithin;
anionic surfactants, such as Na lauryl-sulphate, fatty
alcohol ether-sulphates and mono/dialkyl polyglycol ether
orthophosphoric acid ester monoethanolamine salt; and
cationic surfactants, such as cetyltrimethylammonium
chloride.
Other auxiliaries which may be mentioned are: substances
which increase the viscosity and stabilise the emulsion,
such as carboxymethylcellulose, methylcellulose and other
cellulose and starch derivatives, polyacrylates, algin-
ates, gelatine, gum arabic, polyvinylpyrrolidone, poly-
~inyl alcohol, copolymers of methyl vinyl ether and
maleic anhydride, polyethylene glycols, waxes, colloidal
lS silicic acid or mixtures of the substances mentioned.
Suspensions can be used orally, dermally or as an injec-
tion. They are prepared by suspending the active compound
in a carrier liquid, if appropriate with addition of
other auxiliaries, such as wetting agents, dyestuffs,
absorption-promotingsubstances, preservatives, antioxid-
ants and light stabilisers.
Carrier liquids which may be mentioned are all the
homogeneous solvents and solvent mixtures.
Wetting agents (dispersing agents) which may be mentioned
are the surfactants mentioned above.
Le A 28 421 - 35 -
2~71~66
Other auxiliaries which may be mentioned are those
mentioned above.
Semi-solid formulations can be administered orally or
dermally. They differ fro~ the suspensions and emulsions
described above only by their higher viscosity.
To prepare solid formulations, the active compound is
mixed with suitable carrier substances, if appropriate
with the addition of auxiliaries, and brought into the
desired form.
Carrier substances which may be mentioned are all the
physiologically tolerated solid inert substances. In-
organic and organic substances are used as these. In-
organic substances are, for example, sodium chloride,
carbonates, such as calcium carbonate, bicarbonates,
aluminium oxides, silicic acids, aluminas, precipitated
or colloidal silicon dioxide and phosphates.
Organic substances are, for example, sugars, cellulose,
food- and feedstuffs, such as milk powder, anLmal meals,
cereal meals and crushed cereal, and starches.
Auxiliaries are the preservatives, antioxidants and
dyestuffs which have already been mentioned above.
Other suitable auxiliaries are lubricants and anti-
friction agents, such as, for example, magnesium
Le A 28 _21 - 36 -
.,, _ .. _ , . .
_ _ , . , . _ _ ,_, . . .. , . . . . . _. _ _ _ _ . . .. . ._ .. ...
23189-7353 2 ~ 7 1 ~ 6 ~
stearate, stearic acid, talc and bentonites, disintegra-
tion-promoting substances, such as starch or cro~linked
polyvinylpyrrolidone, binders, such as, for example,
starch, gelatine or linear polyvinylpyrrol~done, and dry
binders, such as mlcrocrystalline cellulose.
The active compounds can also be present in the formula-
tions as a mixture with synergists or with other active
compounds which act against pathogenic endoparasites.
Such active compounds are, for example, L-2,3,5,6-tetra-
hydro-6-phenylimidazothia~ole, benzimidazole carbamate,
pra~iquantel, pyrantel and febantel.
Ready-to-uqe formulations contain the active compound in
concentrations of 10 ppm - 20 per cent by weight, prefer-
ably 0.1 to 10 per cent by weight.
Formulations which are diluted before use contain the
active compound in concentrations of 0.5 to 90 per cent
by weight, preferably 5 to 50 per cent by weight.
In general, it has proved advantageous to administer
amounts of about 1 to about 100 mg of active compound per
kg of body weight per day to achieve effective results.
The invention also extends to commercial packages contain-
ing a 6(7)-amino-substitute quinoline-5,8-quinone of
formula (I),together with instructions for its use in
combating endoparasites.
. .~ ~. .
Le A 28 421 - 37 -
2071366
Exam~le A
In-vivo nematode test
Trichostrongylus colubriformis / ~heep
Sheep experimentally infected with Trichostrongylus
colubriformis were treated after the end of the pre-
patency period of the parasite. The active compounds were
administered orally as pure active compound in gelatine
capsules.
The degree of effectiveness is determined by quanti-
tatively counting the worm eggs excreted with the faeces,
before and after treatment~
Complete cessation of the excretion of eggs after the
treatment means that the worms have been expelled or are
so severely damaged that they can no longer produce any
eggs (effective dose)O
The active compounds tested and effective doses can be
seen from the following table.
Active compound Effective dose in
Example No. mg/kg
9 10
13 10
48 10
49 10
~e A 28 421 - 38 -
_ _ .
2071~66
Example B
In-vivo nematode test
Haemonchus contortus / sheep
Sheep experimentally infected with Raemonchus contortus
were treated after the end of the pre-patency period of
the parasite. The active compounds were administered
orally as pure active compound in gelatine capsules.
The degree of effectiveness is determined by quanti-
tatively counting the worm eggs excreted with the faeces,
before and after treatment.
Complete cessation of the excretion of eggs after the
treatment means that the worms have been expelled or are
so severely damaged that they can no longer produce any
eggs (effective dose).
The active compounds tested and effective doses can be
seen from the following table.
Active compoundEffective dose in
Example No. mg/kg
12 10
17 10
Le A 28 421 - 39 -
._ _ ",, _ .. ..
2~7~6
Preparation Examples
Example_l
H~ ~ H CF3
5.O g (21.9 ~mol) of 6,7-dichloro-quinoline-5,8-quinone
are înitially introduced into 35 ml of absolute ethanol,
and 10.6 g (65.7 mmol) of 4-trifluoromethyl-aniline are
added. The mixture is then stirred at the reflux tempera-
ture until conversion is complete (2.5 hours) and is
~ubsequently cooled to O~C. The solid which has precipi-
tated out is filtered off with suction and dried. 7.7 gof a crude product of a regio isomer mixture are obtained
and are chromatographed over a silica-gel column (silica
gel 60 - Merck, particle size: 0.040-0.063 mm) with the
mobile solvent toluene: ethanol (15:1).
6-Chloro-7-(4-trifluoromethyl-phenYlamino)-~uinoline-5,8-
auinone
Melting point: 238-239C 1.5 g (19.5% of theory)
1H-NMR (CDCl3, 6): 7.16; 7.63 (2d, 4H, aromatic;
J = 8.4 Hz); 7.73 (dd, lH, HB; JHB,HA: 4.8 HZ; JHB,H~:
7.9 Hz3; 7.85 (s, lH, NH); 8.53 (dd, lH, ~; JH~,
Le A 28 421 - 40 -
2~71~6
HB: 7.9 Hz; JH~,H^: 1.7 Hz); 9.01 (dd, lH, HA; JH~,~:
1.7 Hz; JHA,H8: 4.8 Hz) ppm.
Example 2
qulnone
Melting point: 188-190C 2.3 g (29.9~ of theory)
lH-NMR (DMSO-d&; ~): 7.28; 7.65 (2d, 4H, aromatic;
J = 8.4 Hz~; 7.83 (dd, lH, H~; JH~,HA: 4.8 Hz; JHB,HC:
7.9 Hz); 8.39 (dd, lH, ~; JH~,H~: 7.9 Hz; JHF,H^: 1.7 Hz);
9.00 (dd, lH, H~; JHA,HC: 1.7 Hz; JHA,HB: 4.8 Hz); 9.57 (s,
lH, NH) ppm
Example 3
7-Chloro-6-(2~3-dimethyl-phenylamino!-quinoline-s~8
quinone
HC H3C CH3
Hs ~ H
HA N Cl
O
5.0 g (0.022 mol) of 7-chloro-6-methoxy-quinoline-5,8-
quinone are initially introduced into 100 ml of hot
ethanol, and 5.3 g (0.044 mol) of 2,3-dimethyl-aniline
are added dropwise. The mixture is then stirred at the
reflux temperature until the conversion is complete
Le A 28 421 - 41 -
... _ . . . _ , _ _ , . ~ . . " ;, _ _ . _ _ _ _ , . . ..
2 ~ 7 ~
(16 hours) and the entire mixture is concentrated in
vacuo. The residue is rinsed with a little cold ethanol
and recrystallised from ethanol. 3.9 g (S6.7~ of theory)
of 7-chloro-6-(2,3-dimethyl~phenylamuno)-quinoline-5,8-
quinone are obtained.
Melting point: 202-203C
H-NMR (DMSO-d6, ~): 2.20; 2.33 (2s, 3H, CH3); 6.98-7.10
(m, 3H, aromatic); 7.78 (dd, lH, H3; JH3,H~: 4.8 Hæ;
JH ,H : 7.9 ~z); 8.38 (dd, lH, ~c; JHc ~3 7 9 Hz; J~C HA:
1.7 Hz); 8.97 (dd, lH, HA; JHA,HC: 1.7 Hz; JHA HB: 4 8 H )
9.13 (s, lH, NH) ppm
The compounds of the formula (Ia, Hal = Cl) listed in the
following Table 1 can be prepared analogously.
Le A 28 421 - 42 -
2071~66
Table 1
Examples of the compounds of the formula (Ia)
~ Cl (Ia)
Example Position ~Position-) Physi
No.of Cl NRl data
RZ ._
G~
4 6 (7~
m . p : 192C
7 (6-) NH~
6 (7-) NH~ C~I3
m .p . :183C
7 (6-) NH~ CH3 .
CH3
6 7 (6-) NH~ m.p.: 19~195C
- CH3 F
7 7 (~ ) NH~ m .p .: 197-199C
~3 /
8 7 (6-) NH~ m.p.: 188-189C
Cl
9 7 (6-) NH~ m.p.: 111-114C
Le A ~8 421 - 43 -
,~ ~
2Q71~
Table 1 (Continuation)
Example Position ( Position- ) P~5i~
No. of Cl Rl data
N
____ _ R
r-~
6 (7-)~nH~
C1 m . p .: 188C
7 (~ ) ~nH~
I 1 7 (6-) ~nH~ Cl m. p .: 217-218C
12 6 (7^) ~nH~ Cl
m . p .: 20BC
7 (6-) ~nH~ Cl
13 7 (6-)~nH~ Cl m.p .: 213-216C
Cl
14 7 (6-) ~nH~ Cl m. p .: 246-248C
7 (6-) ~nH~ m.p.: >250C
/=\ o
16 7 (6-~ ~nH~Cl m.p.: æ3-224 C
CF3
17 (6-) Nn~ F3
m . p .: 20QC
7 (6-) ~n~ C~F3
Le A 28 421 - 44 -
-
2Q71~66
Table 1 (Con~inuation)
_ _ _ _
Example Position ~Position-) Phys;~l
No. of Cl Rl data
S N R _
CF3
18 7 (6-) NnH~ m.p.: 208-209C
C~F3
19 7 (6-) NH~ F m.p: 22CL221C
C~H3O
7 (6-)NHb m-p:l75-178C
21 6 (7_) NnH~OCH3
m . p .: 203C
7 (6-) ~nH~ OCH3
22 7 (6-) NnH~OCF3 m.p.:188-190C
23 7 (6-) NnH~ SCF3 m.p.: 192-193C
24 7 (6-) NnH~No2 m.p.: >250C
6 (i~7-) NH
CH3
~ m.p :133C
7 (i)~6-) NH~ J
Le A 28 421 - 45 -
~ .
2~7~
Table 1 (Continuation)
Example Position (Position-) Phy5;~
~o. of Cl ~ Rl data
N
R2
_ . _
~6 6 (+)~7-)NH~
CH3
7 (iH6-) N~ p.:159C
CH3
CH3
27 6 (+) (7-) NH~
CH3
CH3 ~p.:110C
7 (:t)(~) NH
CH3
28 6 (+)-(7~
CH3 CH3
~ nLp.:118C
7 (+) (~ Q J
CH3 CH3
29 6 (+~ (7 ~ NH ~ CH3
CH3
7 ( )( ) Cll/ -CH3 ¦IILp:l3soc
Le A 28 4~1 - 46 -
2~71~6~
Table 1 (Continuation)
.
Example Position (Position-) Physi
No. of Cl N = R1 data
R
6 (+)-(7-) NH ~ C2Hs
CH3 ~p:98C
7 (+)-(6-) NH ~ C2H5
CH3
Cl
31 6 (~{7-) NH
CH3
Cl ,
~ ~p.:165C
7 (i)~6-) N~
CH3
32 6 (+~7-)NH
CH3
Cl~nLp.:118C
7 (+)-(6-)NH ~
CH3
33 6 (i)~7-) N~ ~ Cl
CH3
~p.:172C
NH ~ Cl
Le A 28 421 - 47 -
2~7~66
Table 1 ~Continuation)
Example Position(Position-) Physical
No.of Cl N = R1 data
_ __ R
34 6 (~)-(7-) NH ~ Cl
CH3
Cl l IlLp:128C
7 (-+)~) NH ~ Cl J
CH3
3~; 6 (+)-~7-) --~ Cl
CH3 Cl
m.p.: 152C
7 (+)-(6-) NH~CI
CH3 Cl
F
36 6 (+) (7 ~ NH ~b - - -
CH3
F ¦ 1~Lp :155C
7 (i:)-(6-) NH~b J
CH3
37 6 (i)~7-) ~ F
CH3 ~P 1380C
~ F J
7 (+)~) ~
~H3
Le A 28 421 - 48 -
2~71~6~
Table 1 ( Continuation )
Example Po~:ition( Po~3ition- ) Plysi~
No. of Cl _Rl data
N----
_ --RZ
38 6 (~ 7~) ~
CH3 OCH3
F\ m~p :l25C
7(+)(O NH~
H3 OCH3
(~) NH {~ m.p. :12~125C
453 7 (6-) NH--(CH2)2~ m.p. :129-130C
41 7 (6-) NH-(CH2)2-CF3 Fp.: 160-162C
42 7 (6-) NH-CH(CH3)2 Fp.: 109-110C
43 7 (6-) NH-CH(CH3)-CF3 Fp.: 180-181C
44 7 (6-) NH-CH(CH3)-C2H5 Fp.: 77-78C
7 (6-) NH-CH2-CH(CH3)2 Fp.: 102-103C
46 7 (6-) NH-C(CH3)3 Fp.: 130-131C
Le A 28 421 - 49 ~
_. , _ , . _ . _ _ ,, , - , _ .. . ~ _ _, _ _ .. .. . . _ _
2~7~ 76~
Example 47
6- r 3~5-Bisttrifluoromethyllphenylamino~-7-methylthio-
quinoline-5,8-quinone
~ CF3
H ~ N ~
~A N SCH3 CF3
o
4.6 g (10.9 mmol) of 6-[3,5-bis~trifluoromethyl)phenyl-
amino]-7-chloro-quinoline-5,8-quinone are initially
introduced into 40 ml of ~thanol, and 2.6 g (10.9 mmol~
of disodium sulphide nonahydrate (dissolved in 40 ml of
water) are added at room temperature. During this opera-
tion, the previously red solution becomes blue-blac~ in
colour. The mixture is subsequently stirred at room
temperature for 5 minutes, 1.0 g 110.9 mmol) of dimethyl
sulphate is added dropwise and the mixture is heated
under reflux for a further 15 minutes. After the mixture
has been stirred at room temperature for one hour, the
entire mixture is concentrated in vacuo, the residue is
stirred with water and the solid is separated off and
recrystallised from ethanol. 1.0 g (21.2% of theory) of
6-r3,5-bis(trifluoromethyl)phenylamino]-7-methylthio-
quinoline-5,8-quinone is obtained.
Melting point: 135-137C.
~-NMR (CDCl3, ~: 2.28 (s, 3H, -SCH3); 7.39 (s, 2H,
Le A 28 421 - 50 -
2~ 6
.
aromatic); 7.S3 (s, l~, aromatic); 7.66 (dd, 1~, H8;
JE~A,JH~: 4.8 I~Z; J~,HC: 7.9 ~z); 7.85 (s, lH, NE~); 8.43
(dd, 1~, ~c; JH~, H~: 7.9 ~z; J~,H~: 1.7 Hz); 9.03 (dd,
lH, ~; J~A,~: 1 7 ~z; J~A ~B: 4 8
Example 48
~ rC~3
10 g (28.3 mmol) of a mixture of 6-chloro-7-(4-trifluoro-
methyl-phenylamino)- and 7-chloro-6-(4-trifluoromethyl-
phenylamino)-quinoline-5,8-quinone are initially intro-
duced into 100 ml of absolute tetrahydrofuran, and 3.4 g
(34.6 mmol) of sodium l-propanethiolate are added in
portions. The mixture is stirred at room temperature
until conversion is complete (15 to 20 hours). The entire
reaction mixture is then concentrated in vacuo, the
syrupy residue is taken up in 200 ml of chloroform, the
chloroform mixture is extracted by shaking with 200 ml of
0.5 N hydrochloric acid and the extract is washed with
water. The organic phase is dried o~er sodium sulphate
and the solvent is then distilled off. The crude product
of the regio-isomer mixture which remains can be chroma-
tographed over a silica-gel column (silica gel 60 Merck,
particle size: 0.040-0.063 mm) using the mobile solvent
toluene:ethanol (10:1)~
Le A 2~ 421 - 51 -
~ ~ 7 ~
-
6~ propylthio)~7-(4-trifluoromethyl-phenylamino)
quinoline-5,8-quinone
Melting point: 175-176C 1.8 g (16.2% of theory)
H, NM~ ( DMSO-d6, ~ ): 0.73 ( t, 3H, -CH3; J = 7.3 Hz ); 1.31
(m, 2H, -CH2-; J = 7.3 Hz~; 2.56 (t, 2H, -SCH2-; J
7.3 Hz); 7.20; 7.62 (d, 4H; aromatic J = 8.4 Hz); 7.82
(dd, lH, HB; JH~,HA: 4.8 Hz; JHB, H~: 7.9 Hz); 8.36 (dd,
lH, HC; JHC, H~: 7.9 Hz; JHC, HA: 1.7 Hz); 8.96 (dd, lH,
HA; JHA,HC: 1.7 Hz; JHA,H~: 4.8 Hz); 9.49 (s, lH, NH) ppm.
~xample 49
7 - ( 1-Propylthio)-7-(4-trifluorome~h~l-phenylamino)-
quinoline-5,8-quinone
Melting point: 85-86C 1.65 g (14.796 o f theory)
lH--NMR (DMSO-d6, ~): 0.74 (t, 3H, --CH3; J = 7.3 Hz); 1.32
(m, 2H, -CH2-; J = 7.3 Hz); 2.57 (t, 2H, -SCH2-; J =
7.3 Hz); 7.19; 7.61 (2d, 4H; aromatic J = 8.4 Hz); 7.79
(dd, lH, H~; JHa,HA: 4.8 Hz; JH3,HC: 7.9 Hz); 8.38 (dd, lH,
HC; JHC,H~: 7.9 Hz; JHC,HA: 1.7 Hz); 8.98 (dd, lH, HA;JHA,
HC: 1.7 Hz; JHA,H~: 4.8 Hz); 9.39 (s, lH, NH) ppm.
The compounds of the formula (Ib) listed in the following
Table 2 can be prepared analogously.
L~ A 28 421 - 52 -
~7~66
Table 2
Examples of the compounds of the formula (Ib)
e~ N'R (Ib~
_
Example (Position-) (Position-) P~ysical
No. S-R4 N--n2 data
CH3
(~)S-CH3 (7-)NH~
c~ J m P 1180C
(7-)S-CH3 (6-)
CH3
51 (~) S-C3H7 (7~) NH--b m p.: 94-95C
CH3
52 (7-) S-C3H~ (6-) NH~ m.p.: oil
53 (~) S-CH3 (7-)NH~ Cl m. p .: 208-210C
54 (7-) S-CH3 (6-)NH~ C1 m . p .: 122-124C
(6-) S-C3H7 (7-) N~I~ Cl m . p .: 202-203C
Le A 28 42l - ~3 -
~Q7~66
Table 2 (Continuation~
Example (Position-) (Position-) Pl~
No . S-R4 N--R1 data
~2 ._
56 (7-) S~3H7 (6-) NH~CI m.p. :117-119C
57 (7-) S CH3 (5-) NH~CI m . p -: 86-87C
58 (7-)S-C3H1 (6-)NH~CI m.p. :83-84C
59 (7-)S{~H3 (6-) NH~ m.p. :18~185C
Cl
(7-) S~c2Hs (6-) NH~ m.p.: 132-133C
Cl
61 (~) 5-C~H3 (7-) NH~ m.p. 202-203C
Cl
62 (7-)S~3H7 (6-)NH~CI m.p. :113-114C
Le A 28 421 - 54 -
- 2~156~
Table 2 ( Continuation )
Example (Position-) (Position-) Physi~al
No. S-R4 R1 data
N--
~ R2
Cl
-
63 (6-) S-CH3 (7-) NH~
Cl ~ m.p. :152C
(7-) S-CH3 (6-) NH~ J
64
(6-)S-CH3 (7-)NH~ F m.p.: 158-160C
(7-) S-CH3 (6-)NH~ F m.p. :102-103C
66
(7-) S-C3H7 (6-)NH~ F m . p .: 95- 96C
67
(6-)S-CH3 (7-)NH~CF3 m.p.: 141-143C
68
(7-) S-CH3 (6-)NH~ CF3 m . p .: i 80-181C
69 (~) S-CH3 (7-)NH~CF3 m.p .: 190-192C
CF3
(7-) S-CH3 (6-) NH~ m.p .: 165-167C
Le A 28 421 - 55 -
2~71566
Table 2 ( Continuation ~
Exa}nple (Position-) (Po~ition-) Ply~i~al
No~ S-R4 iRl data
N
--R2
CF3
71 (6-)S-C3H7 (7-) NH~ m.p.: 126-128C
72 (7-) S-C3H7 (6-) NH~CF3 m . p .: 87- 88C
73 (~) S~H3 (7-) NH~ O~F3 m.p.: 15~155C
74 (7-)S-CH3 (6-) NH~oCF3 m.p.: 112-113C
(6-)S-C3H~ (7) NH~oCF3 m.p.: 154-155C
76 (7-)S-C3H7 (6-) NH~OCF3 m.p.: 108-109C
77 (~) S-CH3 (7-) NH~ SCF3
m . p . :143C
(7-) S-CH3 (6-) NH~ SCF3 J
Le A 28 421 - 56 -
2~7~6
Table 2 (Continuation3
. . _ _
Example (Po~ition-) (Position-) Physi
No. S-R4 -Rl data
N
~2
_
78 (6-)S-C3H7 (7~) NH ~ SCF3
~ m.p.:103C
(7-)S-C3H7 (6-) NH ~ SCF3 J
QCH3
79 (7-)S-CH3 (6-) ~ ~ m.p.: 11~115C
OCH3
(7-)S-C3H7 (6-~ ~ ~ m.p : 92C
81 (7-)S-~H3 (6-) ~ ~ ~.p.: oil
82 (6-)S-cH3 (7~) ~ ~ m.p.:105-106C
83 (~)S~C3H7 (7) ~ ~ m.p.:l45-146C
Le A 28 421 - 57 -
- 2~7~6
.
Table 2 (Continuation)
Example (Position-) (Position-) Physi~
No. S-R4 ____-Rl data
N
_ - -R
84 (6^)S-CH3 (7-) N~ 2 ~
~ m.P-:114C
(7-)S-CH3 (6-) NH-CH2 ~
(6-)S-C3H7 (7-) NH-CH2 ~ m.p.:125-127C
86 (7-)S-CH3 ~6-)NH-(cH2)2 ~ m.p.: 91-93C
87 (6-)S-C3H7 (+)-(7-)NH ~ CH3
CH3
m.p.: oil
(7-)S-C3H7 (+)-(6-) NH ~ CH3
CH3
88 (6-)S-C3H7 (+)-(7-)NH ~
CH3 m.p.: oil
(7-)S-C3H7 ~-(6-) NH
CH3F
Le A 28 421 - 58 -
2o7l5~6
Table 2 (Continuation)
Example (Position-3 (Position-) Physi~l
No. S-R~ N = R1 data
89 (7-) S-CH3 (6-) NH-(CH2)2-CF3 mOp~ 142-144C
(7-) S-CH3 (6-) NH-CH(C~I3)2 m.p.: 103-104C
91 (7-) S-CH3 (6-)NH-CH(CH3)-CF3 m.p. 102-103C
92 (7-) S-CH3 (6-) NH-CH(CH3)-C2H5 m.p. ol
93 (7-) S-CH3 (6-) NH-cH2-cH(cH3)2 m.p. 81-83C
94 (7-) S-CH3 (6-) NH-C(CH3)3 ` m.p. 66-68C
Le A 28 421 - 59 -
~715~
Examples of the preparation of the starting substances
Example ~IV-l~
7-Chloro-6-methoxy-quinoline-5,8-quinone
HC O
H~ ~OCH3
2.0 g (0.029 mol) of potassium methanolate are added in
portions to a suspension, cooled to 0 to -5C, of 6.6 g
(0.029 mol) of 6,7-dichloro-quinoline-5,8-quinone in 200 ml
of methanol. During this operation, a slight evolution of
heat occurs. The mixture is then subsequently stirred at
room temperature for a further 2 hours and the entire
mixture is concentrated in vacuo. The residue which
remains is washed with water and recrystallised from a
little methanol. 4.5 g (69.4% of theory) of 7-chloro-6-
methoxy-quinoline-5,8-quinone are obtained.
Melting point: 175-177C
lH-NMR (DMSO-d6, ~): 4.24 (s, 3H, -OCH3); 7.85 (dd, lH,
HB; JE~B,E[A: 4.8 Hz; JH~,HC: 7.9 HZ); 8.38 (dd, lH, HC;
JHC,HB: 7.9 Hz, JE~C,HA: 1.7 HZ); 9.00 (dd, lH, ~IA; JHA,HC:
1.7 HZ; JHA,HB 4.8 Hz) ppm.
Le A 28 421 - 60 -
,
. .